Phenotypic Variability and Anticancer Alkaloid Profiles of Catharanthus roseus Cultivars Grown Under a Vertical Farming System
Abstract
1. Introduction
2. Results
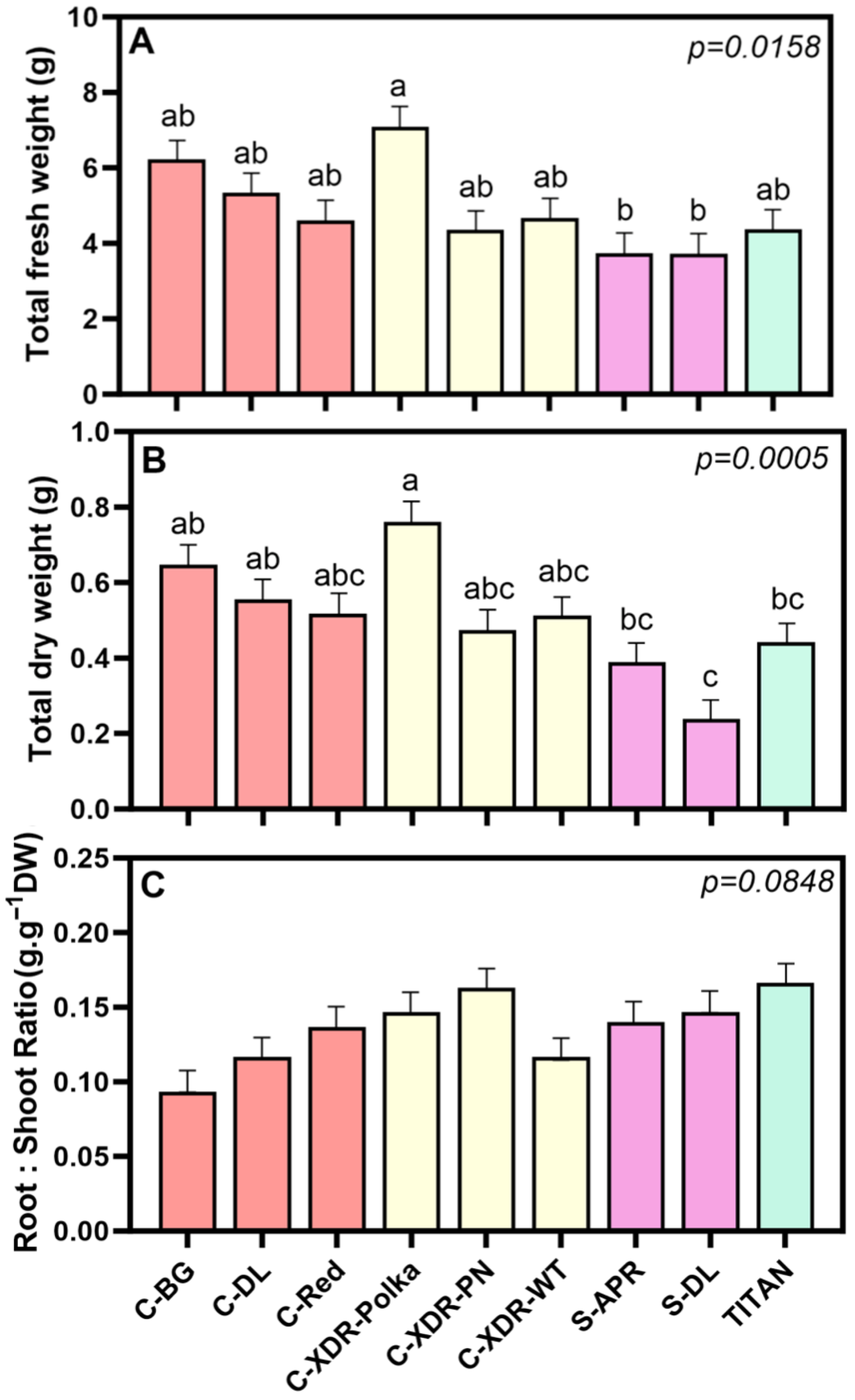
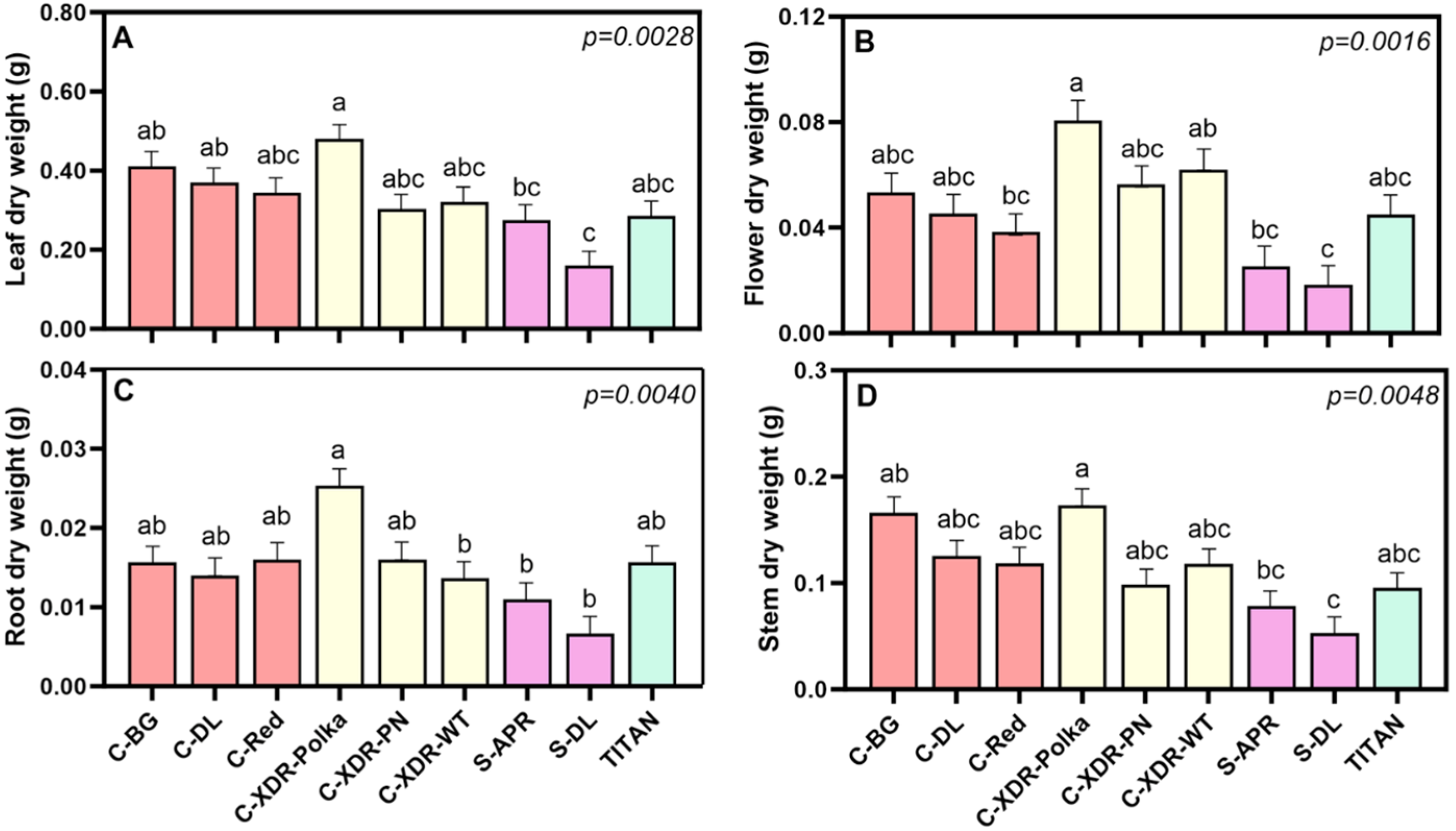
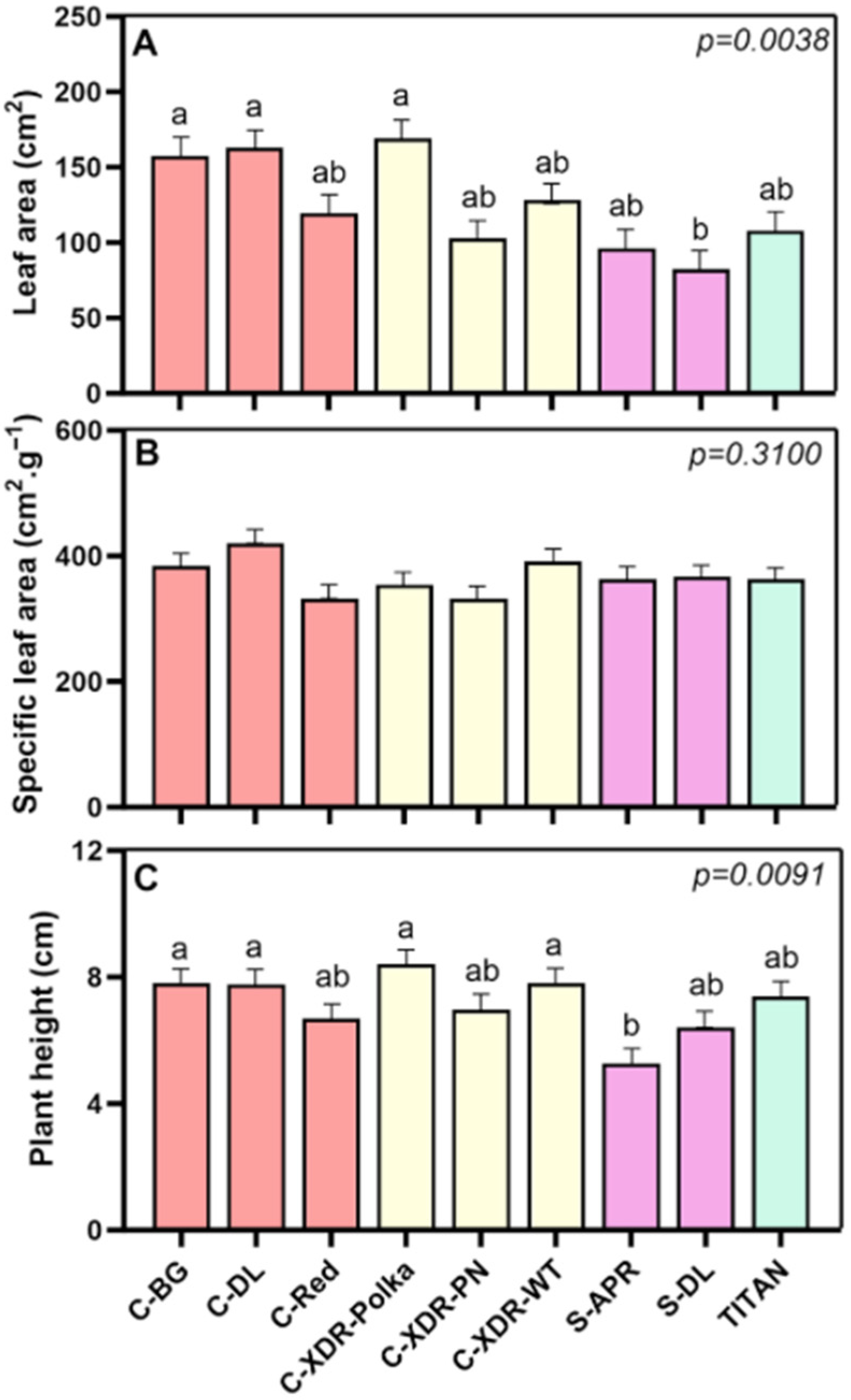
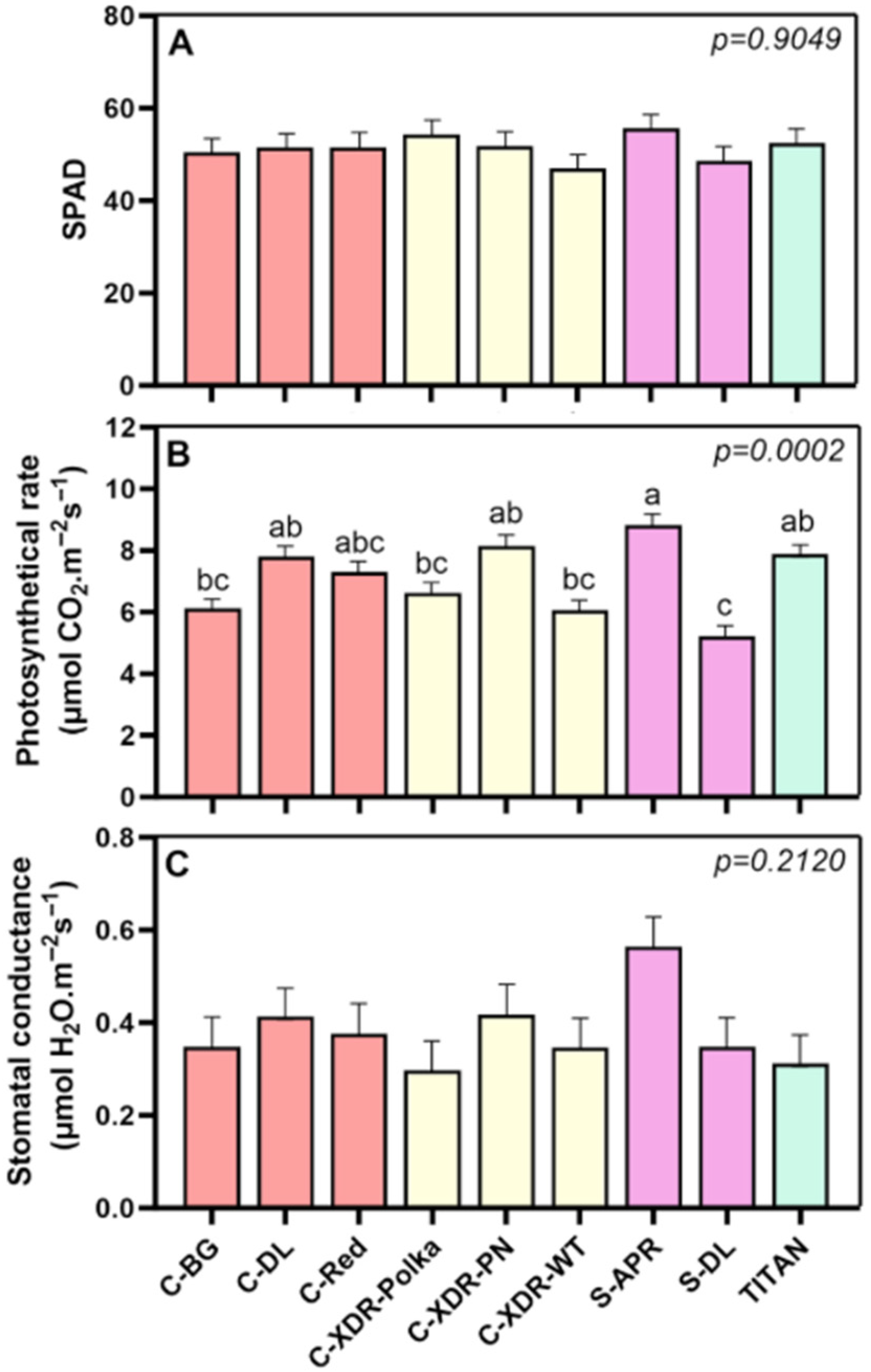
2.1. Morphological and Physiological Parameters
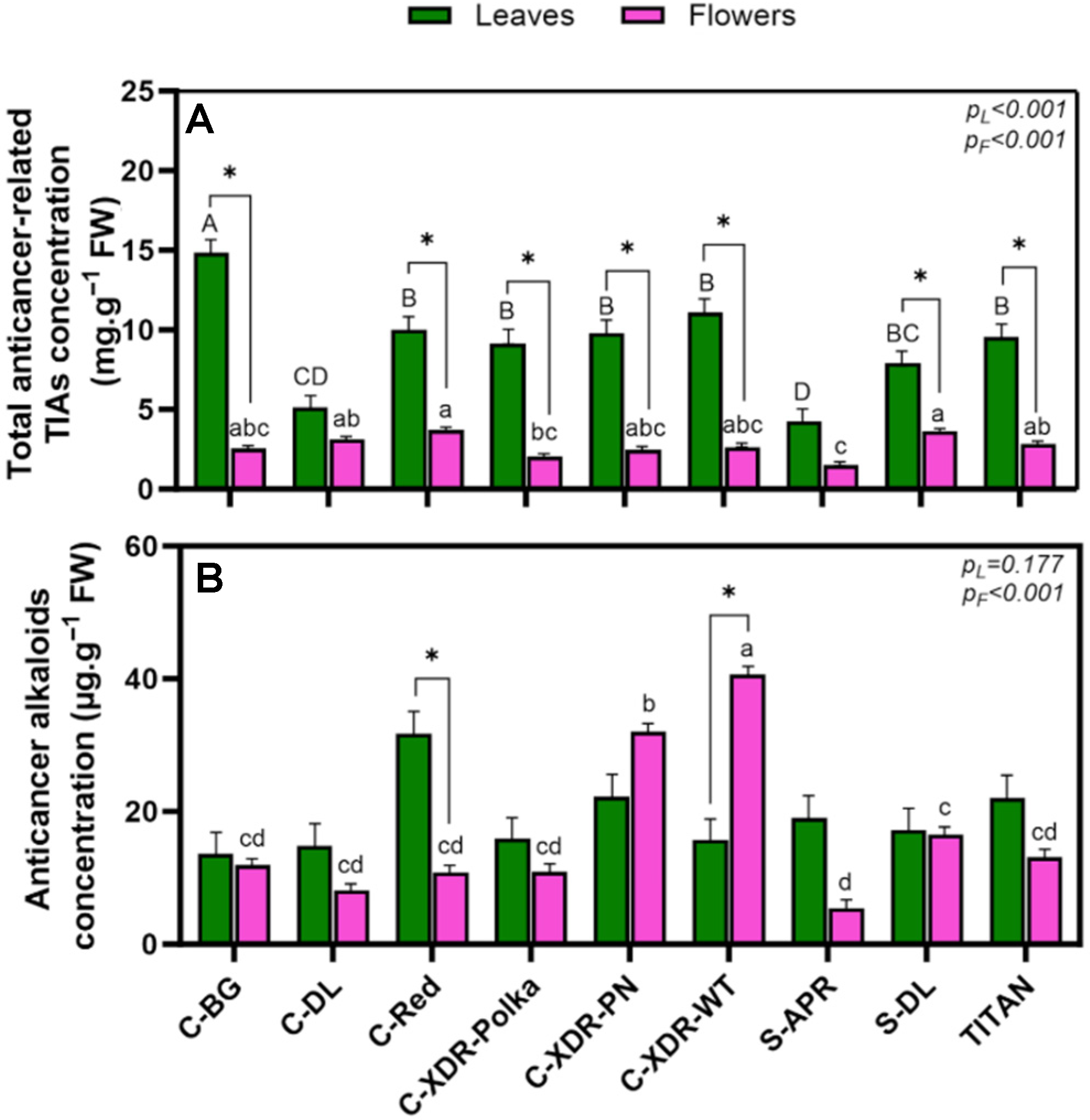
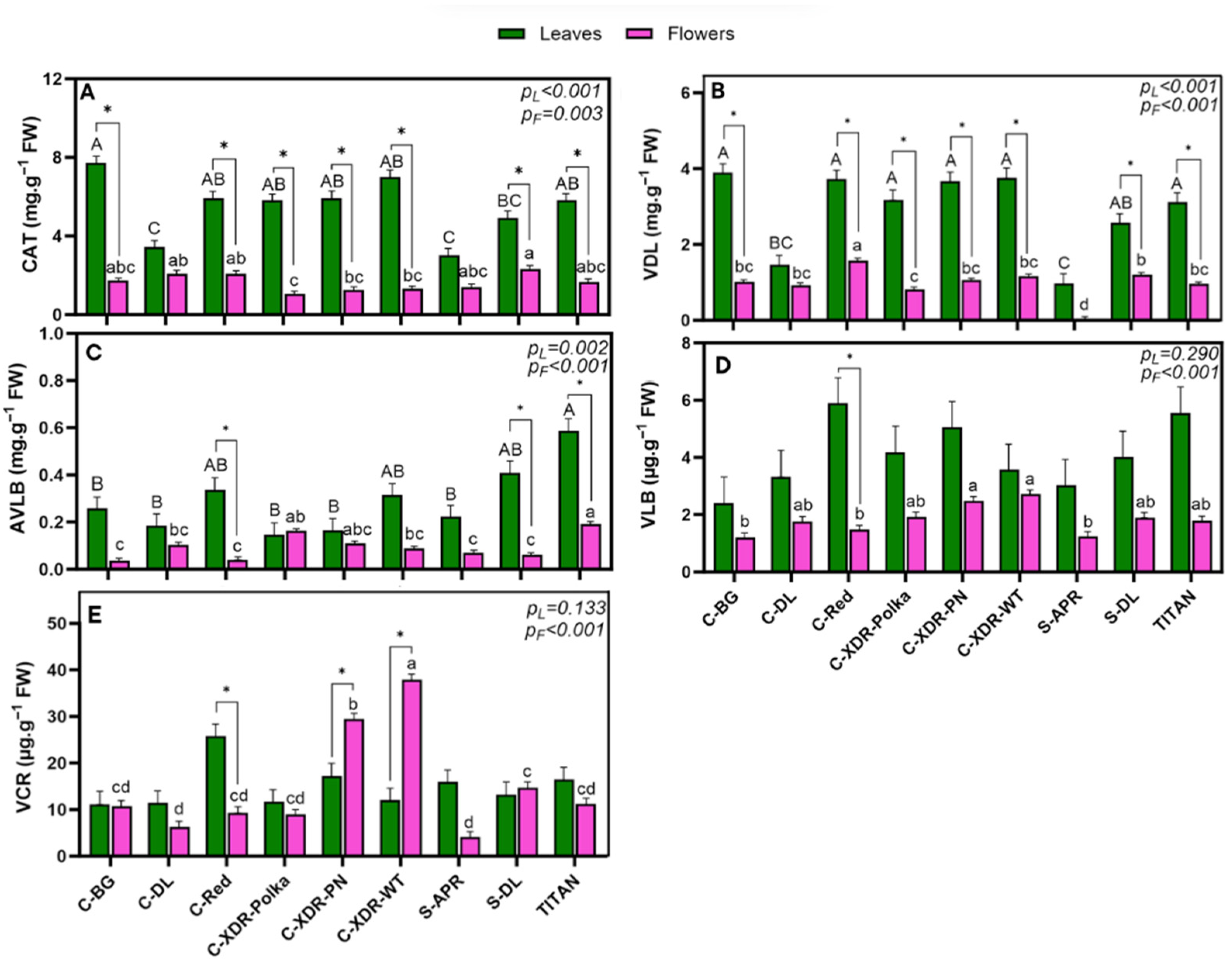
2.2. Alkaloid Profile
3. Discussion
3.1. Morpho-Physiological Diversity in Catharanthus roseus Cultivars
3.2. Variability in Upstream Alkaloid Concentration and Potential Bottlenecks in Anticancer Biosynthesis
3.3. Higher Alkaloid Content from Leaves than from Flowers
3.4. Comparative Analysis of the Alkaloid Concentration with Literature Values
3.5. Toward Molecular Farming of Catharanthus roseus Under Vertical Farming Systems
4. Materials and Methods
4.1. Plant Material and Growing Conditions
4.2. Morphological and Physiological Measurements
4.3. Alkaloid Extraction and LC-MS Methods
4.4. Experimental Design and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AVLB | Anhydrovinblastine |
| CAT | Catharanthine |
| C-BG | Cora Burgundy |
| C-DL | Cora Deep Lavender |
| C-Red | Cora Red |
| C-XDR-Polka | Cora XDR Polka Dot |
| C-XDR-PN | Cora XDR Punch |
| C-XDR-WT | Cora XDR White |
| DAT | Deacetylvindoline 4-O-Acetyltransferase |
| D4H | Deacetoxyvindoline 4-Hydroxylase |
| ESI | Electrospray Ionization Source |
| LA | Leaf Area |
| PMF | Plant Molecular Farming |
| PPFD | Photosynthetic Photon Flux Density |
| PRX1 | Peroxidase 1 |
| S-APR | Sunstorm Apricot |
| S-DL | Sunstorm Deep Lilac |
| SGD | Strictosidine β-Glucosidase |
| SLA | Specific Leaf Area |
| STR | Strictosidine Synthase |
| TDW | Total Dry Weight |
| TFW | Total Fresh Weight |
| TIA | Terpenoid Indole Alkaloid |
| TITAN | Titan Polka Dot |
| VDL | Vindoline |
| VCR | Vincristine |
| VF | Vertical Farming |
| VLB | Vinblastine |
References
- Sowmya, C.; Anand, M.; Indu Rani, C.; Amuthaselvi, G.; Janaki, P. Recent developments and inventive approaches in vertical farming. Front. Sustain. Food Syst. 2024, 8, 1400787. [Google Scholar] [CrossRef]
- Barnum, C.R.; Endelman, B.J.; Shih, P.M. Utilizing plant synthetic biology to improve human health and wellness. Front. Plant Sci. 2021, 12, 691462. [Google Scholar] [CrossRef]
- Guo, M.; Lv, H.; Chen, H.; Dong, S.; Zhang, J.; Liu, W.; He, L.; Ma, Y.; Yu, H.; Chen, S.; et al. Strategies on biosynthesis and production of bioactive compounds in medicinal plants. Chin. Herb. Med. 2024, 16, 13–26. [Google Scholar] [CrossRef]
- Holtz, B.R.; Berquist, B.R.; Bennett, L.D.; Kommineni, V.J.; Munigunti, R.K.; White, E.L. Commercial-scale biotherapeutics manufacturing facility for plant-made pharmaceuticals. Plant Biotechnol. J. 2015, 13, 1180–1190. [Google Scholar] [CrossRef]
- SharathKumar, M.; Heuvelink, E.; Marcelis, L.F.M. Vertical farming: Moving from genetic to environmental modification. Trends Plant Sci. 2020, 25, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Al-Kodmany, K. The vertical farm: A review of developments and implications for the vertical city. Buildings 2018, 8, 24. [Google Scholar] [CrossRef]
- Russo, G.L.; Tedesco, I.; Spagnuolo, C.; Russo, M. Antioxidant polyphenols in cancer treatment: Friend, foe or foil? Semin. Cancer Biol. 2017, 46, 1–13. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Nisar, S.; Masoodi, T.; Prabhu, K.; Kuttikrishnan, S.; Zarif, L.; Khatoon, S.; Ali, S.; Uddin, S.; Al-Shabeeb Akil, A.; Singh, M.; et al. Natural products as chemo-radiation therapy sensitizers in cancers. Biomed. Pharmacother. 2022, 154, 113610. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.; Guan, X. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.; Zotchev, S.; Dirsch, V. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Olofinsan, K.; Abrahamse, H.; George, B. Therapeutic role of alkaloids and alkaloid derivatives in cancer management. Molecules 2023, 28, 5578. [Google Scholar] [CrossRef] [PubMed]
- Falcão, M.; Scopel, R.; Almeida, D.; Espirito Santo, A.; Franceschini, G.; Garcez, J. Supercritical fluid extraction of vinblastine from Catharanthus roseus. J. Supercrit. Fluids 2017, 129, 9–15. [Google Scholar] [CrossRef]
- Magnotta, M.; Murata, J.; Chen, J.; De Luca, V. Identification of a low vindoline accumulating cultivar of Catharanthus roseus (L.) G. Don by alkaloid and enzymatic profiling. Phytochemistry 2006, 67, 1758–1764. [Google Scholar] [CrossRef]
- Guedes, J.G.; Ribeiro, R.; Carqueijeiro, I.; Guimarães, A.L.; Bispo, C.; Archer, J.; Azevedo, H.; Fonseca, N.A.; Sottomayor, M. The leaf idioblastome of the medicinal plant Catharanthus roseus is associated with stress resistance and alkaloid metabolism. J. Exp. Bot. 2024, 75, 274–299. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Kumar Rai, S.; Pandey-Rai, S.; Srivastava, S.; Mishra, R.K.; Sharma, S.; Kumar, S. Predominance of the serpentine route in monoterpenoid indole alkaloid pathway of Catharanthus roseus. Proc. Indian Natl. Sci. Acad. 2008, 74, 97–109. [Google Scholar]
- Iskandar, N.; Prayogo, I. Vinblastine and vincristine production on Madagascar periwinkle (Catharanthus roseus (L.) G. Don) callus culture treated with polyethylene glycol. Makara J. Sci. 2016, 20, 7–16. [Google Scholar] [CrossRef]
- Boon, B.A.; Boger, D.L. Triarylaminium radical cation promoted coupling of catharanthine with vindoline: Diastereospecific synthesis of anhydrovinblastine and reaction scope. J. Am. Chem. Soc. 2019, 141, 14349–14355. [Google Scholar] [CrossRef]
- Courdavault, V.; Papon, N.; Clastre, M.; Giglioli-Guivarc’h, N.; St-Pierre, B.; Burlat, V. A look inside an alkaloid multisite plant: The Catharanthus logistics. Curr. Opin. Plant Biol. 2014, 19, 43–50. [Google Scholar] [CrossRef]
- Sottomayor, M.; Barceló, A.R. The Vinca alkaloids: From biosynthesis and accumulation in plant cells, to uptake, activity and metabolism in animal cells. Stud. Nat. Prod. Chem. 2006, 33, 673–714. [Google Scholar]
- Alam, M.; Naeem, M.; Khan, M.; Uddin, M. Vincristine and vinblastine: Anticancer Catharanthus alkaloids: Pharmacological applications and strategies for yield im-provement. In Catharanthus roseus: Current Research and Future Prospects; Naeem, M., Aftab, T., Khan, M.M., Eds.; Springer: Cham, Germany, 2017; pp. 269–299. [Google Scholar]
- Dugé De Bernonville, T.; Carqueijeiro, I.; Lanoue, A.; Lafontaine, F.; Sánchez Bel, P.; Liesecke, F. Folivory elicits a strong defense reaction in Catharanthus roseus: Metabolomic and transcriptomic analyses reveal distinct local and systemic responses. Sci. Rep. 2017, 7, 40453. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Takahashi, K.; Caputi, L.; Mizuno, H.; Rodriguez-Lopez, C.; Burow, M.; Saito, K. The complexity of intercellular localization of alkaloids revealed by single-cell metabolomics. New Phytol. 2019, 224, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wood, J.; Vu, A.; Hamilton, J.; Rodriguez Lopez, C.; Payne, R.; Serna Guerrero, D.; Gase, K.; Yamamoto, K.; Vaillancourt, B.; et al. Single-cell multi-omics in the medicinal plant Catharanthus roseus. Nat. Chem. Biol. 2023, 19, 1031–1041. [Google Scholar] [CrossRef]
- van Der Heijden, R.; Jacobs, D.I.; Snoeijer, W.; Hallard, D.; Verpoorte, R. The Catharanthus alkaloids: Pharmacognosy and biotechnology. Curr. Med. Chem. 2004, 11, 607–628. [Google Scholar] [CrossRef]
- Pan, Q.; Saiman, M.Z.; Verpoorte, R.; Tang, K. Accumulation of terpenoid indole alkaloids in jasmonic acid elicited Catharanthus roseus plants before and during flowering. Pak. J. Bot. 2018, 50, 1077–1083. [Google Scholar]
- Sottomayor, M.; Lopes Cardoso, I.; Pereira, L.; Ros Barceló, A. Peroxidase and the biosynthesis of terpenoid indole alkaloids in the medicinal plant Catharanthus roseus (L.) G. Don. Phytochem. Rev. 2004, 3, 159–171. [Google Scholar] [CrossRef]
- Barrales-Cureño, H.J.; Reyes Reyes, C.; Vásquez García, I.; López Valdez, L.G.; Gómez De Jesús, A.; Cortés Ruíz, J.A.; Sánchez Herrera, L.M.; Calderón Caballero, M.C.; Salazar Magallón, J.A.; Espinoza Perez, J.; et al. Alkaloids of pharmacological importance in Catharanthus roseus. In Pharmacognosy—Medicinal Plants; IntechOpen: London, UK, 2019; pp. 1–28. [Google Scholar]
- Lourenço, M.S.C.; Prada, J.E.C.; Heuvelink, E.; Carvalho, S.M.P. Production of Catharanthus roseus in vertical farming systems: Dynamic analyses of plant morphological responses of nine cultivars to N-UV supplementation. Acta Hortic. 2022, 1337, 217–224. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, E.H.; Li, M.; Peebles, C.A.M.; Jung, W.S.; Song, H.K.; Ahn, J.K.; San, K.Y. Screening 64 cultivars Catharanthus roseus for the production of vindoline, catharanthine, and serpentine. Biotechnol. Prog. 2011, 26, 1703–1709. [Google Scholar] [CrossRef]
- Vázquez-Flota, F.; De Carolis, E.; Alarco, A.-M.; De Luca, V. Molecular cloning and characterization of desacetoxyvindoline-4-hydroxylase, a 2-oxoglutarate dependent-dioxygenase involved in the biosynthesis of vindoline in Catharanthus roseus (L.) G. Don. Plant Mol. Biol. 1997, 34, 935–948. [Google Scholar] [CrossRef]
- Gantet, P.; Memelink, J. Transcription factors: Tools to engineer the production of pharmacologically active plant metabolites. Trends Pharmacol. Sci. 2002, 23, 563–569. [Google Scholar] [CrossRef]
- Long, S.P.; Zhu, X.G.; Naidu, S.L.; Ort, D.R. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 2006, 29, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R. Improving photosynthesis. Plant Physiol. 2013, 162, 1780–1793. [Google Scholar] [CrossRef]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 167. [Google Scholar] [CrossRef] [PubMed]
- Leister, D. Enhancing the light reactions of photosynthesis: Strategies, controversies, and perspectives. Mol. Plant 2023, 16, 4–22. [Google Scholar] [CrossRef]
- Jaeger, S.R. Vertical farming (plant factory with artificial lighting) and its produce: Consumer insights. Curr. Opin. Food Sci. 2024, 56, 101145. [Google Scholar] [CrossRef]
- Carqueijeiro, I.; Noronha, H.; Duarte, P.; Gerós, H.; Sottomayor, M. Vacuolar transport of the medicinal alkaloids from Catharanthus roseus is mediated by a proton-driven antiport. Plant Physiol. 2013, 162, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, T.; Ohashi-Kaneko, K.; Hirata, K.; Muraoka, M.; Watanabe, H. Effects of ultraviolet A supplemented with red light irradiation on vinblastine production in Catharanthus roseus. Environ. Control Biol. 2017, 55, 65–69. [Google Scholar] [CrossRef]
- St-Pierre, B.; Vazquez-Flota, F.A.; De Luca, V. Multicellular compartmentation of catharanthine and vindoline biosynthesis in Catharanthus roseus. Plant Cell 1999, 11, 887–900. [Google Scholar] [CrossRef]
- Levingston, R.; Zamora, R. Medicine trees of the Tropics. Unasylva 1983, 35, 7–10. [Google Scholar]
- Mall, M.; Verma, R.K.; Gupta, M.M.; Shasany, A.K.; Khanuja, S.P.S.; Shukla, A.K. Influence of seasonal and ontogenic parameters on the pattern of key terpenoid indole alkaloids biosynthesized in the leaves of Catharanthus roseus. S. Afr. J. Bot. 2019, 123, 98–104. [Google Scholar] [CrossRef]
- Ohashi, K.; Fukuyama, T.; Nakai, A.; Usami, H.; Ono, E.; Watanabe, H. Growth and alkaloids production in Madagascar periwinkle plants grown under red LED. IFAC Proc. Vol. 2013, 46, 274–277. [Google Scholar] [CrossRef]
- Liu, D.; Ren, W.; Yin, L. Enhanced accumulation of catharanthine and vindoline in Catharanthus roseus hairy roots by overexpression of transcriptional factor ORCA2. Russ. J. Plant Physiol. 2011, 58, 432–437. [Google Scholar]
- Fukuyama, T.; Okusu, T.; Watanabe, H.; Ohashi-Kaneko, K. Effect of monochromatic red light intensity during growth on production efficiency of vinblastine in Catharanthus roseus with ultraviolet A light irradiation. Environ. Control Biol. 2023, 61, 29–36. [Google Scholar] [CrossRef]
- Wang, C.; Han, Q.; Zhang, T.; Li, C.; Sun, X. Litchi picking points localization in natural environment based on the Litchi-YOSO model and branch morphology reconstruction algorithm. Comput. Electron. Agric. 2024, 226, 109473. [Google Scholar] [CrossRef]
- Carqueijeiro, I.; Guimarães, A.; Bettencourt, S.; Martínez-Cortés, T.; Guedes, J.; Gardner, R.; Rodrigues, J. Isolation of cells specialized in anticancer alkaloid metabolism by fluorescence-activated cell sorting. Plant Physiol. 2016, 171, 2371–2378. [Google Scholar] [CrossRef]
- Jeong, W.T.; Lim, H.B. A UPLC-ESI-Q-TOF method for rapid and reliable identification and quantification of major indole alkaloids in Catharanthus roseus. J. Chromatogr. B 2018, 1080, 27–36. [Google Scholar] [CrossRef]
- Hajibarat, Z.; Saidi, A. Senescence-associated proteins and nitrogen remobilization in grain filling under drought stress condition. J. Genet. Eng. Biotechnol. 2022, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, T.; Ohashi-Kaneko, K.; Watanabe, H. Estimation of optimal red light intensity for production of the pharmaceutical drug components, vindoline and catharanthine, contained in Catharanthus roseus (L.) G. Don. Environ. Control Biol. 2015, 53, 217–220. [Google Scholar] [CrossRef]
- Hirata, K.; Asada, M.; Yatani, E.; Miyamoto, K.; Miura, Y. Effects of near-ultraviolet light on alkaloid production in multiple shoot cultures of Catharanthus roseus plants. Planta Medica 1993, 59, 46–50. [Google Scholar] [CrossRef]


| Measured Terpenoid Indole Alkaloids | Literature Data | References | Present Study | ||
|---|---|---|---|---|---|
| DW Basis | FW Basis | Measured Concentrations | Fold Increase x | ||
| CAT (mg·g−1) | 0.08–6.00 | 0.40–6.50 | [14,30,38,39,41] | 3.01–7.72 mg·g−1 FW | 1.55 |
| VDL (mg·g−1) | 2.08–7.00 | 0.11–1.40 | [14,30,38,39,43] | 0.98–3.90 mg·g−1 FW | 5.85 |
| AVLB (mg·g−1) | ------ | 0.07 | [38] | 0.14–0.59 mg·g−1 FW | 3.46 |
| VLB (µg·g−1) | 5.00–80.0 | ------ | [39,45] | 23.3–57.3 µg·g−1 DW | 0.95 |
| VCR (µg·g−1) | ------ | 1.90 | [41] | 11.1–25.8 µg·g−1 FW | 8.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lourenço, M.S.C.; Freitas, V.; Heuvelink, E.; Carvalho, S.M.P. Phenotypic Variability and Anticancer Alkaloid Profiles of Catharanthus roseus Cultivars Grown Under a Vertical Farming System. Plants 2025, 14, 2576. https://doi.org/10.3390/plants14162576
Lourenço MSC, Freitas V, Heuvelink E, Carvalho SMP. Phenotypic Variability and Anticancer Alkaloid Profiles of Catharanthus roseus Cultivars Grown Under a Vertical Farming System. Plants. 2025; 14(16):2576. https://doi.org/10.3390/plants14162576
Chicago/Turabian StyleLourenço, Marisa S. C., Victor Freitas, Ep Heuvelink, and Susana M. P. Carvalho. 2025. "Phenotypic Variability and Anticancer Alkaloid Profiles of Catharanthus roseus Cultivars Grown Under a Vertical Farming System" Plants 14, no. 16: 2576. https://doi.org/10.3390/plants14162576
APA StyleLourenço, M. S. C., Freitas, V., Heuvelink, E., & Carvalho, S. M. P. (2025). Phenotypic Variability and Anticancer Alkaloid Profiles of Catharanthus roseus Cultivars Grown Under a Vertical Farming System. Plants, 14(16), 2576. https://doi.org/10.3390/plants14162576






