Effects of Shading on Metabolism and Grain Yield of Irrigated Rice During Crop Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Leaf Temperature
2.3. Quantification of Photosynthetic Pigments
2.4. Specific Activity of Invertases
2.5. Quantification of Total Soluble Carbohydrates and Amino Acids
2.6. Nutrient Content in Leaves
2.7. Productivity and Weight of a Thousand Grains
2.8. Statistical Analysis
3. Results
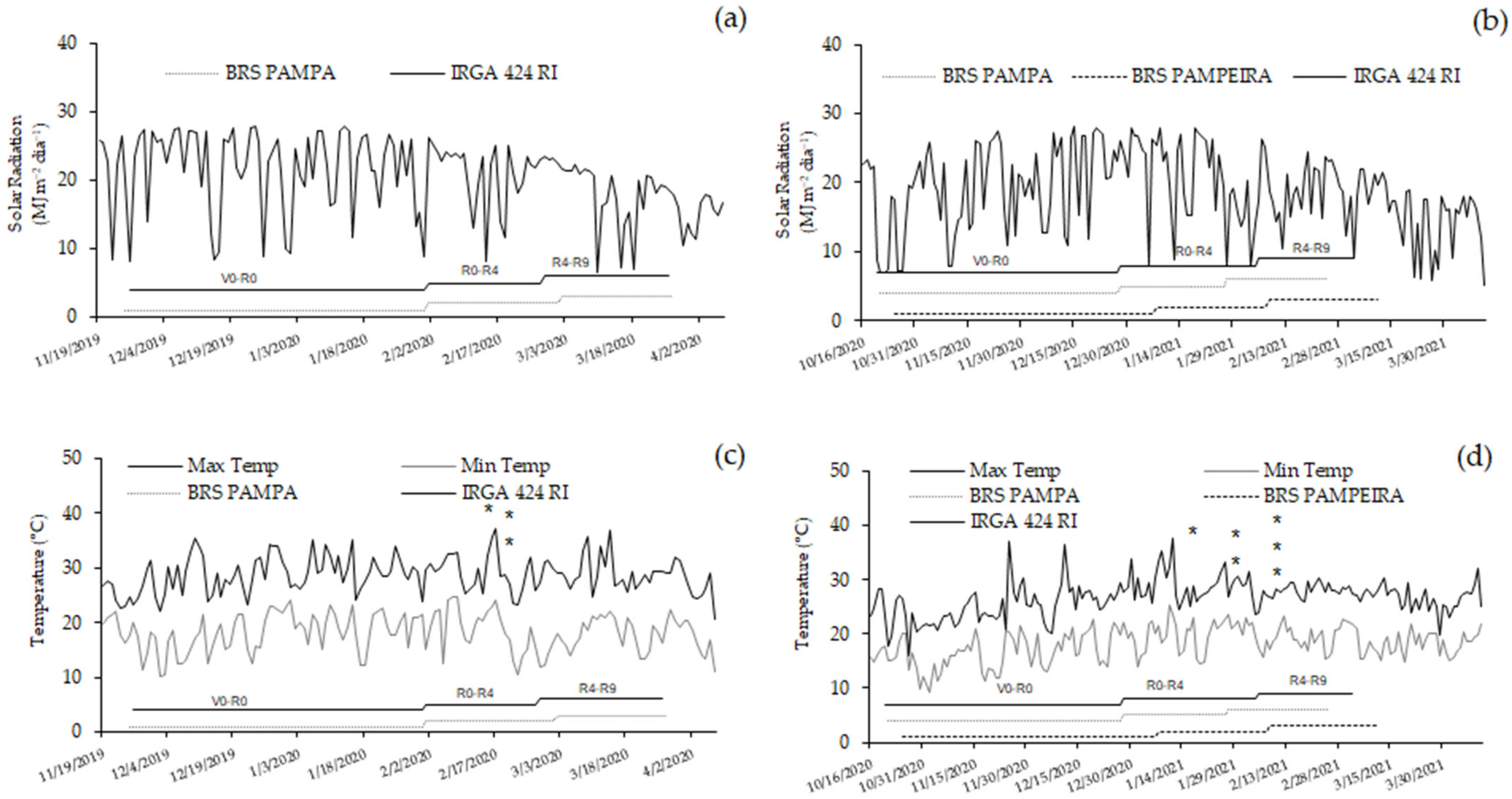
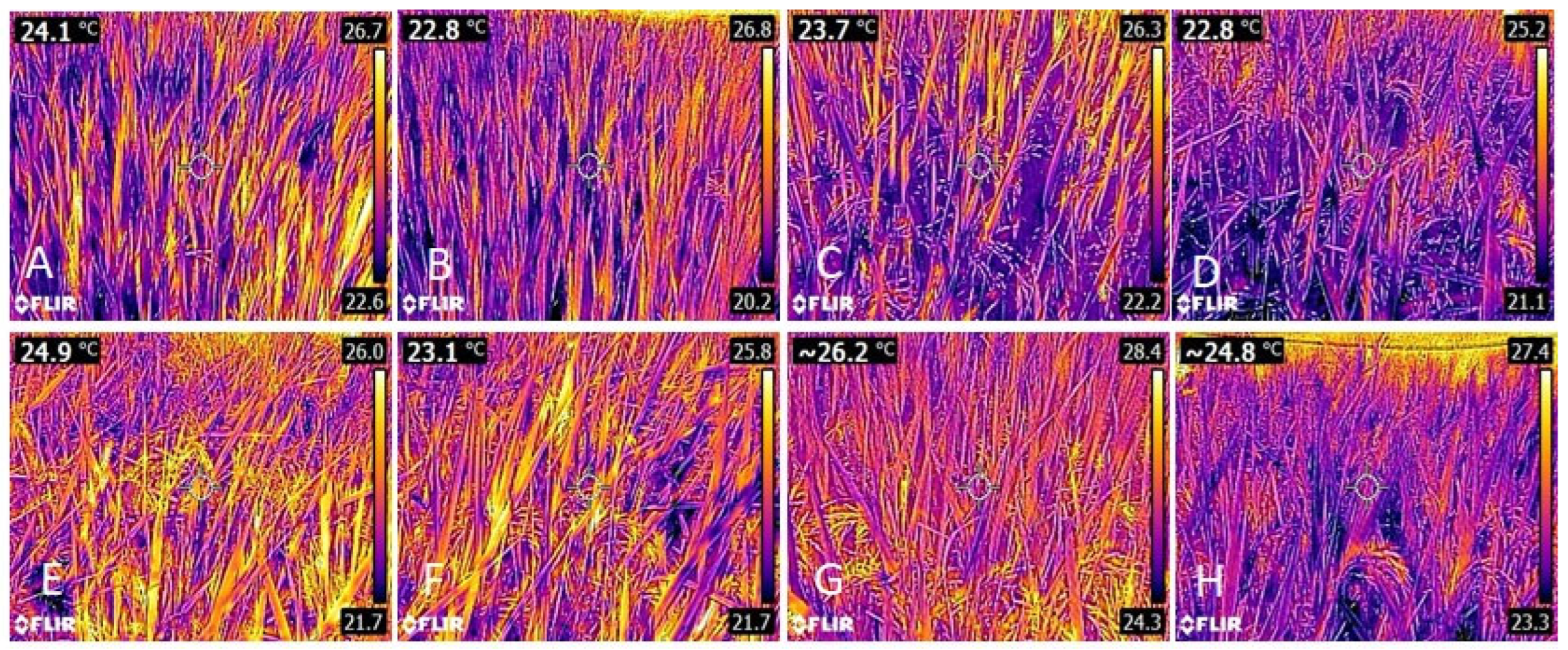
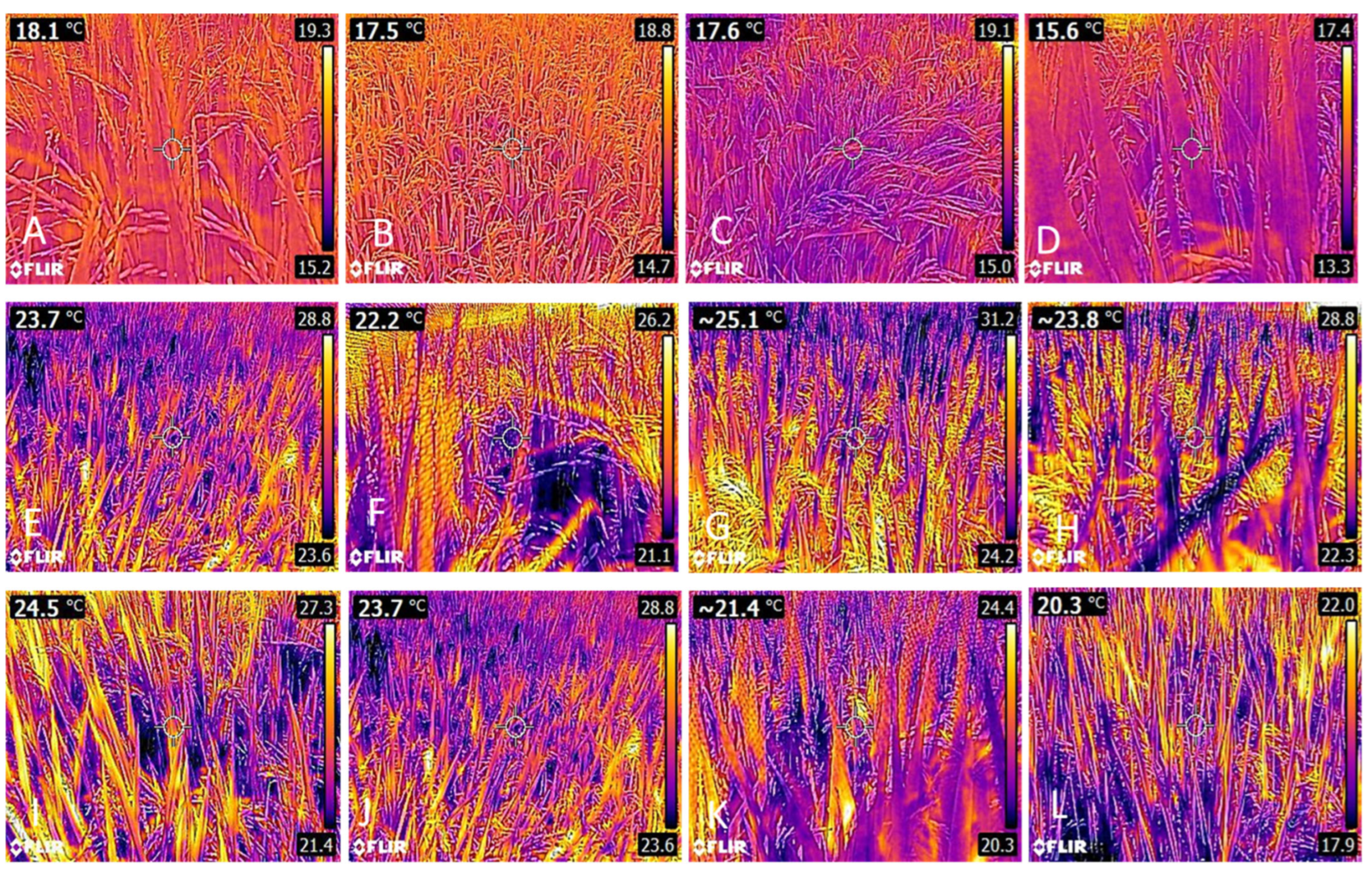
3.1. Leaf Temperature
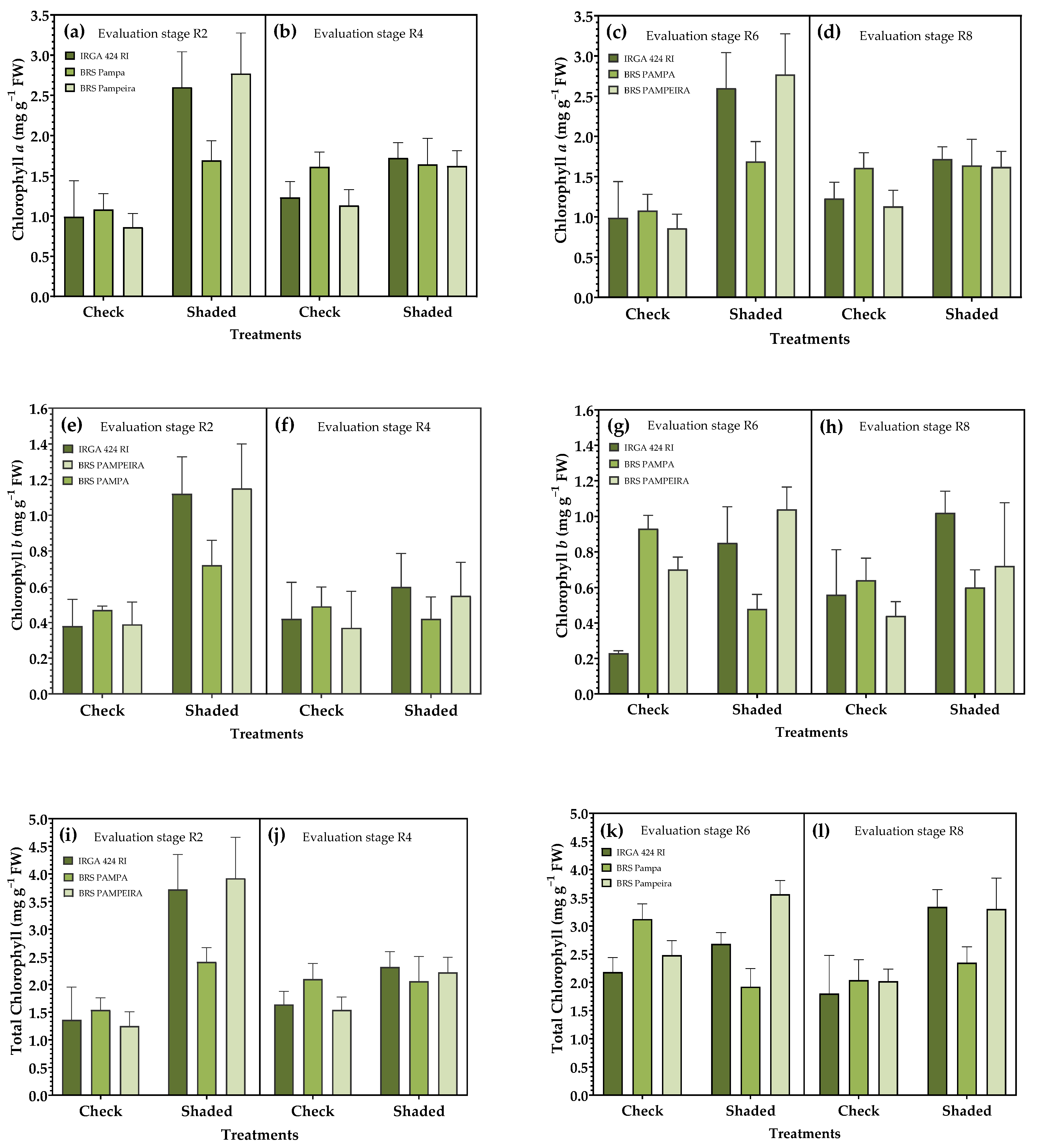
3.2. Photosynthetic Pigments
3.3. Activity of Invertases
3.4. Carbohydrate Metabolism in Leaves
3.5. Nutrient Content in Leaves
Yield, Spikelet Sterility, and Weight of a Thousand Grains
3.6. Productivity, Spikelet Sterility, and a Thousand-Grain Weight
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, L.; Gao, H.; Yoshihiro, H.; Homma, K.; Nakazaki, T.; Liu, T.-s.; Shiraiwa, T.; Xu, Z.-j. Erect panicle super rice varieties enhance yield by harvest index advantages in high nitrogen and density conditions. J. Integr. Agric. 2017, 16, 1467–1473. [Google Scholar] [CrossRef]
- Giller, K.E.; Delaune, T.; Silva, J.V.; Descheemaeker, K.; van de Ven, G.; Schut, A.G.T.; van Wijk, M.; Hammond, J.; Hochman, Z.; Taulya, G.; et al. The future of farming: Who will produce our food? Food Secur. 2021, 13, 1073–1099. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Wang, B.; Feng, P.; He, Q.; Shi, Y.; Liu, K.; Harrison, M.T.; Liu, D.; Yao, N.; et al. Integrating machine learning and environmental variables to constrain uncertainty in crop yield change projections under climate change. Eur. J. Agron. 2023, 149, 126917. [Google Scholar] [CrossRef]
- Conab–National Supply Company. Grain Production Expected to Grow in 2024/2025, Says Conab; Cultivar Magazine: Brasília, Brazil. Available online: https://revistacultivar.com/news/grain-production-should-grow-in-2024-2025-points-out-conab (accessed on 17 September 2024).
- Instituto Rio-Grandense do Arroz (IRGA). Nota Técnica: Relatório Atualizado da Safra 2023/24 de Arroz Irrigado no Rio Grande do Sul, 8 de Maio de 2024; Instituto Rio-Grandense do Arroz (IRGA): Porto Alegre, Brazil, 2024. Available online: https://www.irga.rs.gov.br/nota-tecnica-08-05-24 (accessed on 8 May 2024).
- Sytar, O.; Zivcak, M.; Bruckova, K.; Brestic, M.; Hemmerich, I.; Rauh, C.; Simko, I. Shift in Accumulation of Flavonoids and Phenolic Acids in Lettuce Attributable to Changes in Ultraviolet Radiation and Temperature. Sci. Hortic. 2018, 239, 193–204. [Google Scholar] [CrossRef]
- Xu, J.; Hou, Q.-M.; Khare, T.; Verma, S.K.; Kumar, V. Exploring miRNAs for developing climate-resilient crops: A perspective review. Sci. Total Environ. 2019, 653, 91–104. [Google Scholar] [CrossRef]
- Zhang, B.-L.; Ouyang, Y.-N.; Xu, J.-Y.; Liu, K. Cadmium remobilization from shoot to grain is related to pH of vascular bundle in rice. Ecotoxicol. Environ. Saf. 2018, 147, 913–918. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Zhai, W.; Liu, Y.; Gao, Q.; Liu, J.; Ren, L.; Chen, H.; Zhu, Y. Effects of low light on photosynthetic properties, antioxidant enzyme activity, and anthocyanin accumulation in purple pak-choi (Brassica campestris ssp. Chinensis Makino). PLoS ONE 2017, 12, e0179305. [Google Scholar] [CrossRef]
- Tripathi, S.; Hoang, Q.T.N.; Han, Y.-J.; Kim, J.-I. Regulation of photomorphogenic development by plant phytochromes. Int. J. Mol. Sci. 2019, 20, 6165. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. The dynamic plant: Capture, transformation, and management of energy. Plant Physiol. 2018, 176, 961–966. [Google Scholar]
- Chen-Jing, H.; Wang, Q.; Zhang, H.-B.; Wang, S.-H.; Song, H.-D.; Hao, J.-M.; Dong, H.-Z. Light shading improves the yield and quality of seed in oil-seed peony (Paeonia ostii Feng Dan). J. Integr. Agric. 2018, 17, 1631–1640. [Google Scholar] [CrossRef]
- Yang, H.; Lu, Z.; Duan, L.; Chen, S.; Zhao, Q.; Zhang, F.; Zhang, J. Photosynthetic base of reduced grain yield by shading stress during the early reproductive stage of two wheat cultivars. Sci. Rep. 2020, 10, 14353. [Google Scholar]
- Wang, L.; Deng, F.; Ren, W.; Yang, W. Effects of shading on starch pasting characteristics of indica hybrid rice (Oryza sativa L.). PLoS ONE 2013, 8, e68220. [Google Scholar]
- Huang, W.; Zhang, S.-B.; Liu, T. Moderate photoinhibition of photosystem II significantly affects linear electron flow in the shade-demanding plant Panax notoginseng. Front. Plant Sci. 2018, 9, 637. [Google Scholar] [CrossRef]
- Hussain, S.; Mumtaz, M.; Manzoor, S.; Shuxian, L.; Ahmed, I.; Skalicky, M.; Brestic, M.; Rastogi, A.; Ulhassan, Z.; Shafiq, I.; et al. Foliar application of silicon improves growth of soybean by enhancing carbon metabolism under shading conditions. Plant Physiol. Biochem. 2021, 159, 43–52. [Google Scholar]
- Wu, Y.; Gong, W.; Yang, W. Shade inhibits leaf size by controlling cell proliferation and enlargement in soybean. Sci. Rep. 2017, 7, 9259. [Google Scholar] [CrossRef]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Delatte, T.; Pourcel, L.; Rouhier, N.; Gakière, B.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar]
- Wang, J.; Wang, F.; Wang, L.; Zhang, D.; Gao, J.; Long, S.; Li, B.; Li, Y.; Zhang, S.; Qin, J.; et al. Carbon partitioning in sugarcane (Saccharum species). Front. Plant Sci. 2013, 4, 201. [Google Scholar]
- Sadras, O.; Lemaire, G. Quantifying crop nitrogen status for comparisons of agronomic practices and genotypes. Field Crops Res. 2014, 164, 54–64. [Google Scholar]
- Singh, S.; Mohanty, S.; Banwasi, R. Nitrogen distribution in plant parts of various rice cultivars under graded nitrogen application. J. Pharmacogn. Phytochem. 2020, 9, 1576–1578. [Google Scholar]
- Sun, Y.; Sun, Y.; Chen, L.; Xu, H.; Ma, J. Effects of different sowing dates and low-light stress at heading stage on the physiological characteristics and grain yield of hybrid rice. J. Appl. Ecol. 2012, 23, 2737–2744. [Google Scholar]
- Sridevi, V.; Chellamuthu, V. Impact of weather on rice—A review. Int. J. Appl. Res. 2015, 1, 825–831. [Google Scholar]
- Irga. Instituto Rio-Grandense do Arroz. Boletim Informativo Sobre a Radiação Solar no Rio Grande do Sul–Dezembro 2020. Available online: https://irga.rs.gov.br/upload/arquivos/202103/11163321-radiacao-solar-na-safra-2020-2021.pdf (accessed on 2 December 2021).
- Boeira, L.S.; Gonçalves, G.M.S.; Bartels, G.K.; da Ferreira Silva, J.F.; Collares, G.L. Influência do fenômeno El-niño oscilação sul no cultivo de arroz irrigado na bacia hidrográfica Mirim-São Gonçalo. Irriga 2021, 1, 344–356. [Google Scholar]
- Embrapa. Boletim Agroclimatológico; Embrapa: Capão do Leão, Brazil, 2021; Available online: http://agromet.cpact.embrapa.br/estacao/boletim.php (accessed on 20 May 2021).
- Sosbai. Sociedade sul-brasileira de arroz irrigado. Arroz irrigado: Recomendações técnicas da pesquisa para o Sul do Brasil. In XXXII Reunião Técnica da Cultura do Arroz Irrigado; Sosbai. Sociedade sul-brasileira de arroz irrigado: Farroupilha, Brazil, 2018. Available online: https://irga.rs.gov.br/irga-disponibiliza-em-seu-site-novas-recomendacoes-tecnicas-da-sosbai?utm_source=chatgpt.com (accessed on 9 December 2021).
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar]
- Zeng, Y.; Wu, Y.; Avigne, W.T.; Koch, K.E. Rapid repression of maize invertases by low oxygen. Invertase/sucrose synthase balance, sugar signaling potential, and seedling survival. Plant Physiol. 1999, 121, 599–608. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Graham, D.; Smydzuk, J. Use of Anthrone in the Quantitative Determination of Hexose Phosphates. Anal. Biochem. 1965, 11, 246–255. [Google Scholar]
- Handel, V. Direct microdetermination sucrose. Anal. Biochem. 1968, 22, 280–283. [Google Scholar]
- Yemm, E.M.; Cocking, E.C. Estimation of amino acids by ninhidrin. Analyst 1955, 80, 209–213. [Google Scholar]
- Kjeldahl, J. A New Method for the Determination of Nitrogen in Organic Matter. Z. Für. Anal. Chem. 1983, 22, 366–382. [Google Scholar] [CrossRef]
- Tedesco, M.; Gianello, C.; Bissiani, C.A.; Bohnem, H.; Volkweiss, S.J. Análise de Solo, Plantas e Outros Materiais; Universidade Federal do Rio Grande do Sul: Porto Alegre, Brazil, 1995. [Google Scholar]
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Regras para Análise de Sementes. Ministério da Agricultura, Pecuária e Abastecimento; Secretaria de Defesa Agropecuária Mapa/ACS: Brasília, Brazil, 2009. Available online: https://www.gov.br/agricultura/pt-br (accessed on 9 December 2024).
- Bhering, L.L. Rbio: A Tool For Biometric And Statistical Analysis Using The R Platform. Crop Breed. Appl. Biotechnol. 2017, 17, 187–190. [Google Scholar] [CrossRef]
- Li, R.; Shi, F.; Fukai, S.; Basnayake, J.; Liu, G.; Liu, H. Effects of cultivar and maternal environment on seed quality in Vicia sativa. Front. Plant Sci. 2017, 8, 1411. [Google Scholar] [CrossRef]
- Hussain, S.; Iqbal, N.; Rahman, T.; Liu, T.; Brestic, M.; Safdar, M.E.; Asghar, M.A.; Farooq, M.U.; Shafiq, I.; Ali, A.; et al. Shade effect on carbohydrates dynamics and stem strength of soybean genotypes. Environ. Exp. Bot. 2019, 162, 374–382. [Google Scholar] [CrossRef]
- Wang, L.; Deng, F.; Ren, W. Shading tolerance in rice is related to better light harvesting and use efficiency and grain filling rate during grain filling period. Field Crops Res. 2015, 180, 54–62. [Google Scholar] [CrossRef]
- Pan, S.; Zhang, Y.; Wang, X.; Liu, L.; Zhao, X.; Xia, X. Effects of nitrogen and shading on root morphologies, nutrient accumulation, and photosynthetic parameters in different rice genotypes. Sci. Rep. 2016, 6, 32148. [Google Scholar] [CrossRef]
- Li, H.; Jiang, D.; Wollenweber, B.; Dai, T.; Cao, W. Effects of shading on morphology, physiology and grain yield of winter wheat. Eur. J. Agron. 2010, 33, 267–275. [Google Scholar] [CrossRef]
- Azarmi, R.; Tabatabaei, S.J.; Chaparzadeh, N. Interactive effects of Mg and shading on the yield, physiology and antioxidant activity in cucumber grown in hydroponics. J. Plant Process Funct. 2018, 22, 63–71. [Google Scholar]
- Zivcak, M.; Brestic, M.; Olsovska, K.; Slamka, P. Photosynthetic responses of sun-and shade-grown barley leaves to high light: Is the lower PSII connectivity in shade leaves associated with protection against excess of light? Photosynth. Res. 2014, 119, 339–354. [Google Scholar] [CrossRef]
- Sergeeva, L.I.; Keurentjes, J.J.B.; Bino, R.J.; Vreugdenhil, D. Vacuolar Invertase INV4 Is Involved in Sucrose Regulation of Germination and Seedling Establishment in Arabidopsis thaliana. Plant Physiol. Biochem. 2006, 44, 701–709. [Google Scholar]
- González, M.C.; Vera, J.; Castro, A.; Reyes-Díaz, M.; Ruiz-Lara, S.; Mora, M.L.; Alberdi, M. Diurnal and developmental expression of vacuolar invertase in petioles of sugar beet (Beta vulgaris L.) plants. Planta 2005, 221, 153–160. [Google Scholar]
- Roitsch, T.; González, M.C. Function and regulation of plant invertases: Sweet sensations. Trends Plant Sci. 2004, 9, 606–613. [Google Scholar] [CrossRef]
- Koch, K.E. Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef]
- Weiszmann, J.; Fürtauer, L.; Weckwerth, W.; Nägele, T. Vacuolar invertase activity shapes photosynthetic stress response of Arabidopsis thaliana and stabilizes central energy supply. bioRxiv 2018, 168617. [Google Scholar] [CrossRef]
- Chen, G.; Jin, Z.; Liu, T.; Qin, S.; Ma, L.; Li, Z.; Liu, W.; Yang, W. Heterogeneous Light Conditions Reduce the Assimilate Translocation Towards Maize Ears. Plants 2020, 9, 987. [Google Scholar] [CrossRef]
- Sturm, A.; Tang, Q. As enzimas de clivagem da sacarose das plantas são cruciais para o desenvolvimento, crescimento e partição de carbono. Tendências Ciência Veg. 1999, 4, 401–407. [Google Scholar]
- Zhang, Y.; Chen, Y.; Liu, J.; Sun, W. Effects of shading on some morphological and physiological characteristics of Begonia semperflorens. Pak. J. Bot. 2018, 50, 2173–2179. [Google Scholar]
- Chen, Y.; Yu, M.; Xu, J.; Chao, Y.; Ma, Y.; Zhang, M.; Lin, J.; Xia, T. Proteolysis of chloroplast proteins is responsible for accumulation of free amino acids in dark-treated tea (Camellia sinensis) leaves. J. Proteom. 2017, 157, 10–17. [Google Scholar]
- Zhou, T.; Gao, Y.; Wang, Z.; Zhao, Y.; Ma, C.; Cui, Z.; Zhao, M. Improved post-silking light interception increases yield and P-use efficiency of maize in maize/soybean relay strip intercropping. Field Crops Res. 2021, 262, 108054. [Google Scholar] [CrossRef]
- Pons, T.L.; Jordi, W.; Kuiper, D. Acclimation of plants to light gradients in leaf canopies: Evidence for a possible role for cytokinins transported in the transpiration stream. J. Exp. Bot. 2001, 52, 1563–1574. [Google Scholar] [CrossRef]
- Deng, F.; Chen, H.; Ren, W.; Zeng, Y.; Li, Q.; Liu, T.; Liu, W.; Yang, W. Effects of different-growing-stage shading on rice grain-filling and yield. J. Sichuan Agric. Univ. 2009, 27, 265–269. [Google Scholar]
- Ren, W.; Yang, W.; Liu, W.; Chen, H.; Zeng, Y.; Li, Q. Effect of low light on dry matter accumulation and yield of rice. Sichuan Nongye Daxue Xuebao 2003, 21, 292–296. [Google Scholar]
- Ren, W.; Yang, W.; Xu, J. Effect of low light on grains growth and quality in rice. Acta Agron. Sin. 2003, 29, 785–790. [Google Scholar]
- Chen, H.; Deng, F.; Zeng, Y.; Ren, W.; Li, Q.; Liu, T.; Liu, W.; Yang, W. Effect of different shading materials on grain yield and quality of rice. Sci. Rep. 2019, 9, 9992. [Google Scholar] [CrossRef]
- Mo, Z.; Tang, X.; Xu, X.; Hu, Y.; Fang, Y. Shading during the grain filling period increases 2-acetyl-1-pyrroline content in fragrant rice. Rice 2015, 8, 9. [Google Scholar] [CrossRef]
- Yoshinaga, S.; Kondo, M.; Sakurai, N.; Kobayashi, K.; Ugaki, M.; Kato, Y. Varietal differences in sink production and grain-filling ability in recently developed high-yielding rice (Oryza sativa L.) varieties in Japan. Field Crops Res. 2013, 150, 74–82. [Google Scholar]
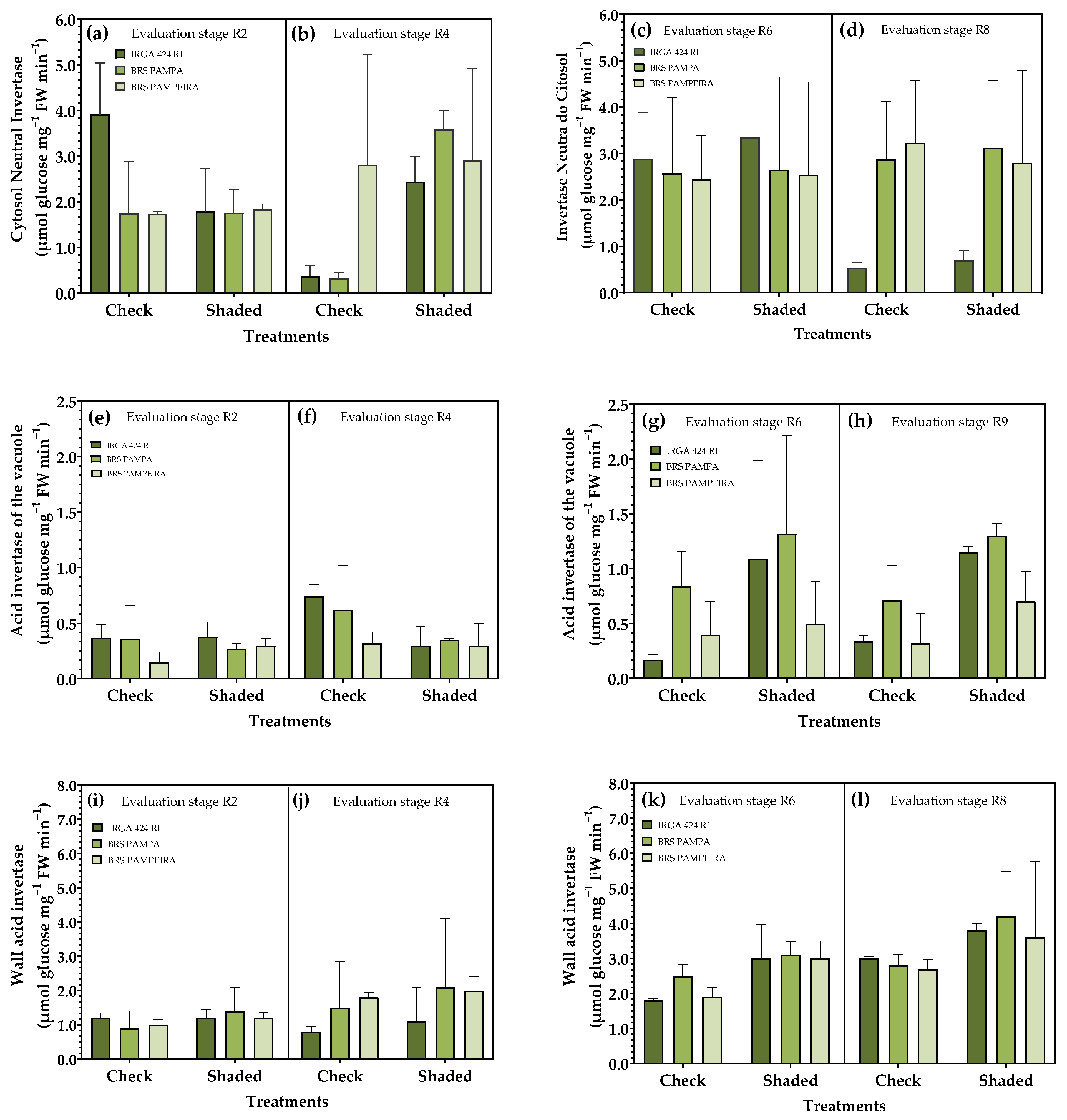
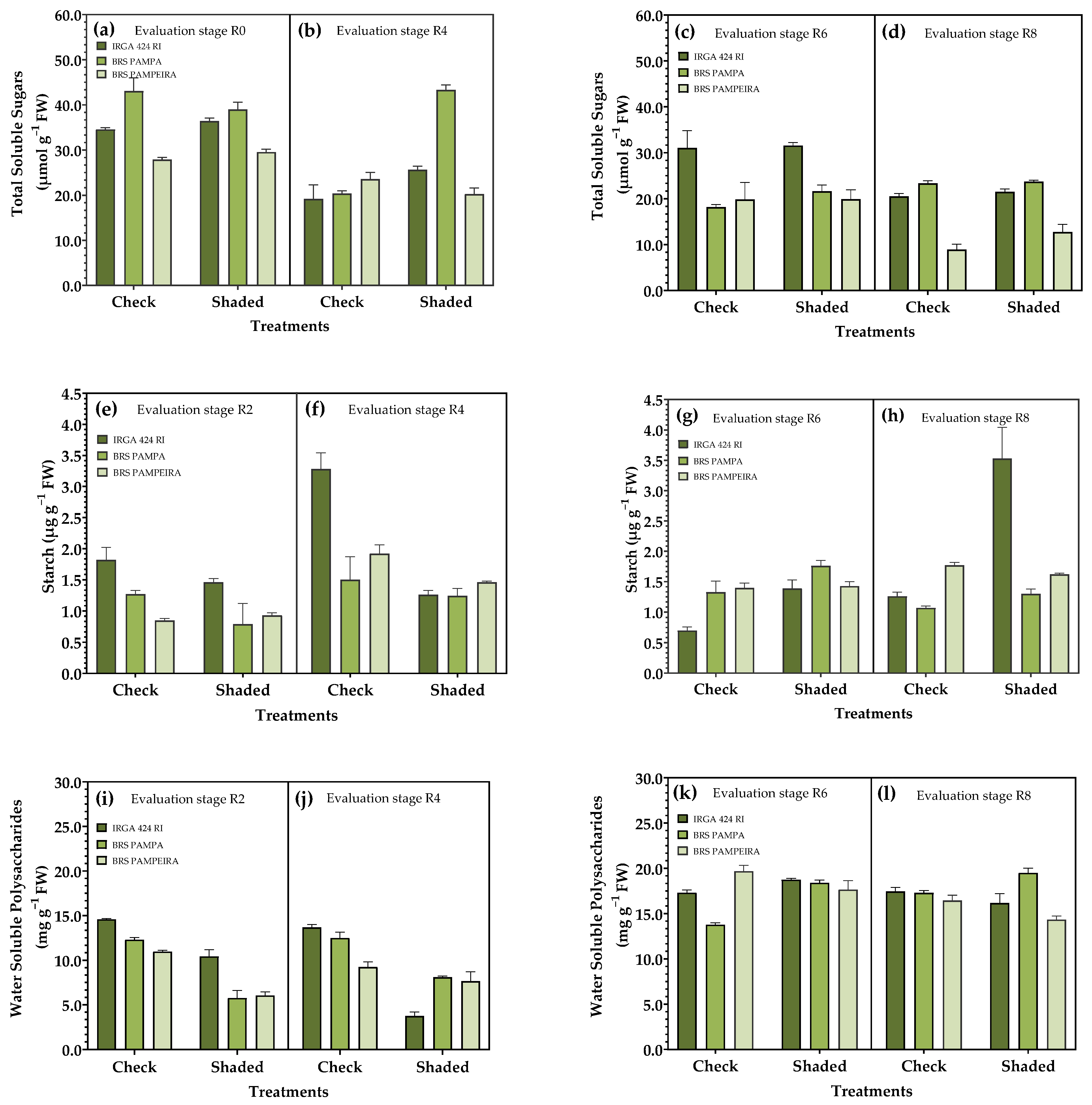
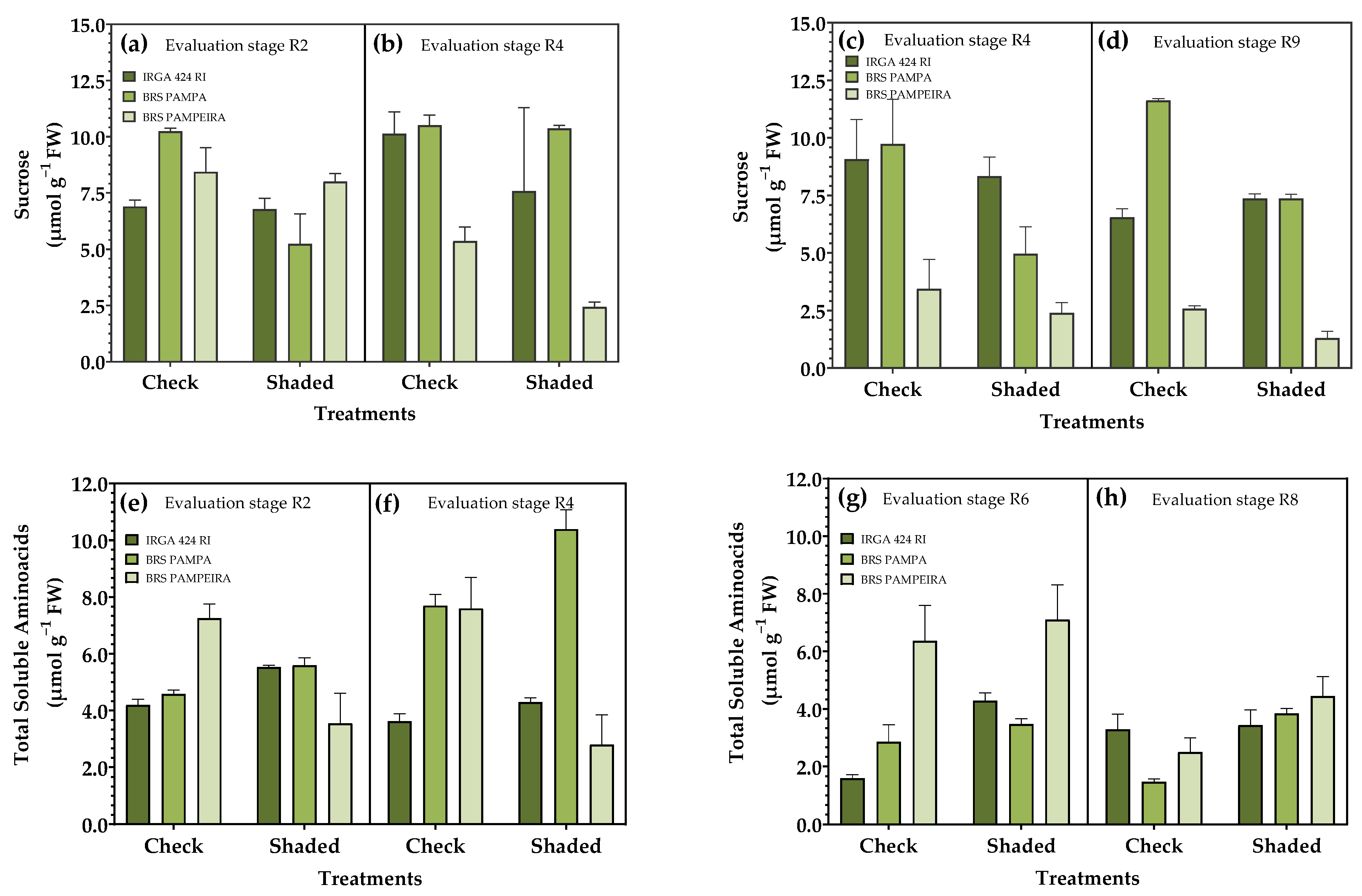
| Treatments | Treatments | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cultivar | Stage | Control | R0–R4 | Av | CV (%) | Stage | Control | R4–R9 | Av | CV (%) | |
| N g kg−1 | IRGA 424 RI | R2 | 36.8 Aa | 25.2 Cb | 31.0 | 3.5 | R6 | 22.1 Ba | 19.3 Bb | 20.7 | 5.5 |
| R4 | 31.0 Aa | 24.0 ABb | 27.5 | R8 | 17.8 Ba | 14.2 Bb | 16.0 | ||||
| BRS PAMPA | R2 | 33.8 Aa | 29.0 Bb | 31.4 | R6 | 26.0 Aa | 21.4 Ab | 23.7 | |||
| R4 | 32.5 Aa | 19.6 Bb | 26.0 | R8 | 22.7 Aa | 18.2 Bb | 20.4 | ||||
| BRS PAMPEIRA | R2 | 30.4 Bb | 28.8 Bb | 29.6 | R6 | 27.3 Aa | 23.3 Ab | 25.3 | |||
| R4 | 24.2 Bb | 27.4 Ab | 25.8 | R8 | 20.8 Aa | 23.8 Aa | 22.3 | ||||
| CV% | 8.5 | CV% | 10.0 | ||||||||
| P g kg−1 | IRGA 424 RI | R2 | 1.8 Bb | 3.0 Aa | 2.4 | 4.7 | R6 | 1.8 Bb | 1.6 Ab | 1.7 | 4.2 |
| R4 | 2.4 Ab | 2.3 Aa | 2.4 | R8 | 0.9 Bb | 1.5 ABa | 1.2 | ||||
| BRS PAMPA | R2 | 2.7 Aa | 2.7 Aa | 2.7 | R6 | 2.0 Aa | 1.6 Ab | 1.8 | |||
| R4 | 2.3 Ab | 2.3 Aa | 2.3 | R8 | 1.4 Aa | 1.4 Ba | 1.4 | ||||
| BRS PAMPEIRA | R2 | 3.4 Aa | 3.0 Aa | 3.2 | R6 | 1.3 Cb | 1.4 Aa | 1.4 | |||
| R4 | 0.9 Bb | 1.5 Ba | 1.2 | R8 | 1.4 Aa | 1.6 Aa | 1.5 | ||||
| CV% | 9.8 | CV% | 7.6 | ||||||||
| K g kg−1 | IRGA 424 RI | R2 | 37.9 Aa | 31.8 Bb | 34.9 | 5.2 | R6 | 12.6 Aa | 11.6 Ab | 12.1 | 3.1 |
| R4 | 17.6 Ba | 12.0 Bb | 14.8 | R8 | 13.4 Aa | 7.2 Cb | 10.3 | ||||
| BRS PAMPA | R2 | 33.3 Ba | 26.3 Cb | 29.8 | R6 | 12.5 Aa | 11.4 ABb | 12.0 | |||
| R4 | 21.2 Aa | 13.3 Bb | 17.2 | R8 | 11.7 Ba | 9.3 Bb | 10.5 | ||||
| BRS PAMPEIRA | R2 | 31.6 Bb | 37.6 Aa | 34.6 | R6 | 8.7 Bb | 10.8 Ba | 9.7 | |||
| R4 | 11.4 Cb | 13.2 Bb | 12.3 | R8 | 12.9 Aa | 11.8 Ab | 12.3 | ||||
| CV% | 9.1 | CV% | 4.1 | ||||||||
| Ca g kg−1 | IRGA 424 RI | R2 | 3.8 Bb | 4.2 Ba | 4.0 | 4.3 | R6 | 2.4 ABb | 2.3 Bb | 2.4 | 9.8 |
| R4 | 3.3 Bb | 2.8 Bb | 3.0 | R8 | 2.6 Bb | 3.5 Aa | 3.1 | ||||
| BRS PAMPA | R2 | 4.9 Ab | 5.3 Aa | 5.1 | R6 | 2.3 Bb | 2.9 Aa | 2.6 | |||
| R4 | 3.6 Bb | 3.2 Bb | 3.4 | R8 | 2.3 Bb | 1.7 Bb | 2.0 | ||||
| BRS PAMPEIRA | R2 | 4.9 Ab | 5.3 Aa | 5.1 | R6 | 2.0 Bb | 3.3 Aa | 2.7 | |||
| R4 | 3.3 Bb | 3.2 Bb | 3.2 | R8 | 2.3 Bb | 1.7 Bb | 2.0 | ||||
| CV% | 4.2 | CV% | 4.7 | ||||||||
| Mg g kg−1 | IRGA 424 RI | R2 | 1.9 Bb | 2.4 Ba | 2.1 | 2.8 | R6 | 2.3 Ab | 2.5 Ab | 2.4 | 6.8 |
| R4 | 2.1 Bb | 2.1 Bb | 2.1 | R8 | 1.4 Bb | 2.3 Aa | 1.8 | ||||
| BRS PAMPA | R2 | 2.6 Ab | 3.2 Aa | 2.9 | R6 | 1.5 Bb | 1.7 Bb | 1.6 | |||
| R4 | 1.7 Bb | 2.6 Ba | 2.2 | R8 | 1.6 Bb | 2.4 Aa | 2.0 | ||||
| BRS PAMPEIRA | R2 | 2.6 Ab | 3.2 Aa | 2.9 | R6 | 1.6 Bb | 2.2 Aa | 1.9 | |||
| R4 | 2.5 Bb | 3.1 Aa | 2.8 | R8 | 1.6 Bb | 1.2 Bb | 1.4 | ||||
| CV% | 3.2 | CV% | 4.9 | ||||||||
| Treatments | Treatments | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cultivar | Stage | Control | R0–R4 | Av | CV (%) | Stage | Control | R4–R9 | Av | CV (%) | |
| Cu mg kg−1 | IRGA 424 RI | R2 | 3.8 Ab | 4.8 Aa | 4.3 | 7.5 | R6 | 3.5 Ab | 4.0 Aa | 3.7 | 7.1 |
| R4 | 4.7 Ab | 4.4 Ab | 4.4 | R8 | 1.6 Bb | 3.7 Ba | 2.7 | ||||
| BRS PAMPA | R2 | 2.6 Bb | 3.5 Ba | 3.3 | R6 | 1.6 Bb | 2.5 Ba | 2.2 | |||
| R4 | 1.4 Cb | 3.2 Ba | 2.2 | R8 | 2.4 Ab | 4.5 Aa | 3.4 | ||||
| BRS PAMPEIRA | R2 | 2.5 Cb | 2.7 Ca | 3.1 | R6 | 1.9 Bb | 3.3 Aa | 2.6 | |||
| R4 | 2.6 Bb | 3.6 Ba | 2.8 | R8 | 2.6 Ab | 4.8 Aa | 3.7 | ||||
| CV% | 6.2 | CV% | 6.2 | ||||||||
| Zn mg kg−1 | IRGA 424 RI | R2 | 15.0 Ab | 15.7 Aa | 15.4 | 3.6 | R6 | 25.5 Ab | 33.3 Aa | 29.4 | 3.7 |
| R4 | 14.5 Ba | 11.2 Cb | 12.8 | R8 | 24.3 Ba | 13.5 Cb | 18.9 | ||||
| BRS PAMPA | R2 | 17.7 Bb | 14.2 Ba | 15.9 | R6 | 15.8 Bb | 20.8 Ba | 18.3 | |||
| R4 | 17.3 Aa | 15.7 Ab | 16.5 | R8 | 13.7 Cb | 24.4 Aa | 19.0 | ||||
| BRS PAMPEIRA | R2 | 12.6 Bb | 14.4 Ba | 13.5 | R6 | 15.2 Bb | 16.5 Cb | 15.9 | |||
| R4 | 13.1 Cb | 13.8 Bb | 13.5 | R8 | 30.6 Aa | 16.1 Bb | 23.3 | ||||
| CV% | 4.0 | CV% | 3.1 | ||||||||
| Fe mg kg−1 | IRGA 424 RI | R2 | 112.2 Aa | 68.8 Bb | 90.5 | 4.7 | R6 | 82.7 Aa | 37.7 Cb | 60.2 | 2.3 |
| R4 | 84.7 Aa | 56.1 Bb | 70.4 | R8 | 81.9 Aa | 66.8 Ab | 74.3 | ||||
| BRS PAMPA | R2 | 91.8 Ba | 69.7 Bb | 80.7 | R6 | 52.3 Ba | 46.6 Bb | 49.5 | |||
| R4 | 66.6 Ca | 47.6 Cb | 57.1 | R8 | 67.6 Ba | 48.5 Bb | 58.1 | ||||
| BRS PAMPEIRA | R2 | 73.2 Cb | 101.6 Aa | 87.4 | R6 | 84.7 Aa | 60.3 Ab | 72.5 | |||
| R4 | 63.2 Bb | 98.6 Aa | 80.9 | R8 | 56.1 Ca | 35.2 Cb | 45.7 | ||||
| CV% | 3.2 | CV% | 3.06 | ||||||||
| Mn mg kg−1 | IRGA 424 RI | R2 | 274.4 Ca | 207.7 Bb | 241 | 1.2 | R6 | 147.5 Ca | 108.9 Cb | 128.2 | 1.5 |
| R4 | 126.7 Ba | 108.2 Bb | 117.4 | R8 | 277.1 ABa | 276.1 Ba | 276.6 | ||||
| BRS PAMPA | R2 | 360.4 Aa | 303.1 Ab | 331.8 | R6 | 212.8 Ba | 129.8 Bb | 171.3 | |||
| R4 | 118.4 Ba | 104.6 Bb | 111.5 | R8 | 266.3 Bb | 313.4 Aa | 289.9 | ||||
| BRS PAMPEIRA | R2 | 354.5 Aa | 329.7 Ab | 342.1 | R6 | 384.3 Aa | 328.4 Ab | 356.3 | |||
| R4 | 326.5 Ab | 365.5 Aa | 346 | R8 | 295.3 Ab | 322.7 Aa | 309.0 | ||||
| CV% | 2.5 | CV% | 3.1 | ||||||||
| Agricultural Year | |||||||
|---|---|---|---|---|---|---|---|
| 2019/20 | 2020/21 | ||||||
| Shading Season | Shading Season | ||||||
| Cultivate | Check | R0–R4 | R4–R9 | Check | R0–R4 | R4–R9 | |
| Productivity (kg ha−1) | IRGA 424 RI | 13.91 Aa | 9.91 Ac | 11.39 Ab | 13.60 Aa | 11.28 Ab | 10.45 Ab |
| BRS PAMPA | 12.11 Ba | 8.64 Bb | 9.89 Bb | 12.16 Ba | 9.95 Bb | 7.89 Bb | |
| BRS PAMPEIRA | - | - | - | 9.76 Ca | 6.68 Cb | 7.81 Bb | |
| Média | 12.70 | 9.27 | 10.64 | 11.86 | 9.30 | 8.71 | |
| CV (%) | 7.66 | 8.0 | |||||
| Spikelet sterility (%) | IRGA 424 RI | 14.26 Ab | 16.01 Ab | 22.18 Aa | 13.81 Bb | 16.70 Bb | 24.02 Ba |
| BRS PAMPA | 13.41 Ab | 18.04 Aa | 17.14 Ba | 12.04 Bb | 14.54 Bb | 24.69 Ba | |
| BRS PAMPEIRA | - | - | - | 24.70 Ab | 37.57 Aa | 36.03 Aa | |
| Média | 13.84 | 17.03 | 19.66 | 14.81 | 20.38 | 25.92 | |
| CV (%) | 6.97 | 8.93 | |||||
| Thousand-grain weight (g) | IRGA 424 RI | 25.55 Aa | 24.94 Ab | 24.45 Ab | 25.05 Aa | 23.59 Ac | 24.49 Ab |
| BRS PAMPA | 25.35 Aa | 24.45 Ab | 24.46 Ab | 25.06 Ba | 24.40 Bb | 24.18 Bb | |
| BRS PAMPEIRA | - | - | - | 25.72 Ba | 24.14 Cb | 24.20 ABb | |
| Média | 25.45 | 24.7 | 24.46 | 25.27 | 24.04 | 24.29 | |
| CV (%) | 2.44 | 1.08 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, S.N.; Souza, F.R.d.; Silva, B.E.P.; Fagundes, N.d.S.; Lucho, S.R.; Avila, L.A.d.; Deuner, S. Effects of Shading on Metabolism and Grain Yield of Irrigated Rice During Crop Development. Plants 2025, 14, 2491. https://doi.org/10.3390/plants14162491
Pires SN, Souza FRd, Silva BEP, Fagundes NdS, Lucho SR, Avila LAd, Deuner S. Effects of Shading on Metabolism and Grain Yield of Irrigated Rice During Crop Development. Plants. 2025; 14(16):2491. https://doi.org/10.3390/plants14162491
Chicago/Turabian StylePires, Stefânia Nunes, Fernanda Reolon de Souza, Bruna Evelyn Paschoal Silva, Natan da Silva Fagundes, Simone Ribeiro Lucho, Luis Antonio de Avila, and Sidnei Deuner. 2025. "Effects of Shading on Metabolism and Grain Yield of Irrigated Rice During Crop Development" Plants 14, no. 16: 2491. https://doi.org/10.3390/plants14162491
APA StylePires, S. N., Souza, F. R. d., Silva, B. E. P., Fagundes, N. d. S., Lucho, S. R., Avila, L. A. d., & Deuner, S. (2025). Effects of Shading on Metabolism and Grain Yield of Irrigated Rice During Crop Development. Plants, 14(16), 2491. https://doi.org/10.3390/plants14162491








