Effects of Different Soil Phosphorus Levels on the Physiological and Growth Characteristics of Phyllostachys edulis (Moso Bamboo) Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set Up
2.2. Harvest and Measurements
2.3. Statistical Analysis
3. Results
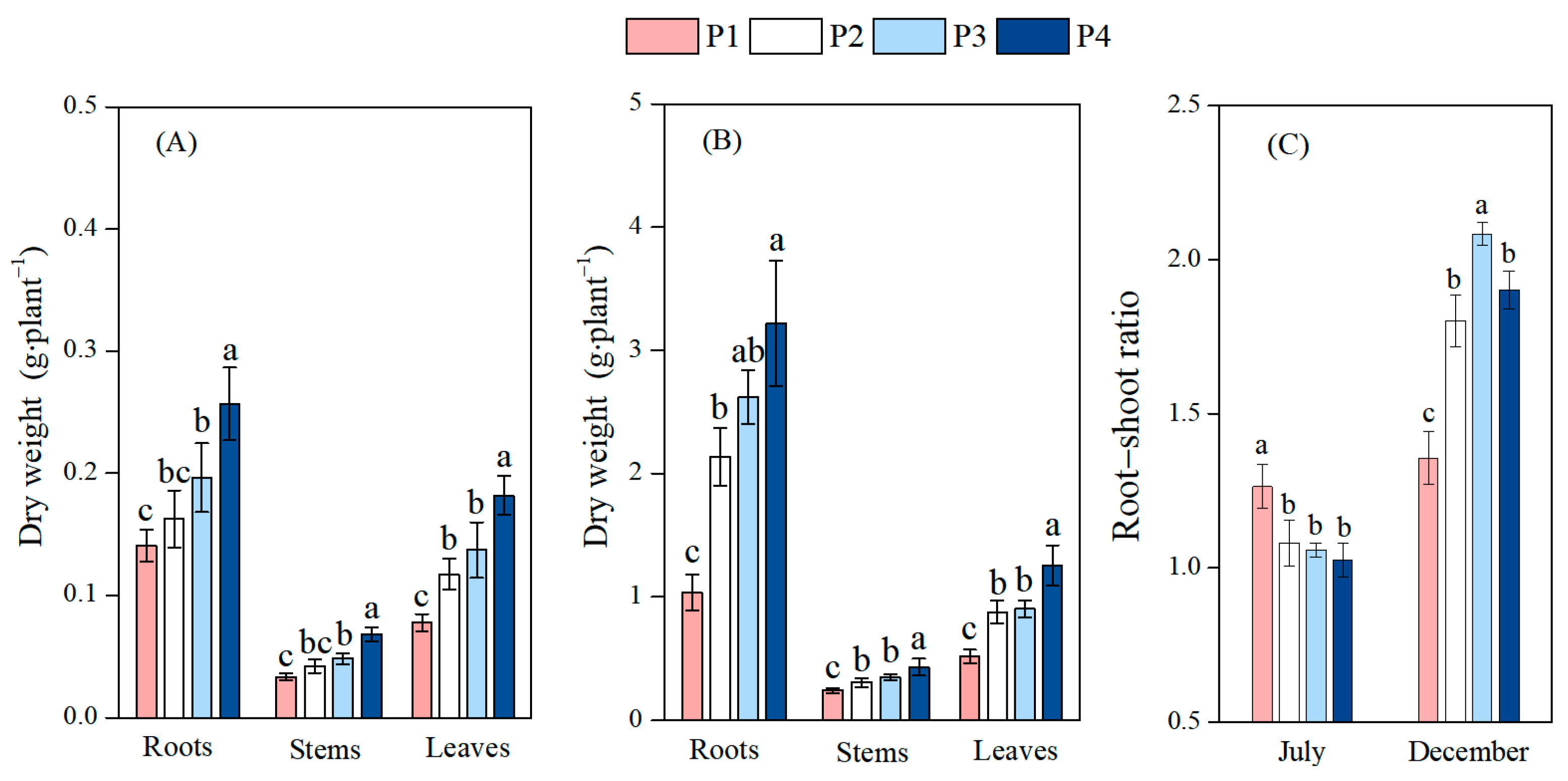
3.1. Effects of Different Soil Phosphorus Levels on the Biomass Allocation of Moso Bamboo Seedlings
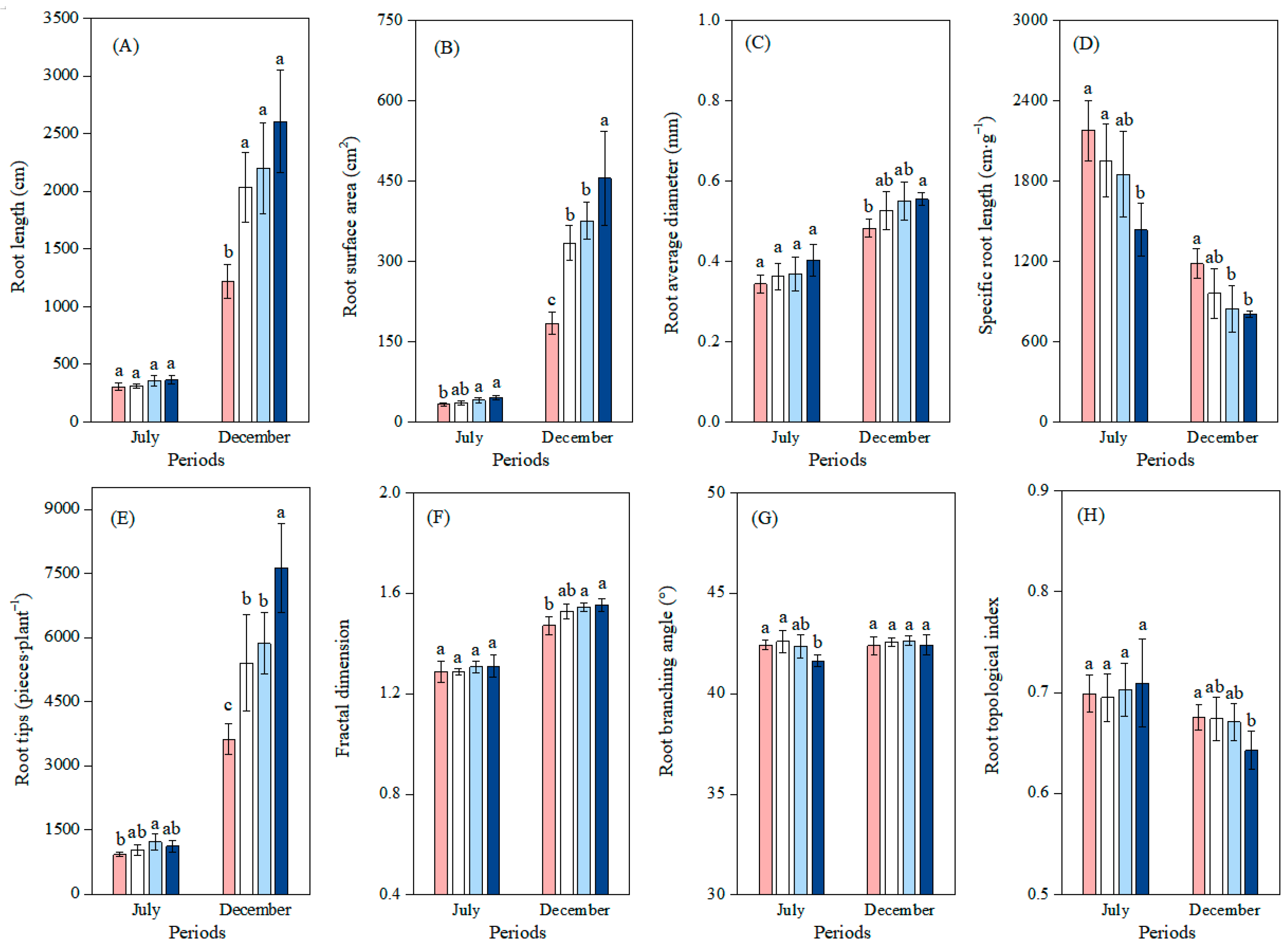
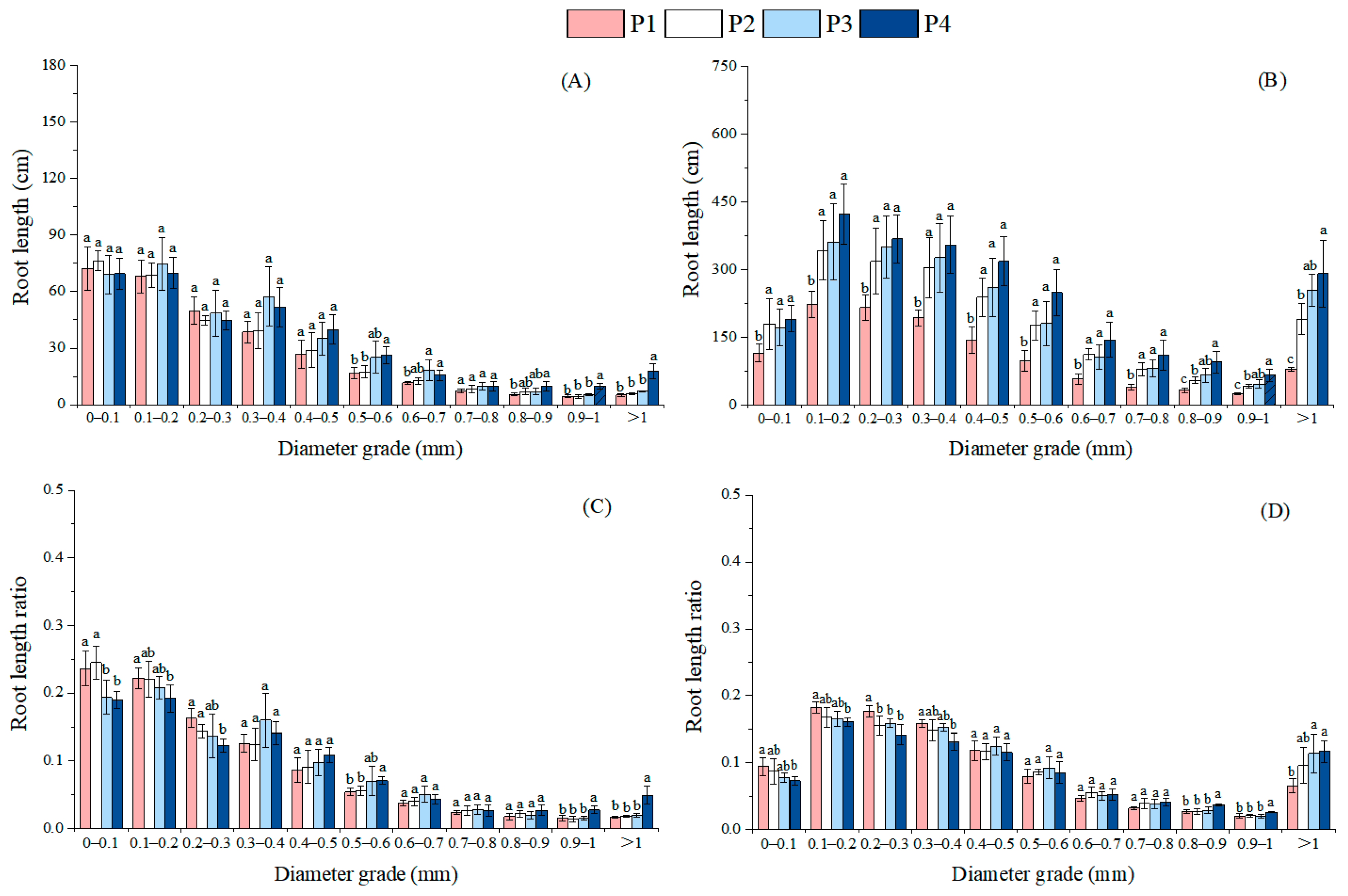
3.2. Effects of Different Soil Phosphorus Levels on the Root Morphology and Architecture of Moso Bamboo Seedlings
3.3. Effects of Different Soil Phosphorus Levels on the Nutrient Elements and Non-Structural Carbohydrates of Moso Bamboo Seedlings
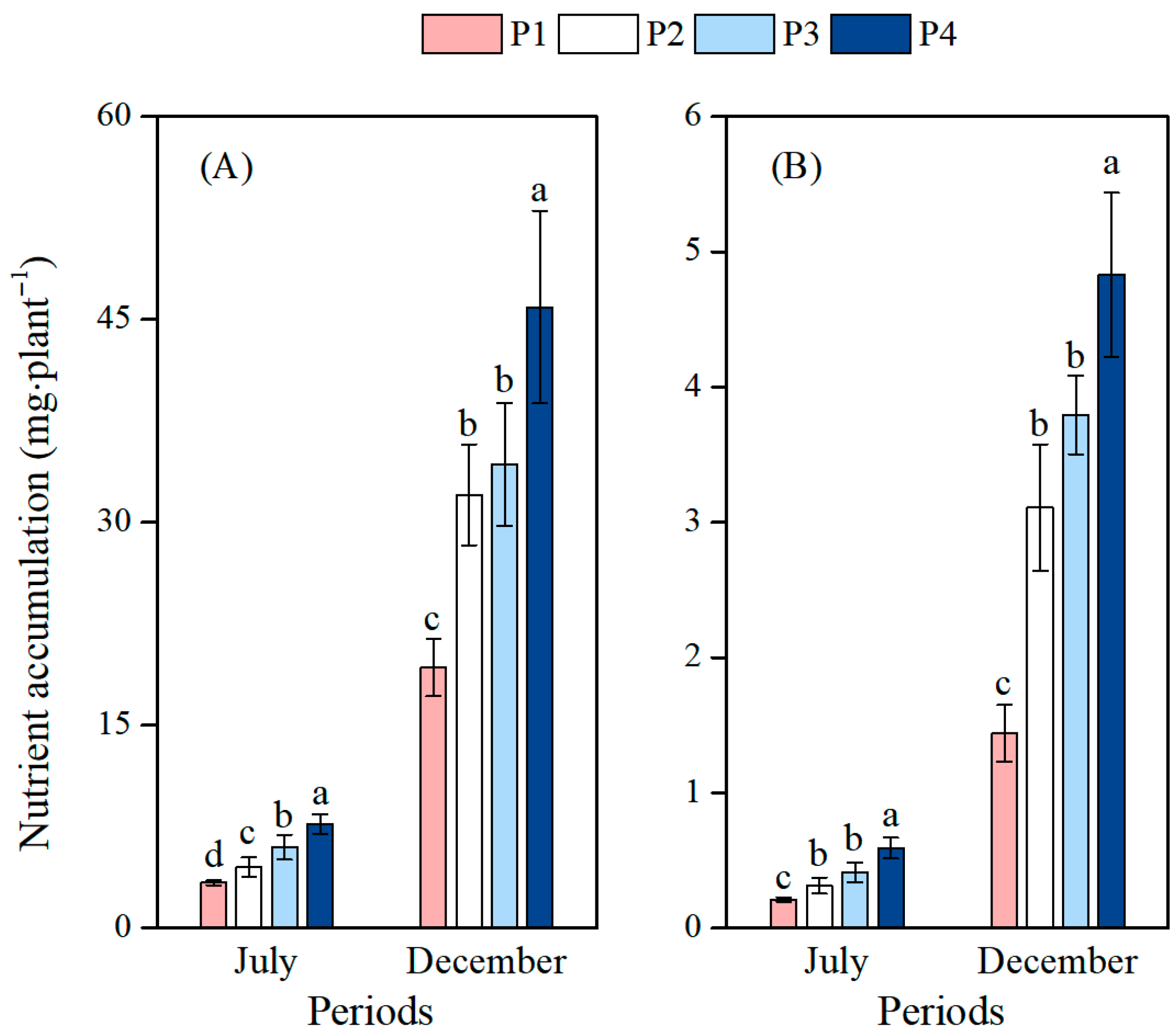
3.4. Effects of Different Soil Phosphorus Levels on the Nutrient Accumulation of Moso Bamboo Seedlings
3.5. The Correlations Among Biomass, Root Morphology and Architecture, and Non-Structural Carbohydrates
4. Discussion
4.1. Responses of Biomass Allocation of Moso Bamboo Seedlings to Different Soil Phosphorus Levels
4.2. Responses of Root Morphology of Moso Bamboo Seedlings to Different Soil Phosphorus Levels
4.3. Responses of Root Architecture of Moso Bamboo Seedlings to Different Soil Phosphorus Levels
4.4. Responses of Nutrient Element and Non-Structural Carbohydrate Allocation of Moso Bamboo Seedlings to Different Soil Phosphorus Levels
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peng, Z.H.; Lu, Y.; Li, L.B.; Zhao, Q.; Feng, Q.; Gao, Z.M.; Lu, H.Y.; Hu, T.; Yao, N.; Liu, K.Y.; et al. The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla). Nat. Genet. 2013, 45, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Bicharanloo, B.; Shirvan, M.B.; Keitel, C.; Dijkstra, F.A. Nitrogen and phosphorus availability affect wheat carbon allocation pathways: Rhizodeposition and mycorrhizal symbiosis. Soil Res. 2020, 58, 125–136. [Google Scholar] [CrossRef]
- Su, M.; Meng, L.Z.; Zhao, L.; Tang, Y.K.; Qiu, J.J.; Tian, D.; Li, Z. Phosphorus deficiency in soils with red color: Insights from the interactions between minerals and microorganisms. Geoderma 2021, 404, 115311. [Google Scholar] [CrossRef]
- Camenzind, T.; Hättenschwiler, S.; Treseder, K.K.; Lehmann, A.; Rillig, M.C. Nutrient limitation of soil microbial processes in tropical forests. Ecol. Monogr. 2018, 88, 4–21. [Google Scholar] [CrossRef]
- Hata, K.J.; Hiradate, S.; Kachi, N. Soil phosphorous is the primary factor determining species-specific plant growth depending on soil acidity in island ecosystems with severe erosion. Sci. Rep. 2023, 13, 12163. [Google Scholar] [CrossRef]
- Li, J.; Yan, X.; Dai, A.; Liu, L.; Li, Z. Root and shoot traits responses to phosphorus deficiency and QTL analysis at seedling stage using introgression lines of rice. J. Genet. Genom. 2009, 36, 173–183. [Google Scholar] [CrossRef]
- Shinde, S.; Naik, D.; Cumming, J.R. Carbon allocation and partitioning in Populus tremuloides are modulated by ectomycorrhizal fungi under phosphorus limitation. Tree Physiol. 2017, 38, 52–65. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Bai, C.; Liu, Y.F.; Ma, M.Z.; Zhang, S.W.; Liu, H.; Bai, R.; Han, X.R.; Yong, J.W.H. Resilient and sustainable production of peanut (Arachis hypogaea) in phosphorus-limited environment by using exogenous gamma-aminobutyric acid to sustain photosynthesis. Ecotoxicol. Environ. Saf. 2023, 263, 115388. [Google Scholar] [CrossRef]
- Xu, W.F.; Zhang, Q.; Yuan, W.; Xu, F.Y.; Aslam, M.M.A.; Miao, R.; Li, Y.; Wang, Q.W.; Li, X.; Zhang, X.; et al. The genome evolution and low-phosphorus adaptation in white lupin. Nat. Commun. 2020, 11, 1069. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Z.; Mo, S.; Liu, J.; Xing, Y.; Wang, Y.; Ge, C.; Wang, Y. Mechanism of low phosphorus inducing the main root lengthening of rice. J. Plant Growth Regul. 2021, 18, 1032–1043. [Google Scholar] [CrossRef]
- Fitter, A.H.; Stickland, T.R.; Harvey, M.L.; Wilson, G.W. Architectural analysis of plant root systems 1. Architectural correlates of exploitation efficiency. New Phytol. 1991, 118, 375–382. [Google Scholar] [CrossRef]
- Sorgonà, A.; Abenavoli, M.R.; Cacco, G. A comparative study between two citrus rootstocks: Effect of nitrate on the root morpho-topology and net nitrate uptake. Plant Soil 2005, 270, 257–267. [Google Scholar] [CrossRef]
- Zhou, J.F.; Shi, W.H.; Pan, K.T.; Ying, Y.Q.; Sun, C. Effect of low phosphorus stress on growth and nutrient physiology of Phyllostachys edulis seedlings. J. Zhejiang A&F Univ. 2022, 39, 1010–1017. [Google Scholar]
- Chen, Y.W.; Gao, J.; Zhang, Y.; Ma, Y.J.; Qi, F.Y. Effects of different phosphorus concentration on growth and development of moso bamboo. J. Trop. Subtrop. Bot. 2013, 21, 78–84. [Google Scholar]
- Yang, Z.Y.; Zhou, B.Z.; Ge, X.G.; Cao, Y.H.; Brunner, I.; Shi, J.X.; Li, M.H. Species-specific responses of root morphology of three co-existing tree species to nutrient patches reflect their root foraging strategies. Front Plant Sci. 2021, 11, 618222. [Google Scholar] [CrossRef]
- Palacio, S.; Maestro, M.; Montserrat-Martí, G. Relationship between shoot-rooting and root-sprouting abilities and the carbohydrate and nitrogen reserves of mediterranean dwarf shrubs. Ann. Bot. 2007, 100, 865–874. [Google Scholar] [CrossRef]
- Chen, J.X.; Lian, W.T.; Li, Z.; Guo, X.; Li, Y.N.; Zhao, H.Y.; Yi, K.K.; Li, X.X.; Liao, H. Natural variation in the GmVPE1 promoter contributes to phosphorus re-translocation to seeds and improves soybean yield. Plant Biotechnol. J. 2025, 23, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.T.; Zou, X.H.; Sun, X.L.; Hu, Y.N.; Wu, P.F.; Ma, X.Q. Differences in morphology and nutrient distribution of Chinese fir with different phosphorus use efficiency under low phosphorus stress. J. Northwest For. Univ. 2021, 4, 94–102. [Google Scholar]
- Xiao, X.L.; Zhang, J.Q.; Satheesh, V.; Meng, F.X.; Gao, W.L.; Dong, J.S.; Zheng, Z.; An, G.Y.; Nussaume, L.; Liu, D.; et al. SHORT-ROOT stabilizes PHOSPHATE1 to regulate phosphate allocation in Arabidopsis. Nat. Plants 2022, 8, 1074–1081. [Google Scholar] [CrossRef]
- Shi, W.H.; Wang, K.C.; Zhou, J.F.; Xiong, R.; Ying, Y.Q. Effects of nitrogen forms on adaptive strategies of Moso bamboo seedlings under low-phosphorus conditions. Adv. Bamboo Sci. 2025, 10, 100133. [Google Scholar] [CrossRef]
- Chen, C.; Xiao, W.Y. The global positive effect of phosphorus addition on soil microbial biomass. Soil Biol. Biochem. 2023, 176, 108882. [Google Scholar] [CrossRef]
- Fabiańska, I.; Gerlach, N.; Almario, J.; Bucher, M. Plant-mediated effects of soil phosphorus on the root-associated fungal microbiota in Arabidopsis thaliana. New phytol. 2019, 221, 2123–2137. [Google Scholar] [CrossRef]
- Xie, C.H.; Xie, F.; Song, Y.; Wang, J.J.; Zhang, R. Response of plant growth and physiological and biochemical characteristics to low phosphorus stress. Acta Agric. Jiangxi 2020, 5, 71–75. [Google Scholar]
- Siedliska, A.; Baranowski, P.; Pastuszka-Woźniak, J.; Zubik, M.; Krzyszczak, J. Identification of plant leaf phosphorus content at different growth stages based on hyperspectral reflectance. BMC Plant Biol. 2021, 21, 28. [Google Scholar] [CrossRef]
- Niu, Y.F.; Chai, R.S.; Jin, G.L.; Wang, H.; Tang, C.X.; Zhang, Y.S. Responses of root architecture development to low phosphorus availability: A review. Ann. Bot. 2013, 112, 391–408. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Jiao, J.Y.; Chen, T.D.; Chen, Y.L.; Lin, H.; Xu, Q.; Cheng, Y.Z.; Zhao, W.T. Soil nutrient evaluation of alluvial fan in the middle and lower reaches of Lhasa River Basin. J. Plant Nutr. Fertil. 2022, 28, 2082–2096. [Google Scholar]
- Bouma, T.J.; Nielsen, K.L.; Hal, J.V.; Koutstaal, B. Root system topology and diameter distribution of species from habitats differing in inundation frequency. Funct Ecol. 2001, 15, 360–369. [Google Scholar] [CrossRef]
- Li, C.Y.; Zheng, L.; Lu, L.H.; Li, L.L. Improvement in the H2SO4-H2O2 digestion method for determining plant total nitrogen. Chin. Agric. Sci. Bull. 2014, 30, 159–162. [Google Scholar]
- Luo, F.; Gong, X.J.; Zhang, T.; Du, X. Effects of nitrogen, phosphorus and potassium on photo-biological characteristics and amino acid components of tea plants in spring. J. Plant Nutr. Fertil. 2015, 21, 147–155. [Google Scholar]
- Li, M.H.; Xiao, W.F.; Wang, S.G.; Cheng, G.W.; Cherubini, P.; Cai, X.H. Mobile carbohydrates in Himalayan treeline trees I. evidence for carbon gain limitation but not for growth limitation. Tree Physiol. 2008, 28, 1287–1296. [Google Scholar] [CrossRef]
- Zhang, C.C.; Gu, R.; Lin, L.X.; Russo, S.E. Functional traits and ecological niches as correlates of the interspecific growth–mortality trade-off among seedlings of 14 tropical tree species. Funct. Ecol. 2024, 38, 1888–1901. [Google Scholar] [CrossRef]
- Hu, D.D.; Zhang, J.Y.; Yang, Y.M.; Yu, D.Y.; Zhang, H.Y.; Zhang, D. Molecular mechanisms underlying plant responses to low phosphate stress and potential applications in crop improvement. New Crops 2025, 2, 100064. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, S.G.; Geng, L.P.; Huo, H.; Liu, W.J. Effects of phosphorus deficiency on root morphology and nutrients concentrations of different crops. J. Plant Nutr. Fertil. 2013, 19, 577–585. [Google Scholar]
- Joshi, S.R.; Morris, J.W.; Tfaily, M.M.; Young, R.P.; McNear, D.H., Jr. Low soil phosphorus availability triggers maize growth stage specific rhizosphere processes leading to mineralization of organic P. Plant Soil 2021, 459, 423–440. [Google Scholar] [CrossRef]
- Lu, L.W.; Yang, F.; Wang, Q.Q. Responses of maize growth to low phosphorus and phosphorus fertilizer application in different types of soil. J. South China Agric. Univ. 2024, 45, 516–524. [Google Scholar]
- Xing, X.Z.; Yang, Z.W.; Du, H.; Li, X.H.; Zhang, C.Y. Functional analysis of transcription factor GmPTF1 for facilitating nodulation and nitrogen-fixation in Soybean. Soybean Sci. 2023, 42, 653–663. [Google Scholar]
- Zheng, L.; Fan, J.B.; He, Y.Q.; Zheng, X.B.; Xu, X.W. Effect of phosphorus on growth, root morphology, phosphorus uptake and utilization efficiency of rice seedlings. Soils 2015, 47, 664–669. [Google Scholar]
- Wu, F.K.; Yahaya, B.S.; Gong, Y.; He, B.; Gou, J.L.; He, Y.F.; Li, J.; Kang, Y.; Xu, J.; Wang, Q.J.; et al. ZmARF1 positively regulates low phosphorus stress tolerance via modulating lateral root development in maize. PLoS Genet. 2024, 20, e1011135. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.G.; Zhao, X.T.; Liang, N.S.; He, L.M.; Yu, L.; Zhan, Y.G. Phosphorus deficiency promotes the lateral root growth of Fraxinus mandshurica seedlings. J. Plant Nutr. Soil Sci. 2019, 182, 552–559. [Google Scholar] [CrossRef]
- Heydari, M.M.; Brook, R.M.; Jones, D.L. The role of phosphorus sources on root diameter, root length and root dry matter of barley (Hordeum vulgare L.). J. Plant Nutr. 2019, 42, 1–15. [Google Scholar] [CrossRef]
- Janes, G.; Wangenheim, D.; Cowling, S.; Kerr, I.; Band, L.; French, A.; Bishopp, A. Cellular patterning of Arabidopsis roots under low phosphate conditions. Front. Plant Sci. 2018, 9, 735. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J. Effects of phosphorus on shoot and root growth, partitioning, and phosphorus utilization efficiency in Lantana. Hortscience 2016, 51, 1001–1009. [Google Scholar] [CrossRef]
- Chu, Y.Y.; Duan, R.C.; Song, H.R.; Zhang, W.S.; Zhou, Y.X.; Ma, Y.T.; Yin, X.T.; Tian, L.N.; Ausin, I. Zhaofen HanAtHD2D is involved in regulating lateral root development and participates in abiotic stress response in Arabidopsis. J. Plant Physiol. 2024, 297, 154242. [Google Scholar] [CrossRef]
- Lin, D.L.; Yao, H.Y.; Jia, L.H.; Tan, J.F.; Xu, Z.H.; Zheng, W.M.; Xue, H.W. Phospholipase D-derived phosphatidic acid promotes root hair development under phosphorus deficiency by suppressing vacuolar degradation of PIN-FORMED2. New Phytol. 2020, 226, 142–155. [Google Scholar] [CrossRef]
- Lei, K.J.; Lin, Y.M.; Ren, J.; Bai, L.; Miao, Y.C.; An, G.Y.; Song, C.P. Modulation of the Phosphate-Deficient Responses by MicroRNA156 and its Targeted SQUAMOSA PROMOTER BINDING PROTEIN-LIKE 3 in Arabidopsis. Plant Cell Physiol. 2016, 57, 192–203. [Google Scholar] [CrossRef]
- Swarup, R.; Perry, P.; Hagenbeek, D.; Van Der Straeten, D.; Beemster, G.T.S.; Sandberg, G.; Bhalerao, R.; Ljung, K.; Bennett, M.J. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 2007, 19, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.; Li, X.F.; Chen, D.; Yang, S.; Liu, Y.; Tang, X.L.; Ling, G.Z. Zunkang ZhaoShallower root spatial distribution induced by phosphorus deficiency contributes to topsoil foraging and low phosphorus adaption in sugarcane (Saccharum officinarum L.). Front Plant Sci. 2022, 12, 797635. [Google Scholar] [CrossRef]
- Shishkova, S.; Huang, L.; Rodríguez, R.E.; Ristova, D.; Ioio, R.D. Editorial: Root development: Towards understanding regulatory networks and complex interactions between cell populations. Front Plant Sci. 2023, 13, 1108367. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Zhao, J.C.; Ni, H.J.; Wang, H.; Zhou, B.Z. Three subtropical species adapt to drought by reallocating biomass and adjusting root architecture. Forests 2023, 14, 806. [Google Scholar] [CrossRef]
- Ni, H.J.; Zhao, J.C.; Yang, Z.Y. Effects of different moso bamboo densities on the physiological Growth of Indocalamus latifolius cultivated in moso bamboo forests. Forests 2025, 16, 636. [Google Scholar] [CrossRef]
- Li, M.H.; Jiang, Y.; Wang, A.; Li, X.B.; Zhu, W.; Yan, C.F.; Du, Z.; Shi, Z.; Lei, J.P.; Schönbeck, L.; et al. Active summer carbon storage for winter persistence in trees at the cold alpine treeline. Tree Physiol. 2018, 38, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Dong, Y.F.; Xu, T.; Wang, Y.P.; Wang, H.T.; Duan, B.L. Root order-dependent seasonal dynamics in the carbon and nitrogen chemistry of poplar fine roots. New Forests 2017, 48, 587–607. [Google Scholar] [CrossRef]
- Fu, K.Y.; Xu, F.F.; Shi, X.Y.; Zhu, Y.Q.; Qin, A.X.; Li, C.; Li, C.Y. Effect of phosphorus on the morphology and quality characteristics of starch granules in Whea. J. Shihezi Univ. Nat. Sci. 2015, 33, 410–416. [Google Scholar]
- Hammond, J.P.; White, P.J. Sugar signaling in root responses to low phosphorus availability. Plant Physiol. 2011, 156, 1033–1040. [Google Scholar] [CrossRef]
- Zhou, K.Q.; Yamagishi, M.; Osaki, M.; Masuda, K. Sugar signalling mediates cluster root formation and phosphorus starvation-induced gene expression in white lupin. J. Exp. Bot. 2008, 59, 2749–2756. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Cao, Y.H.; Zhao, J.C.; Zhou, B.Z.; Ge, X.G.; Li, Q.; Li, M.H. Root response of moso bamboo (Phyllostachys edulis (Carrière) J. Houz.) seedlings to drought with different intensities and durations. Forests 2020, 12, 50. [Google Scholar] [CrossRef]

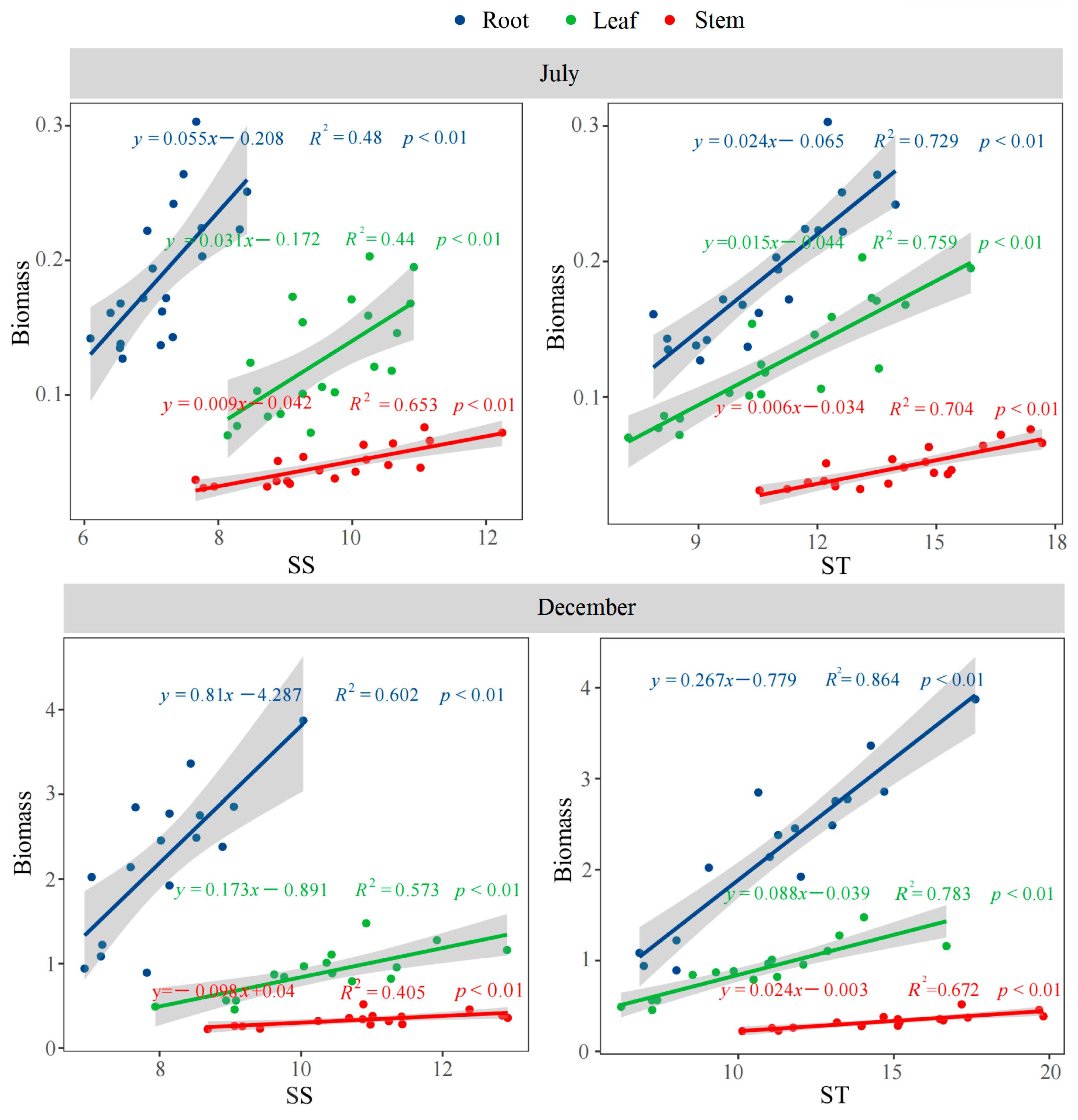
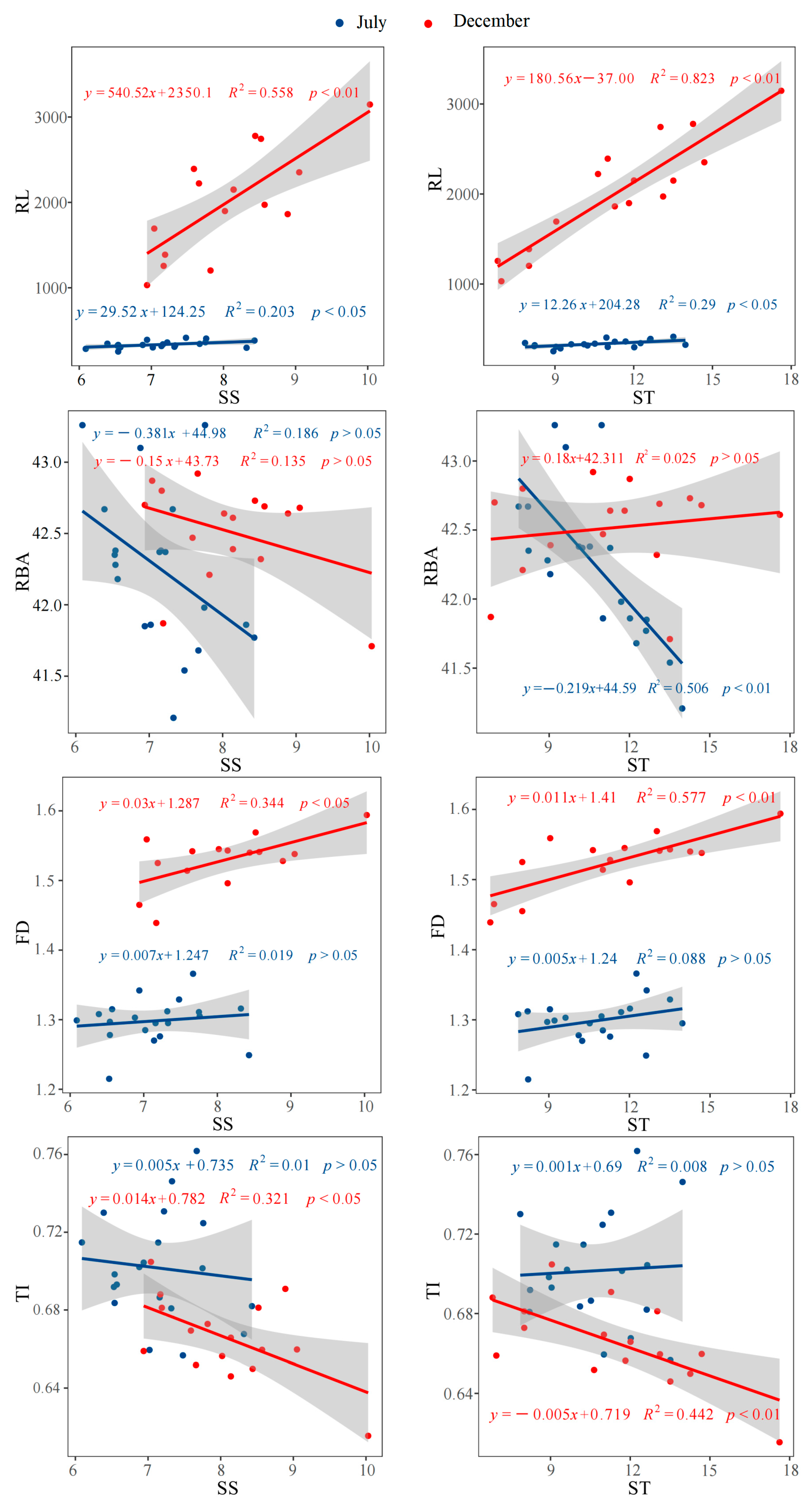
| Treatment | Sampling Time | |||||
|---|---|---|---|---|---|---|
| July | December | |||||
| pH | HN (mg·kg−1) | AP (mg·kg−1) | pH | HN (mg·kg−1) | AP (mg·kg−1) | |
| P1 | 5.01 ± 0.09 | 79.9 ± 3.7 | 4.01 ± 0.20 | 4.95 ± 0.13 | 77.4 ± 3.6 | 3.72 ± 0.12 |
| P2 | 5.11 ± 0.14 | 82.1 ± 3.0 | 8.99 ± 0.62 | 5.06 ± 0.15 | 77.5 ± 3.7 | 8.17 ± 0.47 |
| P3 | 5.17 ± 0.08 | 82.5 ± 2.4 | 14.5 ± 0.84 | 5.08 ± 0.19 | 77.8 ± 2.9 | 13.7 ± 1.1 |
| P4 | 5.09 ± 0.07 | 81.5 ± 4.0 | 29.9 ± 1.7 | 5.07 ± 0.11 | 76.5 ± 1.7 | 27.4 ± 2.0 |
| Abbreviation | Full Form |
|---|---|
| RL | Root length |
| RSA | Root surface area |
| RD | Root average diameter |
| RLR | Root length ratio |
| Rl | Root length of each diameter grade |
| SRL | Specific root length |
| RT | Number of root tips |
| RBA | Root branching angle |
| FD | Fractal dimension |
| TI | Root topological index |
| N | Nitrogen |
| P | Phosphorus |
| NSCs | Non-structural carbohydrates |
| SS | Soluble sugar |
| ST | Starch |
| Period | Nutrient Content and Ratio | Treatment | Leaves | Stems | Roots |
|---|---|---|---|---|---|
| July | P (mg·g−1) | P1 | 1.17 ± 0.08 c | 0.626 ± 0.034 b | 0.696 ± 0.037 b |
| P2 | 1.49 ± 0.11 b | 0.738 ± 0.049 a | 0.733 ± 0.064 ab | ||
| P3 | 1.59 ± 0.08 b | 0.790 ± 0.059 a | 0.789 ± 0.036 a | ||
| P4 | 1.78 ± 0.11 a | 0.835 ± 0.072 a | 0.815 ± 0.037 a | ||
| N (mg·g−1) | P1 | 21.96 ±1.23 c | 7.37 ± 0.55 b | 9.85 ± 0.27 a | |
| P2 | 23.12 ± 1.11 bc | 7.91 ± 0.50 ab | 10.05 ± 0.38 a | ||
| P3 | 25.42 ± 0.62 a | 8.60 ± 0.46 a | 10.59 ± 0.42 a | ||
| P4 | 24.56 ± 0.66 ab | 8.26 ± 0.31 a | 10.38 ± 0.56 a | ||
| N/P | P1 | 18.73 ±0.96 a | 11.77 ± 0.72 a | 14.18 ± 0.65 a | |
| P2 | 15.59 ± 0.58 b | 10.73 ± 0.14 ab | 13.77 ± 0.84 ab | ||
| P3 | 16.00 ± 0.45 b | 10.91 ± 0.37 ab | 13.44 ± 0.74 ab | ||
| P4 | 13.83 ± 0.96 c | 9.96 ± 0.95 b | 12.75 ± 0.59 b | ||
| December | P (mg·g−1) | P1 | 1.27 ± 0.09 b | 0.602 ± 0.057 c | 0.613 ± 0.066 b |
| P2 | 1.49 ± 0.12 a | 0.709 ± 0.045 b | 0.736 ± 0.063 a | ||
| P3 | 1.57 ± 0.05 a | 0.740 ± 0.043 ab | 0.810 ± 0.041 a | ||
| P4 | 1.55 ± 0.13 a | 0.820 ± 0.088 a | 0.791 ± 0.047 a | ||
| N (mg·g−1) | P1 | 16.35 ± 0.72 a | 6.80 ± 0.41 a | 8.84 ± 0.48 a | |
| P2 | 14.23 ± 1.06 b | 6.49 ± 0.49 a | 8.21 ± 0.42 a | ||
| P3 | 12.78 ± 1.09 b | 6.51 ± 0.47 a | 7.78 ± 0.71 a | ||
| P4 | 13.50 ± 0.95 b | 6.48 ± 0.50 a | 8.24 ± 0.31 a | ||
| N/P | P1 | 12.91 ± 1.00 a | 11.40 ± 1.56 a | 14.57 ± 2.05 a | |
| P2 | 9.55 ± 0.50 b | 9.20 ± 1.02 ab | 11.25 ± 1.45 b | ||
| P3 | 8.17 ± 0.76 b | 8.83 ± 1.00 b | 9.62 ± 0.92 b | ||
| P4 | 8.80 ± 1.13 b | 7.61 ± 0.60 b | 10.34 ± 0.64 b |
| Period | Non-Structural Carbohydrate Content and Ratio | Treatment | Leaves | Stems | Roots |
|---|---|---|---|---|---|
| July | Soluble sugar (%) | P1 | 8.69 ± 0.50 b | 8.20 ± 0.56 c | 6.67 ± 0.37 b |
| P2 | 9.33 ± 0.87 ab | 9.25 ± 0.36 bc | 6.73 ± 0.42 b | ||
| P3 | 10.01 ± 0.58 a | 10.22 ± 0.65 ab | 7.48 ± 0.56 ab | ||
| P4 | 10.23 ± 0.74 a | 10.68 ± 0.77 a | 7.73 ± 0.42 a | ||
| Starch (%) | P1 | 8.08 ± 0.53 c | 11.81 ± 0.99 c | 8.46 ± 0.51 c | |
| P2 | 10.38 ± 0.37 b | 13.11 ± 1.22 bc | 10.04 ± 0.68 b | ||
| P3 | 12.06 ± 1.15 ab | 14.69 ± 0.66 ab | 11.48 ± 0.85 a | ||
| P4 | 14.02 ± 1.11 a | 16.53 ± 1.13 a | 12.81 ± 0.93 a | ||
| Sugar/starch | P1 | 1.077 ± 0.044 a | 0.695 ± 0.035 a | 0.791 ± 0.068 a | |
| P2 | 0.898 ± 0.069 b | 0.710 ± 0.066 a | 0.672 ± 0.033 b | ||
| P3 | 0.834 ± 0.060 b | 0.696 ± 0.035 a | 0.654 ± 0.064 b | ||
| P4 | 0.731 ± 0.045 c | 0.670 ± 0.043 a | 0.607 ± 0.065 b | ||
| December | Soluble sugar (%) | P1 | 8.76 ± 0.55 b | 9.08 ± 0.31 b | 7.28 ± 0.38 b |
| P2 | 10.12 ± 0.52 a | 10.83 ± 0.50 a | 7.70 ± 0.50 b | ||
| P3 | 10.78 ± 0.64 a | 11.51 ± 0.95 a | 8.41 ± 0.53 ab | ||
| P4 | 11.55 ± 1.10 a | 11.88 ± 0.89 a | 8.92 ± 0.83 a | ||
| Starch (%) | P1 | 7.02 ±0.53 c | 11.07 ± 0.69 c | 7.45 ± 0.65 c | |
| P2 | 9.85 ± 1.15 b | 14.35 ± 0.96 b | 10.97 ± 1.35 b | ||
| P3 | 11.04 ± 0.93 b | 15.73 ± 0.95 b | 12.02 ± 1.24 b | ||
| P4 | 14.21 ± 1.72 a | 18.51 ± 1.42 a | 15.02 ± 1.80 a | ||
| Sugar/starch | P1 | 1.249 ± 0.028 a | 0.822 ± 0.037 a | 0.981 ± 0.064 a | |
| P2 | 1.034 ± 0.086 b | 0.756 ± 0.036 ab | 0.706 ± 0.048 b | ||
| P3 | 0.979 ± 0.065 b | 0.733 ± 0.055 b | 0.704 ± 0.064 b | ||
| P4 | 0.815 ± 0.059 c | 0.642 ± 0.013 c | 0.595 ± 0.020 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Zhou, B. Effects of Different Soil Phosphorus Levels on the Physiological and Growth Characteristics of Phyllostachys edulis (Moso Bamboo) Seedlings. Plants 2025, 14, 2473. https://doi.org/10.3390/plants14162473
Yang Z, Zhou B. Effects of Different Soil Phosphorus Levels on the Physiological and Growth Characteristics of Phyllostachys edulis (Moso Bamboo) Seedlings. Plants. 2025; 14(16):2473. https://doi.org/10.3390/plants14162473
Chicago/Turabian StyleYang, Zhenya, and Benzhi Zhou. 2025. "Effects of Different Soil Phosphorus Levels on the Physiological and Growth Characteristics of Phyllostachys edulis (Moso Bamboo) Seedlings" Plants 14, no. 16: 2473. https://doi.org/10.3390/plants14162473
APA StyleYang, Z., & Zhou, B. (2025). Effects of Different Soil Phosphorus Levels on the Physiological and Growth Characteristics of Phyllostachys edulis (Moso Bamboo) Seedlings. Plants, 14(16), 2473. https://doi.org/10.3390/plants14162473





