Yield Stability of Soybean Variety Morkhor 60 in Integrated Rotation Systems of Northeastern Thailand
Abstract
1. Introduction
2. Results
2.1. Environmental Variation
2.2. Sources of Variance for Yield Components and Grain Yield of Four Soybean Genotypes Across 13 Environments
2.3. Yield Performance of Four Soybean Genotypes Across 13 Environments
2.4. Yield Stability of Four Soybean Genotypes
2.5. Variation in Chemical Components in Four Soybean Genotypes
3. Discussion
3.1. Environmental Effects and Genotype Performance in Soybean Production
3.2. Soybean–Rice Cropping System Integration
3.3. Soybean-Sugarcane Cropping System Potential
3.4. Seed Rotation System Implementation
3.5. Quality Characteristics and Environmental Benefits
3.6. Yield Levels and Economic Viability
3.7. Future Research Directions
4. Materials and Methods
4.1. Plant Material and Experiment Design
4.2. Data Collection
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, H.W.; Bernard, R.L. Soybean Genetics and Breeding. Adv. Agron. 1962, 14, 149–221. [Google Scholar] [CrossRef]
- USDA Economic Research Service. Soybeans and Oil Crops. 2023. Available online: https://www.ers.usda.gov/topics/crops/soybeans-oil-crops/ (accessed on 13 January 2025).
- USDA Foreign Agricultural Service. World Agricultural Production. 2024. Available online: https://apps.fas.usda.gov/psdonline/circulars/production.pdf (accessed on 13 January 2025).
- FAOSTAT. Database. 2023. Available online: http://www.fao.org/faostat/en/#data/SC (accessed on 13 January 2025).
- Peoples, M.B.; Brockwell, J.; Herridge, D.F.; Rochester, I.J.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M.; Dakora, F.D.; Bhattarai, S.; Maskey, S.L.; et al. The Contributions of Nitrogen-Fixing Crop Legumes to the Productivity of Agricultural Systems. Symbiosis 2009, 48, 1–17. [Google Scholar] [CrossRef]
- Behnke, G.D.; Zuber, S.M.; Pittelkow, C.M.; Nafziger, E.D.; Villamil, M.B. Long-term crop rotation and tillage effects on soil greenhouse gas emissions and crop production in Illinois, USA. Agric. Ecosyst. Environ. 2018, 261, 62–70. [Google Scholar] [CrossRef]
- Alencar, E.R.; Faroni, L.R.D. Storage of Soybeans and Its Effects on Quality of Soybean Sub-Products. In Recent Trends for Enhancing the Diversity and Quality of Soybean Products; Krezhova, D., Ed.; IntechOpen: London, UK, 2011; pp. 48–66. [Google Scholar] [CrossRef]
- Sritongtae, C.; Monkham, T.; Sanitchon, J.; Lodthong, S.; Srisawangwong, S.; Chankaew, S. Identification of Superior Soybean Cultivars through the Indication of Specific Adaptabilities within Duo-Environments for Year-Round Soybean Production in Northeast Thailand. Agronomy 2021, 11, 585. [Google Scholar] [CrossRef]
- Bashir, M.; Adam, A.M.; Shehu, B.M.; Abubakar, M.S. Effects of Soil Texture and Nutrients Application on Soybean Nutrient Uptake, Growth and Yield Response. J. Agric. Food Sci. 2022, 1, 227–241. [Google Scholar] [CrossRef]
- Faé, G.S.; Kemanian, A.R.; Roth, G.W.; White, C.; Watson, J.E. Soybean yield about environmental and soil properties. Agric. Ecosyst. Environ. 2020, 118, 126070. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, X.; Wei, D.; Lin, X.; Qiu, S.; Ciampitti, I.; He, P. Soybean Yield, Nutrient Uptake, and Stoichiometry under Different Climate Regions of Northeast China. Sci. Rep. 2020, 10, 8431. [Google Scholar] [CrossRef]
- Uguru, M.I.; Oyiga, B.C.; Jandong, E.A. Responses of Some Soybean Genotypes to Different Soil pH Regimes in Two Planting Seasons. Afr. J. Plant Sci. Biotechnol. 2012, 6, 26–37. [Google Scholar]
- Mandić, V.; Đorđević, S.; Đorđević, N.; Bijelić, Z.; Krnjaja, V.; Petričević, M.; Brankov, M. Genotype and Sowing Time Effects on Soybean Yield and Quality. Agriculture 2020, 10, 502. [Google Scholar] [CrossRef]
- Arega, A.; Dabessa, A.; Tola, M.; Dabala, C. Genotype and genotype by environment interaction and grain yield stability of medium maturity soybean groups [Glycine max (L.) Merrill] varieties in Western Oromia, Ethiopia. Afr. J. Plant Sci 2018, 12, 227–237. [Google Scholar] [CrossRef]
- Silva, W.J.D.S.; Neto, F.D.A.; Al-Qahtani, W.H.; Okla, M.K.; Al-Hashimi, A.; Vieira, P.F.d.M.J.; Gravina, G.d.A.; Zuffo, A.M.; Dutra, A.F.; Carvalho, L.C.B.; et al. Yield of Soybean Genotypes Identified through GGE Biplot and Path Analysis. PLoS ONE 2022, 17, e0278476. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, A.M.; Azirun, S.M.; Boyce, A.N. Tropical legume crop rotation and nitrogen fertilizer effects on agronomic and nitrogen efficiency of rice. Sci. World J. 2014, 2014, 490841. [Google Scholar] [CrossRef]
- Park, S.E.; Webster, T.J.; Horan, H.L.; James, A.T.; Thorburn, P.J. A legume rotation crop lessens the need for nitrogen fertiliser throughout the sugarcane cropping cycle. Field Crops Res. 2010, 119, 331–341. [Google Scholar] [CrossRef]
- Yan, W.; Hunt, L.A.; Sheng, Q.; Szlavnics, Z. Cultivar Evaluation and Mega-Environment Investigation Based on GGE Biplot. Crop Sci. 2000, 40, 597–605. [Google Scholar] [CrossRef]
- Rodrigues, P.C.; Monteiro, A.; Lourenço, V.M. A robust AMMI model for the analysis of genotype-by-environment data. Bioinformatics 2016, 32, 58–66. [Google Scholar] [CrossRef]
- Kona, P.; Ajay, B.C.; Gangadhara, K.; Kumar, N.; Choudhary, R.R.; Mahatma, M.K.; Singh, S.; Reddy, K.K.; Bera, S.K.; Sangh, C.; et al. AMMI and GGE biplot analysis of genotype by environment interaction for yield and yield contributing traits in confectionery groundnut. Sci. Rep. 2024, 14, 2943. [Google Scholar] [CrossRef]
- Majidian, P.; Masoudi, B.; Ghasemi-Golezani, K.; Rahimzadeh-Khoee, F. Deciphering genotype-by-environment interaction in new soybean lines based on multiple traits using different adaptability and stability methods. Food Sci. Nutr. 2024, 12, 2847–2863. [Google Scholar] [CrossRef]
- Araújo, M.S.; Pereira, G.R.; Chaves, S.F.S.; Blasques, G.M.; Dias, L.A.S.; Silva, F.L. Exploring genotype-by-environment interactions in tropical soybean multi-environment trials. Euphytica 2025, 241, 12. [Google Scholar] [CrossRef]
- Abush, T.; Abebe Adeyinka, S.A.; Moses, A.A.; Aondover, S.; Hapson, M.; Tunrayo, A.; Derera, J.; Agbona, A.; Chigeza, G. Genotype x environment interaction and yield stability of soybean (Glycine max L.) genotypes in multi-environment trials (METs) in Nigeria. Heliyon 2024, 10, e38097. [Google Scholar] [CrossRef] [PubMed]
- Soni, M.; Shrivastava, M.K.; Amrate, P.K.; Singh, Y.; Khare, V. Analysis of Genotype by Environment Interactions using AMMI and GGE Biplot Methods for Soybean Germplasm Lines. Legume Res. 2025, 1–9. [Google Scholar] [CrossRef]
- Wondaferew, D.; Mullualem, D.; Bitewlgn, W.; Kassa, Z.; Abebaw, Y.; Ali, H.; Kebede, K.; Astatkie, T. Cultivating sustainable futures: Multi-environment evaluation and seed yield stability of faba bean (Vicia faba L.) genotypes by using different stability parameters in Ethiopia. BMC Plant Biol. 2024, 24, 1108. [Google Scholar] [CrossRef]
- Amogne, A.; Malede, M.; Tefera, G. AMMI and GGE-Biplot Analysis of Yield Performance and Stability of Soybean [Glycine max (L.) Merrill] Genotypes in Southern and Northwestern Ethiopia. Ethiop. J. Agric. Sci. 2023, 33, 30–40. [Google Scholar]
- Bordeleau, L.M.; Prévost, D. Nodulation and Nitrogen Fixation in Extreme Environments. Plant Soil 1994, 161, 115–125. [Google Scholar] [CrossRef]
- Graham, P.H.; Vance, C.P. Legumes: Importance and Constraints to Greater Use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Hongyu, K.; García-Peña, M.; de Araújo, L.B.; dos Santos Dias, C.T. Statistical Analysis of Yield Trials by AMMI Analysis of Genotype × Environment Interaction. Biometr. Lett. 2014, 51, 89–102. [Google Scholar] [CrossRef]
- Yan, W.; Tinker, N.A. An Integrated Biplot Analysis System for Displaying, Interpreting, and Exploring Genotype × Environment Interaction. Crop Sci. 2005, 45, 1004–1016. [Google Scholar] [CrossRef]
- Yan, W.; Rajcan, I. Biplot Evaluation of Test Sites and Trait Relations of Soybean in Ontario. Crop Sci. 2002, 42, 11–20. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Abdullah, N.; Magaji, U.; Miah, G.; Hussin, G.; Ramli, A. Genotype × Environment interaction and stability analyses of yield and yield components of established and mutant rice genotypes tested in multiple locations in Malaysia. Acta Agric. Scand. Sec. B Soil Plant Sci. 2017, 67, 590–606. [Google Scholar] [CrossRef]
- Ladha, J.K.; Tirol-Padre, A.; Reddy, C.K.; Cassman, K.G.; Verma, S.; Powlson, D.S.; van Kessel, C.; Richter, D.d.B.; Chakraborty, D.; Pathak, H. Global Nitrogen Budgets in Cereals: A 50-Year Assessment for Maize, Rice, and Wheat Production Systems. Sci. Rep. 2016, 6, 19355. [Google Scholar] [CrossRef]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global Inputs of Biological Nitrogen Fixation in Agricultural Systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- McDaniel, M.D.; Tiemann, L.K.; Grandy, A.S. Does Agricultural Crop Diversity Enhance Soil Microbial Biomass and Organic Matter Dynamics? A Meta-Analysis. Ecol. Appl. 2014, 24, 560–570. [Google Scholar] [CrossRef]
- Giller, K.E.; Wilson, K.J. Nitrogen Fixation in Tropical Cropping Systems, 2nd ed.; CABI Publishing: Wallingford, UK, 2001. [Google Scholar]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and Fungal Contributions to Carbon Sequestration in Agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Unkovich, M.; Pate, J. An Appraisal of Recent Field Measurements of Symbiotic N2; Fixation by Annual Legumes. Field Crops Res. 2000, 65, 211–228. [Google Scholar] [CrossRef]
- Mushoriwa, H.; Mathew, I.; Gwata, E.T.; Tongoona, P.; Derera, J. Grain Yield Potential and Stability of Soybean Genotypes of Different Ages across Diverse Environments in Southern Africa. Agronomy 2022, 12, 1147. [Google Scholar] [CrossRef]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef]
- Yang, Y.; Zou, J.; Huang, W.; Olesen, I.E.; Li, W.; Rees, R.M.; Harrison, M.T.; Feng, B.; Feng, Y.; Chen, F.; et al. Drivers of soybean-based rotations synergistically increase crop productivity and reduce GHG emissions. Agric. Ecosyst. Environ. 2024, 372, 109094. [Google Scholar] [CrossRef]
- Wilson, R.F. Seed Composition. In Soybean: Improvement, Production, and Uses, 3rd ed.; Shibles, R.M., Harper, J.E., Wilson, R.F., Shoemaker, R.C., Eds.; American Society of Agronomy: Madison, WI, USA, 2004; pp. 621–677. [Google Scholar] [CrossRef]
- Pathan, S.M.; Vuong, T.; Clark, K.; Lee, J.D.; Shannon, J.G.; Roberts, C.A.; Ellersieck, M.R.; Burton, J.W.; Cregan, P.B.; Hyten, D.L.; et al. Genetic Mapping and Confirmation of Quantitative Trait Loci for Seed Protein and Oil Contents and Seed Weight in Soybean. Crop Sci. 2013, 53, 765–774. [Google Scholar] [CrossRef]
- Guo, B.; Sun, L.; Jiang, S.; Ren, H.; Sun, R.; Wei, Z.; Hong, H.; Luan, X.; Wang, J.; Wang, X.; et al. Soybean genetic resources contributing to sustainable protein production. Theor. Appl. Genet. 2022, 135, 4095–4121. [Google Scholar] [CrossRef] [PubMed]
- Della Chiesa, T.; Northrup, D.; Miguez, F.E.; Archontoulis, S.V.; Baum, M.E.; Venterea, R.T.; Emmett, B.D.; Malone, R.W.; Iqbal, J.; Necpalova, M.; et al. Reducing greenhouse gas emissions from North American soybean production. Nat. Sustain. 2024, 7, 1608–1615. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.A.; Booij, C.J.H.; Tscharntke, T. Sustainable Pest Regulation in Agricultural Landscapes: A Review on Landscape Composition, Biodiversity and Natural Pest Control. Proc. R. Soc. B 2006, 273, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.B. Resilience in Agriculture through Crop Diversification: Adaptive Management for Environmental Change. BioScience 2011, 61, 183–193. [Google Scholar] [CrossRef]
- Pretty, J.; Noble, A.D.; Bossio, D.; Dixon, J.; Hine, R.E.; de Vries, F.W.T.P.; Morison, J.I.L. Resource-Conserving Agriculture Increases Yields in Developing Countries. Environ. Sci. Technol. 2006, 40, 1114–1119. [Google Scholar] [CrossRef]
- Berkelaar, D. Soybeans in the Tropics. ECHO Development Notes No. 92. 2006. Available online: https://www.echocommunity.org/en/resources/bd75ce05-f9c2-4074-aa6e-2cbd1fc08ad9 (accessed on 13 January 2025).
- Ambrosini, V.G.; Ciampitti, I.A.; Fontoura, S.M.V.; Tamagno, S.; de Moraes, R.P.; Schwalbert, R.A.; Urquiaga, S.; Bayer, C. Environmental variables controlling biological nitrogen fixation in soybean. Symbiosis 2024, 93, 43–55. [Google Scholar] [CrossRef]
- Chen, Z.Q. Optimizing soybean yield through integrated agronomic management. Legume Genom. Genet. 2024, 15, 303–314. [Google Scholar] [CrossRef]
- Pendke, M.S.; Asewar, B.V.; Gourkhede, P.H.; Narkhede, W.N.; Abdulraheem, M.I.; Alghamdi, A.G.; Singh, C.; Abdi, G. Impact of tillage and fertilizer management on Soybean-Cotton rotation system: Effects on yield, plant nutrient uptake, and soil fertility for sustainable agriculture. Sci. Rep. 2025, 15, 9991. [Google Scholar] [CrossRef] [PubMed]
- Thai Meteorological Department. The season of Thailand. 2023. Available online: https://thailand.go.th/issue-focus-detail/009_142 (accessed on 30 December 2024).
- Goto, S.; Kuwagata, T.; Konghakote, P.; Polthanee, A.; Ishigooka, Y.; Toritani, H.; Ha-segawa, T. Characteristics of water balance in a rainfed paddy field in Northeast Thai-land. Paddy Water Environ. 2008, 6, 153–157. [Google Scholar] [CrossRef]
- Polthanee, A.; Srisutham, M. Growth, yield and water use of drip irrigated cassava planted in the late rainy season of Northeastern Thailand. Indian J. Agric. Res. 2018, 52, 554–559. [Google Scholar] [CrossRef]
- Kilmer, V.J.; Mullins, J.F. Improved stirring and pipetting apparatus for mechanical analysis for soil. Soil Sci. 1954, 77, 437–441. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis. Part 3. Chemical Methods. Book Ser No. 5; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 475–489. [Google Scholar] [CrossRef]
- Jackson, M.L. Nitrogen determination for soils and plant tissue. In Soil Chemical Analysis; Prentice-Hall of India Private Limited: New Delhi, India, 1967; pp. 83–203. [Google Scholar]
- Bray, R.A.; Kurtz, L.T. Determination of total organic and available form of phosphorus in soil. Soil Sci. 1954, 59, 39–45. [Google Scholar] [CrossRef]
- Pratt, P.E. Potassium. In Method of Soil Analysis, Part II; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1022–1030. [Google Scholar]
- AOAC. Official Methods of Analysis, 15th ed.; The Association of Official Analytical Chemists (AOAC): Arlington, VA, USA, 1991. [Google Scholar]
- Adamu, H.M.; Ushie, O.A.; Gwangwala, A.H.; Yadav, R.P.; Singh, A.; Bhardwaj, A.K.; Lone, P.A.; Dar, M.M.; Parray, J.A.; Shah, K.; et al. Estimation of Total Flavonoids and Tannins in the Stem Bark and Leaves of Anogeisus leiocarpus Plant. Int. J. Tradit. Nat. Med. 2013, 2, 141–148. Available online: https://api.semanticscholar.org/CorpusID:70649841 (accessed on 15 January 2024).
- Sigel, H. Metals in Biological Systems; Marcel Dekker: New York, NY, USA, 1978. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Version 3.2.1. 2015. Available online: https://www.R-project.org/ (accessed on 30 December 2020).
- Yan, W.; Kang, M.S.; Ma, B.; Woods, S.; Cornelius, P.L. GGE biplot vs. AMMI analysis of genotype-by-environment data. Crop Sci. 2007, 47, 641–653. [Google Scholar] [CrossRef]



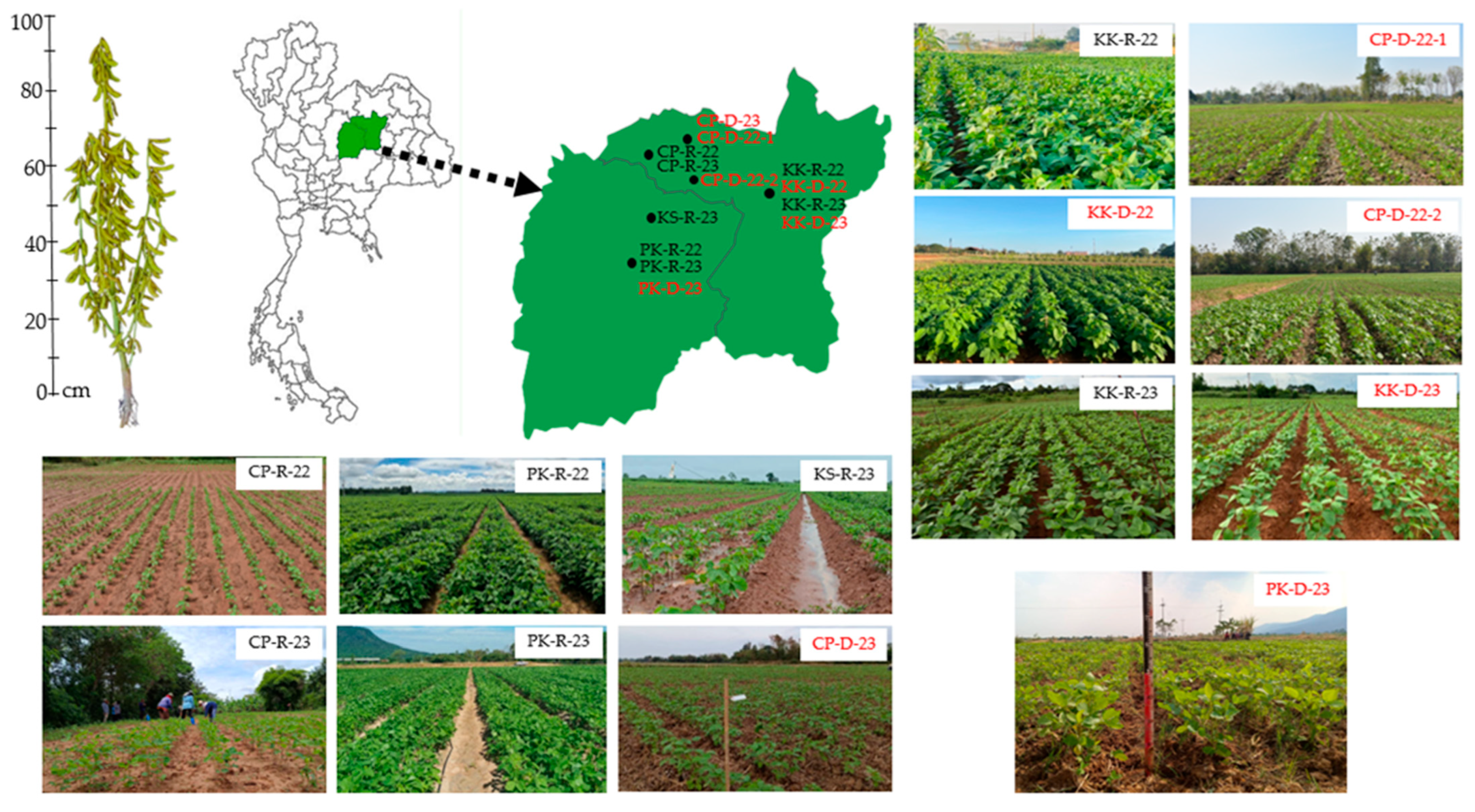
| Locations Code * | Seasons | Soil Texture | pH | Total N | Available P | Exchangeable K | Planting | Mean Grain Yield |
|---|---|---|---|---|---|---|---|---|
| (%) | (mg/kg) | (mg/kg) | Date | (kg/ha) | ||||
| KK-R-22 | Rainy | Loamy Sand | 6.28 | 0.01 | 40.83 | 25.03 | 29-Jul-2022 | 1366 c |
| CP-R-22 | Rainy | Clay | 7.14 | 0.11 | 14.56 | 121.00 | 30-Jul-2022 | 1123 de |
| PK-R-22 | Rainy | Sandy loam | 6.25 | 0.89 | 96.00 | 157.22 | 05-Jul-2022 | 1685 b |
| KK-D-22 | Dry | Loamy Sand | 5.57 | 0.03 | 56.30 | 69.99 | 08-Dec-2022 | 1984 a |
| CP-D-22-1 | Dry | Clay | 7.00 | 0.11 | 2.08 | 182.11 | 24-Dec-2022 | 1444 c |
| CP-D-22-2 | Dry | Clay | 7.13 | 0.11 | 18.00 | 135.25 | 24-Dec-2022 | 1272 cd |
| KK-R-23 | Rainy | Loamy Sand | 5.58 | 0.03 | 57.50 | 128.21 | 24-Jun-2023 | 1374 c |
| CP-R-23 | Rainy | Loam | 7.19 | 0.04 | 11.25 | 59.82 | 12-Aug-2023 | 1001 e |
| PK-R-23 | Rainy | Sandy loam | 6.23 | 0.97 | 87.50 | 113.03 | 07-Jul-2023 | 1979 a |
| KS-R-23 | Rainy | Clay | 7.11 | 0.47 | 5.50 | 830.03 | 12-Aug-2023 | 749 f |
| KK-D-23 | Dry | Loamy Sand | 6.58 | 0.01 | 36.52 | 44.00 | 10-Dec-2023 | 1776 b |
| CP-D-23 | Dry | Clay | 7.01 | 0.11 | 18.33 | 220.05 | 24-Dec-2023 | 1137 de |
| PK-D-23 | Dry | Loam | 5.52 | 0.02 | 81.41 | 137.78 | 25-Dec-2023 | 1025 e |
| Source of Variation | df | Number | 100-Grain Weight | Grain Yield | |||||
|---|---|---|---|---|---|---|---|---|---|
| Nodes | Branches | Pods per Main Stem | Pods per Branch | Pods per Plant | Grains per Pod | ||||
| Envi. (E) | 12 | 190.80 ** | 29.09 ** | 3306.90 ** | 12,618.00 ** | 26,456.00 ** | 0.46 ** | 27.89 ** | 2,374,800 ** |
| Rep./E | 39 | 0.66 | 0.43 | 13.71 | 90.30 | 123.70 | 0.01 | 0.52 | 64,616 |
| Genotype (G) | 3 | 6.06 ** | 37.70 ** | 322.00 ** | 7016.50 ** | 4336.50 ** | 0.76 ** | 58.31 ** | 717,184 ** |
| G × E | 36 | 3.45 ** | 2.29 ** | 61.14 ** | 1186.70 ** | 1448.50 ** | 0.28 ** | 2.31 ** | 142,382 ** |
| error | 117 | 0.23 | 0.25 | 5.55 | 43.40 | 47.10 | 0.01 | 0.31 | 12,079 |
| CV (%) | 3.64 | 12.10 | 8.67 | 22.62 | 12.2 | 4.04 | 4.02 | 7.97 | |
| Varieties/Lines | The Grain Yield (kg/ha) in Rainy and Dry Seasons in 2022 | Mean | |||||
|---|---|---|---|---|---|---|---|
| Rainy Season | Dry Season | ||||||
| KK-R-22 | CP-R-22 | PK-R-22 | KK-D-22 | CP-D-22-1 | CP-D-22-2 | ||
| Morkhor 60 | 1428 AB | 1213 B | 1954 A | 2046 A | 1789 A | 1302 B | 1622 |
| SJ 5 | 1237 BC | 1456 A | 1500 C | 1910 B | 1736 A | 1644 A | 1581 |
| 223*Lh-85 | 1161 C | 856 C | 1589 BC | 1846 B | 1162 B | 1044 C | 1276 |
| CM 60 | 1637 A | 969 C | 1696 B | 2135 A | 1090 B | 1099 C | 1438 |
| Mean | 1366 | 1123 | 1685 | 1984 | 1444 | 1272 | 1479 |
| F-test | ** | ** | ** | ** | ** | ** | |
| CV% | 10.39 | 11.52 | 5.13 | 4.22 | 10.87 | 7.21 | |
| Varieties/Lines | The Grain Yield (kg/ha) in the Rainy and Dry Seasons of 2023 | Mean | ||||||
|---|---|---|---|---|---|---|---|---|
| Rainy Season | Dry Season | |||||||
| KK-R-23 | CP-R-23 | PK-R-23 | KS-R-23 | KK-D-23 | CP-D-23 | PK-D-23 | ||
| Morkhor 60 | 1434 A | 1101 A | 2330 A | 818 A | 1733 B | 1294 A | 979 B | 1384 |
| SJ 5 | 1320 B | 1112 A | 2190 A | 851 A | 1781 B | 1077 BC | 889 B | 1317 |
| 223*Lh-85 | 1300 B | 942 B | 1596 B | 672 B | 1557 B | 975 C | 1208 A | 1179 |
| CM 60 | 1443 A | 850 B | 1798 B | 656 B | 2035 A | 1201 AB | 1024 B | 1287 |
| Mean | 1374 | 1001 | 1979 | 749 | 1776 | 1137 | 1025 | 1308 |
| F-test | ** | ** | ** | ** | ** | ** | ** | |
| CV% | 2.99 | 7.09 | 7.51 | 6.61 | 8.61 | 8.95 | 8.51 | |
| Source of Variation | df | Oil (%) | Protein (%) | Source of Variation | df | Ash (%) | Fiber (%) |
|---|---|---|---|---|---|---|---|
| Envi. (E) | 6 | 27.76 ** | 18.27 ** | Envi. (E) | 6 | 20.77 ** | 76.43 ** |
| Rep./E | 7 | 0.22 | 0.07 | Rep./E | 14 | 0.00 | 1.67 |
| Genotype (G) | 3 | 27.77 ** | 70.80 ** | Genotype (G) | 3 | 2.65 ** | 13.39 ** |
| G × E | 18 | 1.40 ** | 5.69 ** | G × E | 18 | 3.56 ** | 3.72 ** |
| error | 21 | 0.06 | 0.47 | error | 42 | 0.00 | 1.26 |
| CV (%) | 55 | 1.64 | 1.78 | CV (%) | 83 | 0.72 | 7.83 |
| Genotype | Ash (%) | Fiber (%) | Oil (%) | Protein (%) |
|---|---|---|---|---|
| Morkhor 60 | 5.53 C | 14.70 A | 14.66 C | 39.63 B |
| SJ 5 | 5.63 B | 15.29 A | 13.38 D | 40.74 A |
| 223*Lh-85 | 5.66 B | 13.84 B | 15.72 B | 35.53 D |
| CM 60 | 6.31 A | 13.54 B | 16.65 A | 38.20 C |
| Mean | 5.78 | 14.34 | 15.10 | 38.53 |
| Environment (E) | ** | ** | ** | ** |
| Genotype (G) | ** | ** | ** | ** |
| E × G | ** | ** | ** | ** |
| CV (%) | 0.72 | 7.83 | 1.64 | 1.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taiyawong, A.; Monkham, T.; Sanitchon, J.; Choenkwan, S.; Srisawangwong, S.; Khodphuwiang, J.; Reewarabundit, S.; Chankaew, S. Yield Stability of Soybean Variety Morkhor 60 in Integrated Rotation Systems of Northeastern Thailand. Plants 2025, 14, 2503. https://doi.org/10.3390/plants14162503
Taiyawong A, Monkham T, Sanitchon J, Choenkwan S, Srisawangwong S, Khodphuwiang J, Reewarabundit S, Chankaew S. Yield Stability of Soybean Variety Morkhor 60 in Integrated Rotation Systems of Northeastern Thailand. Plants. 2025; 14(16):2503. https://doi.org/10.3390/plants14162503
Chicago/Turabian StyleTaiyawong, Adisak, Tidarat Monkham, Jirawat Sanitchon, Sukanlaya Choenkwan, Sittipong Srisawangwong, Jamnan Khodphuwiang, Suntit Reewarabundit, and Sompong Chankaew. 2025. "Yield Stability of Soybean Variety Morkhor 60 in Integrated Rotation Systems of Northeastern Thailand" Plants 14, no. 16: 2503. https://doi.org/10.3390/plants14162503
APA StyleTaiyawong, A., Monkham, T., Sanitchon, J., Choenkwan, S., Srisawangwong, S., Khodphuwiang, J., Reewarabundit, S., & Chankaew, S. (2025). Yield Stability of Soybean Variety Morkhor 60 in Integrated Rotation Systems of Northeastern Thailand. Plants, 14(16), 2503. https://doi.org/10.3390/plants14162503






