Anatomical and Digital Image Analysis of Flavonoid-Mediated Grain Coloration in Rye (Secale cereale L.)
Abstract
1. Introduction
2. Results
2.1. Color Coordinates Analysis
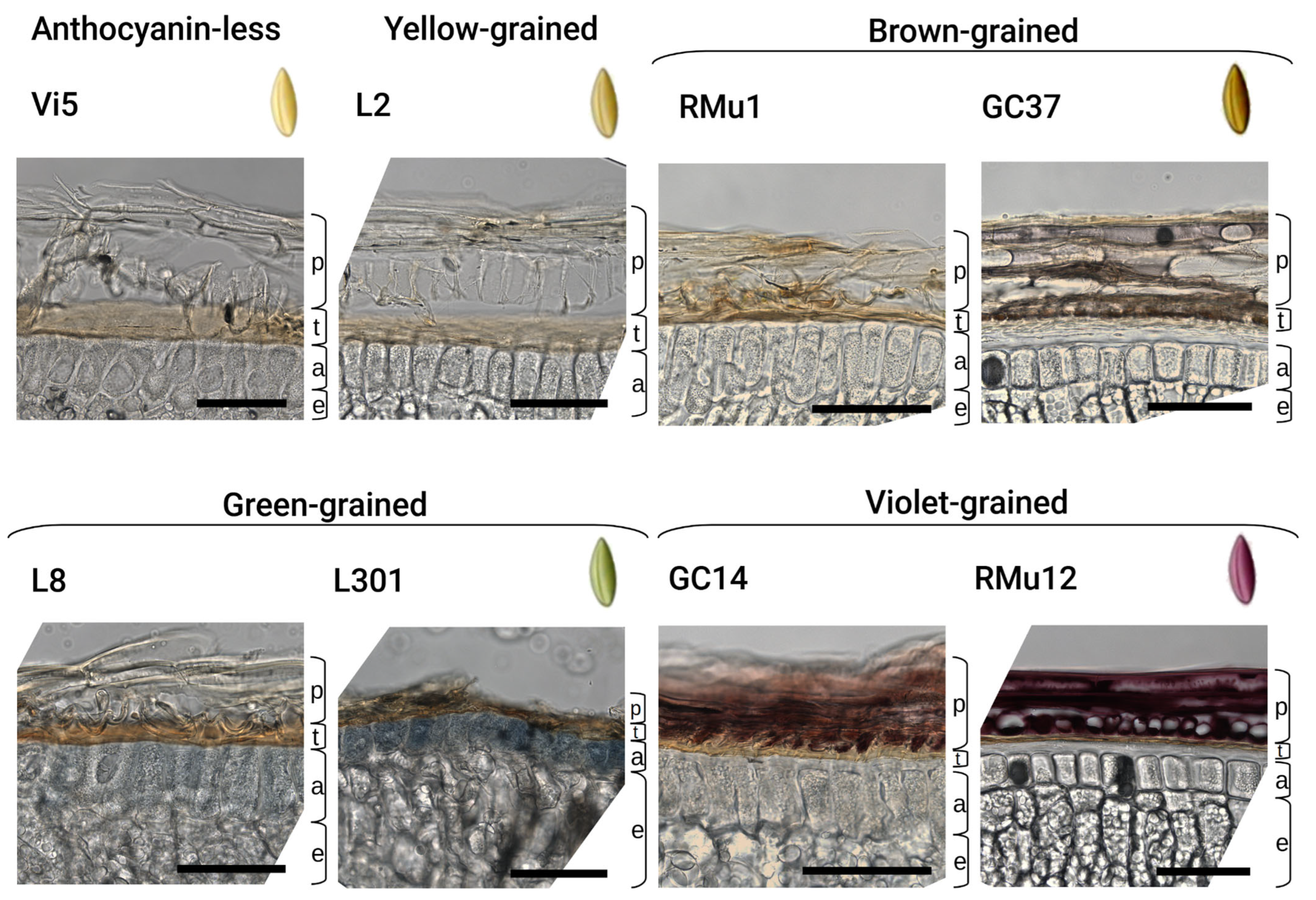
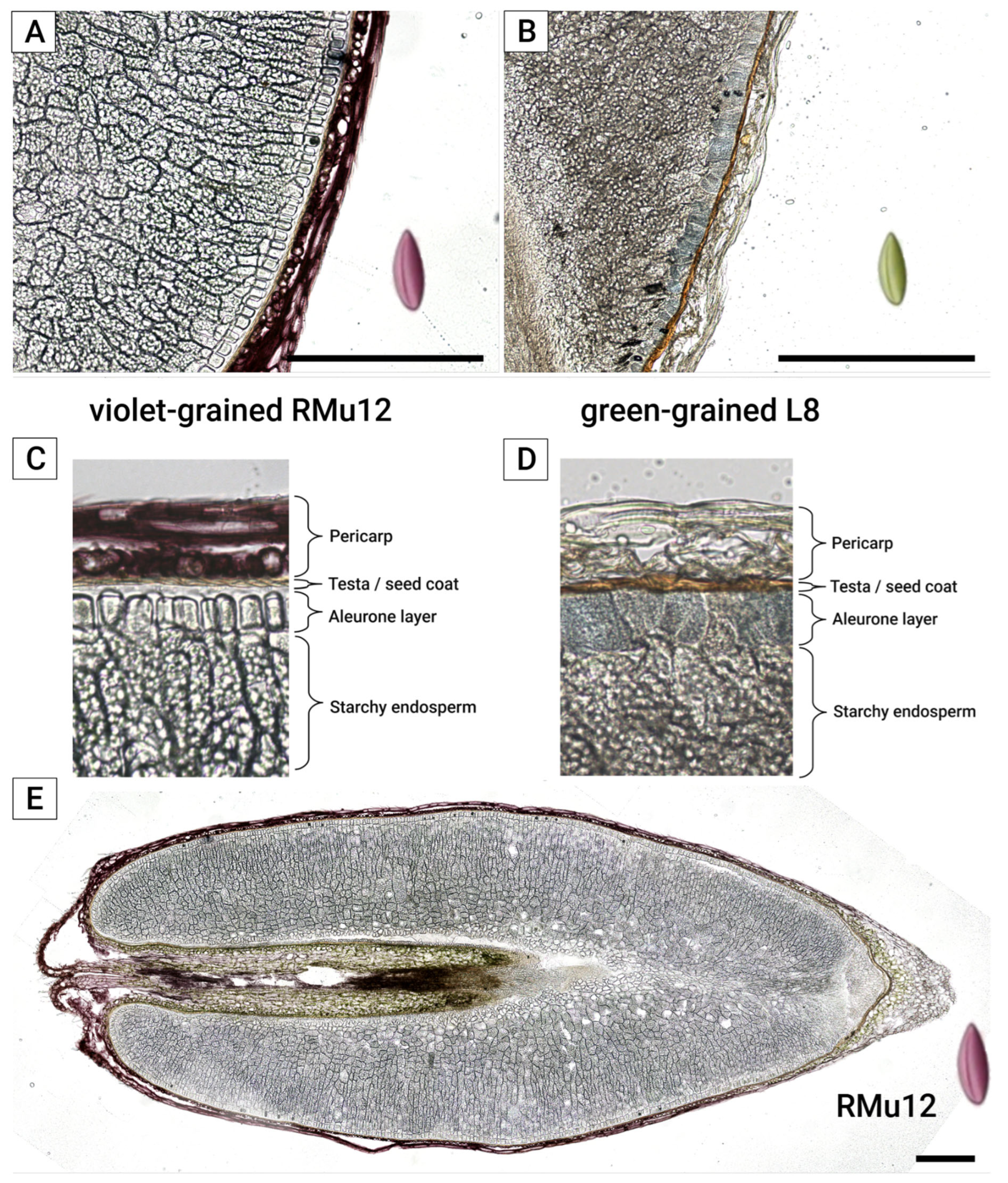
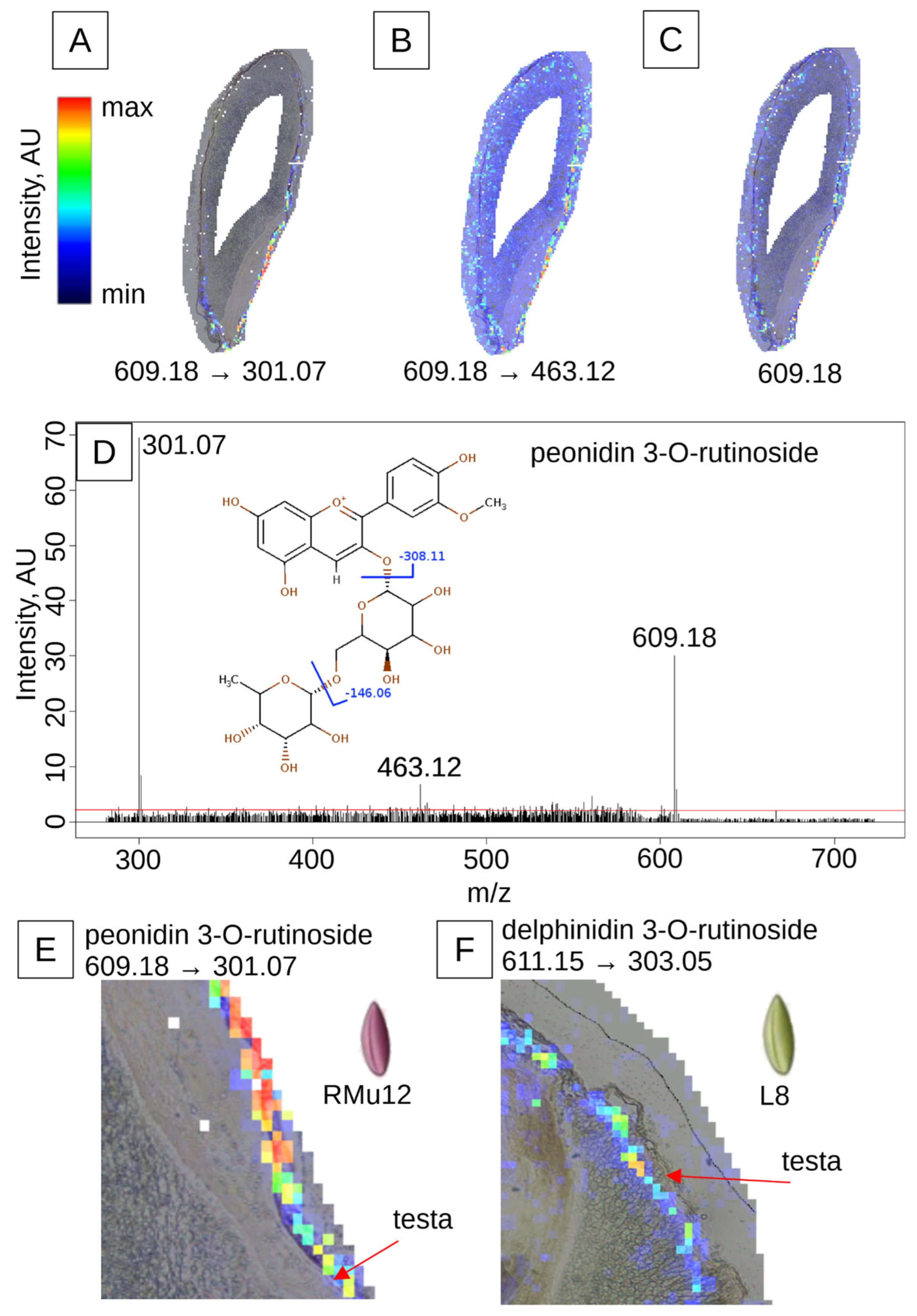
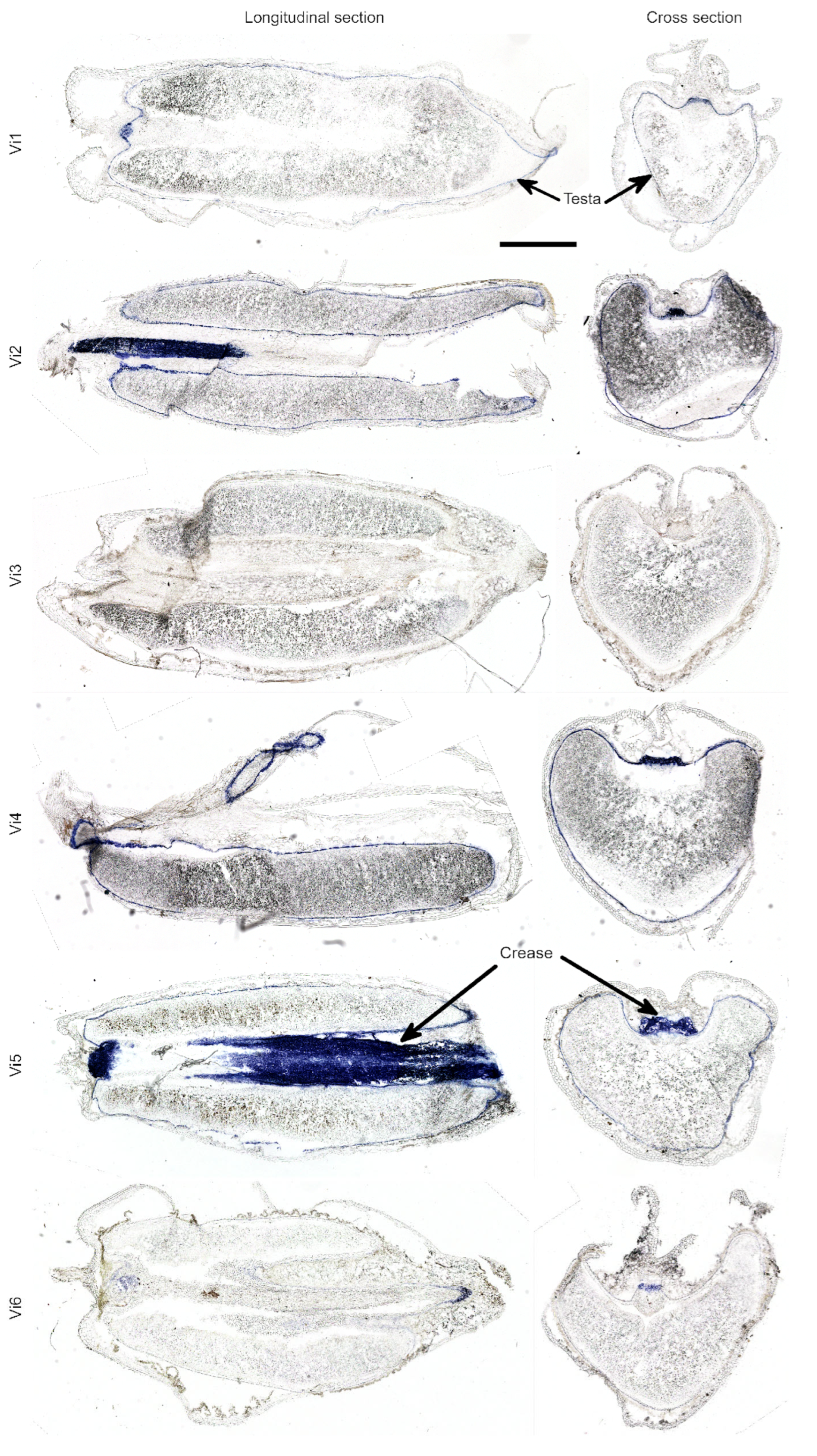
2.2. Anthocyanin and PAs Localization in Rye Kernel
3. Discussion
3.1. Color Coordinates
3.2. Pigment Localization
3.3. Genetic Implications
4. Materials and Methods
4.1. Plant Material
4.2. Color Coordinate Analysis
4.3. Histological Procedures and Microscopy of Pigment Distribution
- Vanillin-HCl Staining: Slices were incubated in absolute ethanol containing 50 mg/mL vanillin (Sigma-Aldrich, St. Louis, MO, USA) for 30 s, followed by the immediate application of a drop of concentrated HCl [46]. The slices were then coverslipped and immediately examined under a microscope.
- DMACA Staining: Slices were incubated for 20 min in a freshly prepared solution of 0.01% (w/v) 4-dimethylaminocinnamaldehyde (Sigma-Aldrich, St. Louis, MO, USA) and 0.8% (v/v) concentrated HCl in absolute ethanol [47]. After incubation, the slices were washed three times with 70% ethanol and coverslipped using self prepared Hoyer’s medium according to Cold Spring Harbour protocol [48].
4.4. MALDI-Imaging of Anthocyanins Localization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abayomi, O.O.; Gan, C.-Y.; Shafie, M.H.; Alenezi, H.; Taiwo, A.E.; Olumide, F.S. Nutritional quality of color cereals and effects of processing on its functional properties. In Functionality and Application of Colored Cereals Nutritional, Bioactive, and Health Aspects; Academic Press: Cambridge, MA, USA, 2023; pp. 27–46. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Liu, J.; Huang, T.; Zhang, X.; Xie, H.; Guo, Y.; Wang, Q.; Zhang, P.; Qin, P. Grain Color Formation and Analysis of Correlated Genes by Metabolome and Transcriptome in Different Wheat Lines at Maturity. Front. Nutr. 2023, 10, 1112497. [Google Scholar] [CrossRef] [PubMed]
- Kobylyansky, V.D. (Ed.) Cultural Flora of the USSR. A Rye. V. 2. Part 1; Agropromizdat: Leningrad, Russia, 1989; 368p. [Google Scholar]
- Lubarsky, L.N. Rozh’ (Biologo-Tekhnologicheskiye Svoystva Zerna) [A Rye (Biological and Technological Properties of Grain)]; Hleboizdat: Moscow, Russia, 1956; 259p. [Google Scholar]
- Lykholay, A.N.; Vladimirov, I.A.; Andreeva, E.A.; Smirnov, V.G.; Voylokov, A.V. Genetics of Anthocyaninless Rye. Russ. J. Genet. 2014, 50, 1102–1106. [Google Scholar] [CrossRef]
- Voylokov, A.V.; Sosnikhina, S.P.; Tikhenko, N.D.; Tsvetkova, N.V.; Mikhailova, E.I.; Smirnov, V.G. Peterhof collection of rye and its use in genetic studies. Ecol. Genet. 2018, 16, 40–49. [Google Scholar] [CrossRef]
- Andreeva, E.; Burlakovskiy, M.; Buzovkina, I.; Chekunova, E.; Dodueva, I.; Golubkova, E.; Matveenko, A.; Rumyantsev, A.; Tsvetkova, N.; Zadorsky, S.; et al. Genetic Collections of St. Petersburg University. Biol. Commun. 2023, 68, 199–214. [Google Scholar] [CrossRef]
- Voylokov, A.V.; Lykholay, A.N.; Smirnov, V.G. Genetic Control of Anthocyanin Coloration in Rye. Russ. J. Genet. Appl. Res. 2015, 5, 262–267. [Google Scholar] [CrossRef]
- Zykin, P.A.; Andreeva, E.A.; Lykholay, A.N.; Tsvetkova, N.V.; Voylokov, A.V. Anthocyanin Composition and Content in Rye Plants with Different Grain Color. Molecules 2018, 23, 948. [Google Scholar] [CrossRef]
- Whan, A.P.; Smith, A.B.; Cavanagh, C.R.; Ral, J.-P.F.; Shaw, L.M.; Howitt, C.A.; Bischof, L. GrainScan: A Low Cost, Fast Method for Grain Size and Colour Measurements. Plant Methods 2014, 10, 23. [Google Scholar] [CrossRef]
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of Objective Color Measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Liang, Z.; Sang, M.; Fan, P.; Wu, B.; Wang, L.; Yang, S.; Li, S. CIELAB Coordinates in Response to Berry Skin Anthocyanins and Their Composition in Vitis. J. Food Sci. 2011, 76, C490–C497. [Google Scholar] [CrossRef]
- Rolle, L.; Guidoni, S. Color and Anthocyanin Evaluation of Red Winegrapes by CIE L*, A*, B* Parameters. OENO One 2007, 41, 193–201. [Google Scholar] [CrossRef]
- Ham, T.-H.; Kwon, S.W.; Ryu, S.-N.; Koh, H.-J. Correlation Analysis between Grain Color and Cyanidin-3-Glucoside Content of Rice Grain in Segregate Population. Plant Breed. Biotechnol. 2015, 3, 160–166. [Google Scholar] [CrossRef]
- Shao, Y.; Hu, Z.; Yu, Y.; Mou, R.; Zhu, Z.; Beta, T. Phenolic Acids, Anthocyanins, Proanthocyanidins, Antioxidant Activity, Minerals and Their Correlations in Non-Pigmented, Red, and Black Rice. Food Chem. 2018, 239, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Chawla, M.; Chunduri, V.; Kumar, R.; Sharma, S.; Sharma, N.K.; Kaur, N.; Kumar, A.; Mundey, J.K.; Saini, M.K.; et al. Transfer of Grain Colors to Elite Wheat Cultivars and Their Characterization. J. Cereal Sci. 2016, 71, 138–144. [Google Scholar] [CrossRef]
- Han, F.-L.; Zhang, W.-N.; Pan, Q.-H.; Zheng, C.-R.; Chen, H.-Y.; Duan, C.-Q. Principal Component Regression Analysis of the Relation Between CIELAB Color and Monomeric Anthocyanins in Young Cabernet Sauvignon Wines. Molecules 2008, 13, 2859–2870. [Google Scholar] [CrossRef]
- Ravisankar, S.; Queiroz, V.A.V.; Awika, J.M. Rye Flavonoids—Structural Profile of the Flavones in Diverse Varieties and Effect of Fermentation and Heat on Their Structure and Antioxidant Properties. Food Chem. 2020, 324, 126871. [Google Scholar] [CrossRef]
- Strygina, K.V. Synthesis of Flavonoid Pigments in Grain of Representatives of Poaceae: General Patterns and Exceptions in N.I. Vavilov’s Homologous Series. Russ. J. Genet. 2020, 56, 1345–1358. [Google Scholar] [CrossRef]
- Bulanov, A.N.; Voylokov, A.V. Adaptive Significance and Origin of Flavonoid Biosynthesis Genes in the Grain of Cultivated Cereals. Russ. J. Genet. 2024, 60, 137–151. [Google Scholar] [CrossRef]
- Jende-Strid, B. Genetic Control of Flavonoid Biosynthesis in Barley. Hereditas 1993, 119, 187–204. [Google Scholar] [CrossRef]
- Kohyama, N.; Chono, M.; Nakagawa, H.; Matsuo, Y.; Ono, H.; Matsunaka, H. Flavonoid Compounds Related to Seed Coat Color of Wheat. Biosci. Biotechnol. Biochem. 2017, 81, 2112–2118. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and Hydrolysable Tannins: Occurrence, Dietary Intake and Pharmacological Effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, N.; Kapoor, P.; Chunduri, V.; Pandey, A.K.; Garg, M. Spotlight on the overlapping routes and partners for anthocyanin transport in plants. Physiol. Plant. 2021, 171, 868–881. [Google Scholar] [CrossRef]
- Himi, E.; Noda, K. Red Grain Colour Gene (R) of Wheat Is a Myb-Type Transcription Factor. Euphytica 2005, 143, 239–242. [Google Scholar] [CrossRef]
- Vaughan, S.P.; Baker, J.M.; Primavesi, L.F.; Patil, A.; King, R.; Hassani-Pak, K.; Kulasekaran, S.; Coghill, J.; Ward, J.L.; Huttly, A.K.; et al. Proanthocyanidin Biosynthesis in the Developing Wheat Seed Coat Investigated by Chemical and RNA-Seq Analysis. Plant Direct 2022, 6, e453. [Google Scholar] [CrossRef]
- Lang, J.; Jiang, H.; Cheng, M.; Wang, M.; Gu, J.; Dong, H.; Li, M.; Guo, X.; Chen, Q.; Wang, J. Variation of TaMyb10 and Their Function on Grain Color and Pre-harvest Sprouting Resistance of Wheat. Plant J. 2024, 118, 1388–1399. [Google Scholar] [CrossRef]
- White, A.D.; Lyon, D.J.; Mallory-Smith, C.; Medlin, C.R.; Yenish, J.P. Feral Rye (Secale cereale) in Agricultural Production Systems. Weed Technol. 2006, 20, 815–823. [Google Scholar] [CrossRef]
- Finch, R.A.; Simpson, E. New colours and complementary colour genes in barley. Z. Pflanzenzüchtung 1978, 81, 40–53. [Google Scholar]
- Jia, Y.; Selva, C.; Zhang, Y.; Li, B.; McFawn, L.A.; Broughton, S.; Zhang, X.; Westcott, S.; Wang, P.; Tan, C.; et al. Uncovering the Evolutionary Origin of Blue Anthocyanins in Cereal Grains. Plant J. 2020, 101, 1057–1074. [Google Scholar] [CrossRef] [PubMed]
- Zeven, A.C. Wheats with Purple and Blue Grains: A Review. Euphytica 1991, 56, 243–258. [Google Scholar] [CrossRef]
- Dedio, W.; Kaltsikes, P.J.; Larter, E.N. The Anthocyanins of Secale cereale. Phytochemistry 1969, 8, 2351–2352. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Young, J.C.; Rabalski, I. Anthocyanin Composition in Black, Blue, Pink, Purple, and Red Cereal Grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef]
- Shoeva, O.Y.; Gordeeva, E.I.; Khlestkina, E.K. The Regulation of Anthocyanin Synthesis in the Wheat Pericarp. Molecules 2014, 19, 20266–20279. [Google Scholar] [CrossRef]
- Shoeva, O.Y.; Mock, H.-P.; Kukoeva, T.V.; Börner, A.; Khlestkina, E.K. Regulation of the Flavonoid Biosynthesis Pathway Genes in Purple and Black Grains of Hordeum vulgare. PLoS ONE 2016, 11, e0163782. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Yamaguchi, T.; Omoteno, M.; Takarada, T.; Fujita, K.; Murata, K.; Iyama, Y.; Kojima, Y.; Morikawa, M.; Ozaki, H.; et al. Genetic Dissection of Black Grain Rice by the Development of a near Isogenic Line. Breed. Sci. 2014, 64, 134–141. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.N.; Sybenga, J. Chromosomal Location of 17 Monogenically Inherited Morphological Markers in Rye (Secale cereale L.) Using the Translocation Tester Set. Z. Pflanzenzüchtung 1984, 92, 117–139. [Google Scholar]
- Khlestkina, E.K.; Röder, M.S.; Börner, A. Mapping Genes Controlling Anthocyanin Pigmentation on the Glume and Pericarp in Tetraploid Wheat (Triticum durum L.). Euphytica 2010, 171, 65–69. [Google Scholar] [CrossRef]
- Francavilla, A.; Joye, I.J. Anthocyanins in Whole Grain Cereals and Their Potential Effect on Health. Nutrients 2020, 12, 2922. [Google Scholar] [CrossRef]
- Mbanjo, E.G.N.; Kretzschmar, T.; Jones, H.; Ereful, N.; Blanchard, C.; Boyd, L.A.; Sreenivasulu, N. The Genetic Basis and Nutritional Benefits of Pigmented Rice Grain. Front. Genet. 2020, 11, 229. [Google Scholar] [CrossRef]
- Furukawa, T.; Maekawa, M.; Oki, T.; Suda, I.; Iida, S.; Shimada, H.; Takamure, I.; Kadowaki, K. The Rc and Rd Genes Are Involved in Proanthocyanidin Synthesis in Rice Pericarp. Plant J. 2007, 49, 91–102. [Google Scholar] [CrossRef]
- Petroni, K.; Tonelli, C. Recent Advances on the Regulation of Anthocyanin Synthesis in Reproductive Organs. Plant Sci. 2011, 181, 219–229. [Google Scholar] [CrossRef]
- Coe, E.H.; Neuffer, M.G.; Hoisington, D.A. The Genetics of Corn. In Corn and Corn Improvement, 3rd ed.; Sprague, G.F., Dudley, J.W., Eds.; American Society of Agronomy: Madison, WI, USA; Crop Science Society of America: Madison, WI, USA; Soil Science Society of America: Madison, WI, USA, 1988; ISBN 978-0-89118-212-2. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 4. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 2 February 2025).
- Gardner, R.O. Vanillin-Hydrochloric Acid as a Histochemical Test for Tannin. Stain Technol. 1975, 50, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.H.; Liu, C.; Xiao, X.; Dixon, R.A. The Transcriptional Repressor MYB2 Regulates Both Spatial and Temporal Patterns of Proanthocyandin and Anthocyanin Pigmentation in Medicago truncatula. Plant Cell 2015, 27, 2860–2879. [Google Scholar] [CrossRef] [PubMed]
- Hoyer’s Medium. Cold Spring Harb. Protoc. 2011, 2011, pdb.rec12429. [CrossRef]
- Edelstein, A.; Amodaj, N.; Hoover, K.; Vale, R.; Stuurman, N. Computer Control of Microscopes Using µManager. Curr. Protoc. Mol. Biol. 2010, 92, 14.20.1–14.20.17. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Kawamoto, T. Use of a New Adhesive Film for the Preparation of Multi-Purpose Fresh-Frozen Sections from Hard Tissues, Whole-Animals, Insects and Plants. Arch. Histol. Cytol. 2003, 66, 123–143. [Google Scholar] [CrossRef]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A Cross-Platform Toolkit for Mass Spectrometry and Proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Race, A.M.; Styles, I.B.; Bunch, J. Inclusive Sharing of Mass Spectrometry Imaging Data Requires a Converter for All. J. Proteom. 2012, 75, 5111–5112. [Google Scholar] [CrossRef]
- Bemis, K.D.; Harry, A.; Eberlin, L.S.; Ferreira, C.; van de Ven, S.M.; Mallick, P.; Stolowitz, M.; Vitek, O. Cardinal: An R Package for Statistical Analysis of Mass Spectrometry-Based Imaging Experiments. Bioinformatics 2015, 31, 2418–2420. [Google Scholar] [CrossRef]
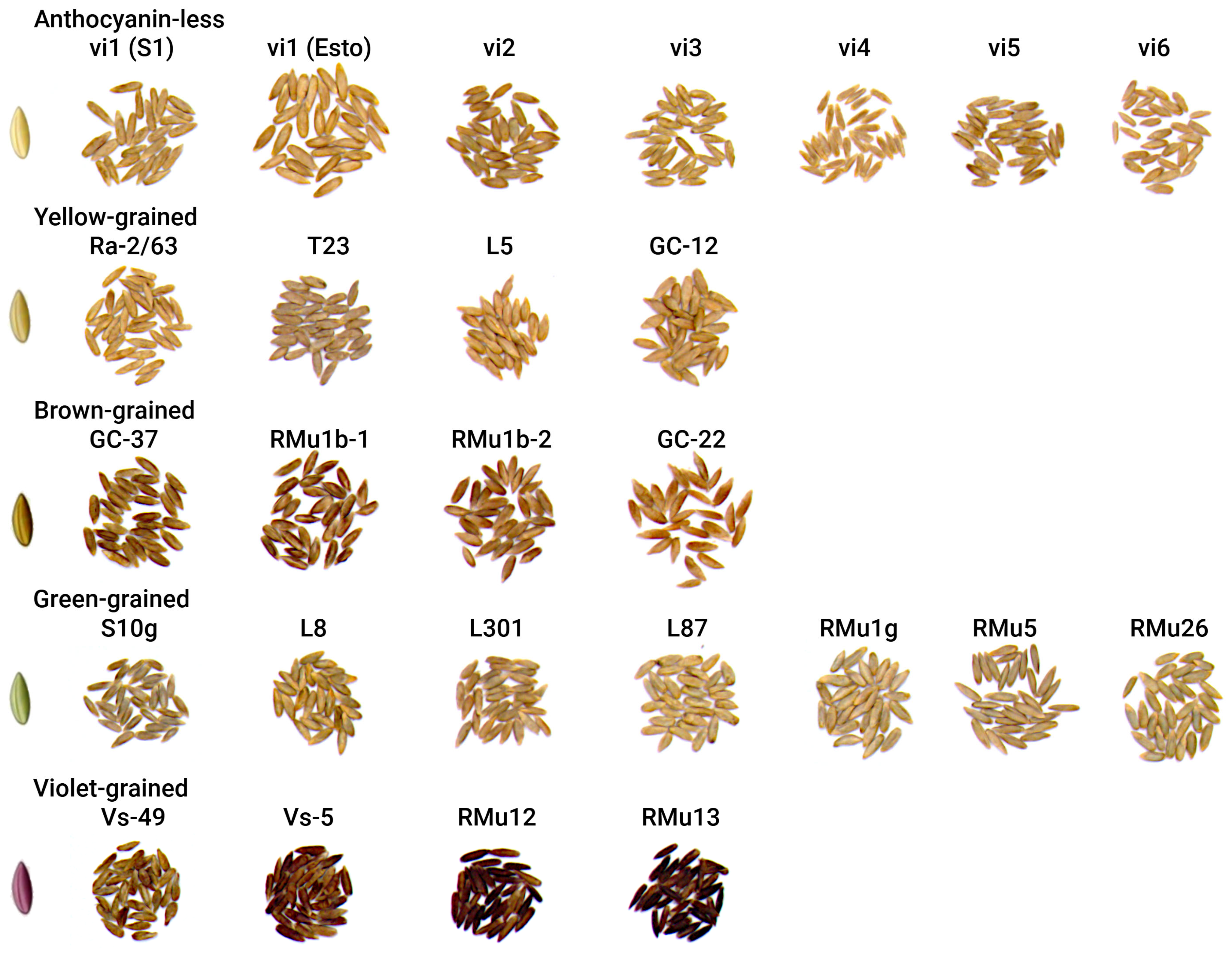

| Accessions 1 | N | L* | a* | b* | C* | h° | Color 3 |
|---|---|---|---|---|---|---|---|
| Anthocyanin-less: | |||||||
| 1. vi1 (S1) | 90 | 49.09 ± 2.12 | 3.69 ± 0.54 | 15.19 ± 0.60 | 15.64 ± 0.64 | 76.36 ± 1.83 | |
| 2. vi1 (Esto) | 98 | 48.24 ± 2.23 | 3.83 ± 0.70 | 15.56 ± 0.82 | 16.04 ± 0.90 | 76.23 ± 2.08 | |
| 3. vi2 | 96 | 45.74 ± 1.96 | 3.86 ± 0.75 | 15.46 ± 0.64 | 15.95 ± 0.73 | 76.06 ± 2.39 | |
| 4. vi3 | 94 | 50.14 ± 1.75 | 3.19 ± 0.48 | 17.27 ± 0.68 | 17.56 ± 0.72 | 79.56 ± 1.39 | |
| 5. vi4 | 94 | 49.24 ± 2.54 | 3.06 ± 0.38 | 14.80 ± 0.52 | 15.11 ± 0.56 | 78.35 ± 1.19 | |
| 6. vi5 | 91 | 48.66 ± 3.18 | 3.81 ± 0.76 | 16.16 ± 1.09 | 16.62 ± 1.18 | 76.80 ± 2.05 | |
| 7. vi6 | 100 | 50.73 ± 2.11 | 3.29 ± 0.83 | 15.66 ± 1.04 | 16.02 ± 1.10 | 78.18 ± 2.64 | |
| Mean 1–7 | 48.83 a 2 | 3.53 b | 15.73 a | 16.13 a | 77.36 b | ||
| Yellow: | |||||||
| 8. Ra-2/63 | 103 | 45.10 ± 2.55 | 3.02 ± 0.57 | 14.66 ± 1.17 | 14.98 ± 1.19 | 78.36 ± 2.03 | |
| 9. T23 | 93 | 46.76 ± 1.95 | 2.93 ± 0.45 | 13.35 ± 0.85 | 13.67 ± 0.90 | 77.64 ± 1,48 | |
| 10. L5 | 106 | 46.07 ± 2.22 | 3.82 ± 0.91 | 14.74 ± 0.96 | 15.25 ± 1.05 | 75.57 ± 3.04 | |
| 11. GC-12 | 100 | 45.04 ± 2.53 | 3.06 ± 0.50 | 14.69 ± 1.16 | 15.01 ± 1.17 | 78.22 ± 1.76 | |
| Mean 8–11 | 45.74 a | 3.21 b | 14.36 ab | 14.73 ab | 77.45 b | ||
| Brown: | |||||||
| 12. GC-37 | 90 | 39.80 ± 4.06 | 4.70 ± 0.78 | 14.65 ± 0.82 | 15.40 ± 0.85 | 72.24 ± 2.78 | |
| 13. RMu1b-1 | 89 | 44.44 ± 2.79 | 4.10 ± 0.62 | 14.87 ± 0.13 | 15.43 ± 1.20 | 74.53 ± 2.40 | |
| 14. RMu1b-2 | 135 | 38.93 ± 3.47 | 4.80 ± 0.69 | 15.40 ± 0.99 | 16.14 ± 1.08 | 72.74 ± 1.94 | |
| 15. GC-22b | 93 | 36.81 ± 3.15 | 5.05 ± 0.80 | 15.72 ± 0.83 | 16.53 ± 0.84 | 72.22 ± 2.44 | |
| Mean 12–15 | 40.00 b | 4.66 a | 15.16 ab | 15.88 ab | 72.81 c | ||
| Green: | |||||||
| 16. S10g | 100 | 48.60 ± 2.27 | 2.18 ± 0.66 | 13.10 ± 0.93 | 13.29 ± 0.98 | 80.65 ± 2.52 | |
| 17. L8 | 100 | 43.99 ± 2.48 | 2.74 ± 0.74 | 15.13 ± 1.02 | 15.38 ± 1.24 | 79.87 ± 4.65 | |
| 18. L301 | 102 | 47.07 ± 2.05 | 1.96 ± 0.42 | 13.51 ± 0.70 | 13.66 ± 0.73 | 81.77 ± 1.55 | |
| 19. L87 | 102 | 49.46 ± 2.32 | 2.02 ± 0.63 | 14.86 ± 0.99 | 15.00 ± 1.06 | 82.36 ± 1.95 | |
| 20. RMu1g | 109 | 49.06 ± 2.32 | 1.87 ± 0.51 | 12.65 ± 1.04 | 12.80 ± 1.06 | 81.64 ± 2.05 | |
| 21. RMu5 | 133 | 46.43 ± 2.09 | 2.27 ± 0.62 | 13.03 ± 0.94 | 13.24 ± 1.01 | 80.24 ± 2.13 | |
| 22. RMu26 | 101 | 45.14 ± 2.68 | 1.91 ± 0.87 | 14.28 ± 1.32 | 14.43 ± 1.40 | 82.58 ± 2.98 | |
| Mean 16–22 | 47.11 a | 2.14 c | 13.79 b | 13.97 bc | 81.30 a | ||
| Violet: | |||||||
| 23. Vs-49 | 64 | 33.53 ± 3.52 | 5.05 ± 0.94 | 13.31 ± 1.06 | 14.26 ± 1.11 | 69.23 ± 3.52 | |
| 24. Vs-5 | 92 | 32.19 ± 2.85 | 6.01 ± 0.76 | 12.11 ± 1.14 | 13.54 ± 1.28 | 63.54 ± 3.3 | |
| 25. RMu12 | 91 | 24.06 ± 2.36 | 3.02 ± 0.97 | 8.24 ± 1.00 | 8.80 ± 1.22 | 70.36 ± 4.69 | |
| 26. RMu13 | 83 | 25.02 ± 3.44 | 4.61 ± 1.20 | 9.06 ± 1.55 | 10.20 ± 1.81 | 63.30 ± 4.33 | |
| Mean 23–26 | 28.70 c | 4.67 a | 10.68 c | 11.71 c | 66.61 d |
| PAs Testa | PAs Crease | Aleurone | Testa | Pericarp | Accessions |
|---|---|---|---|---|---|
| Anthocyanin-less: | |||||
| blue | blue | uncolored | light brown | uncolored | 1. vi1 (S1) |
| uncolored | slight blue | uncolored | brown | uncolored | 2. vi1 (Esto) |
| blue | dark blue | uncolored | light brown | uncolored | 3. vi2 |
| uncolored | uncolored | light greenish | light greenish | uncolored | 4. vi3 |
| light blue | slight blue | uncolored | brown | uncolored | 5. vi4 |
| light blue | blue | uncolored | light brown | uncolored | 6. vi5 |
| uncolored | very slight blue | uncolored | slight yellow | uncolored | 7. vi6 |
| Yellow: | |||||
| blue | blue | uncolored | yellow | uncolored | 8. Ra-2/63 |
| light blue | blue | yellowish | dark brown | uncolored | 9. T23 |
| light blue | blue | uncolored | brown | uncolored | 10. L5 |
| light blue | blue | uncolored | brown | uncolored | 11. GC-12 |
| Brown: | |||||
| blue | blue | uncolored | dark brown | brown | 12. GC-37 |
| blue | blue | uncolored | light brown | light brown | 13. RMu1b-1 |
| blue | blue | uncolored | brown | light brown | 14. RMu1b-2 |
| brown | blue | uncolored | brown | brown | 15. GC-22b |
| Green: | |||||
| dark blue | dark blue | very slightly blue | brown | uncolored | 16. S10g |
| slight blue | slight blue | very slightly blue/blue | light brown | uncolored | 17. L8 |
| slight blue | slight blue | blue | brown | uncolored | 18. L301 |
| dark blue, blue aleurone | dark blue | blue | dark brown | uncolored | 19. L87 |
| very slight blue | slight blue | very slightly blue | brown | uncolored | 20. RMu1g |
| uncolored | uncolored | light-blue | uncolored | uncolored | 21. RMu5 |
| uncolored | uncolored | blue | brown | uncolored | 22. RMu26 |
| Violet: | |||||
| red | slight blue | uncolored | brown | light brown | 23. V-49 |
| slight blue | blue | uncolored | brown | brown-violet | 24. V-5 |
| dark blue | blue | uncolored | dark brown | dark violet | 25. RMu12 |
| slight brown | slight blue | very slightly pink | brown | violet | 26. RMu13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zykin, P.A.; Andreeva, E.A.; Tsvetkova, N.V.; Bulanov, A.N.; Voylokov, A.V. Anatomical and Digital Image Analysis of Flavonoid-Mediated Grain Coloration in Rye (Secale cereale L.). Plants 2025, 14, 2557. https://doi.org/10.3390/plants14162557
Zykin PA, Andreeva EA, Tsvetkova NV, Bulanov AN, Voylokov AV. Anatomical and Digital Image Analysis of Flavonoid-Mediated Grain Coloration in Rye (Secale cereale L.). Plants. 2025; 14(16):2557. https://doi.org/10.3390/plants14162557
Chicago/Turabian StyleZykin, Pavel A., Elena A. Andreeva, Natalia V. Tsvetkova, Andrey N. Bulanov, and Anatoly V. Voylokov. 2025. "Anatomical and Digital Image Analysis of Flavonoid-Mediated Grain Coloration in Rye (Secale cereale L.)" Plants 14, no. 16: 2557. https://doi.org/10.3390/plants14162557
APA StyleZykin, P. A., Andreeva, E. A., Tsvetkova, N. V., Bulanov, A. N., & Voylokov, A. V. (2025). Anatomical and Digital Image Analysis of Flavonoid-Mediated Grain Coloration in Rye (Secale cereale L.). Plants, 14(16), 2557. https://doi.org/10.3390/plants14162557









