Phosphorus-Driven Stem-Biased Allocation: NPK Synergy Optimizes Growth and Physiology in Dalbergia odorifera T. C. Chen Seedlings
Abstract
1. Introduction
2. Results
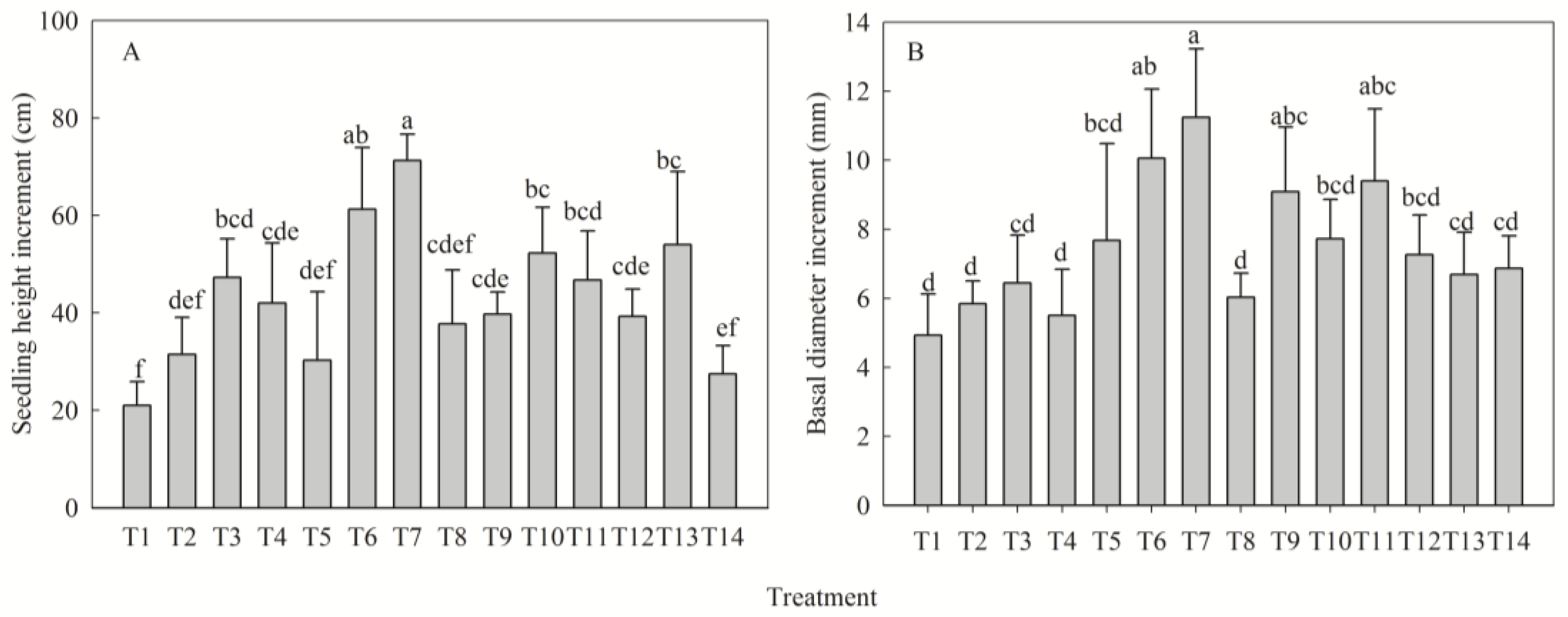
2.1. Regulatory Effects of NPK Fertilization on Seedling Morphogenesis
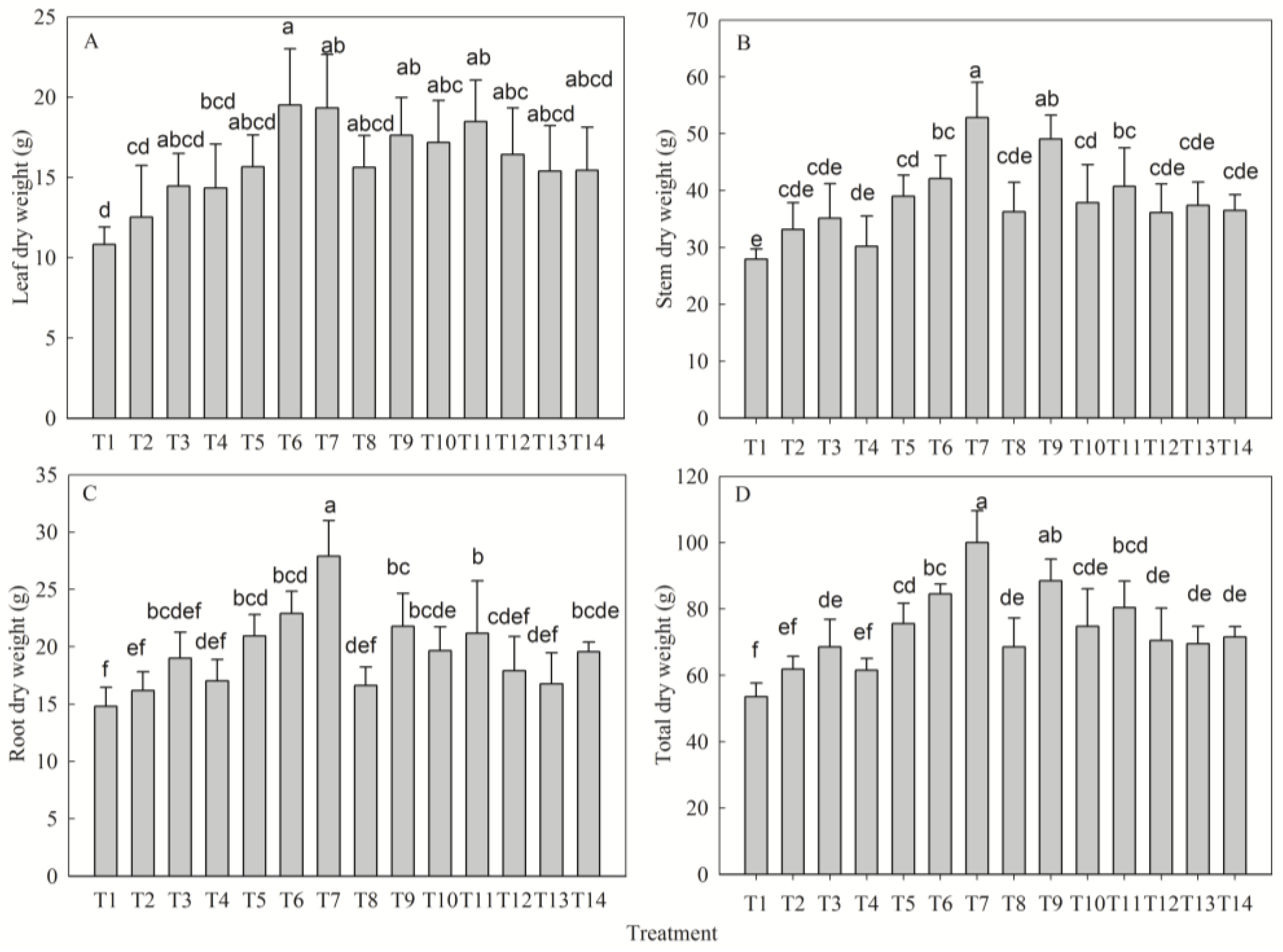
2.2. Nutrient-Driven Patterns of Biomass Accumulation and Allocation
2.3. Response of Photosynthetic Pigment Synthesis to NPK Fertilization
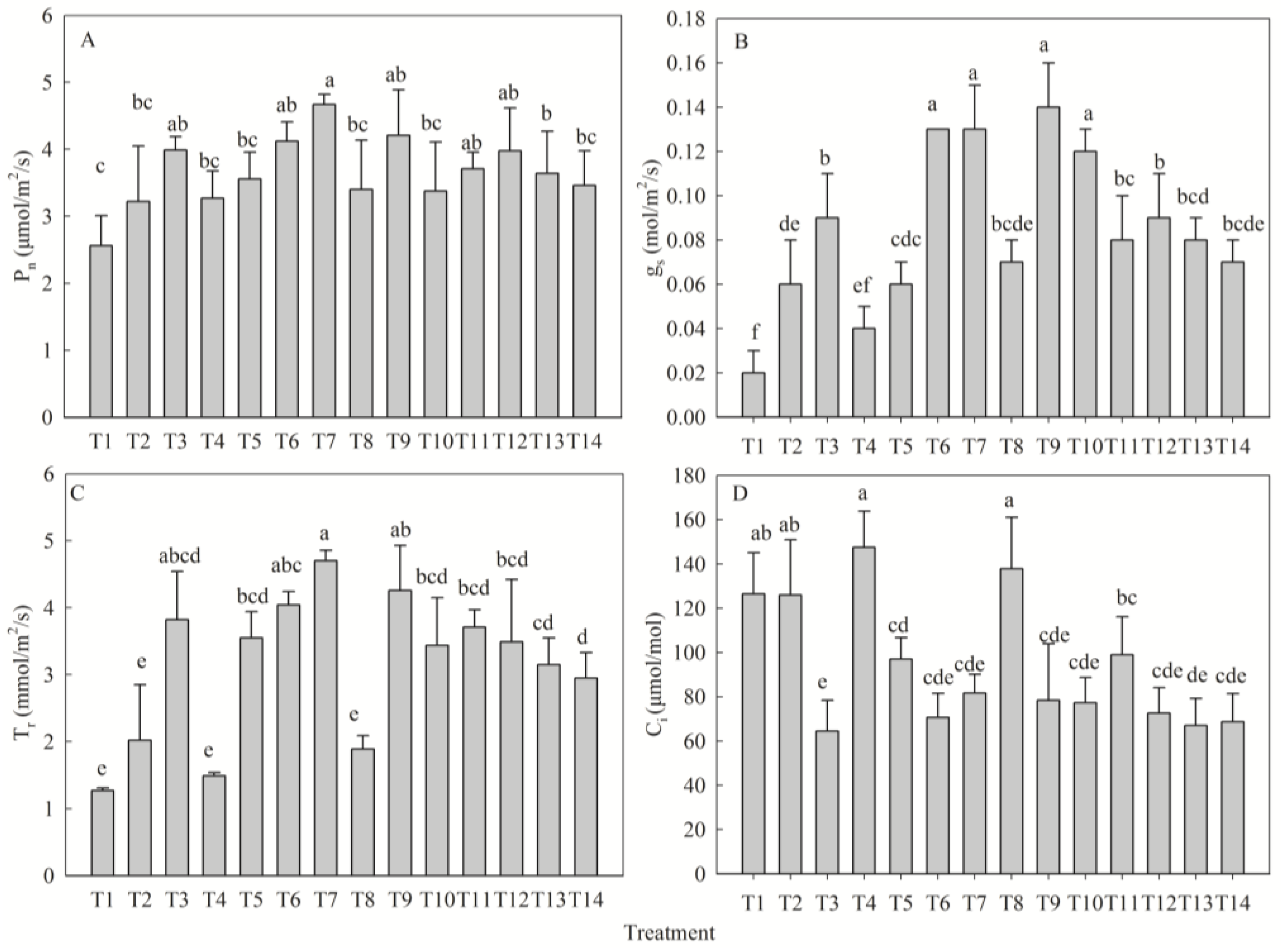
2.4. Synergistic Response of Gas Exchange Parameters to Nutrient Levels
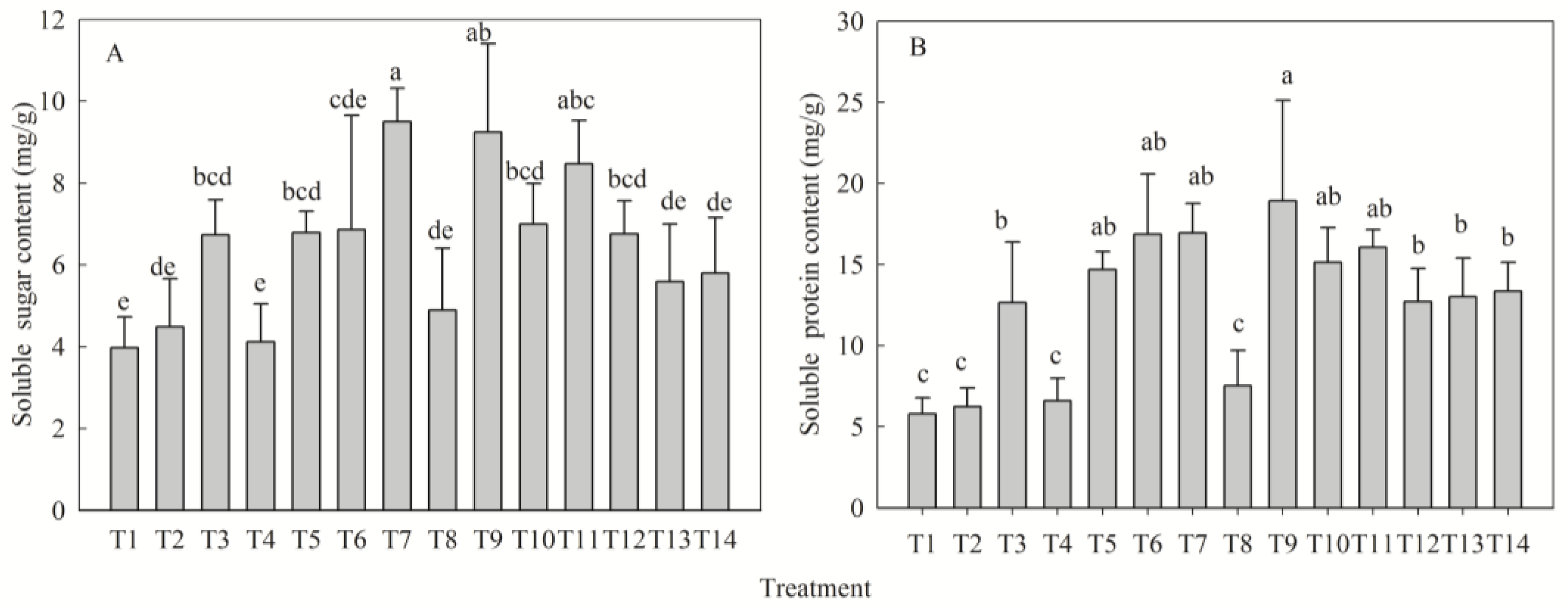
2.5. Fertilization Regulation of Carbon and Nitrogen Metabolism Products
2.6. Quantitative Screening of Optimal Fertilizer Ratios
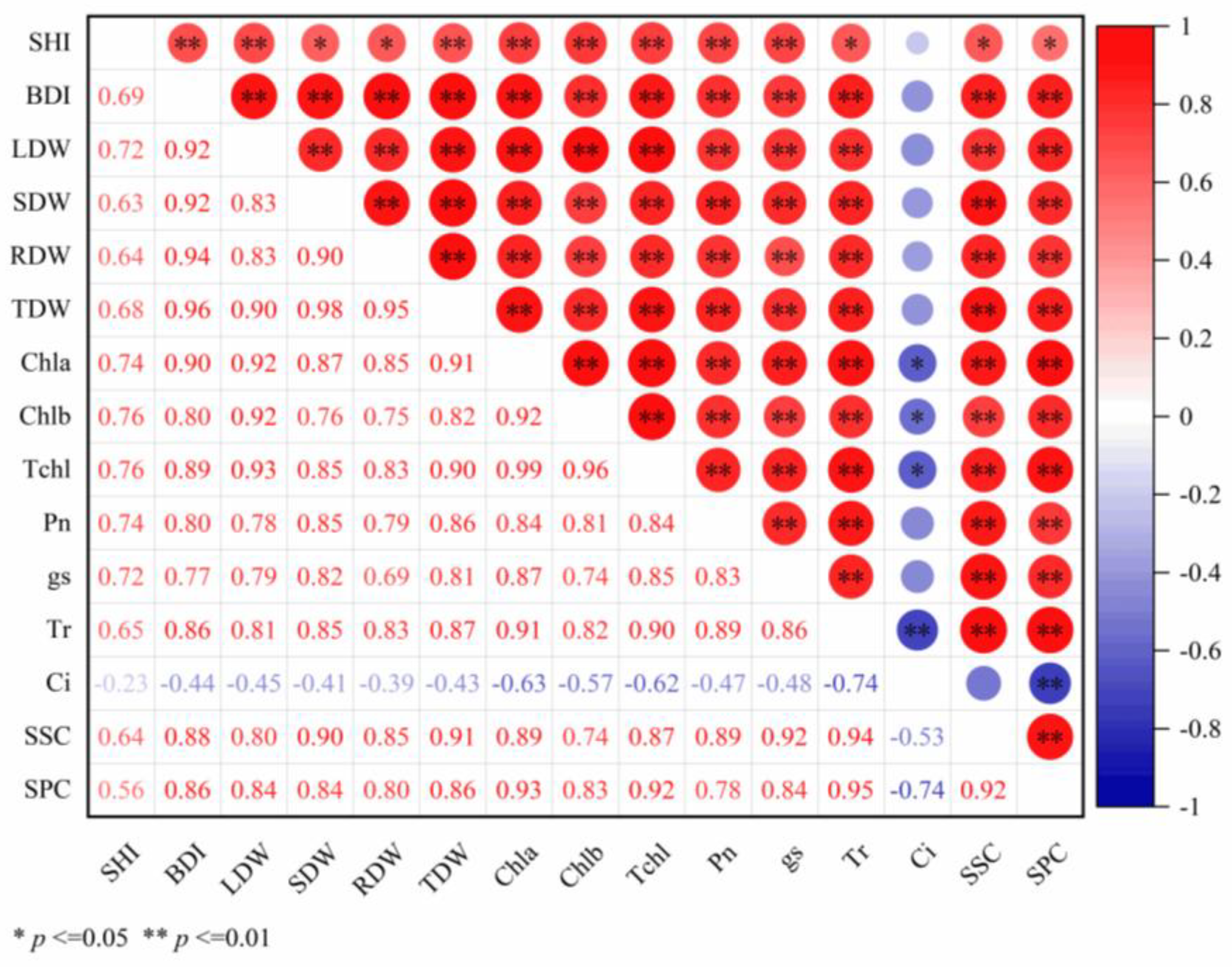
2.6.1. Interaction Network of Growth–Physiological Indicators
- (1)
- Biomass coordination: BDI exhibited strong positive correlations (r = 0.92–0.96) with all biomass components (LDW, SDW, RDW, TDW). TDW functioned as a central hub, showing robust correlations (r = 0.90–0.98) with each organ-specific biomass measure (all correlations p < 0.01).
- (2)
- Photosynthetic and metabolic integration: Photosynthetic pigments (Chla, Chlb, TChl) demonstrated high intercorrelation (r = 0.92–0.99). Chla and TChl correlated positively with Tr (Chla-Tr: r = 0.91; TChl-Tr: r = 0.90) and SPC (Chla-SPC: r = 0.93; TChl-SPC: r = 0.92), while SSC exhibited strong associations with gs (r = 0.92) and Tr (r = 0.94) (all p < 0.01).
- (3)
- Cross-module connectivity: Significant positive associations linked Chla to BDI (r = 0.90), LDW (r = 0.92), and TDW (r = 0.91); Chlb to LDW (r = 0.92); and TChl to TDW (r = 0.90). Similarly, SSC correlated positively with SDW (r = 0.90) and TDW (r = 0.91) (all p < 0.01). Notably, Ci exhibited negative correlations with all measured indicators (r = −0.740 to −0.229), which are significant for Chla, Chlb, and TChl (p < 0.05), and highly significant for Tr and SSC (p < 0.001). Collectively, this network topology underscores tight functional coupling among carbon assimilation, biomass partitioning, and nutrient metabolism.
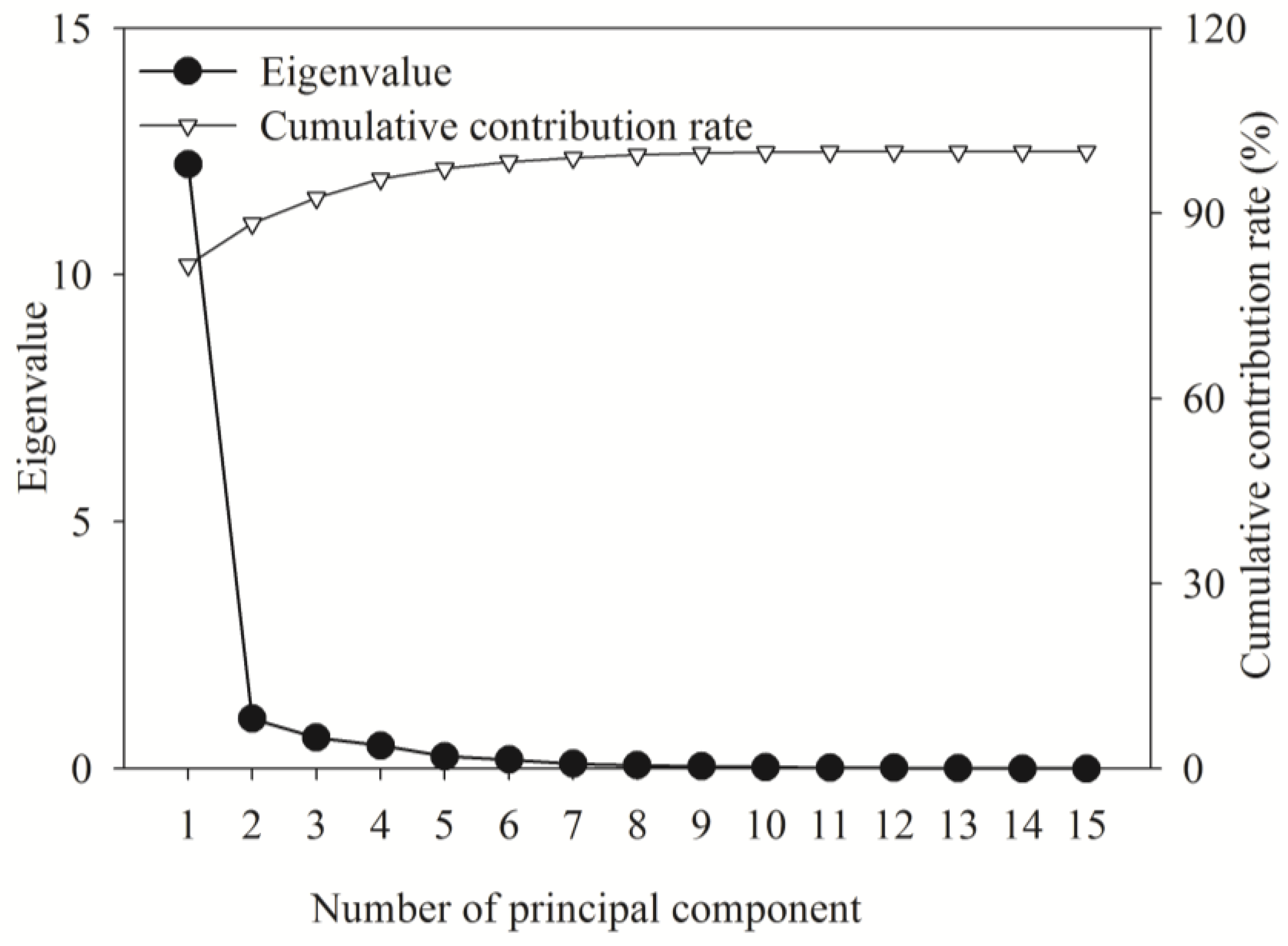
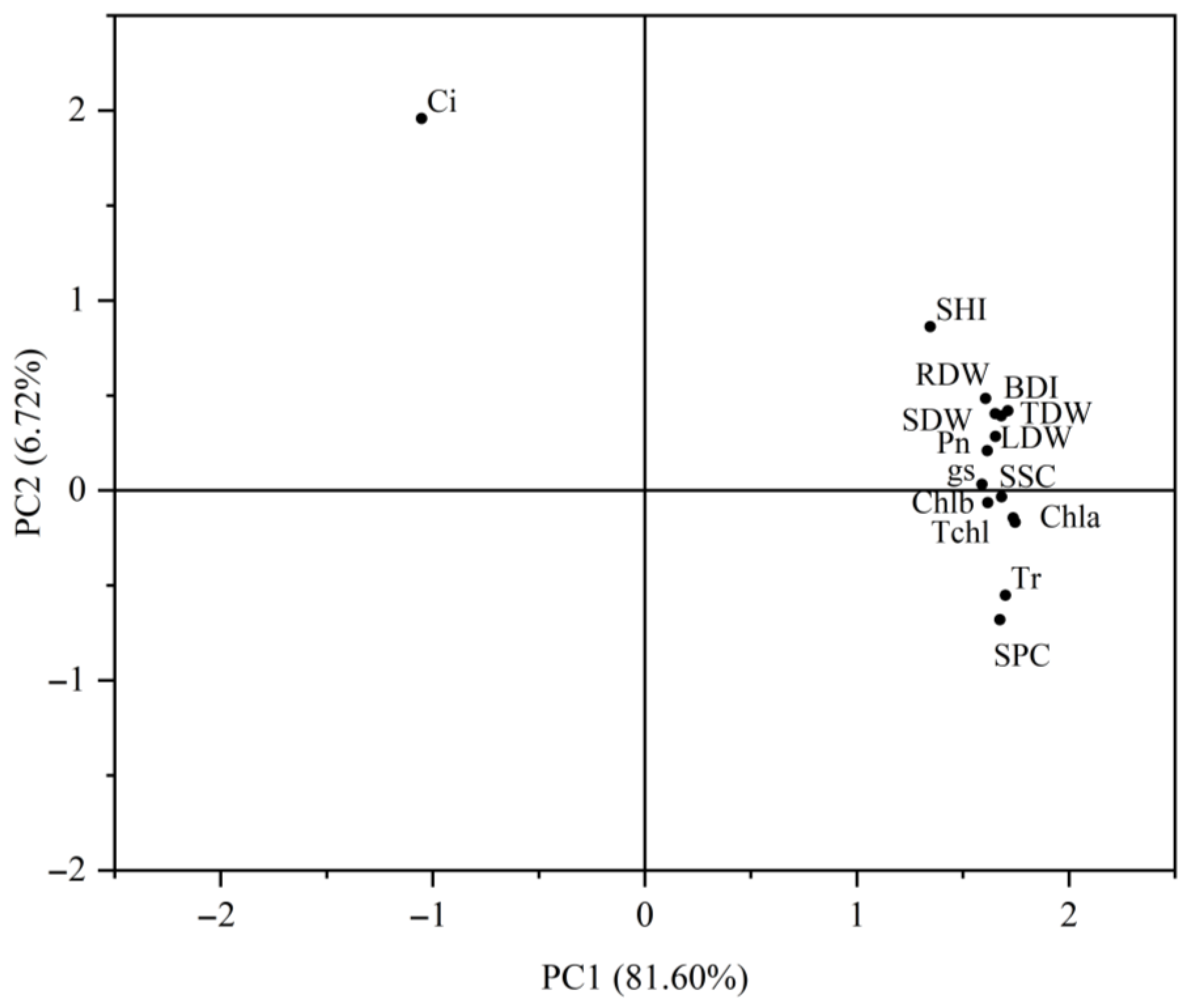
2.6.2. Comprehensive Evaluation of Fertilization Effects Based on Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Site Area
4.2. Materials
4.3. Experimental Design
4.4. Measurement Parameters
- (1)
- Growth and biomass parameters
- (2)
- Leaf physiological parameters
- (3)
- Photosynthetic characterization
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wasan, S.; Thanapat, S.; Saranya, K.; Jindarat, E.; Tadanori, A.; Sophon, B. Growth Enhancement of the Highly Prized Tropical Trees Siamese Rosewood and Burma Padauk. Rhizosphere 2021, 19, 100363. [Google Scholar] [CrossRef]
- Yue, X.; Miao, L.; Yang, F.; Nawaz, M. Morphological and Physiological Responses of Dalbergia odorifera T. Chen Seedlings to Different Culture Substances. PLoS ONE 2020, 15, e0232051. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Zhao, W.; Li, J.; Chen, J. Genome-Wide Identification, Characterization, and Genetic Diversity of CCR Gene Family in Dalbergia odorifera. Front. Plant Sci. 2022, 13, 1064262. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, C.; Meng, H.; Yu, Z.; Yang, M.; Wei, J. Dalbergia odorifera: A Review of Its Traditional Uses, Phytochemistry, Pharmacology, and Quality Control. J. Ethnopharmacol. 2020, 248, 112328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, F.; Huang, C.; Lin, L.; Dong, X.; Chen, L. Analysis of Resource Status and Site Conditions of Dalbergia odorifera Plantations in Hainan Province. Trop. For. 2024, 52, 52–57. [Google Scholar]
- Lin, L.; Zhang, M.; Chen, F.; Huang, C.; Dong, X.; Chen, L. Research Progress on Breeding Techniques of Dalbergia odorifera, a National Second-Class Protected Plant. J. Shanxi Agric. Univ. (Nat. Sci. Ed.) 2024, 44, 1–9. [Google Scholar]
- Ma, R.; Luo, J.; Qiao, M.; Fu, Y.; Zhu, P.; Wei, P.; Liu, Z. Chemical Composition of Extracts from Dalbergia odorifera Heartwood and Its Correlation with Color. Ind. Crops Prod. 2022, 180, 114693. [Google Scholar] [CrossRef]
- Cui, Z. Study on Artificial Promotion of Heartwood Formation in Dalbergia odorifera and Related Signaling Substances. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2018. [Google Scholar]
- Jia, R. Study on Artificial Promotion of Heartwood Formation in Dalbergia odorifera. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2014. [Google Scholar]
- Davis, E.C.; Sohngen, B.; Lewis, D.J. The Effect of Carbon Fertilization on Naturally Regenerated and Planted US Forests. Nat. Commun. 2022, 13, 5490. [Google Scholar] [CrossRef]
- Khan, A.; Yan, L.; Wang, W.; Xu, K.; Zou, G.W.; Liu, X.D.; Fang, X.W. Leaf Traits and Leaf Nitrogen Shift Photosynthesis Adaptive Strategies Among Functional Groups and Diverse Biomes. Ecol. Indic. 2022, 141, 109098. [Google Scholar] [CrossRef]
- Xiang, Y.X.; Pan, P.; Ouyang, X.Z.; Zang, H.; Rao, J.F. The Chemical Stoichiometry Characteristics of Plant-Soil Carbon and Nitrogen in Subtropical Pinus massoniana Natural Forests. Sci. Rep. 2024, 14, 5031. [Google Scholar] [CrossRef]
- Shalizi, M.N.; Goldfarb, B.; Burney, O.T.; Shear, T.H. Effects of Five Growing Media and Two Fertilizer Levels on Polybag-Raised Camden Whitegum (Eucalyptus benthamii Maiden & Cambage) Seedling Morphology and Drought Hardiness. Forests 2019, 10, 543. [Google Scholar]
- Garrish, V.; Cernusak, L.A.; Winter, K.; Turner, B.L. Nitrogen to Phosphorus Ratio of Plant Biomass versus Soil Solution in a Tropical Pioneer Tree, Ficus insipida. J. Exp. Bot. 2010, 61, 3735–3748. [Google Scholar] [CrossRef] [PubMed]
- Venterink, H.O.; Wassen, M.J.; Verkroost, A.W.M.; de Ruiter, P.C. Species Richness-Productivity Patterns Differ between N-, P-, and K-Limited Wetlands. Ecology 2003, 84, 2191–2199. [Google Scholar] [CrossRef]
- Schnbeck, L.; Gessler, A.; Schaub, M.; Rigling, A.; Hoch, G.; Kahmen, A.; Li, M. Soil Nutrients and Lowered Source:Sink Ratio Mitigate Effects of Mild but Not of Extreme Drought in Trees. Environ. Exp. Bot. 2020, 169, 103905. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, M.; Huang, Z.; Zou, H.; Qin, X.; Yu, Y.; Bao, Y.; Zeng, S.; Mo, Q. Non-Structural Carbohydrates Regulated by Nitrogen and Phosphorus Fertilization Varied with Organs and Fertilizer Levels in Moringa oleifera Seedlings. J. Plant Growth Regul. 2020, 40, 1777–1786. [Google Scholar] [CrossRef]
- Sloan, J.L.; Uscola, M.; Jacobs, D.F. Nitrogen Recovery in Planted Seedlings, Competing Vegetation, and Soil in Response to Fertilization on a Boreal Mine Reclamation Site. For. Ecol. Manag. 2016, 360, 60–68. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, W.; Cao, F.; Feng, G.; Li, Y.; Peng, F. Effects of Formula Fertilization on Nutrient Content and Photosynthesis of Carya illinoinensis Seedlings. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2019, 43, 169–174. [Google Scholar]
- Zhang, M.; Yang, H.; Bao, L.; Wang, T.; Zhang, N. Effects of N, P, K Combined Fertilization on Growth and Nutrient Uptake in Leaves of Moringa oleifera. J. For. Res. 2019, 32, 114–120. [Google Scholar]
- Wang, X.; Bai, J.; He, Q.; Qiu, Q.; Wu, J.; Wang, J.; Li, J. Effects of N, P, K Formula Fertilization on Growth and Nutrient Utilization of Catalpa bungei Seedlings. For. Environ. Sci. 2021, 37, 40–46. [Google Scholar] [CrossRef]
- Luo, X.; Mo, R.; Ding, G.; Chen, L. Effects of Different Fertilization Ratios on Growth Characteristics of Pinus massoniana Seedlings. Guihaia 2022, 42, 608–616. [Google Scholar]
- Tang, L.; Cheng, C.; Wan, K.; Li, R.; Wang, D.; Tao, Y.; Pan, J.; Xie, J.; Chen, F. Impact of Fertilizing Pattern on the Biodiversity of a Weed Community and Wheat Growth. PLoS ONE 2014, 9, e84370. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, D.; Ji, L.; Zhang, J.; Yang, L. Effects of Formula Fertilization on Growth, Nutrient Accumulation and Root Morphology of Tilia amurensis Seedlings. J. Cent. S. Univ. For. Technol. 2021, 41, 63–70. [Google Scholar]
- Chen, L.; Lu, L.; Meng, C. Effects of Nitrogen, Phosphorus and Potassium on Growth and Photosynthesis of Manglietia glauca Seedlings. J. Northwest For. Univ. 2017, 32, 16–21. [Google Scholar]
- Wang, X.; Wei, X.; Wu, G.; Chen, S. High Nitrate or Ammonium Applications Alleviated Photosynthetic Decline of Phoebe bournei Seedlings under Elevated Carbon Dioxide. Forests 2020, 11, 293. [Google Scholar] [CrossRef]
- Liu, J.; Chen, W.; Wang, H.; Peng, F.; Chen, M.; Liu, S.; Chu, G. Effects of NPK Fertilization on Photosynthetic Characteristics and Nutrients of Pecan at the Seedling Stage. J. Soil Sci. Plant Nutr. 2021, 21, 2425–2435. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, X.; Grossnickle, S.C.; Chen, L.; Yu, X.; El-Kassaby, Y.A.; Feng, J. Formula Fertilization Promotes Phoebe bournei Robust Seedling Cultivation. Forests 2020, 11, 781. [Google Scholar] [CrossRef]
- Guo, J.; Wu, Y.; Wang, B.; Lu, Y.; Cao, F.; Wang, G. The Effects of Fertilization on the Growth and Physiological Characteristics of Ginkgo biloba L. Forests 2016, 7, 293. [Google Scholar] [CrossRef]
- Yang, Z.; Feng, J.; Wu, X.; Chen, L.; Yu, X.; Chen, L. Effects of N, P, K Fertilization on Nutrient Absorption and Utilization in Phoebe bournei Seedlings. Chin. J. Ecol. 2021, 40, 998–1011. [Google Scholar]
- Li, Y.; Liu, X.; Xu, D.; Yang, Z.; Zhang, N.; Cui, Z. Effects of Different Fertilization Levels on Seedling Growth and Leaf Nutrient Status of Dalbergia odorifera. Chin. J. Trop. Crops 2021, 42, 481–487. [Google Scholar]
- Wang, L.; Liu, X.; Cui, Z.; Xu, D.; Yang, Z.; Zhang, N.; Hong, Z.; Chen, J.; Huang, W. Effects of Fertilization on Vegetative and Reproductive Growth of Dalbergia odorifera. Bull. Bot. Res. 2018, 38, 225–231. [Google Scholar]
- Wu, G.; Wang, L.; Liang, H.; Li, Y.; Hao, J. Effects of N, P, K Combined Fertilization on Growth and Physiology of Dalbergia odorifera Seedlings. J. Zhejiang AF Univ. 2012, 29, 296–300. [Google Scholar]
- Yang, Z.; Wu, X.; Chen, L.; Huang, L.; Chen, Y.; Wu, J.; El-Kassaby, Y.A.; Grossnickle, S.C.; Feng, J. Fertilization Regulates Accumulation and Allocation of Biomass and Nutrients in Phoebe bournei Seedlings. Agriculture 2021, 11, 1187. [Google Scholar] [CrossRef]
- Chen, K.; Ma, L.; Chen, C.; Liu, N.; Wang, B.; Bao, Y.; Liu, Z.; Zhou, G. Long-Term Impact of N, P, K Fertilizers in Different Rates on Yield and Quality of Anisodus tanguticus (Maximowicz) Pascher. Plants 2023, 12, 2102. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yang, M.; Chen, W.; Pan, Y.; Zeng, S.; Wang, W.; He, R.; Yan, B. Effects of N, P, K Fertilization on Growth and Physiology of Quercus gilva Blume Container Seedlings. J. Cent. S. Univ. For. Technol. 2024, 44, 74–85. [Google Scholar]
- Zhao, X.; Xu, D.; Liu, X.; Zhang, N.; Yang, Z. Effects of Phosphorus Nutrition on Seedling Growth and Leaf Nutrient Status of Dalbergia odorifera. Bull. Bot. Res. 2018, 38, 218–224. [Google Scholar]
- Duan, R.; Wei, X.; Zhang, Z.; Feng, Y. Formula Fertilization Experiment on Container Seedlings of Precious Tree Species Ormosia henryi. J. For. Environ. 2017, 37, 225–230. [Google Scholar]
- Zheng, S.; Teng, W.; Hu, H.; Wang, Y.; Wang, L.; Wei, J. Effects of N, P, K Fertilization on Growth and Physiological Characteristics of Jatropha curcas Seedlings. Jiangsu Agric. Sci. 2015, 43, 171–174. [Google Scholar]
- Sayed, R.M.M.; Ebeid, A.F.A.; Rabie, A.R. Effect of NPK-Fertilization on Growth, Chemical Composition and Wood Specific Gravity of Some Timber Trees Grown in Aswan Governorate, Egypt. Assiut J. Agric. Sci. 2010, 41, 43–60. [Google Scholar]
- Zhu, M.; Lu, X.; Wei, X. Formula Fertilization of Rare Tree Species Ormosia hosiei Seedlings. J. Cent. S. Univ. For. Technol. 2024, 44, 74–83. [Google Scholar]
- He, T.; Hong, Z.; Xu, D.; Mai, B.; Luo, M.; Peng, F. Effects of Formula Fertilization on Growth Characteristics of Dalbergia cochinchinensis Seedlings. For. Environ. Sci. 2024, 40, 57–68. [Google Scholar]
- Cao, S.; Hu, H.; Zhang, H.; Zhou, C.; Liu, B. Causes and Countermeasures of Soil Available Phosphorus Deficiency in Plantations of Southern China. World For. Res. 2019, 32, 78–84. [Google Scholar]
- Tang, J.; Sun, B.; Cheng, R.; Shi, Z.; Luo, D.; Liu, S.; Centritto, M. Variation in Leaf N Allocation and Mesophyll Conductance to CO2 in Four Tree Species Under Low Soil P Stress in Subtropical China. Acta Physiol. Plant. 2024, 46, 167. [Google Scholar] [CrossRef]
- Jiao, X.; Liu, J.; Zhou, X.; Shi, X. Effects of Combined N, P, K Fertilization on Growth and Physiological Characteristics of Cyclobalanopsis Container Seedlings. J. Ecol. Rural Environ. 2025; in press. [Google Scholar]
- Lei, J.; Wang, L.; Li, Y.; Jian, J.; Zhang, B.; Guan, J.; Zhu, Y. Effects of Formula Fertilization on Growth and Physiological Characteristics of Different Phellodendron chinense Provenances. J. Cent. S. Univ. For. Technol. 2024, 44, 62–72. [Google Scholar]
- Gong, L.; Zhai, B.; Zheng, W.; Liu, J.; Zheng, Z.; Zhao, Z.; Li, Z.; Wang, Z. Effects of Grass Cover Combined with Different Fertilization Regimes on Soil Nutrients and Enzyme Activities in Apple Orchard in Weibei Dryland, China. Chin. J. Appl. Ecol. 2018, 29, 205–212. [Google Scholar]
- Yu, Q.; Xu, F.; Wang, W. Effects of Nitrogen and Phosphorus Fertilizers on Biomass and Nutrient Allocation in Cunninghamia lanceolata Seedlings. J. Plant Nutr. Fertil. 2014, 20, 118–128. [Google Scholar]
- Yan, Z.; Eziz, A.; Tian, D.; Li, X.; Hou, X.; Peng, H.; Han, W.; Guo, Y.; Fang, J. Biomass Allocation in Response to Nitrogen and Phosphorus Availability: Insight from Experimental Manipulations of Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Long, X.; Chen, M.; Zhou, X.; Zhang, C.; Tong, Q.; Ren, S. Effects of N, P, K Formula Fertilization on Physiological Characteristics and Nutrient Accumulation of Torreya grandis Seedlings. Mol. Plant Breed. 2025; in press. [Google Scholar]
- Lai, H.; Li, H.; Song, Z.; Su, T.; Hou, Z.; Liu, A. Effects of Formula Fertilization on Growth and Antioxidant Characteristics of Cunninghamia lanceolata Seedlings. Acta Agric. Univ. Jiangxiensis 2021, 43, 598–609. [Google Scholar]
- Wang, X.; Zhou, Z.; Han, Q.; Pan, D.; Wang, D.; Huang, G. Effects of Nitrogen Application Methods on Growth and Root Development of Camphora micrantha Seedlings. J. Plant Nutr. Fertil. 2023, 29, 2381–2388. [Google Scholar]
- Wang, Y.; Feng, J.; Wu, X.; Huang, L.; Wu, J.; Chen, Y.; Yang, Z. Effects of Fertilization on Photosynthetic Carbon Fixation in Phoebe bournei Seedlings. Sci. Silvae Sin. 2022, 58, 40–52. [Google Scholar]
- Liu, Y.; Liu, W.; Huang, M.; Zhang, X.; Wan, S. Synergistic Effects of Water and Fertilizer on Growth and Resource Use Efficiency of Brassica rapa. N. Hortic 2024, 10, 9–16. [Google Scholar]
- Wang, X.; Yang, J.; Wu, B.; Cao, X.; Li, Y.; Zou, G.; Cao, Z. Effects of Combined Nitrogen and Phosphorus Application on Growth and Nutrient Content of Eucalyptus maidenii Seedlings. J. Yunnan Univ. (Nat. Sci. Ed.) 2022, 44, 171–178. [Google Scholar]
- Zheng, S.; Jin, Y.; Dong, Q.; Zhao, Q.; Wei, X.; Wang, Y.; Shi, S. Effects of Different N, P, K Fertilization Ratios on Growth and Leaf Nutrient Content of Solanum betaceum Seedlings. Soil Fertil. Sci. China 2024, 7, 108–122. [Google Scholar]
- Yin, M.; Yang, Y.; Tang, Y.; Li, Z.; Ye, C.; Qin, P.; Wu, X. Effects of Formula Fertilization on Growth and Physiological Characteristics of Gardenia jasminoides Seedlings. J. Cent. S. Univ. For. Technol. 2022, 42, 83–90+100. [Google Scholar]
- Liu, W.; Rao, D.; Wu, E.; Han, Y.; Chen, Y. Effects of Shading on Leaf Traits and Photosynthetic Characteristics of Alsophila spinulosa Seedlings. J. Shanxi Agric. Univ. (Nat. Sci. Ed.) 2024, 44, 52–62. [Google Scholar]
- Zhang, P.; Pang, S.; Liu, S.; Chen, H.; Duan, R.; Zeng, Q. Effects of Shading on Growth and Chlorophyll Fluorescence Parameters of Keteleeria cyclolepis Seedlings. Acta Bot. Boreal.-Occident. Sin. 2023, 43, 1716–1722. [Google Scholar]
- Yang, J.; Zhu, N.H.; Yang, H.J.; Xie, G.L. Effect of Formula Fertilization on Growth and Physiological-Biochemical Characteristics of Red-Heart Cunninghamia lanceolate Seedlings. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2024, 53, 48–55. [Google Scholar]
- Wang, X.K.; Huang, J.L. Principles and Techniques of Plant Physiological and Biochemical Experiments, 3rd ed.; Higher Education Press: Beijing, China, 2015; pp. 54–96. [Google Scholar]


| Parameter | Factor | Level 0 | Level 1 | Level 2 | Level 3 | Range Value |
|---|---|---|---|---|---|---|
| Seedling height increment (cm) | N | 31.50 ± 7.57 b | 47.25 ± 7.95 ab | 61.25 ± 12.68 a | 46.75 ± 10.03 ab | 29.75 |
| P | 42.00 ± 12.35 bc | 30.25 ± 5.40 c | 61.25 ± 12.68 ab | 71.25 ± 14.10 a | 41.00 | |
| K | 37.75 ± 11.03 b | 39.75 ± 4.55 b | 61.25 ± 12.68 a | 52.25 ± 9.42 ab | 23.50 | |
| Basal diameter increment (mm) | N | 5.85 ± 0.66 b | 6.45 ± 1.39 b | 10.06 ± 2.09 a | 9.40 ± 2.00 a | 4.21 |
| P | 5.51 ± 1.34 b | 7.67 ± 1.98 ab | 10.06 ± 2.09 a | 11.25 ± 2.81 a | 5.74 | |
| K | 6.03 ± 0.69 b | 9.09 ± 1.86 a | 10.06 ± 2.09 a | 7.72 ± 1.14 ab | 3.37 |
| Parameter | Factor | Level 0 | Level 1 | Level 2 | Level 3 | Range Value |
|---|---|---|---|---|---|---|
| Leaf dry weight (g) | N | 12.53 ± 3.23 b | 14.46 ± 2.04 ab | 19.51 ± 3.50 a | 18.48 ± 2.59 ab | 6.98 |
| P | 14.34 ± 2.74 b | 15.66 ± 1.97 b | 19.51 ± 3.49 a | 19.33 ± 3.32 a | 5.17 | |
| K | 15.63 ± 1.98 a | 17.62 ± 2.36 a | 19.51 ± 3.49 a | 17.18 ± 2.61 a | 3.88 | |
| Stem dry weight (g) | N | 33.16 ± 4.70 b | 35.14 ± 6.08 ab | 42.11 ± 4.05 a | 40.75 ± 6.77 a | 8.95 |
| P | 30.21 ± 5.31 c | 38.99 ± 3.72 bc | 42.11 ± 4.05 b | 52.82 ± 6.25 a | 22.61 | |
| K | 36.29 ± 5.18 b | 49.06 ± 4.21 a | 42.11 ± 4.05 a | 37.89 ± 6.66 b | 12.77 | |
| Root dry weight (g) | N | 16.18 ± 1.65 b | 19.01 ± 2.26 ab | 22.9 ± 1.94 a | 21.18 ± 4.57 ab | 6.72 |
| P | 17.00 ± 1.89 b | 20.94 ± 1.87 b | 22.9 ± 1.94 a | 27.88 ± 3.12 a | 10.88 | |
| K | 16.63 ± 1.62 c | 21.79 ± 2.86 ab | 22.9 ± 1.94 a | 19.66 ± 2.07 bc | 6.28 | |
| Total dry weight (g) | N | 61.87 ± 3.87 b | 68.61 ± 8.26 b | 84.52 ± 3.00 a | 80.40 ± 8.04 a | 22.65 |
| P | 61.56 ± 3.52 c | 75.59 ± 6.15 b | 84.52 ± 3.00 ab | 100.03 ± 9.59 a | 38.47 | |
| K | 68.54 ± 8.77 b | 88.47 ± 6.61 a | 84.52 ± 3.00 a | 74.73 ± 11.32 ab | 19.93 |
| Parameter | Factor | Level 0 | Level 1 | Level 2 | Level 3 | Range Value |
|---|---|---|---|---|---|---|
| Chlorophyll a (mg/g) | N | 1.64 ± 0.14 b | 1.91 ± 0.17 ab | 2.28 ± 0.23 a | 2.18 ± 0.40 a | 0.64 |
| P | 1.69 ± 0.10 c | 2.00 ± 0.10 b | 2.28 ± 0.23 ab | 2.49 ± 0.14 a | 0.80 | |
| K | 1.79 ± 0.18 b | 2.23 ± 0.30 a | 2.28 ± 0.23 a | 2.22 ± 0.13 a | 0.49 | |
| Chlorophyll b (mg/g) | N | 0.61 ± 0.04 a | 0.76 ± 0.14 a | 0.87 ± 0.25 a | 0.86 ± 0.06 a | 0.26 |
| P | 0.72 ± 0.03 a | 0.79 ± 0.06 a | 0.87 ± 0.25 a | 0.91 ± 0.10 a | 0.19 | |
| K | 0.75 ± 0.05 a | 0.80 ± 0.05 a | 0.87 ± 0.25 a | 0.81 ± 0.12 a | 0.12 | |
| Total chlorophyll (mg/g) | N | 2.25 ± 0.10 b | 2.67 ± 0.23 ab | 3.16 ± 0.38 a | 3.04 ± 0.45 a | 0.91 |
| P | 2.41 ± 0.07 c | 2.79 ± 0.04 b | 3.16 ± 0.38 ab | 3.40 ± 0.09 a | 0.99 | |
| K | 2.54 ± 0.16 b | 3.04 ± 0.30 ab | 3.16 ± 0.38 a | 3.03 ± 0.24 ab | 0.62 |
| Parameter | Factor | Level 0 | Level 1 | Level 2 | Level 3 | Range Value |
|---|---|---|---|---|---|---|
| Net photosynthetic rate (μmol/m2/s) | N | 3.22 ± 0.83 b | 3.99 ± 0.20 ab | 4.12 ± 0.29 a | 3.71 ± 0.25 ab | 0.9 |
| P | 3.27 ± 0.41 c | 3.56 ± 0.40 bc | 4.12 ± 0.29 ab | 4.67 ± 0.15 a | 1.4 | |
| K | 3.40 ± 0.74 a | 4.21 ± 0.68 a | 4.12 ± 0.29 a | 3.38 ± 0.73 a | 0.81 | |
| Stomatal conductance (mol/m2/s) | N | 0.06 ± 0.02 bc | 0.09 ± 0.02 b | 0.13 ± 0.02 a | 0.08 ± 0.02 c | 0.06 |
| P | 0.04 ± 0.01 b | 0.06 ± 0.01 b | 0.13 ± 0.02 a | 0.14 ± 0.02 a | 0.09 | |
| K | 0.07 ± 0.01 b | 0.14 ± 0.02 a | 0.13 ± 0.02 a | 0.12 ± 0.01 a | 0.07 | |
| Transpiration rate (mol/m2/s) | N | 2.02 ± 0.83 b | 3.82 ± 0.72 a | 4.04 ± 0.20 a | 3.71 ± 0.26 a | 2.02 |
| P | 1.49 ± 0.05 d | 3.55 ± 0.39 c | 4.04 ± 0.20 b | 4.70 ± 0.16 a | 3.21 | |
| K | 1.89 ± 0.20 b | 4.26 ± 0.67 a | 4.04 ± 0.20 a | 3.44 ± 0.71 a | 2.37 | |
| Intercellular CO2 concentration (μmol/m2/s) | N | 125.95 ± 24.88 a | 64.45 ± 13.87 c | 70.63 ± 10.89 bc | 98.98 ± 17.08 ab | 61.50 |
| P | 147.55 ± 16.31 a | 97.05 ± 9.61 b | 70.63 ± 10.89 bc | 81.73 ± 8.35 b | 76.93 | |
| K | 137.80 ± 23.30 a | 78.40 ± 25.48 a | 70.63 ± 10.89 bc | 77.28 ± 11.43 c | 60.52 |
| Parameter | Factor | Level 0 | Level 1 | Level 2 | Level 3 | Range Value |
|---|---|---|---|---|---|---|
| Soluble sugar content (mg/g) | N | 4.49 ± 1.18 b | 6.73 ± 0.86 ab | 6.86 ± 2.79 ab | 8.46 ± 2.79 a | 3.97 |
| P | 4.12 ± 0.93 b | 6.79 ± 0.52 ab | 6.86 ± 2.79 ab | 9.50 ± 0.82 a | 5.38 | |
| K | 4.90 ± 1.50 b | 9.24 ± 2.16 a | 6.86 ± 2.79 ab | 6.99 ± 1.00 ab | 4.34 | |
| Soluble protein content (mg/g) | N | 6.25 ± 1.13 b | 12.66 ± 3.72 ab | 16.86 ± 3.72 a | 16.06 ± 1.09 a | 10.61 |
| P | 6.62 ± 1.38 b | 14.69 ± 1.12 a | 16.86 ± 3.72 a | 16.94 ± 1.81 a | 10.32 | |
| K | 7.53 ± 2.19 b | 18.94 ± 6.18 a | 16.86 ± 3.72 a | 15.13 ± 2.16 a | 11.41 |
| Fertilization Treatment | 1st Principal Component Score (Y1) | 2nd Principal Component Score (Y1) | Aggregate Score (Y) | Rank |
|---|---|---|---|---|
| T1 | −6.685580906 | 0.025042903 | −5.093657638 | 14 |
| T2 | −4.214583572 | 0.587257653 | −3.155666934 | 13 |
| T3 | −0.117797928 | −1.063754402 | −0.192858634 | 9 |
| T4 | −3.935690246 | 1.490184221 | −2.855595941 | 12 |
| T5 | 0.169408269 | −0.759485148 | 0.055544937 | 7 |
| T6 | 3.355158235 | 0.612888541 | 2.616852135 | 3 |
| T7 | 6.415965173 | 1.801764641 | 5.065142429 | 1 |
| T8 | −2.529796481 | 1.281857014 | −1.804138241 | 11 |
| T9 | 3.667974009 | −0.31744776 | 2.765155675 | 2 |
| T10 | 1.368564003 | −0.470855918 | 0.997566681 | 5 |
| T11 | 2.574127645 | −0.135080961 | 1.949040803 | 4 |
| T12 | 0.330418012 | −0.907324911 | 0.163950419 | 6 |
| T13 | 0.061868 | −0.861239638 | −0.036286625 | 8 |
| T14 | −0.460048996 | −1.283797544 | −0.475059492 | 10 |
| Number | Fertilization Treatment | N (g/plant) | P2O5 (g/plant) | K2O (g/plant) |
|---|---|---|---|---|
| T1 | N0P0K0 | 0.00 | 0.00 | 0.00 |
| T2 | N0P2K2 | 0.00 | 20.83 | 0.83 |
| T3 | N1P2K2 | 2.16 | 20.83 | 0.83 |
| T4 | N2P0K2 | 4.31 | 0.00 | 0.83 |
| T5 | N2P1K2 | 4.31 | 10.42 | 0.83 |
| T6 | N2P2K2 | 4.31 | 20.83 | 0.83 |
| T7 | N2P3K2 | 4.31 | 31.25 | 0.83 |
| T8 | N2P2K0 | 4.31 | 20.83 | 0.00 |
| T9 | N2P2K1 | 4.31 | 20.83 | 0.42 |
| T10 | N2P2K3 | 4.31 | 20.83 | 1.25 |
| T11 | N3P2K2 | 6.47 | 20.83 | 0.83 |
| T12 | N1P1K2 | 2.16 | 10.42 | 0.83 |
| T13 | N1P2K1 | 2.16 | 20.83 | 0.42 |
| T14 | N2P1K1 | 2.16 | 10.42 | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Huang, C.; Lin, L.; Chen, L.; Yang, X.; Dong, X.; Song, J.; Chen, F. Phosphorus-Driven Stem-Biased Allocation: NPK Synergy Optimizes Growth and Physiology in Dalbergia odorifera T. C. Chen Seedlings. Plants 2025, 14, 2545. https://doi.org/10.3390/plants14162545
Zhang M, Huang C, Lin L, Chen L, Yang X, Dong X, Song J, Chen F. Phosphorus-Driven Stem-Biased Allocation: NPK Synergy Optimizes Growth and Physiology in Dalbergia odorifera T. C. Chen Seedlings. Plants. 2025; 14(16):2545. https://doi.org/10.3390/plants14162545
Chicago/Turabian StyleZhang, Mengwen, Chuanteng Huang, Ling Lin, Lin Chen, Xiaoli Yang, Xiaona Dong, Jiaming Song, and Feifei Chen. 2025. "Phosphorus-Driven Stem-Biased Allocation: NPK Synergy Optimizes Growth and Physiology in Dalbergia odorifera T. C. Chen Seedlings" Plants 14, no. 16: 2545. https://doi.org/10.3390/plants14162545
APA StyleZhang, M., Huang, C., Lin, L., Chen, L., Yang, X., Dong, X., Song, J., & Chen, F. (2025). Phosphorus-Driven Stem-Biased Allocation: NPK Synergy Optimizes Growth and Physiology in Dalbergia odorifera T. C. Chen Seedlings. Plants, 14(16), 2545. https://doi.org/10.3390/plants14162545







