Out of the Niche: A Bird’s-Eye View of the Molecular Networks Controlling Root Stem Cells
Abstract
1. Introduction
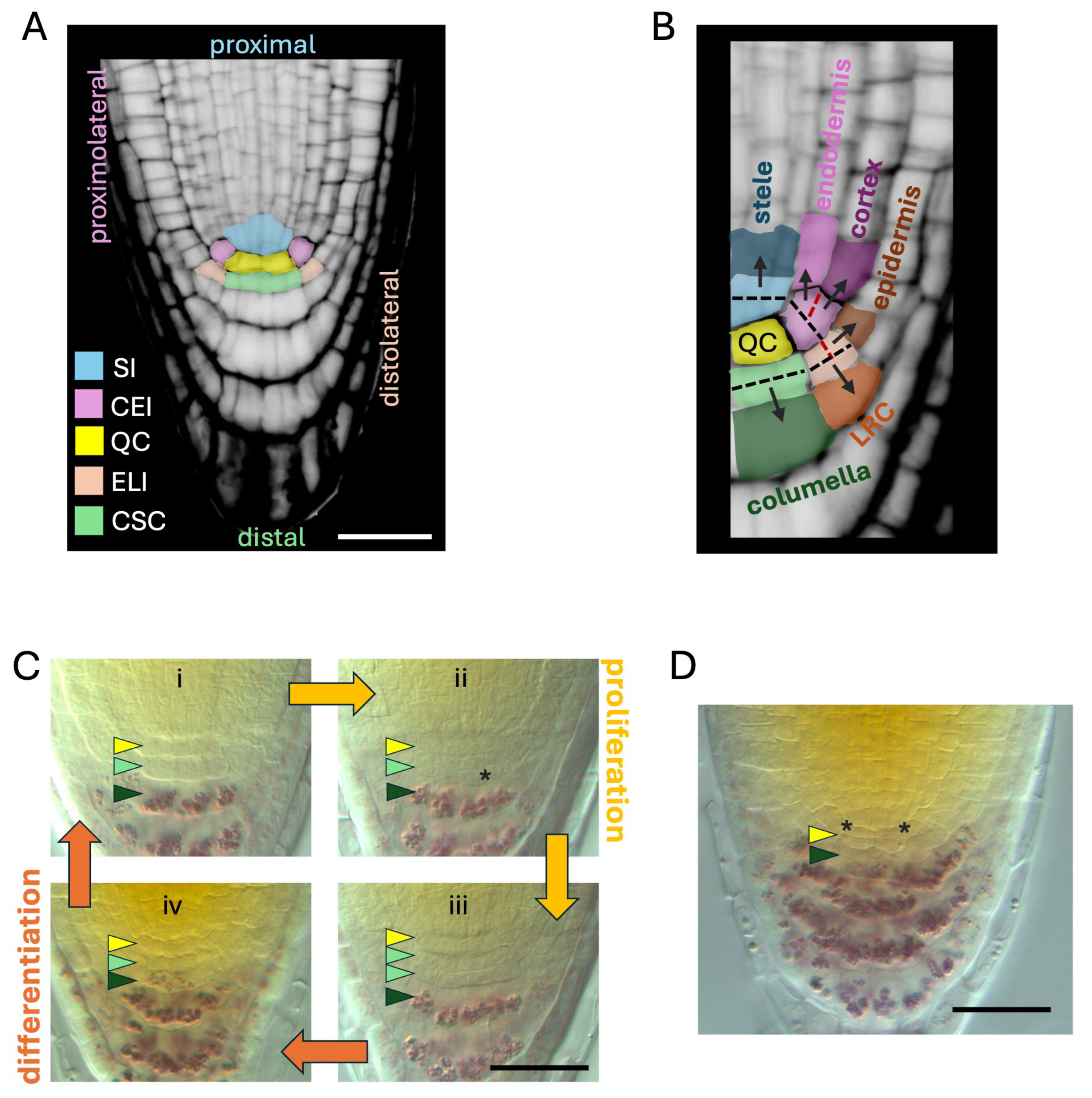
2. Structure and Function of the Root Stem Cell Niche
3. The QC-CSC Interdependence
4. Multiple Regulatory Networks Control DSC Maintenance and QC Quiescence
5. WOX5: The Master Regulator of DSCs
5.1. Cell-Autonomous and Non-Cell-Autonomous Functions of WOX5
5.2. WOX5 as a Key Hub in SCN Maintenance
6. Auxin: The SCN Morphogen
6.1. The Instructive Role of the Auxin Gradient in the SCN
6.2. The PLT Family in IAA-Mediated SCN Patterning
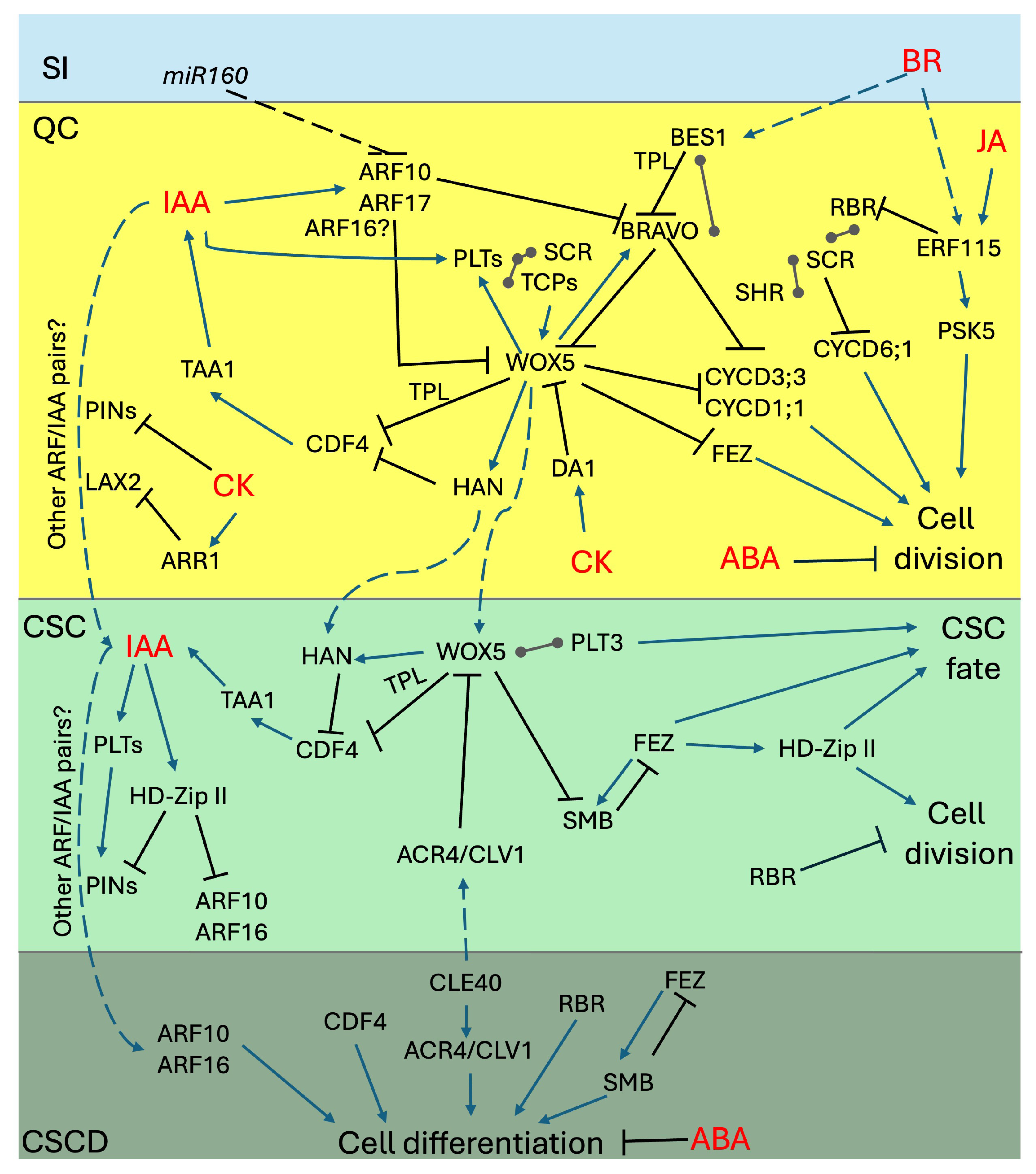
7. The FEZ and SOMBRERO Loop: The Pacemaker of CSC Divisions
8. SHR-SCR-RBR1: Regulators of Asymmetric Cell Divisions
9. Peptides and Receptors: Setting the Boundaries Between Stemness and Differentiation
10. Other Factors Involved in SCN Regulation
10.1. Brassinosteroids
10.2. Cytokinin
10.3. Ethylene, Abscisic Acid, Jasmonic Acid, and Salicylic Acid
| Gene | Function | Role in SCN | References |
|---|---|---|---|
| IAA | |||
| TAA1, TAR2 | Tryptophan Amino Transferases | SCN maintenance | [41,43] |
| PIN3, 4, 7 | Auxin efflux carriers | CSC maintenance | [25,40,42,45,46] |
| ARF10, 16, 17 | C-class ARF TFs | QC quiescence, CSC maintenance | [49,52] |
| IAA5 | Canonical AUX/IAA TF | CSC maintenance | [49] |
| IAA33 | Non-Canonical AUX/IAA TF | CSC maintenance | [49] |
| HAT3, ATHB2, ATHB4 | HD-Zip II TFs | CSC maintenance | [29] |
| PLT1, 2, 3, 4 | AP2/ERF TFs | QC quiescence, CSC maintenance | [20,25,56,57] |
| CK | |||
| AHK2, 4 | Histidine Receptor Kinase | QC quiescence | [92] |
| ARR3-9, 15 | A-type ARR TFs | SCN establishment, QC quiescence | [89,93] |
| ARR1, 12 | B-type ARR TFs | QC quiescence | [92] |
| CKX3, 5 | CK oxidase/dehydrogenase | QC quiescence | [92] |
| LAX2 | Auxin influx carrier | QC quiescence | [92] |
| DA1 | Protease | QC quiescence | [94] |
| BR | |||
| BRI1, BRL1, BRL3 | LRR Receptor Kinases | QC quiescence | [80,81,82,83] |
| BES1, BZR1 | BES1/BZR1 TFs | QC quiescence | [80,81,82] |
| BRAVO | R2-R3 MYB TF | QC quiescence | [24] |
| ERF115 | AP2/ERF TF | QC quiescence | [23] |
| ABA | |||
| ABI1, 2 | Protein Phosphatase 2C | QC quiescence | [98] |
| ABI3 | B3 TF | QC quiescence | [98] |
| ABI5 | bZIP TF | QC quiescence | [98] |
| JA | |||
| SHR, SCR | GRAS TFs | QC quiescence | [99] |
| RBR | Cell Cycle Regulator | QC quiescence | [99] |
| ERF115 | AP2/ERF TF | QC quiescence | [99] |
| SA | |||
| NPR1, 3, 4 | BTB/POZ–Ankyrin Receptor | QC quiescence | [100] |
| ET | |||
| ETO1 | Negative Regulator of ACS5 | QC quiescence | [96] |
11. Concluding Remarks and Future Perspectives
- (1)
- Cell-to-cell Communication: While the nature of the QC-derived mobile signal(s) is still under debate, the contribution of symplastic signaling in SCN maintenance has emerged [78,102]. Tools for blocking plasmodesmata are currently available [102,103], and they could be used to better understand how the QC communicates with the stem cells through the exchange of mobile regulators. Relevantly, mobile regulators might not be limited to protein and peptides, as even BR intermediates and microRNAs have been shown to move via plasmodesmata [52,104].
- (2)
- Complexes’ Complexity: As we discussed above, several interactions among key SCN regulators have been uncovered, although the exact functions of the resulting multimeric complexes are not yet clear. While some complexes were shown to be transcriptionally active, others were postulated to function through subtracting active monomers from their DNA binding functions [36,74,85]. Also, the exact stoichiometry of the proteins involved within a specific cell type—and possibly their cell-to-cell-movement—might alter the complexes’ composition and activity [17,74]. A precise biochemical characterization of these complexes, along with a combination of FRET-FLIM and fluorescence correlation spectroscopy analyses, will help reveal their functionality, allowing for a fine mapping of SCN’s regulatory circuitry.
- (3)
- Interpreting the Auxin Readout: While the instructive role of the auxin gradient is not under debate, most of our knowledge comes from the use of DR5-based transcriptional reporters. In recent years, several lines of evidence have shown that the auxin transcriptional readout is determined by the combinatorial action of activator and repressor ARFs binding to different architectures of ARE pairs in the promoters of auxin-responsive genes [54,105,106]. Considering that many ARFs are expressed in the SCN [107], the current interpretation of DR5-based gradients might be too simplistic. Alternative reporters with different ARE architectures are now available [54], and these tools could help finely dissect the transcriptional readout of IAA gradient in the SCN.
- (4)
- Unexpected Players: How Many? While most of the players discussed above are root-specific regulators, in recent years, several TFs previously implicated in other developmental processes were shown to contribute to SCN maintenance. For instance, HD-Zip II TFs were previously implicated in light signaling and shoot development [29,108], NO TRANSMITTING TRACT/WIP-DOMAIN CONTAINING proteins were shown to regulate reproductive development [109], SENSITIVE TO PROTON RIZOTOXICITY 1 was involved in stress responses [110], and C-REPEAT BINDING FACTOR 3 was previously associated with cold acclimation [111]. While some of these factors may play a more general role in regulating hormone homeostasis [112], they may also represent specific signaling hubs that perceive and integrate external cues to synchronize stem cell activity to changing environmental conditions. Understanding how stem cell activity is influenced by the environment will be key to responding to the threats posed by global climate changes.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scheres, B. Stem-Cell Niches: Nursery Rhymes Across Kingdoms. Nat. Rev. Mol. Cell Biol. 2007, 8, 345–354. [Google Scholar] [CrossRef]
- Aichinger, E.; Kornet, N.; Friedrich, T.; Laux, T. Plant Stem Cell Niches. Annu. Rev. Plant Biol. 2012, 63, 615–636. [Google Scholar] [CrossRef]
- Wybouw, B.; Zhang, X.; Mähönen, A.P. Vascular Cambium Stem Cells: Past, Present and Future. New Phytol. 2024, 243, 851–865. [Google Scholar] [CrossRef]
- Wittmer, J.; Heidstra, R. Appreciating Animal Induced Pluripotent Stem Cells to Shape Plant Cell Reprogramming Strategies. J. Exp. Bot. 2024, 75, 4373–4393. [Google Scholar] [CrossRef]
- Eljebbawi, A.; Dolata, A.; Strotmann, V.I.; Stahl, Y. Stem Cell Quiescence and Dormancy in Plant Meristems. J. Exp. Bot. 2024, 75, 6022–6036. [Google Scholar] [CrossRef]
- Reinhardt, D.; Frenz, M.; Mandel, T.; Kuhlemeier, C. Microsurgical and Laser Ablation Analysis of Interactions Between the Zones and Layers of the Tomato Shoot Apical Meristem. Development 2003, 130, 4073–4083. [Google Scholar] [CrossRef]
- Xu, J.; Hofhuis, H.; Heidstra, R.; Sauer, M.; Friml, J.; Scheres, B. A Molecular Framework for Plant Regeneration. Science 2006, 311, 385–388. [Google Scholar] [CrossRef]
- Sena, G.; Wang, X.; Liu, H.-Y.; Hofhuis, H.; Birnbaum, K.D. Organ Regeneration Does Not Require a Functional Stem Cell Niche in Plants. Nature 2009, 457, 1150–1153. [Google Scholar] [CrossRef] [PubMed]
- Strotmann, V.I.; Stahl, Y. At the Root of Quiescence: Function and Regulation of the Quiescent Center. J. Exp. Bot. 2021, 72, 6716–6726. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, C.; Bonnot, C.; Desnos, T.; Nussaume, L. The Root Cap at the Forefront. C. R. Biol. 2010, 333, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Dolan, L.; Janmaat, K.; Willemsen, V.; Linstead, P.; Poethig, S.; Roberts, K.; Scheres, B. Cellular Organisation of the Arabidopsis Thaliana Root. Development 1993, 119, 71–84. [Google Scholar] [CrossRef]
- Bennett, T.; van den Toorn, A.; Willemsen, V.; Scheres, B. Precise Control of Plant Stem Cell Activity Through Parallel Regulatory Inputs. Development 2014, 141, 4055–4064. [Google Scholar] [CrossRef]
- Sassi, M.; Lu, Y.; Zhang, Y.; Wang, J.; Dhonukshe, P.; Blilou, I.; Dai, M.; Li, J.; Gong, X.; Jaillais, Y.; et al. COP1 Mediates the Coordination of Root and Shoot Growth by Light Through Modulation of PIN1- and PIN2-Dependent Auxin Transport in Arabidopsis. Development 2012, 139, 3402–3412. [Google Scholar] [CrossRef]
- Dello Ioio, R.; Linhares, F.S.; Scacchi, E.; Casamitjana-Martinez, E.; Heidstra, R.; Costantino, P.; Sabatini, S. Cytokinins Determine Arabidopsis Root-Meristem Size by Controlling Cell Differentiation. Curr. Biol. 2007, 17, 678–682. [Google Scholar] [CrossRef]
- Willemsen, V.; Bauch, M.; Bennett, T.; Campilho, A.; Wolkenfelt, H.; Xu, J.; Haseloff, J.; Scheres, B. The NAC Domain Transcription Factors FEZ and SOMBRERO Control the Orientation of Cell Division Plane in Arabidopsis Root Stem Cells. Dev. Cell 2008, 15, 913–922. [Google Scholar] [CrossRef]
- Van Den Berg, C.; Willemsen, V.; Hendriks, G.; Weisbeek, P.; Scheres, B. Short-Range Control of Cell Differentiation in the Arabidopsis Root Meristem. Nature 1997, 390, 287–289. [Google Scholar] [CrossRef]
- Strotmann, V.I.; García-Gómez, M.L.; Stahl, Y. Root Stem Cell Homeostasis in Arabidopsis Involves Cell-Type Specific Transcription Factor Complexes. EMBO Rep. 2025, 26, 2323–2346. [Google Scholar] [CrossRef]
- Cruz-Ramírez, A.; Díaz-Triviño, S.; Wachsman, G.; Du, Y.; Arteága-Vázquez, M.; Zhang, H.; Benjamins, R.; Blilou, I.; Neef, A.B.; Chandler, V.; et al. A SCARECROW-RETINOBLASTOMA Protein Network Controls Protective Quiescence in the Arabidopsis Root Stem Cell Organizer. PLoS Biol. 2013, 11, e1001724. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Canher, B.; Bisht, A.; Heyman, J.; De Veylder, L. Three-Dimensional Quantitative Analysis of the Arabidopsis Quiescent Centre. J. Exp. Bot. 2021, 72, 6789–6800. [Google Scholar] [CrossRef] [PubMed]
- Burkart, R.C.; Strotmann, V.I.; Kirschner, G.K.; Akinci, A.; Czempik, L.; Dolata, A.; Maizel, A.; Weidtkamp-Peters, S.; Stahl, Y. PLETHORA-WOX5 Interaction and Subnuclear Localization Control Arabidopsis Root Stem Cell Maintenance. EMBO Rep. 2022, 23, e54105. [Google Scholar] [CrossRef] [PubMed]
- Wein, A.; Le Gac, A.-L.; Laux, T. Stem Cell Ageing of the Root Apical Meristem of Arabidopsis thaliana. Mech. Ageing Dev. 2020, 190, 111313. [Google Scholar] [CrossRef]
- Timilsina, R.; Kim, J.H.; Nam, H.G.; Woo, H.R. Temporal Changes in Cell Division Rate and Genotoxic Stress Tolerance in Quiescent Center Cells of Arabidopsis Primary Root Apical Meristem. Sci. Rep. 2019, 9, 3599. [Google Scholar] [CrossRef]
- Heyman, J.; Cools, T.; Vandenbussche, F.; Heyndrickx, K.S.; Van Leene, J.; Vercauteren, I.; Vanderauwera, S.; Vandepoele, K.; De Jaeger, G.; Van Der Straeten, D.; et al. ERF115 Controls Root Quiescent Center Cell Division and Stem Cell Replenishment. Science 2013, 342, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Vilarrasa-Blasi, J.; González-García, M.-P.; Frigola, D.; Fàbregas, N.; Alexiou, K.G.; López-Bigas, N.; Rivas, S.; Jauneau, A.; Lohmann, J.U.; Benfey, P.N.; et al. Regulation of Plant Stem Cell Quiescence by a Brassinosteroid Signaling Module. Dev. Cell 2014, 30, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Friml, J. Auxin Regulates Distal Stem Cell Differentiation in Arabidopsis Roots. Proc. Natl. Acad. Sci. USA 2010, 107, 12046–12051. [Google Scholar] [CrossRef]
- Hong, J.H.; Savina, M.; Du, J.; Devendran, A.; Kannivadi Ramakanth, K.; Tian, X.; Sim, W.S.; Mironova, V.V.; Xu, J. A Sacrifice-for-Survival Mechanism Protects Root Stem Cell Niche from Chilling Stress. Cell 2017, 170, 102–113. [Google Scholar] [CrossRef]
- Pardal, R.; Heidstra, R. Root Stem Cell Niche Networks: It’s Complexed! Insights from Arabidopsis. J. Exp. Bot. 2021, 72, 6727–6738. [Google Scholar] [CrossRef] [PubMed]
- Berckmans, B.; Kirschner, G.; Gerlitz, N.; Stadler, R.; Simon, R. CLE40 Signaling Regulates Root Stem Cell Fate. Plant Physiol. 2020, 182, 1776–1792. [Google Scholar] [CrossRef]
- Possenti, M.; Sessa, G.; Alfè, A.; Turchi, L.; Ruzza, V.; Sassi, M.; Morelli, G.; Ruberti, I. HD-Zip II Transcription Factors Control Distal Stem Cell Fate in Arabidopsis Roots by Linking Auxin Signaling to the FEZ/SOMBRERO Pathway. Development 2024, 151, dev.202586. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Luijten, M.; Miyashima, S.; Lenhard, M.; Hashimoto, T.; Nakajima, K.; Scheres, B.; Heidstra, R.; Laux, T. Conserved Factors Regulate Signalling in Arabidopsis Thaliana Shoot and Root Stem Cell Organizers. Nature 2007, 446, 811–814. [Google Scholar] [CrossRef]
- Forzani, C.; Aichinger, E.; Sornay, E.; Willemsen, V.; Laux, T.; Dewitte, W.; Murray, J.A.H. WOX5 Suppresses CYCLIN D Activity to Establish Quiescence at the Center of the Root Stem Cell Niche. Curr. Biol. 2014, 24, 1939–1944. [Google Scholar] [CrossRef]
- Pi, L.; Aichinger, E.; van der Graaff, E.; Llavata-Peris, C.I.; Weijers, D.; Hennig, L.; Groot, E.; Laux, T. Organizer-Derived WOX5 Signal Maintains Root Columella Stem Cells through Chromatin-Mediated Repression of CDF4 Expression. Dev. Cell 2015, 33, 576–588. [Google Scholar] [CrossRef]
- Sharma, M.; Friedrich, T.; Oluoch, P.; Zhang, N.; Peruzzo, F.; Jha, V.; Pi, L.; Groot, E.P.; Kornet, N.; Follo, M.; et al. A Coherent Feed-Forward Loop in the Arabidopsis Root Stem Cell Organizer Regulates Auxin Biosynthesis and Columella Stem Cell Maintenance. Nat. Plants 2024, 10, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Bitterli, P.; Oluoch, P.; Hermann, M.; Aichinger, E.; Groot, E.P.; Laux, T. Deciphering the Molecular Logic of WOX5 Function in the Root Stem Cell Organizer. EMBO J. 2024, 44, 281–303. [Google Scholar] [CrossRef]
- Hong, L.; Fletcher, J.C. Stem Cells: Engines of Plant Growth and Development. Int. J. Mol. Sci. 2023, 24, 14889. [Google Scholar] [CrossRef]
- Betegón-Putze, I.; Mercadal, J.; Bosch, N.; Planas-Riverola, A.; Marquès-Bueno, M.; Vilarrasa-Blasi, J.; Frigola, D.; Burkart, R.C.; Martínez, C.; Conesa, A.; et al. Precise Transcriptional Control of Cellular Quiescence by BRAVO/WOX5 Complex in Arabidopsis Roots. Mol. Syst. Biol. 2021, 17, e9864. [Google Scholar] [CrossRef]
- Sassi, M.; Vernoux, T. Auxin and Self-Organization at the Shoot Apical Meristem. J. Exp. Bot. 2013, 64, 2579–2592. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Chen, S.; Xu, T. The Central Role of Auxin in Orchestrating Apical Stem Cells in Plants. Plant Cell Environ. 2025, 48, 5053–5072. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, S.; Beis, D.; Wolkenfelt, H.; Murfett, J.; Guilfoyle, T.; Malamy, J.; Benfey, P.; Leyser, O.; Bechtold, N.; Weisbeek, P.; et al. An Auxin-Dependent Distal Organizer of Pattern and Polarity in the Arabidopsis Root. Cell 1999, 99, 463–472. [Google Scholar] [CrossRef]
- Dubreuil, C.; Jin, X.; Grönlund, A.; Fischer, U. A Local Auxin Gradient Regulates Root Cap Self-Renewal and Size Homeostasis. Curr. Biol. 2018, 28, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, A.N.; Robertson-Hoyt, J.; Yun, J.; Benavente, L.M.; Xie, D.-Y.; Dolezal, K.; Schlereth, A.; Jürgens, G.; Alonso, J.M. TAA1-Mediated Auxin Biosynthesis Is Essential for Hormone Crosstalk and Plant Development. Cell 2008, 133, 177–191. [Google Scholar] [CrossRef]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN Auxin Efflux Facilitator Network Controls Growth and Patterning in Arabidopsis Roots. Nature 2005, 433, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Brumos, J.; Robles, L.M.; Yun, J.; Vu, T.C.; Jackson, S.; Alonso, J.M.; Stepanova, A.N. Local Auxin Biosynthesis Is a Key Regulator of Plant Development. Dev. Cell 2018, 47, 306–318.e5. [Google Scholar] [CrossRef]
- Friml, J.; Vieten, A.; Sauer, M.; Weijers, D.; Schwarz, H.; Hamann, T.; Offringa, R.; Jürgens, G. Efflux-Dependent Auxin Gradients Establish the Apical-Basal Axis of Arabidopsis. Nature 2003, 426, 147–153. [Google Scholar] [CrossRef]
- Grieneisen, V.A.; Xu, J.; Marée, A.F.M.; Hogeweg, P.; Scheres, B. Auxin Transport Is Sufficient to Generate a Maximum and Gradient Guiding Root Growth. Nature 2007, 449, 1008–1013. [Google Scholar] [CrossRef]
- Friml, J.; Benková, E.; Blilou, I.; Wisniewska, J.; Hamann, T.; Ljung, K.; Woody, S.; Sandberg, G.; Scheres, B.; Jürgens, G.; et al. AtPIN4 Mediates Sink-Driven Auxin Gradients and Root Patterning in Arabidopsis. Cell 2002, 108, 661–673. [Google Scholar] [CrossRef]
- Savina, M.S.; Pasternak, T.; Omelyanchuk, N.A.; Novikova, D.D.; Palme, K.; Mironova, V.V.; Lavrekha, V.V. Cell Dynamics in WOX5-Overexpressing Root Tips: The Impact of Local Auxin Biosynthesis. Front. Plant Sci. 2020, 11, 560169. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Wabnik, K.; Niu, T.; Li, H.; Yu, Q.; Pollmann, S.; Vanneste, S.; Govaerts, W.; Rolčík, J.; Geisler, M.; et al. WOX5-IAA17 Feedback Circuit-Mediated Cellular Auxin Response Is Crucial for the Patterning of Root Stem Cell Niches in Arabidopsis. Mol. Plant 2014, 7, 277–289. [Google Scholar] [CrossRef]
- Lv, B.; Yu, Q.; Liu, J.; Wen, X.; Yan, Z.; Hu, K.; Li, H.; Kong, X.; Li, C.; Tian, H.; et al. Non-Canonical AUX/IAA Protein IAA33 Competes with Canonical AUX/IAA Repressor IAA5 to Negatively Regulate Auxin Signaling. EMBO J. 2020, 39, e101515. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Chen, X.; Tang, W.; Yu, Z.; Xu, T.; Tian, H.; Ding, Z. Dual Regulations of Cell Cycle Regulator DPa by Auxin in Arabidopsis Root Distal Stem Cell Maintenance. Proc. Natl. Acad. Sci. USA 2023, 120, e2218503120. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Wang, L.J.; Mao, Y.B.; Cai, W.J.; Xue, H.W.; Chen, X.Y. Control of Root Cap Formation by MicroRNA-Targeted Auxin Response Factors in Arabidopsis. Plant Cell 2005, 17, 2204–2216. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, H.; Mu, C.; Chen, Y.; He, C.; Liu, M.; Laux, T.; Pi, L. A Mobile miR160-Triggered Transcriptional Axis Controls Root Stem Cell Niche Maintenance and Regeneration in Arabidopsis. Dev. Cell 2024, 60, 459–471. [Google Scholar] [CrossRef]
- Morffy, N.; Van den Broeck, L.; Miller, C.; Emenecker, R.J.; Bryant, J.A.; Lee, T.M.; Sageman-Furnas, K.; Wilkinson, E.G.; Pathak, S.; Kotha, S.R.; et al. Identification of Plant Transcriptional Activation Domains. Nature 2024, 632, 166–173. [Google Scholar] [CrossRef]
- Martin-Arevalillo, R.; Guillotin, B.; Schön, J.; Hugues, A.; Gerentes, M.-F.; Tang, K.; Lucas, J.; Thévenon, E.; Dreuillet, M.; Vissers, G.; et al. Synthetic Deconvolution of an Auxin-Dependent Transcriptional Code. Cell 2025, 188, 2872–2889.e24. [Google Scholar] [CrossRef] [PubMed]
- Sessa, G.; Carabelli, M.; Sassi, M. The Ins and Outs of Homeodomain-Leucine Zipper/Hormone Networks in the Regulation of Plant Development. Int. J. Mol. Sci. 2024, 25, 5657. [Google Scholar] [CrossRef]
- Aida, M.; Beis, D.; Heidstra, R.; Willemsen, V.; Blilou, I.; Galinha, C.; Nussaume, L.; Noh, Y.-S.; Amasino, R.; Scheres, B. The PLETHORA Genes Mediate Patterning of the Arabidopsis Root Stem Cell Niche. Cell 2004, 119, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Galinha, C.; Hofhuis, H.; Luijten, M.; Willemsen, V.; Blilou, I.; Heidstra, R.; Scheres, B. PLETHORA Proteins as Dose-Dependent Master Regulators of Arabidopsis Root Development. Nature 2007, 449, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Mähönen, A.P.; Tusscher, K.T.; Siligato, R.; Smetana, O.; Díaz-Triviño, S.; Salojärvi, J.; Wachsman, G.; Prasad, K.; Heidstra, R.; Scheres, B. PLETHORA Gradient Formation Mechanism Separates Auxin Responses. Nature 2014, 515, 125–129. [Google Scholar] [CrossRef]
- Santuari, L.; Sanchez-Perez, G.F.; Luijten, M.; Rutjens, B.; Terpstra, I.; Berke, L.; Gorte, M.; Prasad, K.; Bao, D.; Timmermans-Hereijgers, J.L.P.M.; et al. The PLETHORA Gene Regulatory Network Guides Growth and Cell Differentiation in Arabidopsis Roots. Plant Cell 2016, 28, 2937–2951. [Google Scholar] [CrossRef]
- Shimotohno, A.; Heidstra, R.; Blilou, I.; Scheres, B. Root Stem Cell Niche Organizer Specification by Molecular Convergence of PLETHORA and SCARECROW Transcription Factor Modules. Genes. Dev. 2018, 32, 1085–1100. [Google Scholar] [CrossRef]
- Marhava, P.; Hoermayer, L.; Yoshida, S.; Marhavý, P.; Marhavý, M.; Benková, E.; Friml, J. Re-Activation of Stem Cell Pathways for Pattern Restoration in Plant Wound Healing. Cell 2019, 177, 957–969. [Google Scholar] [CrossRef]
- Bennett, T.; Van Den Toorn, A.; Sanchez-Perez, G.F.; Campilho, A.; Willemsen, V.; Snel, B.; Scheres, B. SOMBRERO, BEARSKIN1, and BEARSKIN2 Regulate Root Cap Maturation in Arabidopsis. Plant Cell 2010, 22, 640–654. [Google Scholar] [CrossRef]
- Fendrych, M.S.; Hautegem, T.V.; Durme, M.V.; Olvera-Carrillo, Y.; Huysmans, M.; Karimi, M.; Lippens, S.; Gué, C.J.; Krebs, M.; Schumacher, K.; et al. Programmed Cell Death Controlled by ANAC033/SOMBRERO Determines Root Cap Organ Size in Arabidopsis. Curr. Biol. 2014, 24, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Sozzani, R.; Cui, H.; Moreno-Risueno, M.A.; Busch, W.; Van Norman, J.M.; Vernoux, T.; Brady, S.M.; Dewitte, W.; Murray, J.A.H.; Benfey, P.N. Spatiotemporal Regulation of Cell-Cycle Genes by SHORTROOT Links Patterning and Growth. Nature 2010, 466, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Ramírez, A.; Díaz-Triviño, S.; Blilou, I.; Grieneisen, V.A.; Sozzani, R.; Zamioudis, C.; Miskolczi, P.; Nieuwland, J.; Benjamins, R.; Dhonukshe, P.; et al. A Bistable Circuit Involving SCARECROW-RETINOBLASTOMA Integrates Cues to Inform Asymmetric Stem Cell Division. Cell 2012, 150, 1002–1015. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.M.; Szekely, P.; Popov, V.; Belcher, H.; Carter, R.; Jones, M.; Fraser, S.E.; Truong, T.V.; Benfey, P.N. SHR and SCR Coordinate Root Patterning and Growth Early in the Cell Cycle. Nature 2024, 626, 611–616. [Google Scholar] [CrossRef]
- Sabatini, S.; Heidstra, R.; Wildwater, M.; Scheres, B. SCARECROW Is Involved in Positioning the Stem Cell Niche in the Arabidopsis Root Meristem. Genes. Dev. 2003, 17, 354–358. [Google Scholar] [CrossRef]
- Clark, N.M.; Fisher, A.P.; Berckmans, B.; Van den Broeck, L.; Nelson, E.C.; Nguyen, T.T.; Bustillo-Avendaño, E.; Zebell, S.G.; Moreno-Risueno, M.A.; Simon, R.; et al. Protein Complex Stoichiometry and Expression Dynamics of Transcription Factors Modulate Stem Cell Division. Proc. Natl. Acad. Sci. USA 2020, 117, 15332–15342. [Google Scholar] [CrossRef]
- Desvoyes, B.; Gutierrez, C. Roles of Plant Retinoblastoma Protein: Cell Cycle and Beyond. EMBO J. 2020, 39, e105802. [Google Scholar] [CrossRef]
- Wildwater, M.; Campilho, A.; Perez-Perez, J.M.; Heidstra, R.; Blilou, I.; Korthout, H.; Chatterjee, J.; Mariconti, L.; Gruissem, W.; Scheres, B. The RETINOBLASTOMA-RELATED Gene Regulates Stem Cell Maintenance in Arabidopsis Roots. Cell 2005, 123, 1337–1349. [Google Scholar] [CrossRef]
- Horvath, B.M.; Kourova, H.; Nagy, S.; Nemeth, E.; Magyar, Z.; Papdi, C.; Ahmad, Z.; Sanchez-Perez, G.F.; Perilli, S.; Blilou, I.; et al. Arabidopsis RETINOBLASTOMA RELATED Directly Regulates DNA Damage Responses through Functions beyond Cell Cycle Control. EMBO J. 2017, 36, 1261–1278. [Google Scholar] [CrossRef]
- Zamora-Zaragoza, J.; Klap, K.; Sánchez-Pérez, J.; Vielle-Calzada, J.-P.; Willemsen, V.; Scheres, B. Developmental Cues Are Encoded by the Combinatorial Phosphorylation of Arabidopsis RETINOBLASTOMA-RELATED Protein RBR1. EMBO J. 2024, 43, 6656–6678. [Google Scholar] [CrossRef]
- Zhai, H.; Zhang, X.; You, Y.; Lin, L.; Zhou, W.; Li, C. SEUSS Integrates Transcriptional and Epigenetic Control of Root Stem Cell Organizer Specification. EMBO J. 2020, 39, e105047. [Google Scholar] [CrossRef]
- Long, Y.; Stahl, Y.; Weidtkamp-Peters, S.; Postma, M.; Zhou, W.; Goedhart, J.; Sánchez-Pérez, M.I.; Gadella, T.W.J.; Simon, R.; Scheres, B.; et al. In Vivo FRET–FLIM Reveals Cell-Type-Specific Protein Interactions in Arabidopsis Roots. Nature 2017, 548, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.M.; Buckner, E.; Fisher, A.P.; Nelson, E.C.; Nguyen, T.T.; Simmons, A.R.; de Luis Balaguer, M.A.; Butler-Smith, T.; Sheldon, P.J.; Bergmann, D.C.; et al. Stem-Cell-Ubiquitous Genes Spatiotemporally Coordinate Division Through Regulation of Stem-Cell-Specific Gene Networks. Nat. Commun. 2019, 10, 5574. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, P.; Li, X.; Xiao, W.; Pi, L.; Liang, Y.-K. BIG Coordinates Auxin and SHORT ROOT to Promote Asymmetric Stem Cell Divisions in Arabidopsis Roots. Plant Cell Rep. 2024, 43, 188. [Google Scholar] [CrossRef] [PubMed]
- Stahl, Y.; Wink, R.H.; Ingram, G.C.; Simon, R. A Signaling Module Controlling the Stem Cell Niche in Arabidopsis Root Meristems. Curr. Biol. 2009, 19, 909–914. [Google Scholar] [CrossRef]
- Stahl, Y.; Grabowski, S.; Bleckmann, A.; Kühnemuth, R.; Weidtkamp-Peters, S.; Pinto, K.G.; Kirschner, G.K.; Schmid, J.B.; Wink, R.H.; Hülsewede, A.; et al. Moderation of Arabidopsis Root Stemness by CLAVATA1 and ARABIDOPSIS CRINKLY4 Receptor Kinase Complexes. Curr. Biol. 2013, 23, 362–371. [Google Scholar] [CrossRef]
- Pallakies, H.; Simon, R. The CLE40 and CRN/CLV2 Signaling Pathways Antagonistically Control Root Meristem Growth in Arabidopsis. Mol. Plant 2014, 7, 1619–1636. [Google Scholar] [CrossRef] [PubMed]
- González-García, M.-P.; Vilarrasa-Blasi, J.; Zhiponova, M.; Divol, F.; Mora-García, S.; Russinova, E.; Caño-Delgado, A.I. Brassinosteroids Control Meristem Size by Promoting Cell Cycle Progression in Arabidopsis Roots. Development 2011, 138, 849–859. [Google Scholar] [CrossRef]
- Lee, H.-S.; Kim, Y.; Pham, G.; Kim, J.W.; Song, J.-H.; Lee, Y.; Hwang, Y.-S.; Roux, S.J.; Kim, S.-H. Brassinazole Resistant 1 (BZR1)-Dependent Brassinosteroid Signalling Pathway Leads to Ectopic Activation of Quiescent Cell Division and Suppresses Columella Stem Cell Differentiation. J. Exp. Bot. 2015, 66, 4835–4849. [Google Scholar] [CrossRef]
- Lozano-Elena, F.; Planas-Riverola, A.; Vilarrasa-Blasi, J.; Schwab, R.; Caño-Delgado, A.I. Paracrine Brassinosteroid Signaling at the Stem Cell Niche Controls Cellular Regeneration. J. Cell Sci. 2018, 131, jcs204065. [Google Scholar] [CrossRef]
- Fàbregas, N.; Li, N.; Boeren, S.; Nash, T.E.; Goshe, M.B.; Clouse, S.D.; de Vries, S.; Caño-Delgado, A.I. The BRASSINOSTEROID INSENSITIVE1–LIKE3 Signalosome Complex Regulates Arabidopsis Root Development. Plant Cell 2013, 25, 3377–3388. [Google Scholar] [CrossRef]
- Espinosa-Ruiz, A.; Martínez, C.; De Lucas, M.; Fàbregas, N.; Bosch, N.; Caño-Delgado, A.I.; Prat, S. TOPLESS Mediates Brassinosteroid Control of Shoot Boundaries and Root Meristem Development in Arabidopsis Thaliana. Development 2017, 144, 1619–1628. [Google Scholar] [CrossRef]
- Mercadal, J.; Betegón-Putze, I.; Bosch, N.; Caño-Delgado, A.I.; Ibañes, M. BRAVO Self-Confined Expression Through WOX5 in the Arabidopsis Root Stem-Cell Niche. Development 2022, 149, dev200510. [Google Scholar] [CrossRef]
- Chaiwanon, J.; Wang, Z.-Y. Spatiotemporal Brassinosteroid Signaling and Antagonism with Auxin Pattern Stem Cell Dynamics in Arabidopsis Roots. Curr. Biol. 2015, 25, 1031–1042. [Google Scholar] [CrossRef]
- Takahashi, N.; Suita, K.; Koike, T.; Ogita, N.; Zhang, Y.; Umeda, M. DNA Double-Strand Breaks Enhance Brassinosteroid Signaling to Activate Quiescent Center Cell Division in Arabidopsis. J. Exp. Bot. 2024, 75, 1364–1375. [Google Scholar] [CrossRef]
- Canher, B.; Heyman, J.; Savina, M.; Devendran, A.; Eekhout, T.; Vercauteren, I.; Prinsen, E.; Matosevich, R.; Xu, J.; Mironova, V.; et al. Rocks in the Auxin Stream: Wound-Induced Auxin Accumulation and ERF115 Expression Synergistically Drive Stem Cell Regeneration. Proc. Natl. Acad. Sci. USA 2020, 117, 16667–16677. [Google Scholar] [CrossRef]
- Müller, B.; Sheen, J. Cytokinin and Auxin Interaction in Root Stem-Cell Specification during Early Embryogenesis. Nature 2008, 453, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Efroni, I.; Mello, A.; Nawy, T.; Powers, A.; Satija, R.; Birnbaum, K.D. Root Regeneration Triggers an Embryo-like Sequence Guided by Hormonal Interactions. Cell 2016, 165, 1721–1733. [Google Scholar] [CrossRef]
- Antoniadi, I.; Plačková, L.; Simonovik, B.; Doležal, K.; Turnbull, C.; Ljung, K.; Novák, O. Cell-Type-Specific Cytokinin Distribution within the Arabidopsis Primary Root Apex. Plant Cell 2015, 27, 1955–1967. [Google Scholar] [CrossRef]
- Zhang, W.; Swarup, R.; Bennett, M.; Schaller, G.E.; Kieber, J.J. Cytokinin Induces Cell Division in the Quiescent Center of the Arabidopsis Root Apical Meristem. Curr. Biol. 2013, 23, 1979–1989. [Google Scholar] [CrossRef]
- Zhang, W.; To, J.P.C.; Cheng, C.-Y.; Eric Schaller, G.; Kieber, J.J. Type-A Response Regulators Are Required for Proper Root Apical Meristem Function through Post-Transcriptional Regulation of PIN Auxin Efflux Carriers. Plant J. 2011, 68, 1–10. [Google Scholar] [CrossRef]
- Cui, G.; Zhai, Y.; Li, Y.; Zheng, L.; Li, Y. The Cleavage of WOX5 by the Peptidase DA1 Connects Cytokinin Signaling and Root Stem Cell Regulation. Curr. Biol. 2024, 34, 5187–5196. [Google Scholar] [CrossRef] [PubMed]
- Matosevich, R.; Efroni, I. The Quiescent Center and Root Regeneration. J. Exp. Bot. 2021, 72, 6739–6745. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Martínez, O.; Pernas, M.; Carol, R.J.; Dolan, L. Ethylene Modulates Stem Cell Division in the Arabidopsis Thaliana Root. Science 2007, 317, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Mou, W.; Khare, R.; Polko, J.K.; Taylor, I.; Xu, J.; Xue, D.; Benfey, P.; Van de Poel, B.; Chang, C.; Kieber, J.J. Ethylene-Independent Modulation of Root Development by ACC via Downregulation of WOX5 and Group I CLE Peptide Expression. Proc. Natl. Acad. Sci. USA 2025, 122, e2417735122. [Google Scholar] [CrossRef]
- Zhang, H.; Han, W.; De Smet, I.; Talboys, P.; Loya, R.; Hassan, A.; Rong, H.; Jürgens, G.; Paul Knox, J.; Wang, M.-H. ABA Promotes Quiescence of the Quiescent Centre and Suppresses Stem Cell Differentiation in the Arabidopsis Primary Root Meristem. Plant J. 2010, 64, 764–774. [Google Scholar] [CrossRef]
- Zhou, W.; Lozano-Torres, J.L.; Blilou, I.; Zhang, X.; Zhai, Q.; Smant, G.; Li, C.; Scheres, B. A Jasmonate Signaling Network Activates Root Stem Cells and Promotes Regeneration. Cell 2019, 177, 942–956.e14. [Google Scholar] [CrossRef]
- Wang, Z.; Rong, D.; Chen, D.; Xiao, Y.; Liu, R.; Wu, S.; Yamamuro, C. Salicylic Acid Promotes Quiescent Center Cell Division Through ROS Accumulation and Down-Regulation of PLT1, PLT2, and WOX5. J. Integr. Plant Biol. 2021, 63, 583–596. [Google Scholar] [CrossRef]
- Kong, X.; Tian, H.; Yu, Q.; Zhang, F.; Wang, R.; Gao, S.; Xu, W.; Liu, J.; Shani, E.; Fu, C.; et al. PHB3 Maintains Root Stem Cell Niche Identity through ROS-Responsive AP2/ERF Transcription Factors in Arabidopsis. Cell Rep. 2018, 22, 1350–1363. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, M.; Liang, N.; Zheng, Y.; Yu, Q.; Wu, S. Symplastic Communication Spatially Directs Local Auxin Biosynthesis to Maintain Root Stem Cell Niche in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, 4005–4010. [Google Scholar] [CrossRef] [PubMed]
- Vatén, A.; Dettmer, J.; Wu, S.; Stierhof, Y.-D.; Miyashima, S.; Yadav, S.R.; Roberts, C.J.; Campilho, A.; Bulone, V.; Lichtenberger, R.; et al. Callose Biosynthesis Regulates Symplastic Trafficking During Root Development. Dev. Cell 2011, 21, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Perez-Sancho, J.; Platre, M.P.; Callebaut, B.; Smokvarska, M.; Ferrer, K.; Luo, Y.; Nolan, T.M.; Sato, T.; Busch, W.; et al. Plasmodesmata Mediate Cell-to-Cell Transport of Brassinosteroid Hormones. Nat. Chem. Biol. 2023, 19, 1331–1341. [Google Scholar] [CrossRef]
- Freire-Rios, A.; Tanaka, K.; Crespo, I.; van der Wijk, E.; Sizentsova, Y.; Levitsky, V.; Lindhoud, S.; Fontana, M.; Hohlbein, J.; Boer, D.R.; et al. Architecture of DNA Elements Mediating ARF Transcription Factor Binding and Auxin-Responsive Gene Expression in Arabidopsis. Proc. Natl. Acad. Sci. USA 2020, 117, 24557–24566. [Google Scholar] [CrossRef] [PubMed]
- Cherenkov, P.; Novikova, D.; Omelyanchuk, N.; Levitsky, V.; Grosse, I.; Weijers, D.; Mironova, V. Diversity of Cis-Regulatory Elements Associated with Auxin Response in Arabidopsis Thaliana. J. Exp. Bot. 2018, 69, 329–339. [Google Scholar] [CrossRef]
- Rademacher, E.H.; Möller, B.; Lokerse, A.S.; Llavata-Peris, C.I.; van den Berg, W.; Weijers, D. A Cellular Expression Map of the Arabidopsis AUXIN RESPONSE FACTOR Gene Family. Plant J. 2011, 68, 597–606. [Google Scholar] [CrossRef]
- Turchi, L.; Carabelli, M.; Ruzza, V.; Possenti, M.; Sassi, M.; Peñalosa, A.; Sessa, G.; Salvi, S.; Forte, V.; Morelli, G.; et al. Arabidopsis HD-Zip II Transcription Factors Control Apical Embryo Development and Meristem Function. Development 2013, 140, 2118–2129. [Google Scholar] [CrossRef]
- Crawford, B.C.W.; Sewell, J.; Golembeski, G.; Roshan, C.; Long, J.A.; Yanofsky, M.F. Genetic Control of Distal Stem Cell Fate within Root and Embryonic Meristems. Science 2015, 347, 655–659. [Google Scholar] [CrossRef]
- Liu, J.; Tian, H.; Zhang, M.; Sun, Y.; Wang, J.; Yu, Q.; Ding, Z. STOP1 Attenuates the Auxin Response to Maintain Root Stem Cell Niche Identity. Cell Rep. 2024, 43, 113617. [Google Scholar] [CrossRef]
- Perez-Garcia, P.; Pucciariello, O.; Sanchez-Corrionero, A.; Cabrera, J.; del Barrio, C.; del Pozo, J.C.; Perales, M.; Wabnik, K.; Moreno-Risueno, M.A. The Cold-Induced Factor CBF3 Mediates Root Stem Cell Activity, Regeneration, and Developmental Responses to Cold. Plant Commun. 2023, 4, 100737. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Ramírez, D.; Demesa-Arevalo, E.; Durán-Medina, Y.; Becerra-García, R.E.; Cruz-Valderrama, J.E.; Herrera-Ubaldo, H.; Montes, R.A.C.; Marzo, M.D.; Colombo, L.; Sagasser, M.; et al. The Transcription Factor NO TRANSMITTING TRACT/WIP2 Regulates Cytokinin Homeostasis in Arabidopsis. bioRxiv 2025. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sessa, G.; Morelli, G.; Sassi, M. Out of the Niche: A Bird’s-Eye View of the Molecular Networks Controlling Root Stem Cells. Plants 2025, 14, 2574. https://doi.org/10.3390/plants14162574
Sessa G, Morelli G, Sassi M. Out of the Niche: A Bird’s-Eye View of the Molecular Networks Controlling Root Stem Cells. Plants. 2025; 14(16):2574. https://doi.org/10.3390/plants14162574
Chicago/Turabian StyleSessa, Giovanna, Giorgio Morelli, and Massimiliano Sassi. 2025. "Out of the Niche: A Bird’s-Eye View of the Molecular Networks Controlling Root Stem Cells" Plants 14, no. 16: 2574. https://doi.org/10.3390/plants14162574
APA StyleSessa, G., Morelli, G., & Sassi, M. (2025). Out of the Niche: A Bird’s-Eye View of the Molecular Networks Controlling Root Stem Cells. Plants, 14(16), 2574. https://doi.org/10.3390/plants14162574







