Priority Effects Favor Invasive Bidens frondosa over Its Native Congener Bidens biternata, While Late Arrival Incurs Higher Costs
Abstract
1. Introduction
2. Results
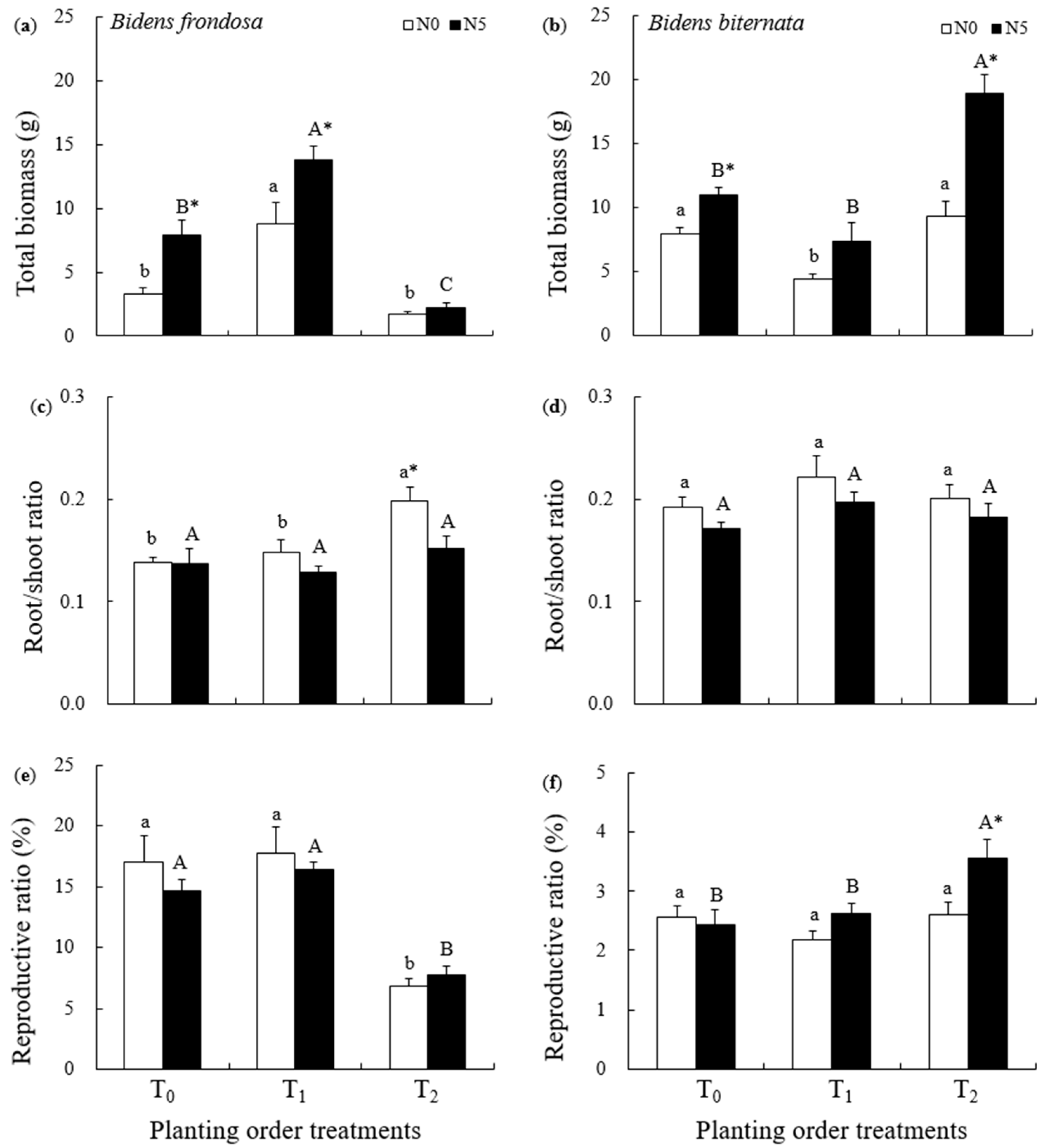
2.1. Growth Comparison of Invasive B. frondosa and Native B. biternata
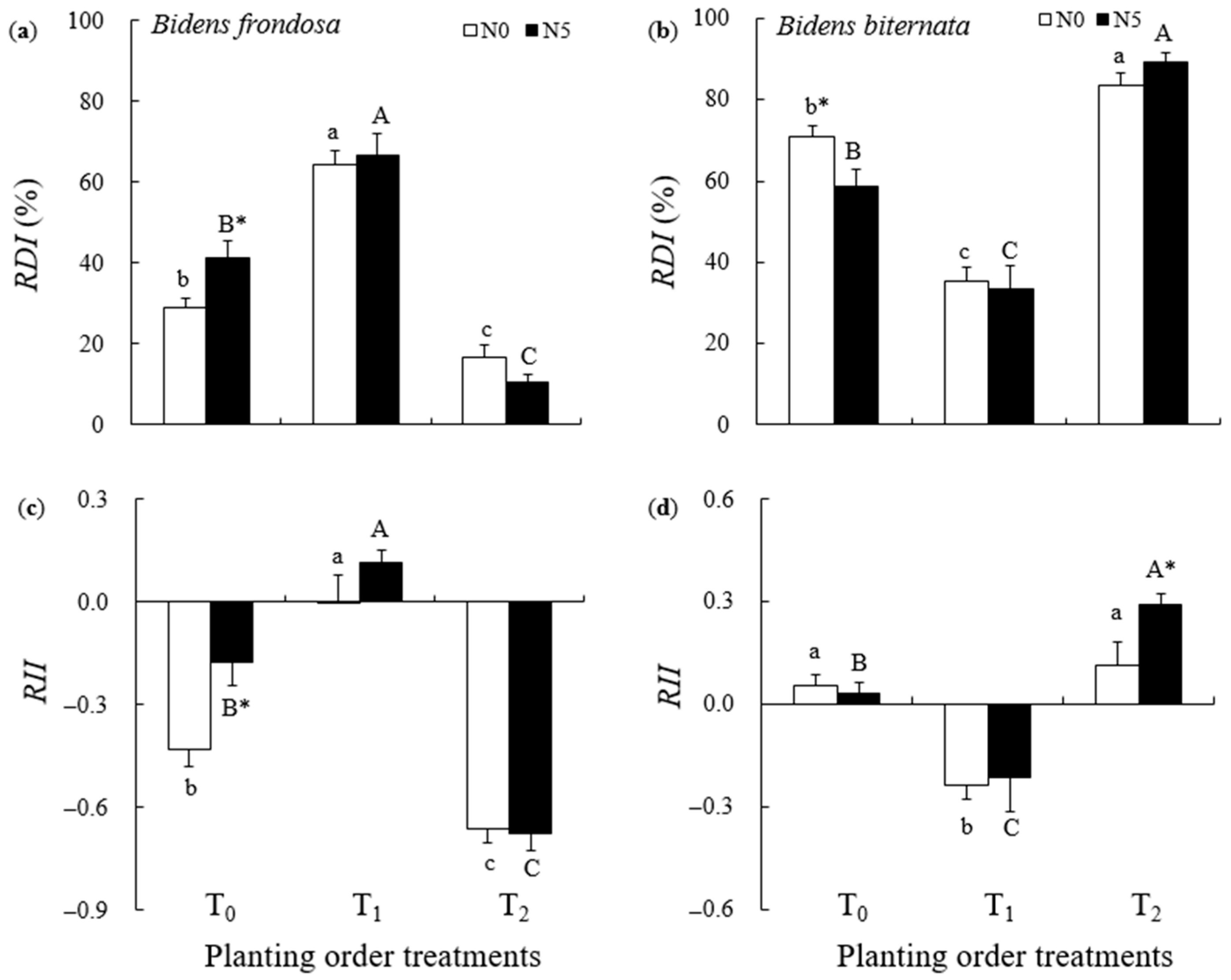
2.2. Competitive Performance of B. frondosa and B. biternata
3. Discussion
3.1. Priority Effects on Growth in the Invasive B. frondosa and Its Native Congener
3.2. Priority Effects on Competitiveness in the Invasive B. frondosa and Its Native Congener
3.3. The Magnitude of Priority Effects for Invasive B. frondosa and Its Native Congener Under N Addition
4. Materials and Methods
4.1. Study Site
4.2. Plant Materials
4.3. Experimental Design
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| RDI | Relative dominance index |
| RII | Relative interaction index |
| RGR | Relative growth rates |
References
- León Cordero, R.; Torchelsen, F.P.; Overbeck, G.E.; Anand, M. Invasive gorse (Ulex europaeus, Fabaceae) changes plant community structure in subtropical forest–grassland mosaics of southern Brazil. Biol. Invasions 2016, 18, 1629–1643. [Google Scholar] [CrossRef]
- Power, G.; Vilas, J.S. Competition between the invasive Impatiens glandulifera and UK native species: The role of soil conditioning and pre-existing resident communities. Biol. Invasions 2020, 22, 152–1537. [Google Scholar] [CrossRef]
- Qin, R.M.; Zheng, Y.L.; Valiente-Banuet, A.; Callaway, R.M.; Barclay, G.F.; Pereyra, C.S.; Feng, Y.L. The evolution of increased competitive ability, innate competitive advantages, and novel biochemical weapons act in concert for a tropical invader. New Phytol. 2012, 197, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.H.; Zhou, B.; Yin, Z.F.; Wang, N.; Zhang, Z.G. Reproductive biological characteristics potentially contributed to invasiveness in an alien invasive plant Bidens frondosa. Plant Spec. Biol. 2015, 31, 107–116. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Burns, J.H.; Liao, Z.L.; Li, W.T.; Li, L. Nutrient fluctuation has different effects on a tropical invader in communities from the native and non-native range. Environ. Exp. Bot. 2020, 178, 104193. [Google Scholar] [CrossRef]
- Wilsey, B.J.; Barber, K.; Martin, L.M. Exotic grassland species have stronger priority effects than natives regardless of whether they are cultivated or wild genotypes. New Phytol. 2015, 205, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Q.; Tang, S.C.; Pan, Y.M.; Wei, C.Q. Effects of light and temperature on germination of heteromorphic achenes of Bidens frondosa L. J. Trop. Subtrop. Bot. 2015, 23, 662–668. [Google Scholar] [CrossRef]
- Montesinos, D. Fast invasives fastly become faster: Invasive plants align largely with the fast side of the plant economics spectrum. J. Ecol. 2021, 110, 1010–1014. [Google Scholar] [CrossRef]
- Fukami, T. Historical contingency in community assembly: Integrating niches, species pools, and priority effects. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 1–23. [Google Scholar] [CrossRef]
- Delory, B.M.; Weidlich, E.W.A.; Kunz, M.; Neitzel, J.; Temperton, V.M. The exotic species Senecio inaequidens pays the price for arriving late in temperate European grassland communities. Oecologia 2019, 191, 657–671. [Google Scholar] [CrossRef]
- Weidlich, E.W.A.; Nelson, C.R.; Maron, J.L.; Callaway, R.M.; Delory, B.M.; Temperton, V.M. Priority effects and ecological restoration. Restor. Ecol. 2020, 29, e13317. [Google Scholar] [CrossRef]
- Young, T.P.; Stuble, K.L.; Balachowski, J.A.; Werner, C.M. Using priority effects to manipulate competitive relationships in restoration. Restor. Ecol. 2016, 25, S114–S123. [Google Scholar] [CrossRef]
- Yan, X.H.; Li, X.L.; Jin, K. Priority effects of forbs arriving early: The role of root interaction and asymmetric competition. Ecol. Process. 2024, 13, 4. [Google Scholar] [CrossRef]
- Weidlich, E.W.A.; de Dechoum, M.d.S. Exploring the potential of using priority effects during ecological restoration to resist biological invasions in the neotropics. Restor. Ecol. 2020, 29, e13295. [Google Scholar] [CrossRef]
- Byun, C. Role of priority effects in invasive plant species management: Early arrival of native seeds guarantees the containment of invasion by giant ragweed. Ecol. Evol. 2023, 13, e9940. [Google Scholar] [CrossRef] [PubMed]
- Aschehoug, E.T.; Brooker, R.; Atwater, D.Z.; Maron, J.L.; Callaway, R.M. The mechanisms and consequences of interspecific competition among plants. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 263–281. [Google Scholar] [CrossRef]
- Tan, J.; Pu, Z.; Ryberg, W.A.; Jiang, L. Species phylogenetic relatedness, priority effects, and ecosystem functioning. Ecology 2012, 93, 1164–1172. [Google Scholar] [CrossRef]
- Vannette, R.L.; Fukami, T. Historical contingency in species interactions: Towards niche-based predictions. Ecol. Lett. 2013, 17, 115–124. [Google Scholar] [CrossRef]
- Stuble, K.L.; Souza, L. Priority effects: Natives, but not exotics, pay to arrive late. J. Ecol. 2016, 104, 987–993. [Google Scholar] [CrossRef]
- Li, X.T.; Chen, J.; Guo, W. A review of the influence factors of plant phenology under different climate types. J. Earth Environ. 2018, 9, 16–27. [Google Scholar] [CrossRef]
- Gioria, M.; Pyšek, P.; Osborne, B.A. Timing is everything: Does early and late germination favor invasions by herbaceous alien plants? J. Plant Ecol. 2018, 11, 4–16. [Google Scholar] [CrossRef]
- Tarsa, E.E.; Holdaway, B.M.; Kettenring, K.M. Tipping the balance: The role of seed density, abiotic filters, and priority effects in seed-based wetland restoration. Ecol. Appl. 2022, 32, e2706. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Sobuj, N.; Byun, C. Native plants do not benefit from arriving early, but invasives pay to arrive late. Ecol. Indic. 2024, 166, 112453. [Google Scholar] [CrossRef]
- Hess, M.C.M.; Mesléard, F.; Buisson, E. Priority effects: Emerging principles for invasive plant species management. Ecol. Eng. 2019, 127, 48–57. [Google Scholar] [CrossRef]
- Dickson, T.L.; Hopwood, J.L.; Wilsey, B.J. Do priority effects benefit invasive plants more than native plants? An experiment with six grassland species. Biol. Invasions 2012, 14, 2617–2624. [Google Scholar] [CrossRef]
- Ferenc, V.; Merkert, C.; Zilles, F.; Sheppard, C.S. Native and alien species suffer from late arrival, while negative effects of multiple alien species on natives vary. Oecologia 2021, 197, 271–281. [Google Scholar] [CrossRef]
- Cleland, E.E.; Esch, E.; McKinney, J. Priority effects vary with species identity and origin in an experiment varying the timing of seed arrival. Oikos 2014, 124, 33–40. [Google Scholar] [CrossRef]
- Kardol, P.; Souza, L.; Classen, A.T. Resource availability mediates the importance of priority effects in plant community assembly and ecosystem function. Oikos 2012, 122, 84–94. [Google Scholar] [CrossRef]
- Goodale, K.M.; Wilsey, B.J. Priority effects are affected by precipitation variability and are stronger in exotic than native grassland species. Plant Ecol. 2018, 219, 429–439. [Google Scholar] [CrossRef]
- Ejrnæs, R.; Bruun, H.H.; Graae, B.J. Community assembly in experimental grasslands: Suitable environment or timely arrival? Ecology 2006, 87, 1225–1233. [Google Scholar] [CrossRef]
- He, W.M.; Montesinos, D.; Thelen, G.C.; Callaway, R.M. Growth and competitive effects of Centaurea stoebe populations in response to simulated nitrogen deposition. PLoS ONE 2012, 7, e36257. [Google Scholar] [CrossRef]
- Wan, L.Y.; Qi, S.S.; Zou, C.B.; Dai, Z.C.; Ren, G.Q.; Chen, Q.; Zhu, B.; Du, D.L. Elevated nitrogen deposition may advance invasive weed, Solidago canadensis, in calcareous soils. J. Plant Ecol. 2019, 12, 846–856. [Google Scholar] [CrossRef]
- Ma, J.S.; Li, H.R. The Checklist of the Alien Invasive Plants in China; Higher Education Press: Beijing, China, 2018. [Google Scholar]
- Yan, X.H.; Zeng, J.J.; Zhou, B.; Wang, N.; Xiang, H.H.; Kang, Y.Y. Allelopathic potential of the extracts from alien invasive plant Bidens frondosa. J. Yangzhou Univ. 2012, 33, 88–94. [Google Scholar] [CrossRef]
- Pan, Y.M.; Tang, S.C.; Wei, C.Q.; Li, X.Q. Effects of global risks-Nitrogen additions on growth and competitive relations among invasive and native congeneric species-Bidens frondosa. Pol. J. Ecol. 2016, 64, 443–452. [Google Scholar] [CrossRef]
- Wei, C.Q.; Tang, S.C.; Pan, Y.M.; Li, X.Q. Plastic responses of invasive Bidens frondosa to water and nitrogen addition. Nord. J. Bot. 2017, 35, 232–239. [Google Scholar] [CrossRef]
- Funk, J.L.; Cleland, E.E.; Suding, K.N.; Zavaleta, E.S. Restoration through reassembly: Plant traits and invasion resistance. Trends Ecol. Evol. 2008, 23, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Yannelli, F.A.; Koch, C.; Jeschke, J.M.; Kollmann, J. Limiting similarity and Darwin’s naturalization hypothesis: Understanding the drivers of biotic resistance against invasive plant species. Oecologia 2017, 183, 775–784. [Google Scholar] [CrossRef]
- Wainwright, C.E.; Cleland, E.E. Exotic species display greater germination plasticity and higher germination rates than native species across multiple cues. Biol. Invasions 2013, 15, 2253–2264. [Google Scholar] [CrossRef]
- Pan, Y.M.; Tang, S.C.; Wei, C.Q.; Li, X.Q. Growth and photosynthetic responses of invasive Bidens frondosa to light and water availability: A comparison with invasive and native congeners. Weed Biol. Manag. 2017, 17, 36–44. [Google Scholar] [CrossRef]
- Schultheis, E.H.; MacGuigan, D.J. Competitive ability, not tolerance, may explain success of invasive plants over natives. Biol. Invasions 2018, 20, 2793–2806. [Google Scholar] [CrossRef]
- Michelan, T.S.; Thomaz, S.M.; Bando, F.M.; Bini, L.M. Competitive effects hinder the recolonization of native species in environments densely occupied by one invasive exotic species. Front. Plant Sci. 2018, 9, 1261. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.; Utz, R. Winners and losers: Competition and the invasive grass Bromus inermis. Acta Oecol. 2024, 124, 104021. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Feng, Y.L.; Liu, W.X.; Liao, Z.Y. Growth, biomass allocation, morphology, and photosynthesis of invasive Eupatorium adenophorum and its native congeners grown at four irradiances. Plant Ecol. 2008, 203, 263–271. [Google Scholar] [CrossRef]
- Liao, Z.Y.; Scheepens, J.F.; Li, W.T.; Wang, R.F.; Zheng, Y.L.; Feng, Y.L. Biomass reallocation and increased plasticity might contribute to successful invasion of Chromolaena odorata. Flora 2019, 256, 79–84. [Google Scholar] [CrossRef]
- Hallett, L.M.; Standish, R.J.; Hobbs, R.J. Seed mass and summer drought survival in a mediterranean-climate ecosystem. Plant Ecol. 2011, 212, 1479–1489. [Google Scholar] [CrossRef]
- Stevens, J.M.; Fehmi, J.S. Early establishment of a native grass reduces the competitive effect of a non-native grass. Restor. Ecol. 2011, 19, 399–406. [Google Scholar] [CrossRef]
- Ulrich, E.; Perkins, L. Bromus inermis and Elymus canadensis but not Poa pratensis demonstrate strong competitive effects and all benefit from priority. Plant Ecol. 2014, 215, 1269–1275. [Google Scholar] [CrossRef]
- Lang, M.; Hanslin, H.M.; Kollmann, J.; Wagner, T. Suppression of an invasive legume by a native grass—High impact of priority effects. Basic Appl. Ecol. 2017, 22, 20–27. [Google Scholar] [CrossRef]
- Luo, Y.J.; Guo, W.H.; Yuan, Y.F.; Liu, J.; Du, N.; Wang, R.Q. Increased nitrogen deposition alleviated the competitive effects of the introduced invasive plant Robinia pseudoacacia on the native tree Quercus acutissima. Plant Soil 2014, 385, 63–75. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Xu, X.; Tian, Y.; Zhang, Z.; van Kleunen, M. The effects of changes in water and nitrogen availability on alien plant invasion into a stand of a native grassland species. Oecologia 2018, 188, 441–450. [Google Scholar] [CrossRef]
- Abraham, J.K.; Corbin, J.D.; D’Antonio, C.M. California native and exotic perennial grasses differ in their response to soil nitrogen, exotic annual grass density, and order of emergence. Plant Ecol. 2009, 201, 445–456. [Google Scholar] [CrossRef]
- Dakshini, K.M.M.; Singh, P.P. Taxonomy of Bidens section psilocarpaea (Asteraceae—Heliantheae—Core0psidinae) in India. Proc. Idian Acd. Sci. 1984, 93, 165–177. [Google Scholar] [CrossRef]
- Lin, D.; Tsuzuki, E.; Sugimoto, Y.; Dong, Y.; Matsuo, M.; Terao, H. Assessment of dwarf lilyturf (Ophiopogon japonicus K.) dried powders for weed control in transplanted rice. Crop Prot. 2003, 22, 431–435. [Google Scholar] [CrossRef]
- Wei, C.Q.; Pan, Y.M.; Tang, S.C.; Lin, C.H.; Liu, M.C. Seed germination of Bidens alba L. and B. biternata L. under different light and temperature regimes. Weed Sci. 2013, 31, 1–4. [Google Scholar] [CrossRef]
- Liu, X.; Duan, L.; Mo, J.; Du, E.; Shen, J.; Lu, X.; Zhang, Y.; Zhou, X.; He, C.; Zhang, F. Nitrogen deposition and its ecological impact in China: An overview. Environ. Pollut. 2011, 159, 2251–2264. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.H.; Bazely, D. Appendix-Some tools for studying plant populations. In Ecology and Control of Introduced Plants; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Armas, C.; Ordiales, R.; Pugnaire, F.I. Measuring plant interactions: A new comparative index. Ecology 2004, 85, 2682–2686. [Google Scholar] [CrossRef]
- Gruntman, M.; Pehl, A.K.; Joshi, S.; Tielbörger, K. Competitive dominance of the invasive plant Impatiens glandulifera: Using competitive effect and response with a vigorous neighbour. Biol. Invasions 2013, 16, 141–151. [Google Scholar] [CrossRef]
- Zhang, H.J.; Chang, R.Y.; Guo, X.; Liang, X.Q.; Wang, R.Q.; Liu, J. Shifts in growth and competitive dominance of the invasive plant Alternanthera philoxeroides under different nitrogen and phosphorus supply. Environ. Exp. Bot. 2017, 135, 118–125. [Google Scholar] [CrossRef]
| Source | df | Total Biomass F-Value | R/S Ratio F-Value | Reproductive Ratio F-Value | RDI F-Value | RII F-Value |
|---|---|---|---|---|---|---|
| (a) B. frondosa | ||||||
| Time of arrival (T) | 2 | 47.04 *** | 6.90 ** | 28.40 ** | 100.50 *** | 82.74 *** |
| N addition (N) | 1 | 18.31 *** | 5.99 * | 0.62 | 0.88 | 6.69 * |
| T × N | 2 | 3.36 * | 2.02 | 0.72 | 3.12 | 2.87 |
| (b) B. biternata | ||||||
| Time of arrival (T) | 2 | 32.47 *** | 2.19 | 5.23 ** | 100.50 *** | 27.58 *** |
| N addition | 1 | 37.96 *** | 4.01 | 5.17 ** | 0.88 | 1.63 |
| T × N | 2 | 6.87 ** | 0.02 | 2.93 | 3.12 | 1.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, C.; Tang, S.; Li, X.; Pan, Y.; Zhou, L. Priority Effects Favor Invasive Bidens frondosa over Its Native Congener Bidens biternata, While Late Arrival Incurs Higher Costs. Plants 2025, 14, 2515. https://doi.org/10.3390/plants14162515
Wei C, Tang S, Li X, Pan Y, Zhou L. Priority Effects Favor Invasive Bidens frondosa over Its Native Congener Bidens biternata, While Late Arrival Incurs Higher Costs. Plants. 2025; 14(16):2515. https://doi.org/10.3390/plants14162515
Chicago/Turabian StyleWei, Chunqiang, Saichun Tang, Xiangqin Li, Yumei Pan, and Longwu Zhou. 2025. "Priority Effects Favor Invasive Bidens frondosa over Its Native Congener Bidens biternata, While Late Arrival Incurs Higher Costs" Plants 14, no. 16: 2515. https://doi.org/10.3390/plants14162515
APA StyleWei, C., Tang, S., Li, X., Pan, Y., & Zhou, L. (2025). Priority Effects Favor Invasive Bidens frondosa over Its Native Congener Bidens biternata, While Late Arrival Incurs Higher Costs. Plants, 14(16), 2515. https://doi.org/10.3390/plants14162515






