The Expression of Genes Involved in Phenylpropanoid Biosynthesis Correlates Positively with Phenolic Content and Antioxidant Capacity in Developing Chickpea (Cicer arietinum L.) Seeds
Abstract
1. Introduction
2. Results and Discussion
2.1. Antioxidant Capacity (AC)
2.2. Phenolic and Flavonoid Content and Their Association with the AC
2.3. Expression of MYB Transcription Factors and Target Genes
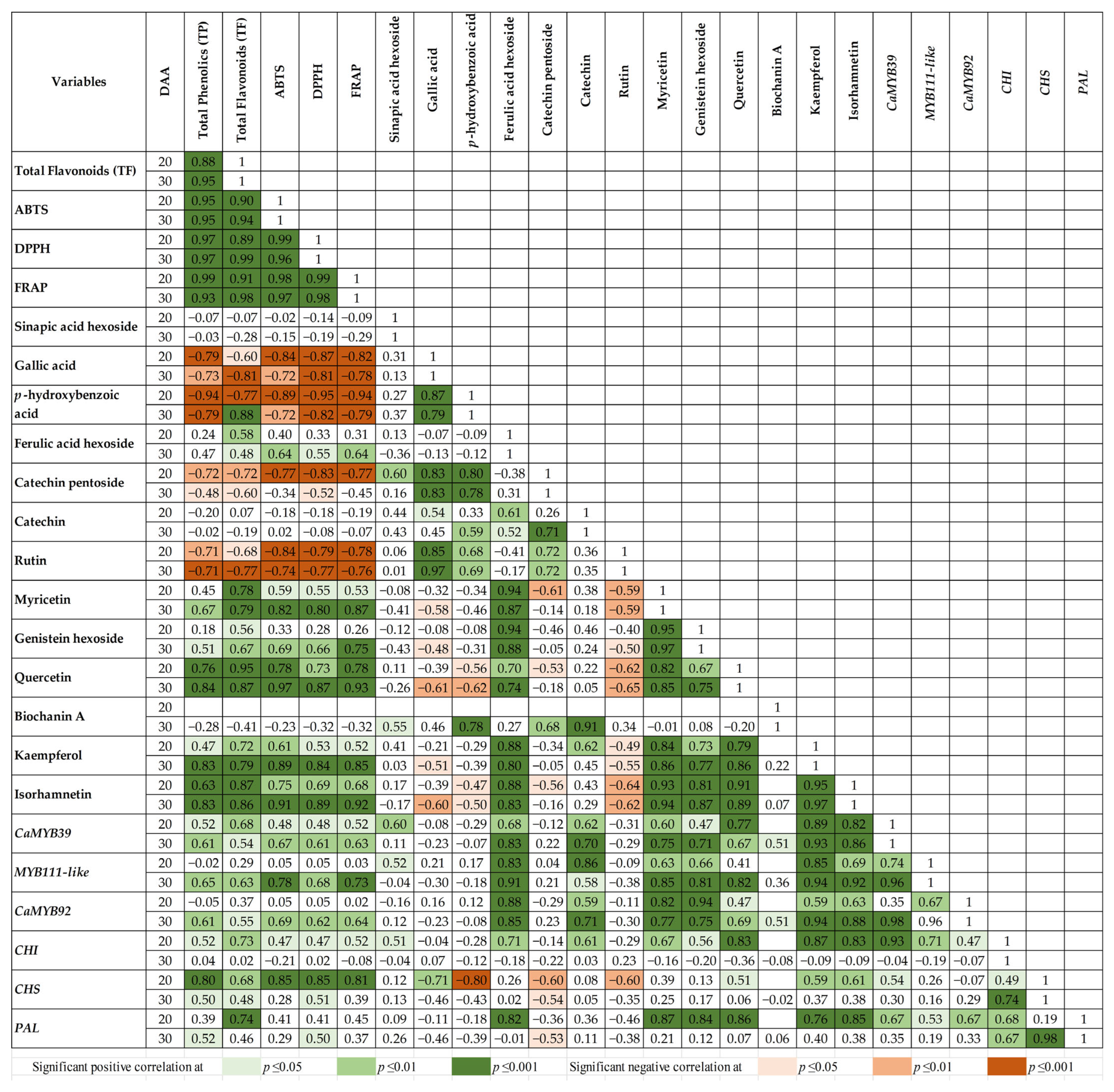
2.4. Associations Between Phenolic Content, Antioxidant Capacity, and Gene Expression
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of Methanol Extracts
3.3. Total Phenolics (TP) and Flavonoids (TF)
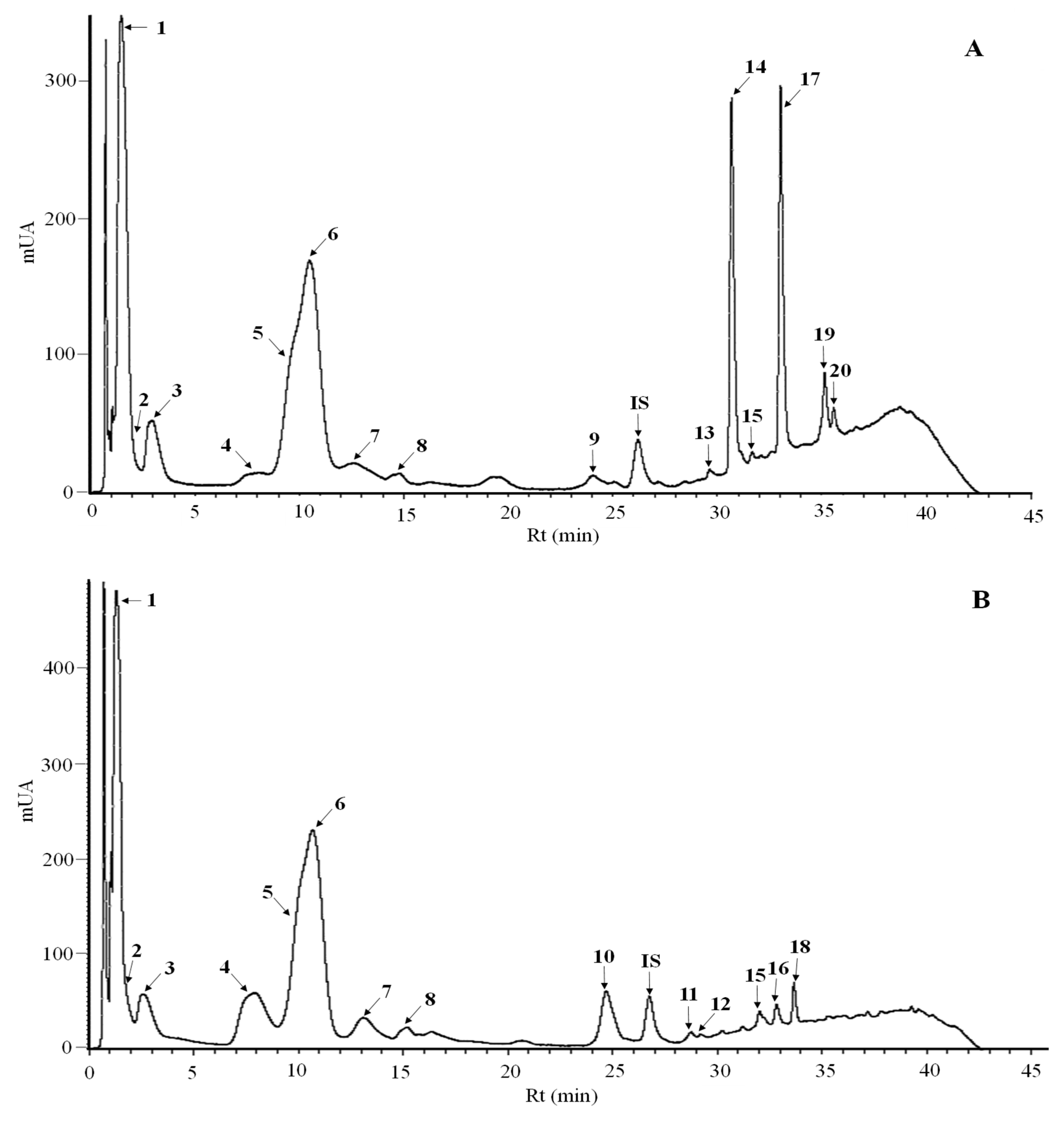
3.4. Phenolic Profiles
3.5. Antioxidant Capacity (AC)
3.6. RNA Isolation
3.7. Gene Expression Analysis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UPLC | Ultra-performance liquid chromatography |
| DAD | Diode array detector |
| MS | Mass spectrometry |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| FRAP | Ferric Reducing Antioxidant Power |
| PAL | Phenylalanine ammonia-lyase |
| CHS | Chalcone synthase |
| CHI | Chalcone isomerase |
| RT-qPCR | Reverse transcription–quantitative polymerase chain reaction |
References
- Kumar, N.; Hong, S.; Zhu, Y.; Garay, A.; Yang, J.; Henderson, D.; Zhang, X.; Xu, Y.; Li, Y. Comprehensive review of chickpea (Cicer arietinum): Nutritional significance, health benefits, techno-functionalities, and food applications. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70152. [Google Scholar] [CrossRef]
- Chavez-Ontiveros, J.; Quintero-Soto, M.F.; Pineda-Hidalgo, K.V.; Lopez-Moreno, H.S.; Reyes Moreno, C.; Garzon-Tiznado, J.A.; Lopez-Valenzuela, J.A. Microsatellite-based genetic diversity and grain quality variation in chickpea genotypes from Mexico and international collections. Agrociencia 2020, 54, 57–73. [Google Scholar]
- Bhagyawant, S.S.; Gautam, A.K.; Narvekar, D.T.; Gupta, N.; Bhadkaria, A.; Srivastava, N.; Upadhyaya, H.D. Biochemical diversity evaluation in chickpea accessions employing mini-core collection. Physiol. Mol. Biol. Plants 2018, 24, 1165–1183. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Soto, M.F.; Saracho-Peña, A.G.; Chavez-Ontiveros, J.; Garzon-Tiznado, J.A.; Pineda-Hidalgo, K.V.; Delgado-Vargas, F.; Lopez-Valenzuela, J.A. Phenolic profiles and their contribution to the antioxidant activity of selected chickpea genotypes from Mexico and ICRISAT collections. Plant Foods Hum. Nutr. 2018, 73, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Bochenek, H.; Francis, N.; Santhakumar, A.B.; Blanchard, C.L.; Chinkwo, K.A. The antioxidant and anticancer properties of chickpea water and chickpea polyphenol extracts in vitro. Cereal Chem. 2023, 100, 895–903. [Google Scholar] [CrossRef]
- Stracke, R.; Werber, M.; Weisshaar, B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 2001, 4, 447–456. [Google Scholar] [CrossRef]
- Stracke, R.; Ishihara, H.; Huep, G.; Barsch, A.; Mehrtens, F.; Niehaus, K.; Weisshaar, B. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 2007, 50, 660–677. [Google Scholar] [CrossRef]
- Rajput, R.; Tyagi, S.; Naik, J.; Pucker, B.; Stracke, R.; Pandey, A. The R2R3-MYB gene family in Cicer arietinum: Genome-wide identification and expression analysis leads to functional characterization of proanthocyanidin biosynthesis regulators in the seed coat. Planta 2022, 256, 67. [Google Scholar] [CrossRef]
- Singh, S.; Pal, L.; Rajput, R.; Chhatwal, H.; Singh, N.; Chattopadhyay, D.; Pandey, A. CaLAP1 and CaLAP2 orchestrate anthocyanin biosynthesis in the seed coat of Cicer arietinum. Planta 2024, 260, 38. [Google Scholar] [CrossRef]
- Saxena, S.; Pal, L.; Naik, J.; Singh, Y.; Verma, P.K.; Chattopadhyay, D.; Pandey, A. The R2R3-MYB-SG7 transcription factor CaMYB39 orchestrates surface phenylpropanoid metabolism and pathogen resistance in chickpea. New Phytol. 2023, 238, 798–816. [Google Scholar] [CrossRef]
- Bulut, M.; Wendenburg, R.; Bitocchi, E.; Bellucci, E.; Kroc, M.; Gioia, T.; Susek, K.; Papa, R.; Fernie, A.R.; Alseekh, S. A comprehensive metabolomics and lipidomics atlas for the legumes common bean, chickpea, lentil and lupin. Plant J. 2023, 116, 1152–1171. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xu, L.; Zhuang, H.; Abd-Eldaim, F.A.; Tang, Z.; Dewer, Y.; Wang, H. Evaluation of skin color supervision genes in chickpea seeds by multiomics. Mol. Biotechnol. 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Bandhiwal, N.; Shah, N.; Kant, C.; Gaur, R.; Bhatia, S. Global transcriptome analysis of developing chickpea (Cicer arietinum L.) seeds. Front. Plant Sci. 2014, 5, 698. [Google Scholar] [CrossRef] [PubMed]
- Ballester, A.-R.; Molthoff, J.; de Vos, R.; te Lintel Hekkert, B.; Orzaez, D.; Fernández-Moreno, J.-P.; Tripodi, P.; Grandillo, S.; Martin, C.; Heldens, J. Biochemical and molecular analysis of pink tomatoes: Deregulated expression of the gene encoding transcription factor SlMYB12 leads to pink tomato fruit color. Plant Physiol. 2010, 152, 71–84. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Romani, A. Rapid tests to assess the antioxidant activity of Phaseolus vulgaris L. dry beans. J. Agric. Food Chem. 2005, 53, 3053–3056. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Holding, R.; Hunter, B.; Chung, T.; Gibbon, B.; Ford, C.; Bharti, A.; Messing, J.; Hamaker, B.; Larkins, B. Genetic analysis of opaque2 modifier loci in quality protein maize. Theor. Appl. Genet. 2008, 117, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


| Chickpea Genotype | Seed Color | Phenolics Content (mg GAE/100 g) | Flavonoids Content (mg CAE/100 g) | Antioxidant Capacity (µmol TE/100 g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ABTS | DPPH | FRAP | |||||||||
| 20 DAA | 30 DAA | 20 DAA | 30 DAA | 20 DAA | 30 DAA | 20 DAA | 30 DAA | 20 DDA | 30 DDA | ||
| Kabuli | |||||||||||
| Bco Sin 92 | Cream | 24.1 ± 0.9 Db | 45.1 ± 4.4 Ea | 28.5 ± 0.8 Ca | 34.9 ± 0.7 Da | 389.6 ± 10.4 Ba | 421.1 ± 3.4 Ea | 145.1 ± 6.5 Ca | 186.6 ± 2.2 Ca | 86.60 ± 1.2 Ea | 102.8 ± 3.6 Ea |
| Blanoro | Cream | 22.2 ± 2.2 Db | 39.4 ± 0.6 Ea | 43.6 ± 2.8 Ca | 37.6 ± 1.9 Da | 240.7 ± 20.9 Cb | 311.8 ± 2.1 Fa | 106.8 ± 6.3 Ca | 125.2 ± 4.5 Da | 40.00 ± 6.7 Fb | 90.9 ± 4.9 Ea |
| Desi | |||||||||||
| ICC 5613 | Green | 147.6 ± 7.7 Ba | 154.7 ± 1.5 Da | 152.4 ± 6.6 Ab | 187.6 ± 7.9 Ba | 900.8 ± 31.4 Ab | 1366.4 ± 41.1 Ca | 736.8 ± 6.9 Ab | 1157.4 ± 55.8 Ba | 545.9 ± 18.3 Bb | 856.6 ± 7.8 Ba |
| ICC 4418 | Black | 124.4 ± 11.5 Cb | 263.6 ± 3.9 Aa | 147.9 ± 6.8 Ab | 220.2 ± 9.9 Aa | 917.7 ± 11.4 Ab | 1794.9 ± 63.8 Aa | 659.3 ± 21.8 Bb | 1590.6 ± 26.8 Aa | 467.8 ± 0.9 Db | 1014.5 ± 17.5 Aa |
| ICC 5383 | Brown | 187.9 ± 1.7 Ab | 226.9 ± 7.5 Ba | 144.9 ± 7.2 Ab | 177.1 ± 7.8 Ca | 959.1 ± 15.7 Ab | 1490.1 ± 17.2 Ba | 765.3 ± 0.9 Ab | 1171.1 ± 9.5 Ba | 622.3 ± 17.2 Ab | 729.1 ± 2.4 Ca |
| ICC 3512 | Brown | 143.6 ± 1.1 Bb | 193.1 ± 1.3 Ca | 94.6 ± 7.0 Bb | 180.9 ± 2.1 Ba | 911.4 ± 17.4 Ab | 1003.8 ± 11.1 Da | 771.2 ± 2.9 Ab | 1175.7 ± 7.4 Ba | 516.1 ± 7.1 Cb | 652.3 ± 36.6 Da |
| Metabolite | DAA | Genotype | ||||||
|---|---|---|---|---|---|---|---|---|
| Bco. Sin. 92 | Blanoro | ICC 5613 | ICC 4418 | ICC 5383 | ICC 3512 | LOD | ||
| Sinapic acid hexoside z | 20 | 124.89 ± 13.50 Ab | 70.50 ± 5.82 Bb | 14.20 ± 1.22 Ca | 143.71 ± 15.89 Aa | 132.37 ± 13.95 Aa | 56.30 ± 5.69 Bb | 0.14 |
| 30 | 171.62 ± 19.94 Aa | 92.72 ± 6.22 Ca | 32.78 ± 3.73 Da | 135.29 ± 16.69 Ba | 130.48 ± 15.77 Ba | 107.38 ± 10.18 Ca | ||
| Gallic acid | 20 | 113.25 ± 8.86 Ba | 169.28 ± 4.71 Ab | 44.66 ± 2.69 Ea | 87.88 ± 7.62 Ca | 69.11 ± 9.27 Da | 10.42 ± 1.83 Fb | 0.14 |
| 30 | 108.96 ± 13.88 Ba | 215.52 ± 5.33 Aa | 39.20 ± 0.69Da | 58.55 ± 6.23 Cb | 70.03 ± 9.33 Ca | 29.68 ± 2.62 Da | ||
| Dihydroxybenzoic acid | 20 | <LOD | 5.61 ± 0.14 b | <LOD | <LOD | <LOD | <LOD | 0.05 |
| 30 | <LOD | 13.41 ± 0.38 a | <LOD | <LOD | <LOD | <LOD | ||
| Vanillic acid | 20 | <LOD | <LOD | 1.79 ± 0.13 Ab | 0.68 ± 0.21 Bb | <LOD | <LOD | 0.04 |
| 30 | <LOD | <LOD | 6.79±1.01 Aa | 2.47 ±0.31 Ba | <LOD | <LOD | ||
| p-Hydroxybenzoic acid | 20 | 41.60 ± 3.15 Ab | 38.91 ± 5.92 Ab | 11.58 ± 0.47 Ca | 22.79 ± 2.64 Ba | 8.59 ± 0.26 Ca | 5.23 ± 0.04 Da | 0.05 |
| 30 | 48.88 ± 3.64 Aa | 45.73 ± 2.51 Aa | 13.20 ± 0.29 Ca | 24.02 ± 0.86 Ba | 10.27 ± 0.08 Ca | 5.26 ± 0.03 Da | ||
| Benzoic acid | 20 | <LOD | 6.44 ± 0.44 Bb | <LOD | 9.48 ± 0.50 Ab | <LOD | <LOD | 0.05 |
| 30 | <LOD | 7.35 ± 0.16 Ba | <LOD | 10.66 ± 0.28 Aa | <LOD | <LOD | ||
| p-Coumaric acid | 20 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 0.12 |
| 30 | <LOD | 34.06 ± 0.37 | <LOD | <LOD | <LOD | <LOD | ||
| Ferulic acid hexoside z | 20 | 0.58 ± 0.07 Ca | 1.26 ± 0.04 Cb | 5.19 ± 0.95 Ba | 9.16 ± 1.10 Aa | 0.95 ± 0.11 Cb | 0.49 ± 0.04 Ca | 0.11 |
| 30 | 1.02 ± 0.03 Ea | 3.71 ± 0.34 Ca | 5.90 ± 0.69 Ba | 7.96 ± 0.23 Ab | 2.77 ± 0.02 Da | 1.26 ± 0.01 Ea | ||
| Metabolite | DAA | Genotype | ||||||
|---|---|---|---|---|---|---|---|---|
| Bco. Sin. 92 | Blanoro | ICC 5613 | ICC 4418 | ICC 5383 | ICC 3512 | LOD | ||
| Catechin pentoside z | 20 | 77.88 ± 6.79 Aa | 72.95 ± 3.88 Ab | 23.74 ± 1.47 Eb | 47.82 ± 1.94 Cb | 57.36 ± 4.58 Ba | 31.86 ± 3.64 Da | 0.09 |
| 30 | 69.58 ± 2.74 Bb | 89.99 ± 5.03 Aa | 58.66 ± 2.09 Ca | 71.88 ± 0.61 Ba | 60.60 ± 6.62 Ca | 38.52 ± 4.06 Da | ||
| Catechin | 20 | 9.93 ± 0.82 Db | 42.64 ± 3.27 Ba | 9.85 ± 1.02 Da | 56.89 ± 3.76 Ab | 18.69 ± 2.23 Ca | 8.87 ± 0.93 Da | 0.09 |
| 30 | 35.54 ± 2.49 Ca | 44.69 ± 4.22 Ba | 12.72 ± 1.32 Ea | 63.13 ± 5.17 Aa | 19.14 ± 0.36 Da | 11.17 ± 1.02 Ea | ||
| Myricetin O-methyl ether hexoside deoxyhexoside pentoside z | 20 | 0.97 ± 0.06 Aa | 0.31 ± 0.04 Bb | <LOD | <LOD | <LOD | <LOD | 0.19 |
| 30 | 0.81 ± 0.01 Ab | 0.41 ± 0.013 Ba | <LOD | <LOD | <LOD | <LOD | ||
| Myricetin O-methyl ether hexoside deoxyhexoside z | 20 | <LOD | 0.45 ± 0.05 b | <LOD | <LOD | <LOD | <LOD | 0.09 |
| 30 | <LOD | 0.62 ± 0.05 a | <LOD | <LOD | <LOD | <LOD | ||
| Rutin | 20 | 0.18 ± 0.01 Ba | 0.41 ± 0.04 Ab | 0.05 ± 0.01 Ea | 0.09 ± 0.01 Da | 0.15 ± 0.005 Ca | 0.09 ± 0.01 Da | 0.02 |
| 30 | 0.20 ± 0.02 Ba | 0.54 ± 0.15 Aa | 0.08 ± 0.013 Ea | 0.10 ± 0.01 Da | 0.14 ± 0.013 Ca | 0.11 ± 0.01 Da | ||
| Myricetin | 20 | 0.06 ± 0.01 Da | 0.85 ± 0.023 Da | 18.26 ± 1.65 Bb | 21.86 ± 0.75 Ab | 4.51 ± 0.37 Ca | 3.82 ± 0.11 Ca | 0.09 |
| 30 | 0.07 ± 0.01 Da | 0.86 ± 0.03 Da | 20.8 ± 0.88 Ba | 23.54 ± 0.47 Aa | 5.68 ± 0.31 Ca | 6.67 ± 0.29 Ca | ||
| Genistein hexoside z | 20 | 1.01 ± 0.09 Ca | 1.66 ± 0.39 Ca | 6.39 ± 0.49 Ba | 7.35 ± 0.28 A a | 0.44 ± 0.02 Da | 0.54 ± 0.04 Da | 0.008 |
| 30 | 0.98 ± 0.06 Ca | 1.09 ± 0.16 Ca | 6.61 ± 0.86 Aa | 6.81 ± 0.86 A a | 1.17 ± 0.03 Ba | 1.81 ± 0.05 Ba | ||
| Isorhamnetin 3-O-β-glucopyranoside z | 20 | 0.23 ± 0.02 Bb | 1.65 ± 0.02 Ab | <LOD | <LOD | <LOD | <LOD | 0.01 |
| 30 | 0.56 ± 0.01 Aa | 2.05 ± 0.03 Aa | <LOD | <LOD | <LOD | <LOD | ||
| Quercetin | 20 | <LOD | <LOD | 16.69 ± 0.28 Bb | 17.89 ± 1.09 Ab | 15.91 ± 0.67 Bb | 2.90 ± 0.16 Cb | 0.03 |
| 30 | <LOD | <LOD | 18.39 ± 0.56 Ba | 22.00 ± 0.15 Aa | 17.63 ± 0.15 Ba | 5.22 ± 0.13 Ca | ||
| Biochanin A | 20 | 11.03 ± 0.16 Bb | <LOD | <LOD | 12.82 ± 0.35 Ab | <LOD | <LOD | 0.01 |
| 30 | 13.08 ± 0.18 Ba | 9.84 ± 0.11 C | <LOD | 14.86 ± 0.21 Aa | <LOD | <LOD | ||
| Kaempferol | 20 | 0.25 ± 0.01 Db | 0.24 ± 0.01 Eb | 1.29 ± 0.02 Bb | 3.78 ± 0.03 Ab | 1.48 ± 0.01 Bb | 0.79 ± 0.02 Cb | 0.07 |
| 30 | 0.63 ± 0.03 Da | 0.43 ± 0.01 Ea | 2.65 ± 0.05 Ba | 6.32 ± 0.19 Aa | 2.58 ± 0.05 Ba | 1.68 ± 0.01 Ca | ||
| Isorhamnetin | 20 | <LOD | <LOD | 23.19 ± 0.49 Bb | 38.06 ± 0.56 Ab | 17.05 ± 0.06 Ca | 9.48 ± 0.09 Db | 0.01 |
| 30 | <LOD | 0.089 ± 0.01 E | 29.24 ± 0.83 Ba | 51.18 ± 2.23 Aa | 18.11 ± 0.28 Ca | 15.28 ± 0.57 Da | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pineda-Hidalgo, K.V.; Flores-Paredes, G.; Garzón-Tiznado, J.A.; Salazar-Salas, N.Y.; Chávez-Ontiveros, J.; López-Angulo, G.; Delgado-Vargas, F.; Lopez-Valenzuela, J.A. The Expression of Genes Involved in Phenylpropanoid Biosynthesis Correlates Positively with Phenolic Content and Antioxidant Capacity in Developing Chickpea (Cicer arietinum L.) Seeds. Plants 2025, 14, 2489. https://doi.org/10.3390/plants14162489
Pineda-Hidalgo KV, Flores-Paredes G, Garzón-Tiznado JA, Salazar-Salas NY, Chávez-Ontiveros J, López-Angulo G, Delgado-Vargas F, Lopez-Valenzuela JA. The Expression of Genes Involved in Phenylpropanoid Biosynthesis Correlates Positively with Phenolic Content and Antioxidant Capacity in Developing Chickpea (Cicer arietinum L.) Seeds. Plants. 2025; 14(16):2489. https://doi.org/10.3390/plants14162489
Chicago/Turabian StylePineda-Hidalgo, Karen V., Gamaliel Flores-Paredes, José A. Garzón-Tiznado, Nancy Y. Salazar-Salas, Jeanett Chávez-Ontiveros, Gabriela López-Angulo, Francisco Delgado-Vargas, and José A. Lopez-Valenzuela. 2025. "The Expression of Genes Involved in Phenylpropanoid Biosynthesis Correlates Positively with Phenolic Content and Antioxidant Capacity in Developing Chickpea (Cicer arietinum L.) Seeds" Plants 14, no. 16: 2489. https://doi.org/10.3390/plants14162489
APA StylePineda-Hidalgo, K. V., Flores-Paredes, G., Garzón-Tiznado, J. A., Salazar-Salas, N. Y., Chávez-Ontiveros, J., López-Angulo, G., Delgado-Vargas, F., & Lopez-Valenzuela, J. A. (2025). The Expression of Genes Involved in Phenylpropanoid Biosynthesis Correlates Positively with Phenolic Content and Antioxidant Capacity in Developing Chickpea (Cicer arietinum L.) Seeds. Plants, 14(16), 2489. https://doi.org/10.3390/plants14162489










