In Vitro Shoot Cultures of Micromeria graeca: Micropropagation and Evaluation of Methanolic Extracts for Anticancer and Antimicrobial Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Micropropagation
2.1.1. Shoot Culture Establishment
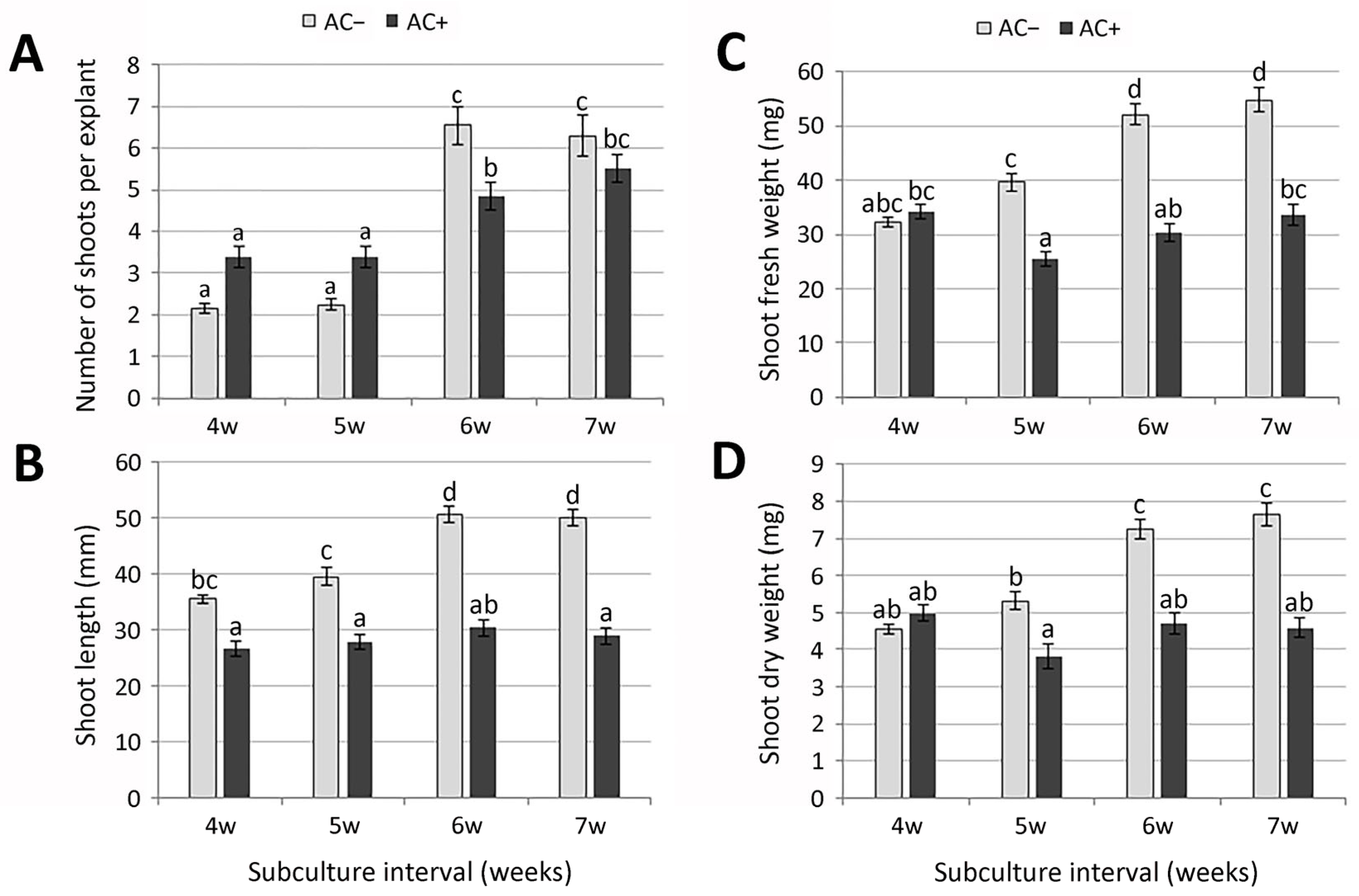
2.1.2. Shoot Multiplication
2.1.3. Rooting and Acclimatization
2.2. Determination of Phenolic Acids by HPLC
2.3. Antimicrobial Activity
2.4. Anti-Hepatoma Activity
2.4.1. Mg Extracts and RA Exert Anti-Hepatoma Effect Against HepG2 Cells
2.4.2. Mg Extracts Induce Oxidative Stress and Apoptosis, RA Inhibits the Proliferation of HepG2 Cells
3. Materials and Methods
3.1. Micropropagation of Micromeria graeca
3.1.1. Plant Material, Shoot Culture Establishment and Maintenance
3.1.2. Shoot Multiplication and Biomass Production
3.1.3. Acclimatization
3.2. HPLC Analysis
3.2.1. Preparation of Methanolic Extracts
3.2.2. HPLC Conditions
3.3. Antimicrobial Activity
3.3.1. Microbial Strains
3.3.2. Micro-Well Dilution Assay
3.4. Cytotoxic Activity of M. graeca Methanolic Extracts Against HepG2 Cell Line
3.4.1. Cell Line
3.4.2. Treatments
3.4.3. Cell Viability Assays
3.4.4. Flow Cytometric Analyses
Apoptosis/Necrosis Assessment
3.4.5. Intracellular ROS Production
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPLC | High-performance liquid chromatography |
| HepG2 | Human hepatocellular carcinoma cell line |
| RA | Rosmarinic acid |
| MS | Murashige and Skoog medium |
| AC | Activated charcoal |
| AC− | MS medium without AC |
| AC+ | MS medium with 0.1% AC |
| PGR | Plant growth regulator |
| MIC | Minimum inhibitory concentration |
| MMC | Minimum microbicidal concentration |
| Mg | Methanolic extracts from 4-week-old M. graeca shoots |
| Mg− | Methanolic extract from M. graeca shoots cultured on AC− medium for four weeks |
| Mg+ | Methanolic extract from M. graeca shoots cultured on AC+ medium for four weeks |
| DMSO | Dimethyl sulfoxide |
| CV | Crystal violet |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| Ann | Annexin-V |
| PI | Propidium iodide |
| DHR | Dihydrorhodamine 123 |
| ROS | Reactive oxygen species |
| ATCC | American Type Culture Collection |
References
- Bräuchler, C.; Ryding, O.; Heubl, G. The genus Micromeria (Lamiaceae), a synoptical update. Willdenowia 2008, 38, 363–410. [Google Scholar] [CrossRef]
- Bräuchler, C.; Meimberg, H.; Heubl, G. Molecular phylogeny of Menthinae (Lamiaceae, Nepetoideae, Mentheae)—Taxonomy, biogeography and conflicts. Mol. Phylogenet. Evol. 2010, 55, 501–523. [Google Scholar] [CrossRef]
- Kremer, D.; Dunkić, V.; Radosavljević, I.; Bogunić, F.; Ivanova, D.; Ballian, D.; Stešević, D.; Matevski, V.; Ranđelović, V.; Eleftheriadou, E.; et al. Phytochemicals and Their Correlation with Molecular Data in Micromeria and Clinopodium (Lamiaceae) Taxa. Plants 2022, 11, 3407. [Google Scholar] [CrossRef]
- Puppo, P.; Paz, P.L.P.D.; Curto, M.; Meimberg, H. Micromeria tenensis (Lamiaceae), a new species from Tenerife, Canary Islands. Phytotaxa 2023, 626, 1–7. [Google Scholar] [CrossRef]
- Harley, R.M.; Atkins, S.; Budantsev, A.; Cantino, P.D.; Conn, B.J.; Grayer, R.; Harley, M.M.; De Kok, R.; Krestovskaja, T.; Morales, R.; et al. Labiatae. In The Families and Genera of Vascular Plants, 1st ed.; Kubitzki, K., Ed.; Springer: Berlin, Germany, 2004; Volume 7, pp. 167–275. [Google Scholar]
- Chater, A.O.; Guinea, E. Micromeria. In Flora Europaea; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1972; Volume 3, pp. 167–170. [Google Scholar]
- Davis, P.H. Micromeria Bentham. In Flora of Turkey and the East Aegean Islands; Edinburgh University Press: Edinburgh, UK, 1982; Volume 7, pp. 335–346. [Google Scholar]
- Güzel, Y.; Güzelşemme, M.; Miski, M. Ethnobotany of medicinal plants used in Antakya: A multicultural district in Hatay Province of Turkey. J. Ethnopharmacol. 2015, 174, 118–152. [Google Scholar] [CrossRef] [PubMed]
- Malamas, M.; Marselos, M. The tradition of medicinal plants in Zagori, Epirus (northwestern Greece). J. Ethnopharmacol. 1992, 37, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, P.M.; Salerno, G.; Caneva, G. Folk phytotherapeutical plants from Maratea area (Basilicata, Italy). J. Ethnopharmacol. 2005, 99, 367–378. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Licata, M.; Leto, C.; Gargano, M.L.; Venturella, G.; La Bella, S. Plant genetic resources and traditional knowledge on medicinal use of wild shrub and herbaceous plant species in the Etna Regional Park (Eastern Sicily, Italy). J. Ethnopharmacol. 2014, 155, 1362–1381. [Google Scholar] [CrossRef] [PubMed]
- Akdad, M.; Eddouks, M. Cardiovascular Effects of Micromeria graeca (L.) Benth. ex Rchb in Normotensive and Hypertensive Rats. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1253–1261. [Google Scholar] [CrossRef]
- Akdad, M.; Azzane, A.; El Ouady, F.; Moujani, A.; El Khallouki, F.; Eddouks, M. Antihyperglycemic Activity of Micromeria graeca Aqueous Extract in Streptozotocin-Induced Diabetic Rats. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 887–894. [Google Scholar] [CrossRef]
- Viegi, L.; Pieroni, A.; Guarrera, P.M.; Vangelisti, R. A review of plants used in folk veterinary medicine in Italy as basis for a databank. J. Ethnopharmacol. 2003, 89, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Brahmi, F.; Guendouze, N.; Hauchard, D.; Okusa, P.; Kamagaju, L.; Madani, K.; Duez, P. Phenolic profile and biological activities of Micromeria graeca (L.) Benth. ex Rchb. Int. J. Food Prop. 2017, 20, 2070–2083. [Google Scholar] [CrossRef]
- Benali, T.; Habbadi, K.; Bouyahya, A.; Khabbach, A.; Marmouzi, I.; Aanniz, T.; Chtibi, H.; Mrabti, H.N.; Achbani, E.H.; Hammani, K. Phytochemical Analysis and Study of Antioxidant, Anticandidal, and Antibacterial Activities of Teucrium polium subsp. polium and Micromeria graeca (Lamiaceae) Essential Oils from Northern Morocco. Evid. Based Complement. Altern. Med. 2021, 2021, 6641720. [Google Scholar] [CrossRef]
- El Kamari, F.; El Omari, H.; El-Mouhdi, K.; Chlouchi, A.; Harmouzi, A.; Lhilali, J.; El Amrani, J.; Zahouani, C.; Hajji, Z.; Ousaaid, D. Effects of Different Solvents on the Total Phenol Content, Total Flavonoid Content, Antioxidant, and Antifungal Activities of Micromeria graeca L. from Middle Atlas of Morocco. Biochem. Res. Int. 2024, 2024, 9027997. [Google Scholar] [CrossRef]
- Laghmari, M.; Touhtouh, J.; Aanniz, T.; Zengin, G.; Bouyahya, A.; Ullah, R.; Alotaibi, A.; Akhazzane, M.; Benali, T.; Hammani, K. Micromeria graeca L., essential oils: In vitro and in silico evaluation. Biochem. Syst. Ecol. 2025, 123, 105071. [Google Scholar] [CrossRef]
- Schippmann, U.; Leaman, D.J.; Cunningham, A.B. Impact of Cultivation and Gathering of Medicinal Plants on Biodiversity: Global Trends and Issues. In FAO. Biodiversity and the Ecosystem Approach in Agriculture, Forestry and Fisheries, Proceedings of the Satellite event on the occasion of the Ninth Regular Session of the Commission on Genetic Resources for Food and Agriculture, Rome, Italy, 12–13 October 2002; Inter-Departmental Working Group on Biological Diversity for Food and Agriculture: Rome, Italy, 2002; Available online: https://www.fao.org/4/aa010e/AA010e00.htm (accessed on 13 June 2025).
- Volk, G.M.; Bonnart, R.M.; Henk, A.D.; Chen, K.Y.; Bettoni, J.C.; Wang, Q.-C.; Kreckel, H.D.; Levinger, N.E. Fundamentals of plant cryopreservation: Dormant bud two-step cooling and shoot tip vitrification. Acta Hortic. 2025, 1421, 117–124. [Google Scholar] [CrossRef]
- Canter, P.H.; Thomas, H.; Ernst, E. Bringing medicinal plants into cultivation: Opportunities and challenges for biotechnology. Trends Biotechnol. 2005, 23, 180–185. [Google Scholar] [CrossRef]
- Kikowska, M.; Thiem, B. In Vitro Systems of Selected Eryngium Species (E. planum, E. campestre, E. maritimum, and E. alpinum) for Studying Production of Desired Secondary Metabolites (Phenolic Acids, Flavonoids, Triterpenoid Saponins, and Essential Oil). In Plant Cell and Tissue Differentiation and Secondary Metabolites, Reference Series in Phytochemistry; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 869–901. [Google Scholar] [CrossRef]
- Petersen, M.; Simmonds, M.S. Rosmarinic acid. Phytochem. 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Murthy, H.N.; Lee, E.-J.; Paek, K.-Y. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tiss. Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Trendafilova, A.; Jadranin, M.; Gorgorov, R.; Stanilova, M. Bioactive Compounds in Wild, In vitro Obtained, Ex vitro Adapted, and Acclimated Plants of Centaurea davidovii (Asteraceae). Nat. Prod. Commun. 2015, 10, 839–841. [Google Scholar] [CrossRef] [PubMed]
- Boggetti, B.; Jasik, J.; Mantell, S.H. In vitro multiplication of cashew (Anacardium occidentale L.) using shoot node explants of glasshouse-raised plants. Plant Cell Rep. 1999, 18, 456–461. [Google Scholar] [CrossRef]
- Stojičić, D.; Budimir, S.; Ćulafić, L. Micropropagation of Pinus heldreichii. Plant Cell Tiss. Organ Cult. 1999, 59, 147–150. [Google Scholar] [CrossRef]
- Stojičić, D.; Budimir, S. Cytokinin mediated axillary shoot formation in Pinus heldreichii. Biol. Plant. 2004, 49, 477–479. [Google Scholar] [CrossRef]
- Zdravković-Korać, S.; Muhovski, Y.; Druart, P.; Ćalić, D.; Radojević, L. Agrobacterium rhizogenes-mediated DNA transfer to Aesculus hippocastanum L. and the regeneration of transformed plants. Plant Cell Rep. 2004, 22, 698–704. [Google Scholar] [CrossRef]
- Soniya, E.V.; Sujitha, M. An efficient in vitro propagation of Aristolochia indica. Biol. Plant. 2006, 50, 272–274. [Google Scholar] [CrossRef]
- Danova, K.; Stanoeva, J.P.; Aneva, I.; Alipieva, K.; Stefova, M. Plant Growth Regulators and Activated Charcoal Selectively Affect Phenylethanoid and Flavone Glycoside Accumulation in Sideritis scardica Griseb. Tissue Culture. Plants 2023, 12, 2541. [Google Scholar] [CrossRef]
- Dörnenburg, H.; Knorr, D. Strategies for the improvement of secondary metabolite production in plant cell cultures. Enz. Microb. Technol. 1995, 17, 674–684. [Google Scholar] [CrossRef]
- Jin, B.; Liu, J.; Gao, D.; Xu, Y.; He, L.; Zang, Y.; Li, N.; Lin, D. Detailed studies on the anticancer action of rosmarinic acid in human Hep-G2 liver carcinoma cells: Evaluating its effects on cellular apoptosis, caspase activation and suppression of cell migration and invasion. J. BUON. 2020, 25, 1383–1389. [Google Scholar] [PubMed]
- Hung, S.S.; Zheng, R.L. Rosmarinic acid inhibits angiogenesis and its mechanism of action in vitro. Cancer Lett. 2006, 239, 271–280. [Google Scholar] [CrossRef]
- Han, Y.-H.; Kee, J.-Y.; Hong, S.-H. Rosmarinic Acid Activates AMPK to Inhibit Metastasis of Colorectal Cancer. Front. Pharmacol. 2018, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Badisa, R.B.; Tzakou, O.; Couladis, M.; Pilarinou, E. Cytotoxic activities of some Greek Labiatae herbs. Phytother. Res. 2003, 17, 472–476. [Google Scholar] [CrossRef]
- Uzelac, B.; Stojičić, D.; Budimir, S.; Tošić, S.; Zlatković, B.; Blagojević, S.; Manić, B.; Janjanin, M.; Slavkovska, V. Essential oils as potential biocontrol products against plant pathogens and weeds: In vitro culture approach. In Proceedings of the 27th Biotechnology Conference with International Participation, Čačak, Serbia, 25–26 March 2022. [Google Scholar] [CrossRef]
- George, E.F. Plant Propagation by Tissue Culture. Part 1: The Technology, 2nd ed.; Exegetics Limited: Eversley, UK, 1993. [Google Scholar]
- Tošić, S.; Stojičić, D.; Slavkovska, V.; Mihajilov-Krstev, T.; Zlatković, B.; Budimir, S.; Uzelac, B. Phytochemical composition and biological activities of native and in vitro-propagated Micromeria croatica (Pers.) Schott (Lamiaceae). Planta 2019, 249, 1365–1377. [Google Scholar] [CrossRef]
- Stojičić, D.; Tošić, S.; Slavkovska, V.; Zlatković, B.; Budimir, S.; Janošević, D.; Uzelac, B. Glandular trichomes and essential oil characteristics of in vitro propagated Micromeria pulegium (Rochel) Benth. (Lamiaceae). Planta 2016, 244, 393–404. [Google Scholar] [CrossRef]
- Kaul, S.; Das, S.; Srivastava, P.S. Micropropagation of Ajuga bracteosa, a medicinal herb. Physiol. Mol. Biol. Plants 2013, 19, 289–296. [Google Scholar] [CrossRef]
- Bakhtiar, Z.; Mirjalili, M.H.; Sonboli, A.; Moridi Farimani, M.; Ayyari, M. In vitro propagation, genetic and phytochemical assessment of Thymus persicus—A medicinally important source of pentacyclic triterpenoids. Biologia 2014, 69, 594–603. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Wysokińska, H. The effect of cytokinins on shoot proliferation, secondary metabolite production and antioxidant potential in shoot cultures of Scutellaria alpina. Plant Cell Tiss. Organ Cult. 2015, 122, 699–708. [Google Scholar] [CrossRef]
- Martini, A.N.; Vlachou, G.; Papafotiou, M. Effect of Explant Origin and Medium Plant Growth Regulators on In Vitro Shoot Proliferation and Rooting of Salvia tomentosa, a Native Sage of the Northeastern Mediterranean Basin. Agronomy 2022, 12, 1889. [Google Scholar] [CrossRef]
- Thomas, T.D. The role of activated charcoal in plant tissue culture. Biotechnol. Adv. 2008, 26, 618–631. [Google Scholar] [CrossRef]
- Patel, K.R.; Thorpe, T.A. In vitro regeneration of plantlets from embryonic and seedling explants of Engelmaun spruce (Picea engelmanii Parry). Tree Physiol. 1986, 1, 289–301. [Google Scholar] [CrossRef]
- Quoirin, M.; Da Silva, M.C.; Martins, K.G.; De Oliveira, D.E. Multiplication of juvenile black wattle by microcuttings. Plant Cell Tiss. Organ Cult. 2001, 66, 199–205. [Google Scholar] [CrossRef]
- Pan, M.J.; Van Staden, J. The use of charcoal in in vitro culture—A review. Plant Growth Regul. 1998, 26, 155–163. [Google Scholar] [CrossRef]
- Borges, M.; Ceiro, W.; Meneses, S.; Aguilera, N.; Vázquez, J.; Infante, Z.; Fonseca, M. Regeneration and multiplication of Dioscorea alata germplasm maintained in vitro. Plant Cell Tissue Organ Cult. 2004, 76, 87–90. [Google Scholar] [CrossRef]
- Marco-Medina, A.; Casas, J.L. In vitro multiplication and essential oil composition of Thymus moroderi Pau ex Martinez, an endemic Spanish plant. Plant Cell Tiss. Organ Cult. 2015, 120, 99–108. [Google Scholar] [CrossRef]
- Komalavalli, N.; Rao, M.V. In vitro micropropagation of Gymnema sylvestre—A multipurpose medicinal plant. Plant Cell Tiss. Organ Cult. 2000, 61, 97–105. [Google Scholar] [CrossRef]
- Vladimir-Knežević, S.; Blažeković, B.; Bival Štefan, M.; Alegro, A.; Kőszegi, T.; Petrik, J. Antioxidant Activities and Polyphenolic Contents of Three Selected Micromeria Species from Croatia. Molecules 2011, 16, 1454–1470. [Google Scholar] [CrossRef] [PubMed]
- Askun, T.; Tekwu, E.M.; Satil, F.; Modanlioglu, S.; Aydeniz, H. Preliminary antimycobacterial study on selected Turkish plants (Lamiaceae) against Mycobacterium tuberculosis and search for some phenolic constituents. BMC Complement. Altern. Med. 2013, 13, 365. [Google Scholar] [CrossRef]
- Vladimir-Knežević, S.; Blažeković, B.; Kindl, M.; Vladić, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase Inhibitory, Antioxidant and Phytochemical Properties of Selected Medicinal Plants of the Lamiaceae Family. Molecules 2014, 19, 767–782. [Google Scholar] [CrossRef]
- Vladimir-Knežević, S.; Cvijanović, O.; Blažeković, B.; Kindl, M.; Bival Štefan, M.; Domitrović, R. Hepatoprotective effects of Micromeria croatica ethanolic extract against CCl4–induced liver injury in mice. BMC Complement. Altern. Med. 2015, 15, 233. [Google Scholar] [CrossRef]
- Al-Hamwi, M.; Aboul-Ela, M.; El-Lakany, A.; El-Achi, N.; Ghanem, N.; El Hamaoui, B.; Bakkour, Y.; El-Omar, F. Chemical Composition, Antimicrobial and Antioxidant Activities of the Ethanolic Extract of Micromeria fruticosa Growing in Lebanon. Int. J. Chem. Sci. 2015, 13, 325–335. [Google Scholar]
- Scognamiglio, M.; D’Abrosca, B.; Esposito, A.; Fiorentino, A. Chemical Composition and Seasonality of Aromatic Mediterranean Plant Species by NMR-based Metabolomics. J. Anal. Methods Chem. 2015, 2015, 258570. [Google Scholar] [CrossRef] [PubMed]
- Abu-Reidah, I.M.; Arráez-Román, D.; Al-Nuri, M.; Warad, I.; Segura-Carretero, A. Untargeted metabolite profiling and phytochemical analysis of Micromeria fruticosa L. (Lamiaceae) leaves. Food Chem. 2019, 279, 128–143. [Google Scholar] [CrossRef]
- Taskin, T.; Oksuz, M.; Rayaman, E.; Ermanoglu, M.; Taskin, D.; Acar, A.G.; Kiliç, Ö.; Çalışkan Salihi, E.; Yılmaz Nur, B.; Elçioğlu, H. Phytochemical analysis and in vitro biological activity assessment of extracts from Micromeria myrtifolia. Int. J. Environ. Health Res. 2024, 35, 1833–1845. [Google Scholar] [CrossRef]
- El Khoury, R.; Caceres, I.; Puel, O.; Bailly, S.; Atoui, A.; Oswald, I.P.; El Khoury, A.; Bailly, J.-D. Identification of the Anti-Aflatoxinogenic Activity of Micromeria graeca and Elucidation of Its Molecular Mechanism in Aspergillus flavus. Toxins 2017, 9, 87. [Google Scholar] [CrossRef]
- Brahmi, F.; Amri, S.; Bentaleb, S.; Elsebai, M.F.; Yalaoui-Guellal, D.; Madani, K. Comparison of Phenolic Contents and Biological Potential of Different Polar Extracts of Micromeria graeca from Algeria. Curr. Nutr. Food Sci. 2019, 15, 148–155. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Güllüce, M.; Sökmen, M.; Şahin, F.; Sökmen, A.; Adigüzel, A.; Özer, H. Biological activities of the essential oil and methanolic extract of Micromeria fruticosa (L.) Druce ssp serpyllifolia (Bieb) PH Davis plants from the eastern Anatolia region of Turkey. J. Sci. Food Agric. 2004, 84, 735–741. [Google Scholar] [CrossRef]
- Yousef, M.M.; Zohri, A.-N.A.; Darwish, A.M.G.; Shamseldin, A.; Kabeil, S.A.; Abdelkhalek, A.; Binsuwaidan, R.; Jaremko, M.; Alshwyeh, H.A.; Hafez, E.E.; et al. Exploring the antibacterial potential of plant extracts and essential oils against Bacillus thermophilus in beet sugar for enhanced sucrose retention: A comparative assessment and implications. Front. Microbiol. 2023, 14, 1219823. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Al-Nuri, M.A.; Yaghmour, R.M.-R.; Faidi, Y.R. Antimicrobial activity of Micromeria nervosa from the Palestinian area. J. Ethnopharmacol. 1997, 58, 143–147. [Google Scholar] [CrossRef]
- Tabanca, N.; Kirimer, N.; Demirci, B.; Demirci, F.; Başer, K.H. Composition and antimicrobial activity of the essential oils of Micromeria cristata subsp. phrygia and the enantiomeric distribution of borneol. J. Agric. Food Chem. 2001, 49, 4300–4303. [Google Scholar] [CrossRef]
- Duru, M.E.; Öztürk, M.; Uğur, A.; Ceylan, Ö. The constituents of essential oil and in vitro antimicrobial activity of Micromeria cilicica from Turkey. J. Ethnopharmacol. 2004, 94, 43–48. [Google Scholar] [CrossRef]
- Tošić, S.; Stojičić, D.; Stankov-Jovanović, V.; Mitić, V.; Mihajilov-Krstev, T.; Zlatković, B. Chemical composition, antioxidant and antimicrobial activities of micropropagated and native Micromeria pulegium (Lamiaceae) extracts. Oxid. Commun. 2015, 38, 55–66. [Google Scholar]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- El-Huneidi, W.; Shehab, N.G.; Bajbouj, K.; Vinod, A.; El-Serafi, A.; Shafarin, J.; Bou Malhab, L.J.; Abdel-Rahman, W.M.; Abu-Gharbieh, E. Micromeria fruticosa Induces Cell Cycle Arrest and Apoptosis in Breast and Colorectal Cancer Cells. Pharmaceuticals 2020, 13, 115. [Google Scholar] [CrossRef]
- Shehab, N.G.; Abu-Gharbieh, E. Constituents and biological activity of the essential oil and the aqueous extract of Micromeria fruticosa (L.) Druce subsp. serpyllifolia. Pak. J. Pharm. Sci. 2012, 25, 687–692. [Google Scholar]
- Al-Yousef, H.M.; Fantoukh, O.I.; El-Sayed, M.A.; Amina, M.; Adel, R.; Hassan, W.H.B.; Abdelaziz, S. Metabolic profiling and biological activities of the aerial parts of Micromeria imbricata Forssk. growing in Saudi Arabia. Saudi J. Biol. Sci. 2021, 28, 5609–5616. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous antioxidants--Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Chimento, A.; De Luca, A.; D’Amico, M.; De Amicis, F.; Pezzi, V. The Involvement of Natural Polyphenols in Molecular Mechanisms Inducing Apoptosis in Tumor Cells: A Promising Adjuvant in Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 1680. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Gruz, J.; Durgo, K.; Kremer, D.; Kosalec, I.; Žulj, L.V.; Martinez, S.; Salopek-Sondi, B.; Piljac-Žegarac, J. Molecular and cellular approach in the study of antioxidant/pro-oxidant properties of Micromeria croatica (Pers.) Schott. Nat. Prod. Res. 2015, 29, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, N.; Luo, M.; Zu, Y.; Efferth, T. Antibacterial Activity and Anticancer Activity of Rosmarinus officinalis L. Essential Oil Compared to That of Its Main Components. Molecules 2012, 17, 2704–2713. [Google Scholar] [CrossRef]
- Vuko, E.; Dunkić, V.; Bezić, N.; Ruščić, M.; Kremer, D. Chemical composition and antiphytoviral activity of essential oil of Micromeria graeca. Nat. Prod. Commun. 2012, 7, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Tzakou, O.; Couladis, M. The essential oil of Micromeria graeca (L.) Bentham et Reichenb. growing in Greece. Flavour Fragr. J. 2001, 16, 107–109. [Google Scholar] [CrossRef]
- Slavkovska, V.; Couladis, M.; Bojovic, S.; Tzakou, O.; Pavlovic, M.; Lakusic, B.; Jancic, R. Essential oil and its systematic significance in species of Micromeria Bentham from Serbia & Montenegro. Plant Syst. Evol. 2005, 255, 1–15. [Google Scholar] [CrossRef]
- Kremer, D.; Stabentheiner, E.; Dunkić, V.; Müller, I.D.; Vujić, L.; Kosalec, I.; Ballian, D.; Bogunić, F.; Bezić, N. Micromorphological and Chemotaxonomical Traits of Micromeria croatica (Pers.) Schott. Chem. Biodiversity 2012, 9, 755–768. [Google Scholar] [CrossRef]
- Slavkovska, V.; Zlatković, B.; Bräuchler, C.; Stojanović, D.; Tzakou, O.; Couladis, M. Variations of essential oil characteristics of Clinopodium pulegium (Lamiaceae) depending on phenological stage. Bot. Serb. 2013, 37, 97–104. [Google Scholar]
- Silva, B.I.M.; Nascimento, E.A.; Silva, C.J.; Silva, T.G.; Aguiar, J.S. Anticancer activity of monoterpenes: A systematic review. Mol. Biol. Rep. 2021, 48, 5775–5785. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, H.; Wang, C.; Shi, X.; Li, K. Rosmarinic acid inhibits proliferation and invasion of hepatocellular carcinoma cells SMMC 7721 via PI3K/AKT/mTOR signal pathway. Biomed. Pharmacother. 2019, 120, 109443. [Google Scholar] [CrossRef] [PubMed]
- Messeha, S.S.; Zarmouh, N.O.; Asiri, A.; Soliman, K.F.A. Rosmarinic acid-induced apoptosis and cell cycle arrest in triple-negative breast cancer cells. Eur. J. Pharmacol. 2020, 885, 173419. [Google Scholar] [CrossRef]
- Da Silva, G.B.; Manica, D.; da Silva, A.P.; Marafon, F.; Moreno, M.; Bagatini, M.D. Rosmarinic acid decreases viability, inhibits migration and modulates expression of apoptosis-related CASP8/CASP3/NLRP3 genes in human metastatic melanoma cells. Chem. Biol. Interact. 2023, 375, 110427. [Google Scholar] [CrossRef]
- Ijaz, S.; Iqbal, J.; Abbasi, B.A.; Ullah, Z.; Yaseen, T.; Kanwal, S.; Mahmood, T.; Sydykbayeva, S.; Ydyrys, A.; Almarhoon, Z.M.; et al. Rosmarinic acid and its derivatives: Current insights on anticancer potential and other biomedical applications. Biomed. Pharmacother. 2023, 162, 114687. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Test for Bacteria That Grow Aerobically (M07-A9, 9th edition); Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Despotović, A.; Mirčić, A.; Misirlić-Denčić, S.; Harhaji-Trajković, L.; Trajković, V.; Zogović, N.; Tovilović-Kovačević, G. Combination of Ascorbic Acid and Menadione Induces Cytotoxic Autophagy in Human Glioblastoma Cells. Oxid. Med. Cell. Longev. 2022, 2022, 2998132. [Google Scholar] [CrossRef] [PubMed]

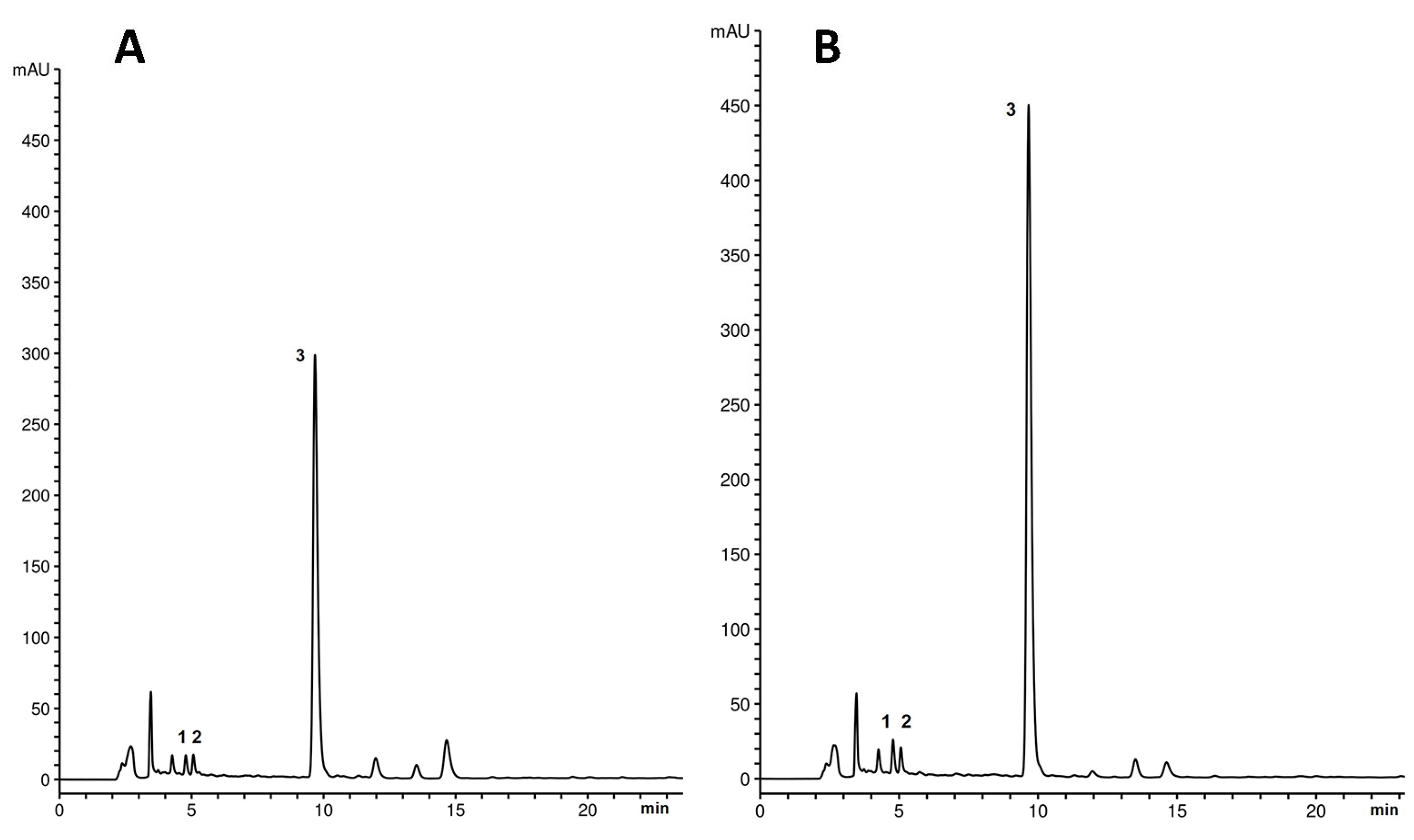


| Shoot Culture Designation * | Subculture Interval (Weeks) | Caffeic Acid (mg/g DW Extract) | Syringic Acid (mg/g DW Extract) | Rosmarinic Acid (mg/g DW Extract) |
|---|---|---|---|---|
| AC− | 4 | 1.52 ± 0.05 a | 0.52 ± 0.05 a | 51.68 ± 0.26 b |
| 5 | 1.68 ± 0.07 ab | 0.54 ± 0.02 a | 51.39 ± 0.15 b | |
| 6 | 1.34 ± 0.07 a | 0.55 ± 0.03 a | 28.92 ± 0.54 a | |
| 7 | 1.69 ± 0.03 ab | 0.81 ± 0.02 b | 52.77 ± 0.65 b | |
| AC+ | 4 | 2.17 ± 0.07 c | 0.78 ± 0.07 b | 157.75 ± 0.79 d |
| 5 | 3.54 ± 0.08 e | 1.37 ± 0.04 d | 123.24 ± 0.44 c | |
| 6 | 2.93 ± 0.09 d | 1.15 ± 0.01 c | 122.21 ± 0.47 c | |
| 7 | 2.02 ± 0.05 bc | 0.93 ± 0.01 b | 52.40 ± 0.23 b |
| Microbial Strains from ATCC Collection | MICs (mg mL−1) of M. graeca Methanolic Extracts | MIC/MMC * (µg mL−1) of Reference Antibiotics 1 | MIC/MMC (µg mL−1) of Reference Fungicide 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AC+ | AC− | Str | Chl | Nys | |||||||
| 4w | 5w | 6w | 7w | 4w | 5w | 6w | 7w | ||||
| Gram (−) bacteria | |||||||||||
| Escherichia coli ATCC 25922 | >54.68 | >44.10 | >59.20 | >75.20 | >68.80 | >43.90 | >57.50 | >58.20 | 16.0/16.0 | 16.0/16.0 | - |
| Pseudomonas aeruginosa ATCC 9027 | >54.68 | >44.10 | >59.20 | >75.20 | >68.80 | >43.90 | >57.50 | >58.20 | 8.0/8.0 | 4.0/16.0 | - |
| Salmonella enteritidis ATCC 13076 | >54.68 | >44.10 | >59.20 | >75.20 | >68.80 | >43.90 | >57.50 | >58.20 | 4.0/4.0 | 4.0/8.0 | - |
| Enterobacter aerogenes ATCC 13048 | >54.68 | >44.10 | >59.20 | >75.20 | >68.80 | >43.90 | >57.50 | >58.20 | 0.5/0.5 | 1.0/1.0 | - |
| Gram (+) bacteria | |||||||||||
| Staphylococcus aureus ATCC 25923 | >54.68 | >44.10 | >59.20 | >75.20 | >68.80 | >43.90 | >57.50 | >58.20 | 0.5/0.5 | 1.0/8.0 | - |
| Bacillus cereus ATCC 11778 | >54.68 | >44.10 | >59.20 | >75.20 | >68.80 | >43.90 | >57.50 | >58.20 | 0.5/0.5 | 1.0/4.0 | - |
| Enterococcus faecalis ATCC 29212 | >54.68 | >44.10 | >59.20 | >75.20 | >68.80 | >43.90 | >57.50 | >58.20 | 16.0/16.0 | 16.0/16.0 | - |
| Yeast | |||||||||||
| Candida albicans ATCC 10231 | >54.68 | >44.10 | >59.20 | >75.20 | >68.80 | >43.90 | >57.50 | >58.20 | - | - | 16.0/16.0 |
| Shoot Culture Designation * | Subculture Interval (Weeks) | Sample Dilution Range (mg mL−1) |
|---|---|---|
| AC+ | 4 | 54.68–0.03 |
| 5 | 44.10–0.02 | |
| 6 | 59.20–0.03 | |
| 7 | 75.20–0.04 | |
| AC− | 4 | 68.80–0.03 |
| 5 | 43.90–0.02 | |
| 6 | 57.50–0.03 | |
| 7 | 58.20–0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzelac, B.; Janjanin, M.; Krstić-Milošević, D.; Tovilović-Kovačević, G.; Ignjatović, Đ.; Mihajilov-Krstev, T.; Stojičić, D. In Vitro Shoot Cultures of Micromeria graeca: Micropropagation and Evaluation of Methanolic Extracts for Anticancer and Antimicrobial Activity. Plants 2025, 14, 2592. https://doi.org/10.3390/plants14162592
Uzelac B, Janjanin M, Krstić-Milošević D, Tovilović-Kovačević G, Ignjatović Đ, Mihajilov-Krstev T, Stojičić D. In Vitro Shoot Cultures of Micromeria graeca: Micropropagation and Evaluation of Methanolic Extracts for Anticancer and Antimicrobial Activity. Plants. 2025; 14(16):2592. https://doi.org/10.3390/plants14162592
Chicago/Turabian StyleUzelac, Branka, Mirjana Janjanin, Dijana Krstić-Milošević, Gordana Tovilović-Kovačević, Đurđica Ignjatović, Tatjana Mihajilov-Krstev, and Dragana Stojičić. 2025. "In Vitro Shoot Cultures of Micromeria graeca: Micropropagation and Evaluation of Methanolic Extracts for Anticancer and Antimicrobial Activity" Plants 14, no. 16: 2592. https://doi.org/10.3390/plants14162592
APA StyleUzelac, B., Janjanin, M., Krstić-Milošević, D., Tovilović-Kovačević, G., Ignjatović, Đ., Mihajilov-Krstev, T., & Stojičić, D. (2025). In Vitro Shoot Cultures of Micromeria graeca: Micropropagation and Evaluation of Methanolic Extracts for Anticancer and Antimicrobial Activity. Plants, 14(16), 2592. https://doi.org/10.3390/plants14162592






