Effects of Elevated Temperature and Water Deficiency on Functional Traits of Vitis vinifera L. cv. Assyrtiko Leaves
Abstract
1. Introduction
2. Results
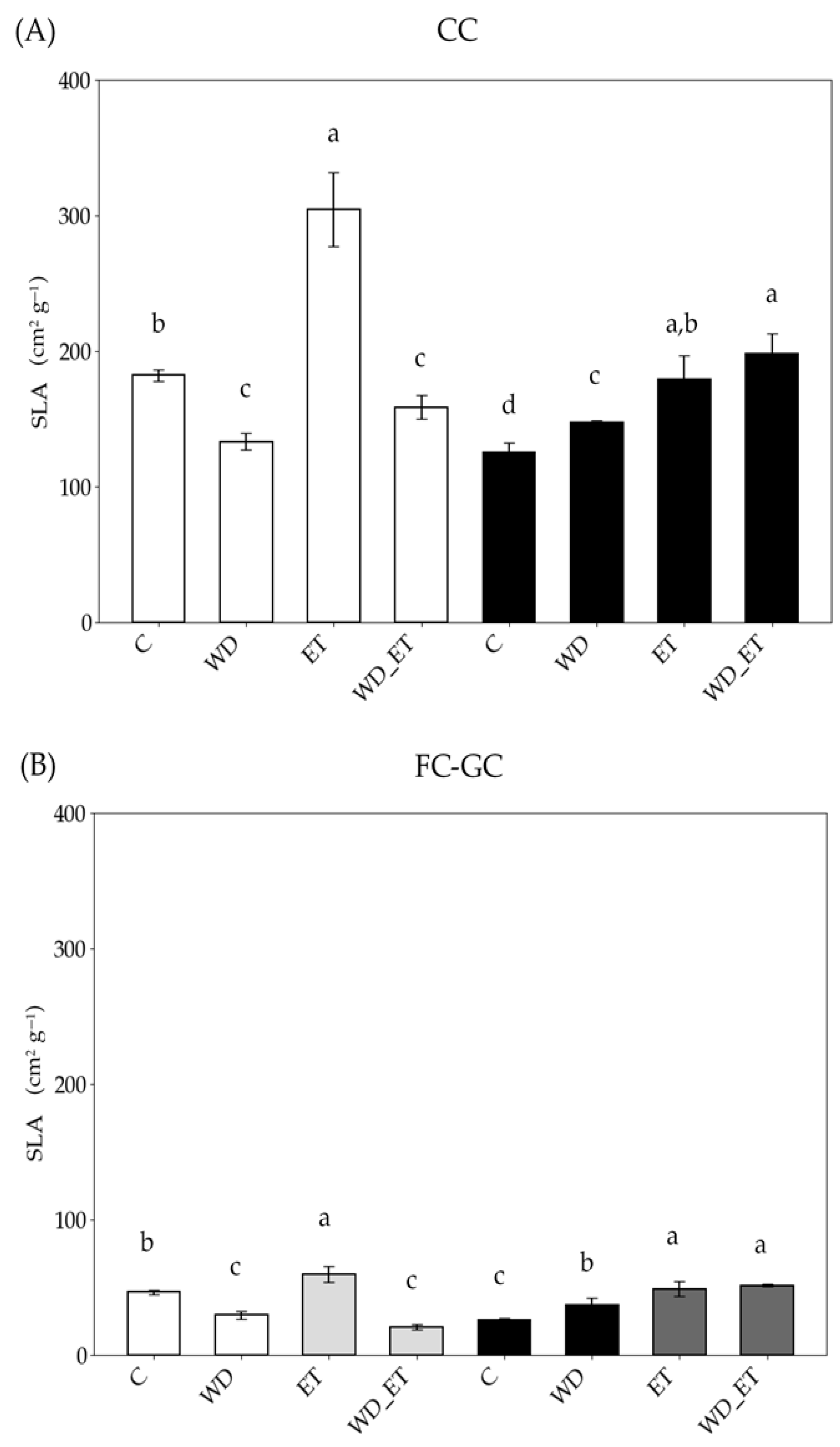
2.1. Specific Leaf Area
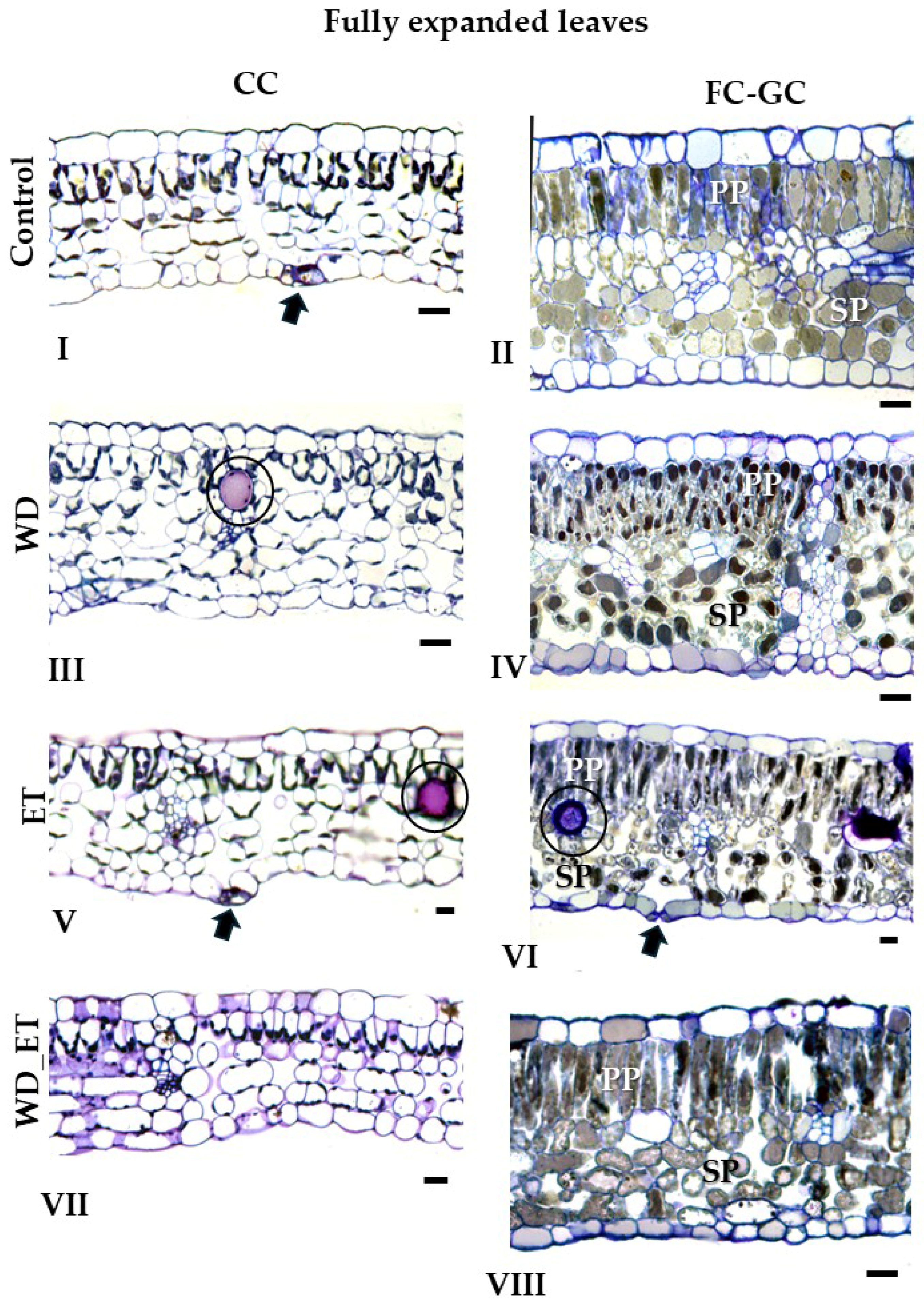
2.2. Anatomical Traits
2.2.1. CCs
2.2.2. FC-GCs
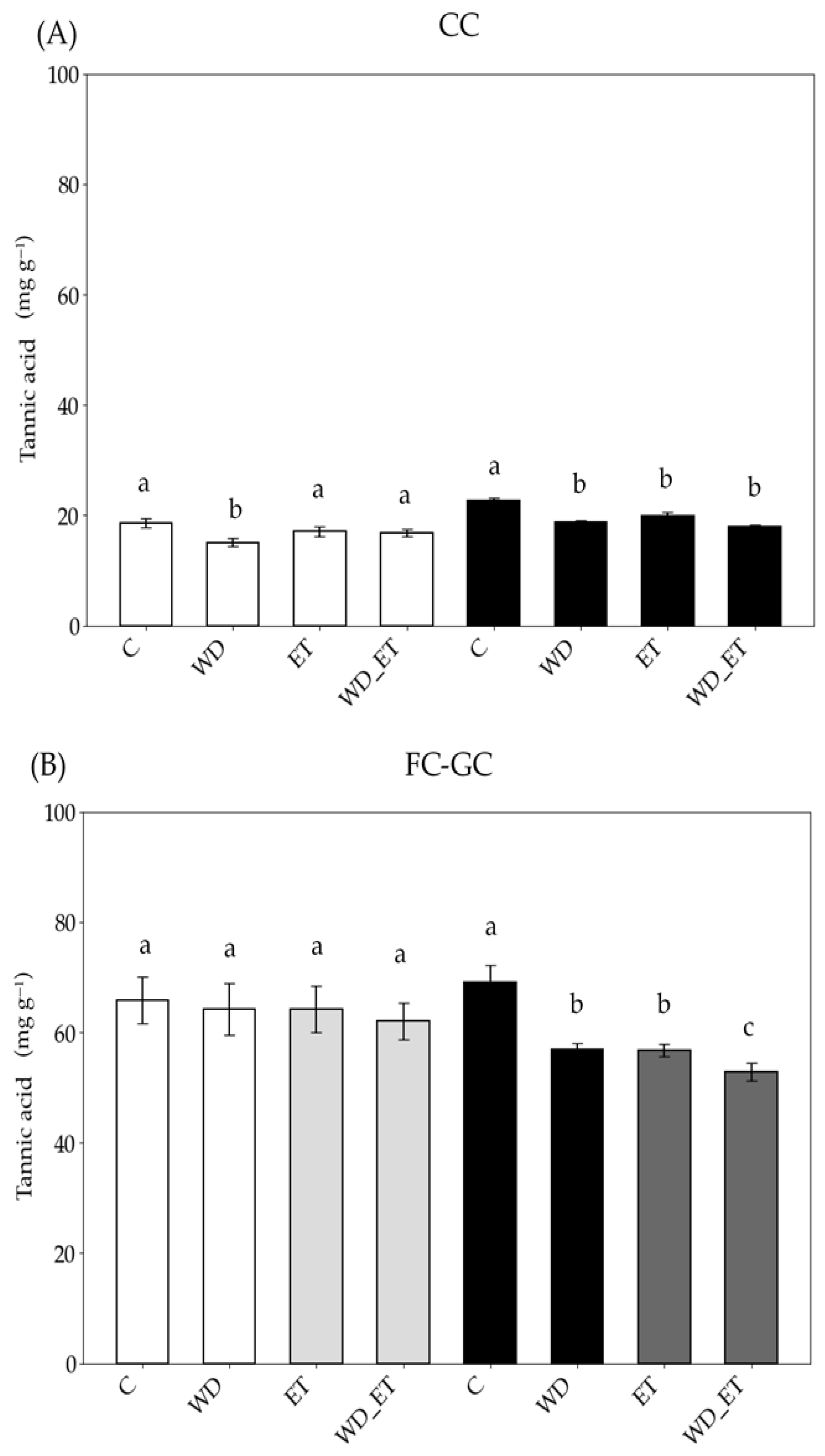
2.3. Metabolic Traits
2.3.1. Phenolic Content
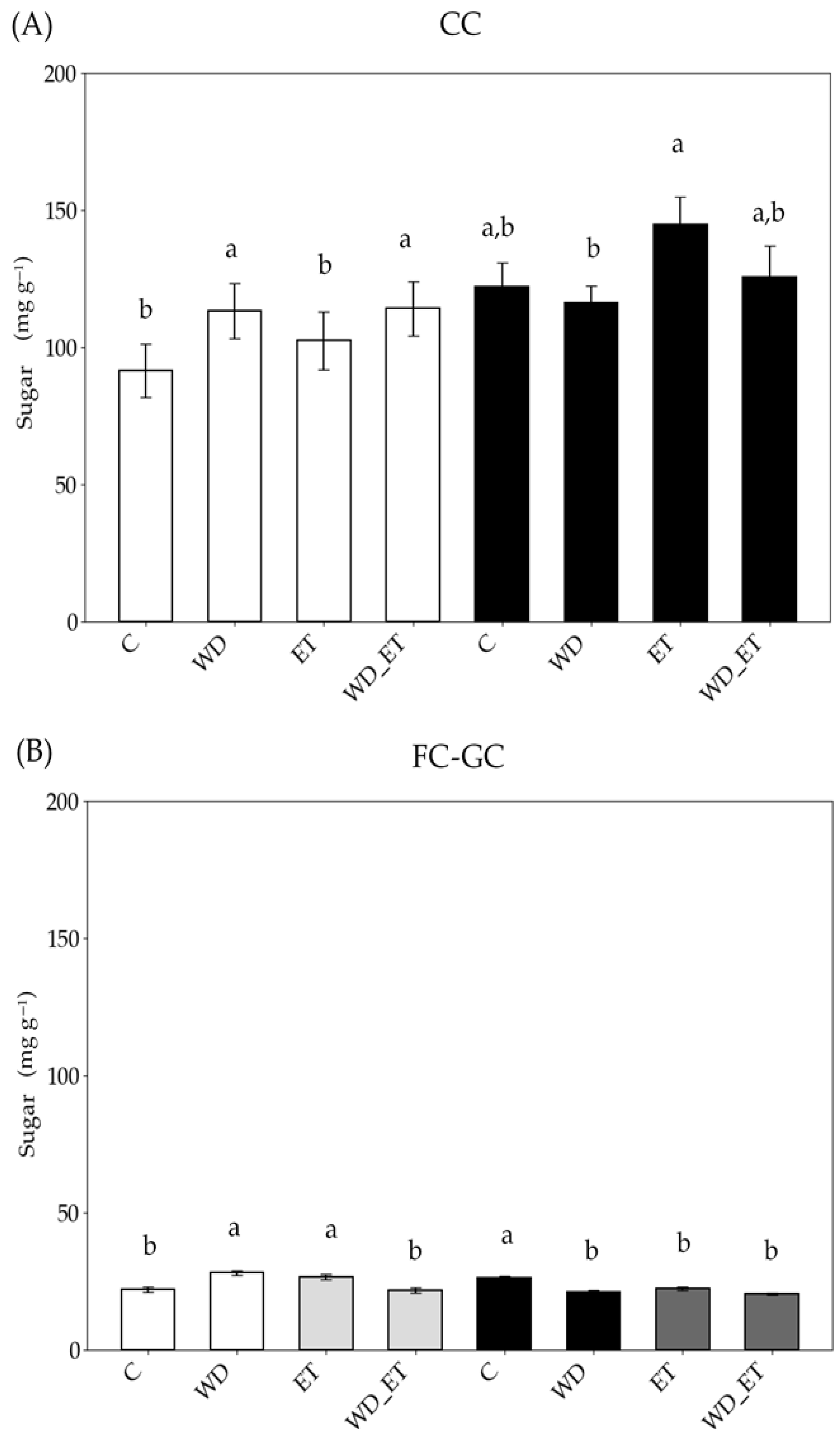
2.3.2. Soluble Sugars
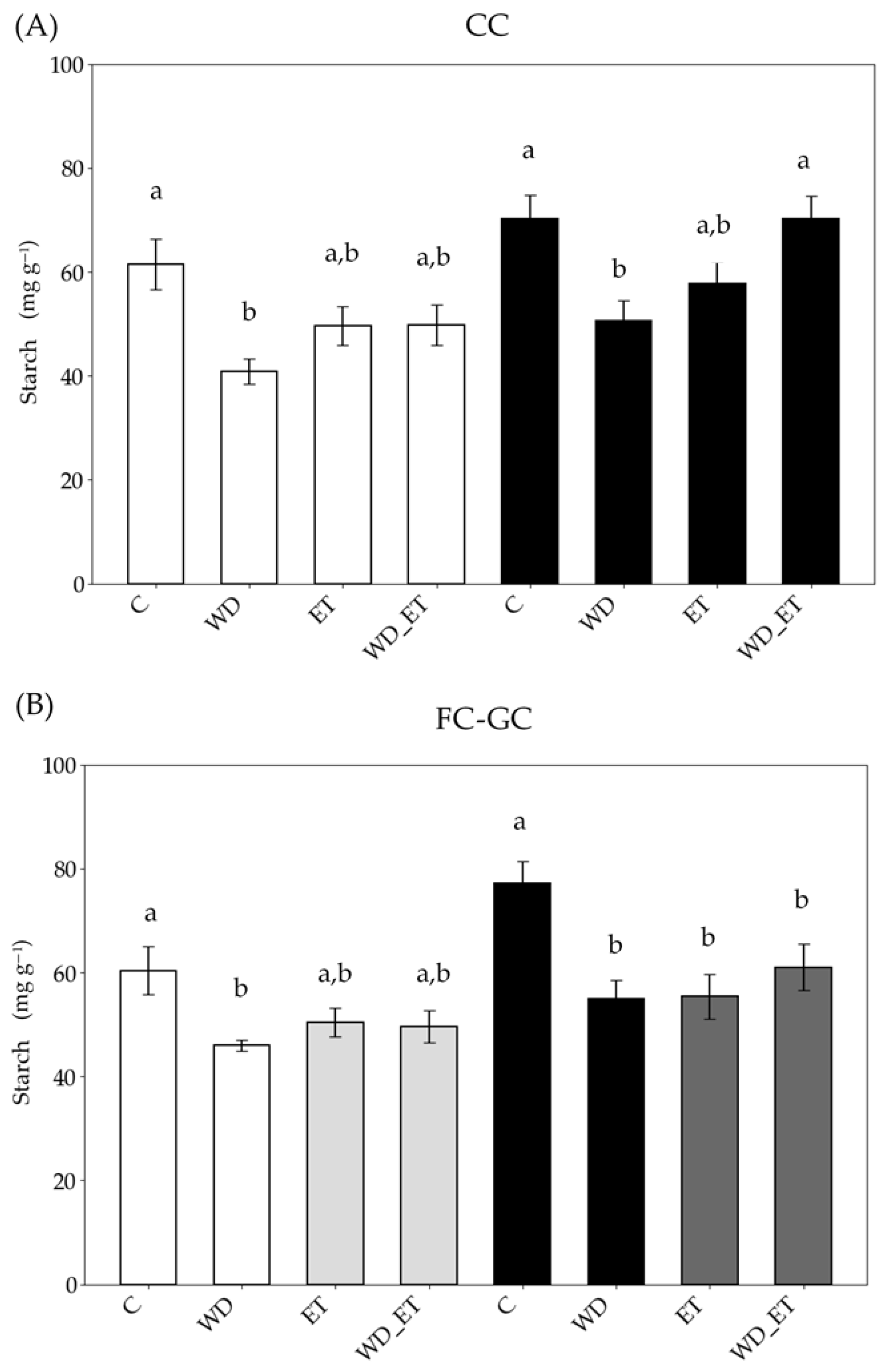
2.3.3. Starch
2.4. Plasticity Index
2.5. Mixed-Effect Analysis
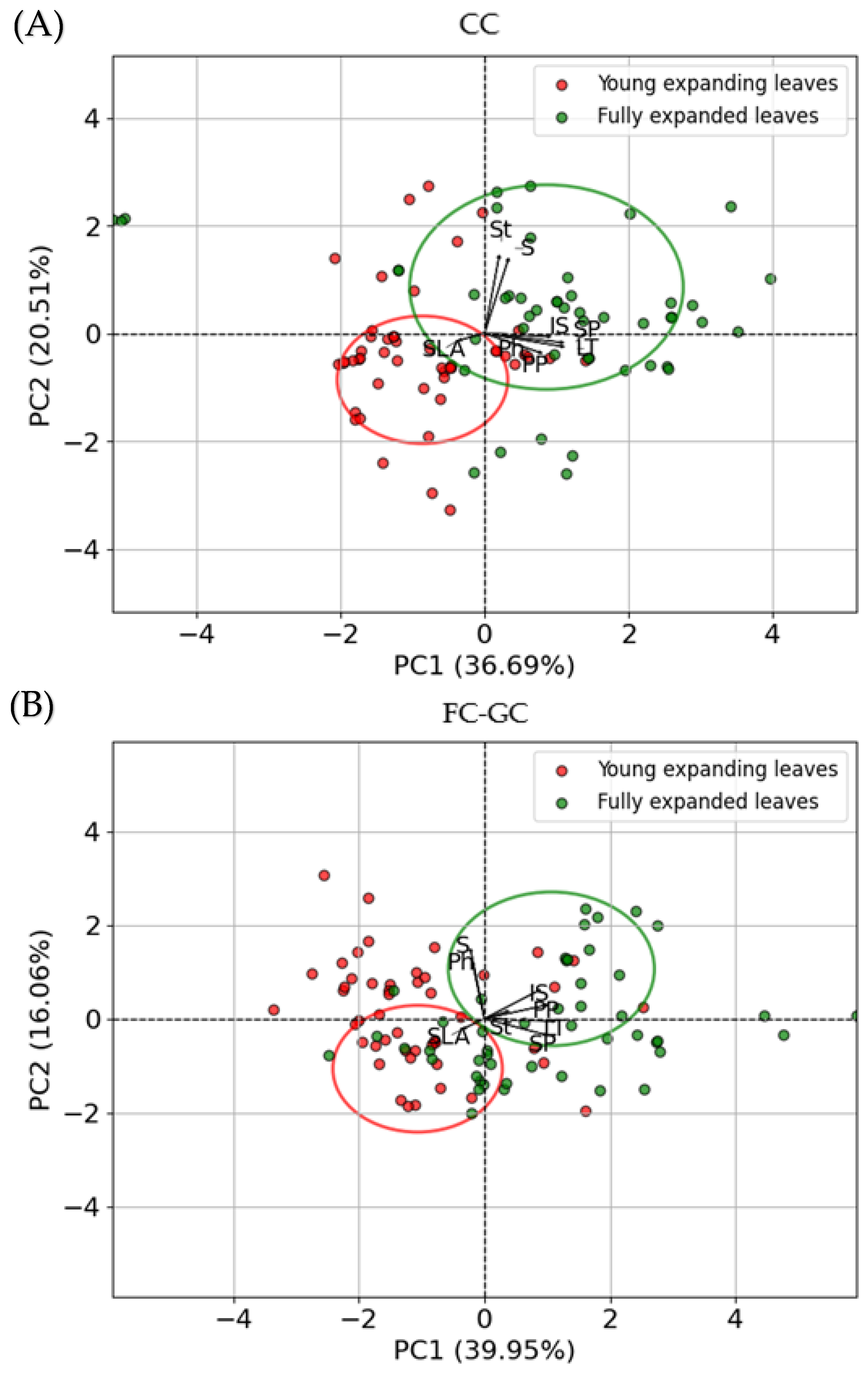
2.6. Principal Component Analysis
3. Discussion
4. Materials and Methods
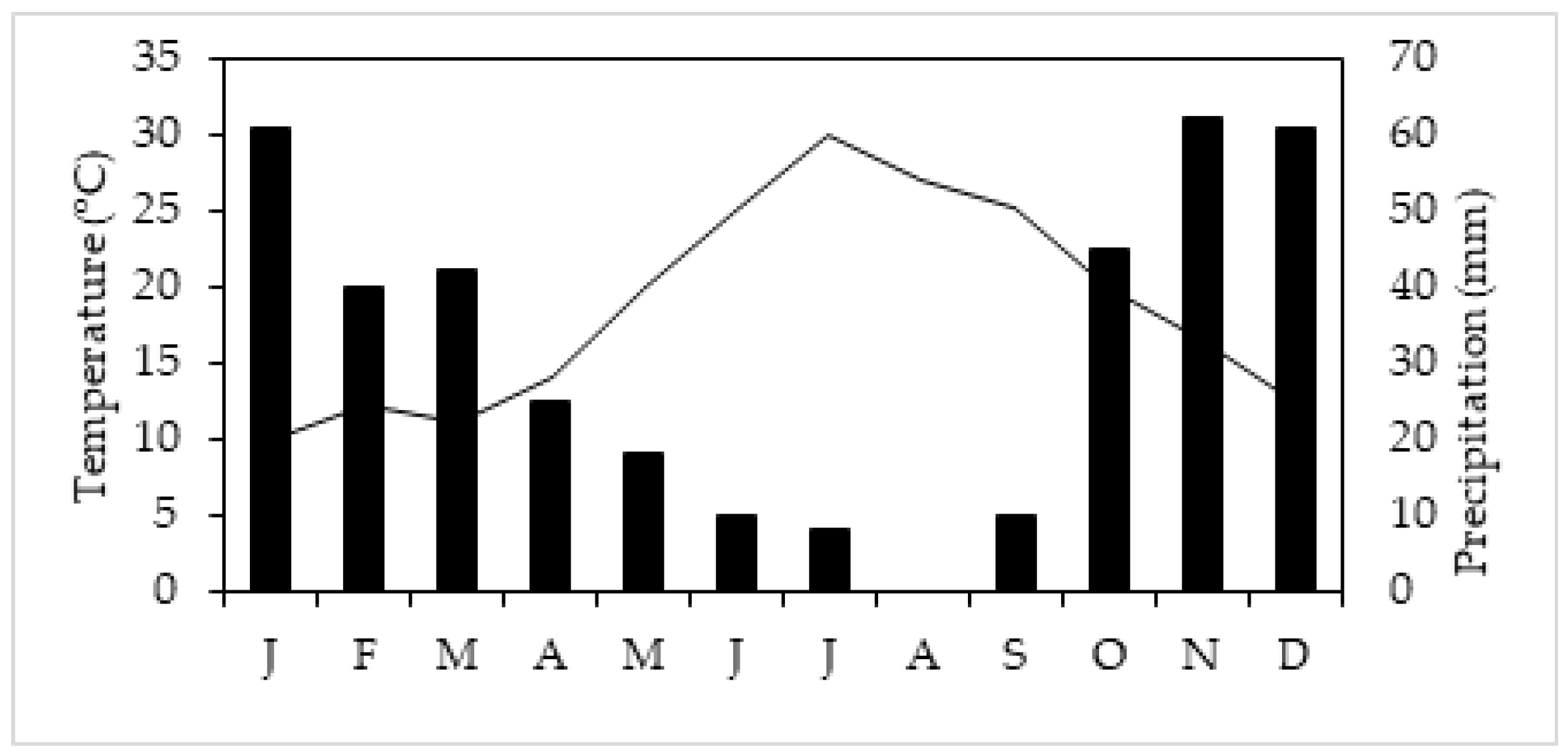
4.1. Plant Material and Experimental Conditions
4.1.1. Controlled Conditions
4.1.2. Greenhouse and Field Conditions
4.2. Leaf Material
4.3. Leaf Water Potential
4.4. Microscopy
4.5. Specific Leaf Area
4.6. Soluble Sugars and Starch
4.7. Phenolic Content
4.8. Leaf Plasticity Index
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar]
- Brogli, R.; Sørland, S.L.; Kröner, N.; Schär, C. Causes of future Mediterranean precipitation decline depend on the season. Environ. Res. Lett. 2019, 14, 114017. [Google Scholar] [CrossRef]
- del Pozo, A.; Brunel-Saldias, N.; Engler, A.; Ortega-Farias, S.; Acevedo-Opazo, C.; Lobos, G.A.; Jara-Rojas, R.; Molina-Montenegro, M.A. Climate change impacts and adaptation strategies of agriculture in Mediterranean-climate regions (MCRs). Sustainability 2019, 11, 2769. [Google Scholar] [CrossRef]
- Lazoglou, G.; Papadopoulos-Zachos, A.; Georgiades, P.; Zittis, G.; Velikou, K.; Manios, E.M.; Anagnostopoulou, C. Identification of climate change hotspots in the Mediterranean. Sci. Rep. 2024, 14, 29817. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Bota, J.; Escalona, J.M.; Sampol, B.; Medrano, H. Effects of drought on photosynthesis in grapevines under field conditions: An evaluation of stomatal and mesophyll limitations. Funct. Plant Biol. 2002, 29, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Bertamini, M.; Zulini, L.; Muthuchelian, K.; Nedunchezhian, N. Effect of water deficit on photosynthetic and other physiological responses in grapevine (Vitis vinifera L. cv. Riesling) plants. Photosynthetica 2006, 44, 151–154. [Google Scholar] [CrossRef]
- Gómez del Campo, M.; Baeza, P.; Ruiz, C.; Lissarrague, J.R. Water-stress induced physiological changes in leaves of four container-grown grapevine cultivars (Vitis vinifera L.). Vitis-J. Grapevine Res. 2015, 43, 99–105. [Google Scholar]
- Molitor, D.; Junk, J. Climate change is implicating a two-fold impact on air temperature increase in the ripening period under the conditions of the Luxembourgish grape growing region. OENO One 2019, 53, 409–422. [Google Scholar] [CrossRef]
- Koufos, G.C.; Mavromatis, T.; Koundouras, S.; Jones, G.V. Adaptive capacity of winegrape varieties cultivated in Greece to climate change: Current trends and future projections. OENO One 2020, 54, 1201–1219. [Google Scholar] [CrossRef]
- Mosedale, J.R.; Abernethy, K.E.; Smart, R.E.; Wilson, R.J.; Maclean, I.M. Climate change impacts and adaptive strategies: Lessons from the grapevine. Glob. Change Biol. 2016, 22, 3814–3828. [Google Scholar] [CrossRef]
- Alba, V.; Russi, A.; Caputo, A.R.; Gentilesco, G. Climate change and viticulture in Italy: Historical trends and future scenarios. Atmosphere 2024, 15, 885. [Google Scholar] [CrossRef]
- Morales-Castilla, I.; García de Cortázar-Atauri, I.; Cook, B.I.; Lacombe, T.; Parker, A.; Van Leeuwen, C.; Nicholas, K.A.; Wolkovich, E.M. Diversity buffers wine-growing regions from climate change losses. Proc. Natl. Acad. Sci. USA 2020, 117, 2864–2869. [Google Scholar] [CrossRef]
- Naulleau, A.; Gary, C.; Prévot, L.; Hossard, L. Evaluating strategies for adaptation to climate change in grapevine production–A systematic review. Front. Plant Sci. 2021, 11, 607859. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; García de Cortázar-Atauri, I.; Morales-Castilla, I.; Nicholas, K.A.; Lacombe, T. From Pinot to Xinomavro in the world’s future wine-growing regions. Nat. Clim. Change 2018, 8, 29–37. [Google Scholar] [CrossRef]
- Puga, G.; Anderson, K.; Jones, G.; Tchatoka, F.D.; Umberger, W. A climatic classification of the world’s wine regions. OENO One 2022, 56, 165–177. [Google Scholar] [CrossRef]
- Prada, J.; Dinis, L.T.; Soriato, E.; Vandelle, E.; Soletkin, O.; Uysal, Ş.; Dihazi, A.; Santos, C.; Santos, J.A. Climate change impact on Mediterranean viticultural regions and site-specific climate risk-reduction strategies. Mitig. Adapt. Strateg. Glob. Change 2024, 29, 52. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Darriet, P. The impact of climate change on viticulture and wine quality. J. Wine Econ. 2016, 11, 150–167. [Google Scholar] [CrossRef]
- Faralli, M.; Bontempo, L.; Bianchedi, P.L.; Moser, C.; Bertamini, M.; Lawson, T.; Camin, F.; Stefanini, M.; Varotto, C. Natural variation in stomatal dynamics drives divergence in heat stress tolerance and contributes to seasonal intrinsic water-use efficiency in Vitis vinifera (subsp. sativa and sylvestris). J. Exp. Bot. 2022, 73, 3238–3250. [Google Scholar] [CrossRef]
- Baltazar, M.; Castro, I.; Gonçalves, B. Adaptation to climate change in viticulture: The role of varietal selection—A review. Plants 2025, 14, 104. [Google Scholar] [CrossRef]
- Degu, A.; Hochberg, U.; Wong, D.C.J.; Alberti, G.; Lazarovitch, N.; Peterlunger, E.; Castellarin, S.D.; Herrera, J.C.; Fait, A. Swift metabolite changes and leaf shedding are milestones in the acclimation process of grapevine under prolonged water stress. BMC Plant Biol. 2019, 19, 69. [Google Scholar] [CrossRef]
- Barry, P. Jim Barry Wines to release Australia’s first commercial Assyrtiko this year. Wine Vitic. J. 2016, 31, 55–56. [Google Scholar]
- Kolyva, F.; Nikolopoulos, D.; Bresta, P.; Liakopoulos, G.; Karabourniotis, G.; Rhizopoulou, S. Acclimation of the grapevine Vitis vinifera L. cv. Assyrtiko to water deficit: Coordination of structural and functional leaf traits and the dynamic of calcium oxalate crystals. Plants 2023, 12, 3992. [Google Scholar] [CrossRef] [PubMed]
- Xyrafis, E.G.; Gambetta, G.A.; Biniari, K. A comparative study on training systems and vine density in Santorini Island: Physiological, microclimate, yield and quality attributes. OENO One 2023, 57, 141–152. [Google Scholar] [CrossRef]
- Sigala, M. Scarecrows: An Art Exhibition at Domaine Sigalas Inspiring Transformational Wine Tourism Experiences. In Management and Marketing of Wine Tourism Business; Sigala, M., Robinson, R.N.S., Eds.; Palgrave Macmillan: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Koufos, G.C.; Mavromatis, T.; Koundouras, S.; Fyllas, N.M.; Theocharis, S.; Jones, G.V. Greek wine quality assessment and relationships with climate: Trends, future projections and uncertainties. Water 2022, 14, 573. [Google Scholar] [CrossRef]
- Xyrafis, E.G.; Deloire, A.; Petoumenou, D.; Paraskevopoulos, I.; Biniari, K. The unique and extreme vineyards of Santorini Island (Cyclades). IVES Tech. Rev. Vine Wine 2021. [Google Scholar] [CrossRef]
- Frankel, C. Santorini. In Volcanoes and Wine: From Pompeii to Napa; University of Chicago Press: Chicago, IL, USA, 2019; pp. 13–36. [Google Scholar]
- Alatzas, A.; Theocharis, S.; Miliordos, D.E.; Tsaktsarlis, V.; Chronis, I.; Kotseridis, Y.; Koundouras, S.; Hatzopoulos, P. Defoliation at berry set alters grape composition and gene expression during berry development in two Greek Vitis vinifera L. cultivars. OENO One 2024, 58, 1–15. [Google Scholar] [CrossRef]
- Liakopoulos, G.; Nikolopoulos, D.; Klouvatou, A.; Vekkos, K.A.; Manetas, Y.; Karabourniotis, G. The photoprotective role of epidermal anthocyanins and surface pubescence in young leaves of grapevine (Vitis vinifera). Ann. Bot. 2006, 98, 257–265. [Google Scholar] [CrossRef]
- Chitwood, D.H. The shapes of wine and table grape leaves: An ampelometric study inspired by the methods of Pierre Galet. Plants People Planet 2021, 3, 155–170. [Google Scholar] [CrossRef]
- Carvalho, M.L.; Carneiro, C.E. Leaf morphoanatomy of an endemic massaranduba from Chapada Diamantina, Bahia, Brazil. Biota Neotrop. 2021, 21, e20201187. [Google Scholar] [CrossRef]
- Baumgartner, A.; Donahoo, M.; Chitwood, D.H.; Peppe, D.J. The influences of environmental change and development on leaf shape in Vitis. Am. J. Bot. 2020, 107, 676–688. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Chrysargyris, A.; Aziz, A. Adaptive response of a native Mediterranean grapevine cultivar upon short-term exposure to drought and heat stress in the context of climate change. Agronomy 2020, 10, 249. [Google Scholar] [CrossRef]
- MacMillan, P.; Teixeira, G.; Lopes, C.M.; Monteiro, A. The role of grapevine leaf morphoanatomical traits in determining capacity for coping with abiotic stresses: A review. Cienc. Tec. Vitivinic. 2021, 36, 75–88. [Google Scholar] [CrossRef]
- Sadras, V.O.; Reynolds, M.P.; De la Vega, A.J.; Petrie, P.R.; Robinson, R. Phenotypic plasticity of yield and phenology in wheat, sunflower and grapevine. Field Crops Res. 2009, 110, 242–250. [Google Scholar] [CrossRef]
- Suter, B.; Destrac Irvine, A.; Gowdy, M.; Dai, Z.; van Leeuwen, C. Adapting wine grape ripening to global change requires a multi-trait approach. Front. Plant Sci. 2021, 12, 624867. [Google Scholar] [CrossRef] [PubMed]
- Stotz, C.C.; Salgado-Luarte, C.; Escobedo, V.M.; Valladares, F.; Gianoli, E. Phenotypic plasticity and the leaf economics spectrum: Plasticity is positively associated with specific leaf area. Oikos 2022, 2022, e09342. [Google Scholar] [CrossRef]
- Chitwood, D.H.; Rundell, S.M.; Li, D.Y.; Woodford, Q.L.; Yu, T.T.; Lopez, J.R.; Greenblatt, D.; Kang, J.; Londo, J.P. Climate and developmental plasticity: Interannual variability in grapevine leaf morphology. Plant Physiol. 2016, 170, 1480–1491. [Google Scholar] [CrossRef]
- Xyrafis, E.G.; Biniari, K.; Stavrakaki, M. Particle film treatments on ‘Assyrtiko’ grapevines enhance physiology and grape attributes in Santorini Island. Not. Bot. Horti Agrobot. Cluj-Napoca 2024, 52, 13425. [Google Scholar] [CrossRef]
- Alatzas, A.; Theocharis, S.; Miliordos, D.E.; Leontaridou, K.; Kanellis, A.K.; Kotseridis, Y.; Hatzopoulos, P.; Koundouras, S. The effect of water deficit on two Greek Vitis vinifera L. cultivars: Physiology, grape composition and gene expression during berry development. Plants 2021, 10, 1947. [Google Scholar] [CrossRef]
- Xyrafis, E.G.; Fraga, H.; Nakas, C.T.; Koundouras, S. A study on the effects of climate change on viticulture on Santorini Island. OENO One 2022, 56, 259–273. [Google Scholar] [CrossRef]
- Zufferey, V.; Murisier, F.; Vivin, P.; Belcher, S.; Lorenzini, F.; Spring, J.L.; Viret, O. Carbohydrate reserves in grapevine (Vitis vinifera L. ‘Chasselas’): The influence of the leaf to fruit ratio. Vitis 2012, 51, 103–110. [Google Scholar]
- Król, A.; Amarowicz, R.; Weidner, S. Changes in the composition of phenolic compounds and antioxidant properties of grapevine roots and leaves (Vitis vinifera L.) under continuous long-term drought stress. Acta Physiol. Plant. 2014, 36, 1491–1499. [Google Scholar] [CrossRef]
- Dayer, S.; Prieto, J.A.; Galat, E.; Peña, J.P. Leaf carbohydrate metabolism in Malbec grapevines: Combined effects of regulated deficit irrigation and crop load. Aust. J. Grape Wine Res. 2016, 22, 115–123. [Google Scholar] [CrossRef]
- Théroux-Rancourt, G.; Herrera, J.C.; Voggeneder, K.; De Berardinis, F.; Luijken, N.; Nocker, L.; Savi, T.; Scheffknecht, S.; Schneck, M.; Tholen, D. Analyzing anatomy over three dimensions unpacks the differences in mesophyll diffusive area between sun and shade Vitis vinifera leaves. AoB Plants 2023, 15, plad001. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Abudureheman, R.; Zhong, H.; Yadav, V.; Zhang, C.; Ma, Y.; Liu, X.; Zhang, F.; Zha, Q.; Wang, X. The impact of high temperatures in the field on leaf tissue structure in different grape cultivars. Horticulturae 2023, 9, 731. [Google Scholar] [CrossRef]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osório, M.L.; Pinheiro, C. How plants cope with water stress in the field. Photosynthesis and growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef]
- Nardini, A. Hard and tough: The coordination between leaf mechanical resistance and drought tolerance. Flora 2022, 288, 152023. [Google Scholar] [CrossRef]
- Pantin, F.; Simonneau, T.; Muller, B. Coming of leaf age: Control of growth by hydraulics and metabolics during leaf ontogeny. New Phytol. 2012, 196, 349–366. [Google Scholar] [CrossRef]
- Gago, P.; Conejero, G.; Martínez, M.C.; This, P.; Verdeil, J.L. Comparative anatomy and morphology of the leaves of Grenache Noir and Syrah grapevine cultivars. S. Afr. J. Enol. Vitic. 2019, 40, 1–9. [Google Scholar] [CrossRef]
- Xu, F.; Guo, W.; Xu, W.; Wei, Y.; Wang, R. Leaf morphology correlates with water and light availability: What consequences for simple and compound leaves? Prog. Nat. Sci. 2009, 19, 1789–1798. [Google Scholar] [CrossRef]
- Leigh, A.; Sevanto, S.; Ball, M.C.; Close, J.D.; Ellsworth, D.S.; Knight, C.A.; Nicotra, A.B.; Vogel, S. Do thick leaves avoid thermal damage in critically low wind speeds? New Phytol. 2012, 194, 477–487. [Google Scholar] [CrossRef]
- Wahid, A.; Farooq, M.; Hussain, I.; Rasheed, R.; Galani, S. Responses and management of heat stress in plants. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, A., Wani, M.R., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 135–157. [Google Scholar] [CrossRef]
- Trovato, M.; Brini, F.; Mseddi, K.; Rhizopoulou, S.; Jones, M.A. A holistic and sustainable approach linked to drought tolerance of Mediterranean crops. Front. Plant Sci. 2023, 14, 1167376. [Google Scholar] [CrossRef]
- Salem-Fnayou, A.B.; Bouamama, B.; Ghorbel, A.; Mliki, A. Investigations on the leaf anatomy and ultrastructure of grapevine (Vitis vinifera) under heat stress. Microsc. Res. Tech. 2011, 74, 756–762. [Google Scholar] [CrossRef]
- Petoumenou, D.G.; Liava, V. Sustainable Foliar Applications to Improve Grapevine Responses to Drought, High Temperatures, and Salinity: Impacts on Physiology, Yields, and Berry Quality. Plants 2025, 14, 2157. [Google Scholar] [CrossRef] [PubMed]
- Doupis, G.; Bosabalidis, A.M.; Patakas, A. Comparative effects of water deficit and enhanced UV-B radiation on photosynthetic capacity and leaf anatomy traits of two grapevine (Vitis vinifera L.) cultivars. Theor. Exp. Plant Physiol. 2016, 28, 131–141. [Google Scholar] [CrossRef]
- Tomás, M.; Medrano, H.; Brugnoli, E.; Escalona, J.M.; Martorell, S.; Pou, A.; Ribas-Carbó, M.; Flexas, J. Variability of mesophyll conductance in grapevine cultivars under water stress conditions in relation to leaf anatomy and water use efficiency. Aust. J. Grape Wine Res. 2014, 20, 272–280. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Ul-Allah, S.; Siddique, K.H. Physiological and agronomic approaches for improving water-use efficiency in crop plants. Agric. Water Manag. 2019, 219, 95–108. [Google Scholar] [CrossRef]
- Weiler, C.S.; Merkt, N.; Hartung, J.; Graeff-Hönninger, S. Variability among young table grape cultivars in response to water deficit and water use efficiency. Agronomy 2019, 9, 135. [Google Scholar] [CrossRef]
- Koundouras, S.; Tsialtas, I.T.; Zioziou, E.; Nikolaou, N. Rootstock effects on the adaptive strategies of grapevine (Vitis vinifera L. cv. Cabernet–Sauvignon) under contrasting water status: Leaf physiological and structural responses. Agric. Ecosyst. Environ. 2008, 128, 86–96. [Google Scholar] [CrossRef]
- Moustaka, J.; Tanou, G.; Adamakis, I.-D.; Eleftheriou, E.P.; Moustakas, M. Leaf age-dependent photoprotective and antioxidative response mechanisms to paraquat-induced oxidative stress in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 13989–14006. [Google Scholar] [CrossRef]
- Kamimori, M.; Miwa, Y.; Isobe, T.; Hosomi, A. Estimation of leaf emergence in ‘Delaware’ grape from daily mean temperature to predict the optimal timing for gibberellic acid application to achieve seedlessness. Hort. J. 2021, 90, 181–189. [Google Scholar] [CrossRef]
- Bartlett, M.K. The Ecological Impacts of Leaf Drought Tolerance. Ph.D. Thesis, University of California, Los Angeles, CA, USA, 2016. [Google Scholar]
- Meletiou-Christou, M.S.; Rhizopoulou, S. Constraints of photosynthetic performance and water status of four evergreen species co-occurring under field conditions. Bot. Stud. 2012, 53, 325–334. [Google Scholar]
- Meletiou-Christou, M.S.; Rhizopoulou, S. Leaf functional traits of four evergreen species growing in Mediterranean environmental conditions. Acta Physiol. Plant. 2017, 39, 34. [Google Scholar] [CrossRef]
- Jones, H.G.; Higgs, K.H. Resistance to water loss from the mesophyll cell surface in plant leaves. J. Exp. Bot. 1980, 31, 545–553. [Google Scholar] [CrossRef]
- Rodrigues, M.; Chaves, M.M.; Wendler, R.; David, M.; Quick, W.P.; Leegood, R.C.; Pereira, J.S. Osmotic adjustment in water-stressed grapevine leaves in relation to carbon assimilation. Funct. Plant Biol. 1993, 20, 569–579. [Google Scholar] [CrossRef]
- Satisha, J.; Prakash, G.S.; Bhatt, R.M.; Sampathkumar, P. Effect of soil moisture stress on physiological response in grape (Vitis vinifera L.) varieties. J. Hortic. Sci. 2006, 1, 99–103. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Li, C.; Zhang, Z.; Ma, F.; Li, M. Response of sugar metabolism in apple leaves subjected to short-term drought stress. Plant Physiol. Biochem. 2019, 141, 164–171. [Google Scholar] [CrossRef] [PubMed]
- MacNeill, G.J.; Mehrpouyan, S.; Minow, M.A.; Patterson, J.A.; Tetlow, I.J.; Emes, M.J. Starch as a source, starch as a sink: The bifunctional role of starch in carbon allocation. J. Exp. Bot. 2017, 68, 4433–4453. [Google Scholar] [CrossRef]
- Dong, S.; Beckles, D.M. Dynamic changes in the starch-sugar interconversion within plant source and sink tissues promote a better abiotic stress response. J. Plant Physiol. 2019, 234, 80–93. [Google Scholar] [CrossRef]
- Gutiérrez-Gordillo, S.; Pérez-González, J.M.; Sancho-Galán, P.; Amores-Arrocha, A.; Palacios, V.; Jiménez-Cantizano, A. Physiological, enological and agronomic characterization of Pedro Ximénez grapevine cultivar under organic farming in a warm climate zone. Agronomy 2023, 13, 1732. [Google Scholar] [CrossRef]
- Candar, S.; Seçkin, G.U.; Kizildeniz, T.; Korkutal, İ.; Bahar, E. Variations of chlorophyll, proline, and abscisic acid (ABA) contents in grapevines (Vitis vinifera L.) under water deficit conditions. Erwerbs-Obstbau 2023, 65, 1965–1977. [Google Scholar] [CrossRef]
- Niu, L.; Wu, X.; Liu, H.; Hu, X.; Wang, W. Leaf starch degradation by β-amylase ZmBAM8 influences drought tolerance in maize. Carbohydr. Polym. 2024, 345, 122555. [Google Scholar] [CrossRef]
- Düring, H. Evidence for osmotic adjustment to drought in grapevines (Vitis vinifera L.). Vitis-J. Grapevine Res. 2016, 23, 1–10. [Google Scholar]
- Scalabrelli, G.; Saracini, E.; Remorini, D.; Massai, R.; Tattini, M. Changes in leaf phenolic compounds in two grapevine varieties (Vitis vinifera L.) grown in different water conditions. Acta Hortic. 2007, 754, 295–300. [Google Scholar] [CrossRef]
- Dorado, J.F.; Pinto, G.; Monteiro, P.; Chaves, N.; Alías, J.C.; Rodrigo, S.; Camison, A.; Solla, A. Heat stress and recovery effects on the physiology and biochemistry of Castanea sativa Mill. Front. For. Glob. Change 2023, 5, 1072661. [Google Scholar] [CrossRef]
- Sokolova, E.; Krol, T.; Adamov, G.; Minyazeva, Y.; Baleev, D.; Sidelnikov, N. Total content and composition of phenolic compounds from Filipendula genus plants and their potential health-promoting properties. Molecules 2024, 29, 2013. [Google Scholar] [CrossRef] [PubMed]
- Zapata, C.; Deléens, E.; Chaillou, S.; Magné, C. Partitioning and mobilization of starch and N reserves in grapevine (Vitis vinifera L.). J. Plant Physiol. 2004, 161, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Klopotek, Y.; Kläring, H.P. Accumulation and remobilisation of sugar and starch in the leaves of young tomato plants in response to temperature. Sci. Hortic. 2014, 180, 262–267. [Google Scholar] [CrossRef]
- Walker, R.P.; Bonghi, C.; Varotto, S.; Battistelli, A.; Burbidge, C.A.; Castellarin, S.D.; Chen, Z.-H.; Darriet, P.; Moscatello, S.; Rienth, M.; et al. Sucrose metabolism and transport in grapevines, with emphasis on berries and leaves, and insights gained from a cross-species comparison. Int. J. Mol. Sci. 2021, 22, 7794. [Google Scholar] [CrossRef]
- Politi, N.; Vlachogiannis, D.; Sfetsos, A.; Nastos, P.T. High resolution projections for extreme temperatures and precipitation over Greece. Clim. Dyn. 2023, 61, 633–667. [Google Scholar] [CrossRef]
- Giannakopoulos, C.; Le Sager, P.; Bindi, M.; Moriondo, M.; Kostopoulou, E.; Goodess, C.M. Climatic changes and associated impacts in the Mediterranean resulting from a 2 °C global warming. Glob. Planet. Change 2009, 68, 209–224. [Google Scholar] [CrossRef]
- Zittis, G.; Hadjinicolaou, P.; Klangidou, M.; Proestos, Y.; Lelieveld, J. A multi-model, multi-scenario, and multi-domain analysis of regional climate projections for the Mediterranean. Reg. Environ. Change 2019, 19, 2621–2635. [Google Scholar] [CrossRef]
- Proutsos, N.; Liakatas, A.; Alexandris, S.; Nwokolo, S.C.; Solomou, A.D.; Amadi, S.O. Assessing the impact of atmospheric attributes on the effectiveness of solar irradiance for photosynthesis of urban vegetation in Attica, Greece. Theor. Appl. Climatol. 2024, 155, 1415–1427. [Google Scholar] [CrossRef]
- Biniari, K.; Xenaki, M.; Daskalakis, I.; Rusjan, D.; Bouza, D.; Stavrakaki, M. Polyphenolic compounds and antioxidants of skin and berry grapes of Greek Vitis vinifera cultivars in relation to climate conditions. Food Chem. 2020, 307, 125518. [Google Scholar] [CrossRef] [PubMed]
- Carrara, M.; Catania, P.; Pipitone, F.; Vallone, M.; Piraino, S.; Salvia, M.; Paolino, C. Temperature and relative humidity distribution inside a greenhouse using wireless sensors. Acta Hortic. 2008, 801, 595–599. [Google Scholar] [CrossRef]
- Reid, K.; Oki, L. Irrigation and climate zone trials of perennial plants for sustainable landscapes. Acta Hortic. 2013, 980, 95–102. [Google Scholar] [CrossRef]
- Anderson, K.; Bows, A. Beyond ‘dangerous’ climate change: Emission scenarios for a new world. Philos. Transact. A Math. Phys. Eng. Sci. 2011, 369, 20–44. [Google Scholar] [CrossRef]
- Joel, G.; Cramer, W. Climate change: The 2015 Paris Agreement thresholds and Mediterranean basin ecosystems. Science 2016, 354, 465–468. [Google Scholar] [CrossRef]
- Kay, A.L.; Lane, R.A.; Bell, V.A. Grid-based simulation of soil moisture in the UK: Future changes in extremes and wetting and drying dates. Environ. Res. Lett. 2022, 17, 074029. [Google Scholar] [CrossRef]
- Boso Alonso, S.; Alonso-Villaverde, I.V.; Santiago Blanco, J.L.; Gago Montaña, P.; Dürrenberger, M.; Düggelin, M.; Kassemeyer, H.H.; Martínez Rodríguez, M.D.C. Macro- and microscopic leaf characteristics of six grapevine genotypes (Vitis spp.) with different susceptibilities to grapevine downy mildew. Vitis 2010, 49, 43–50. [Google Scholar]
- Groenveld, T.; Obiero, C.; Yu, Y.; Flury, M.; Keller, M. Predawn leaf water potential of grapevines is not necessarily a good proxy for soil moisture. BMC Plant Biol. 2023, 23, 369. [Google Scholar] [CrossRef]
- Zhu, J.; Dai, Z.; Vivin, P.; Gambetta, G.A.; Henke, M.; Peccoux, A.; Ollat, N.; Delrot, S. A 3-D functional–structural grapevine model that couples the dynamics of water transport with leaf gas exchange. Ann. Bot. 2018, 121, 833–848. [Google Scholar] [CrossRef]
- Adamakis, I.D.S.; Eleftheriou, E.P.; Rost, T.L. Effects of sodium tungstate on the ultrastructure and growth of pea (Pisum sativum) and cotton (Gossypium hirsutum) seedlings. Environ. Exp. Bot. 2008, 63, 416–425. [Google Scholar] [CrossRef]
- O’Brien, T.; Feder, N.M.E.M.; McCully, M.E. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Meidani, C.; Ntalli, N.G.; Giannoutsou, E.; Adamakis, I.-D.S. Cell wall modifications in giant cells induced by the plant parasitic nematode Meloidogyne incognita in wild-type (Col-0) and the fra2 Arabidopsis thaliana katanin mutant. Int. J. Mol. Sci. 2019, 20, 5465. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, M.D.; Magelhaes, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Buysse, J.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 4, 1627–1629. [Google Scholar] [CrossRef]
- McCready, R.M.; Guggolz, J.; Silviera, V.; Owens, H.S. Determination of starch and amylose in vegetables. Anal. Chem. 1950, 22, 1156–1158. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Katalinic, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic profile, antioxidant capacity, and antimicrobial activity of leaf extracts from six Vitis vinifera L. varieties. Int. J. Food Prop. 2013, 16, 45–60. [Google Scholar] [CrossRef]
- Šuković, D.; Knežević, B.; Gašić, U.; Sredojević, M.; Ćirić, I.; Todić, S.; Mutić, J.; Tešić, Ž. Phenolic profiles of leaves, grapes and wine of grapevine variety vranac (Vitis vinifera L.) from Montenegro. Foods 2020, 9, 138. [Google Scholar] [CrossRef]
- Kedrina-Okutan, O.; Novello, V.; Hoffmann, T.; Hadersdorfer, J.; Occhipinti, A.; Schwab, W.; Ferrandino, A. Constitutive polyphenols in blades and veins of grapevine (Vitis vinifera L.) healthy leaves. J. Agric. Food Chem. 2018, 66, 10977–10990. [Google Scholar] [CrossRef]
- Valladares, F.; Chico, J.M.; Aranda, I.; Balaguer, L.; Dizengremel, P.; Manrique, E.; Dreyer, E. The greater seedling high-light tolerance of Quercus robur over Fagus sylvatica is linked to a greater physiological plasticity. Trees 2002, 16, 395–403. [Google Scholar] [CrossRef]
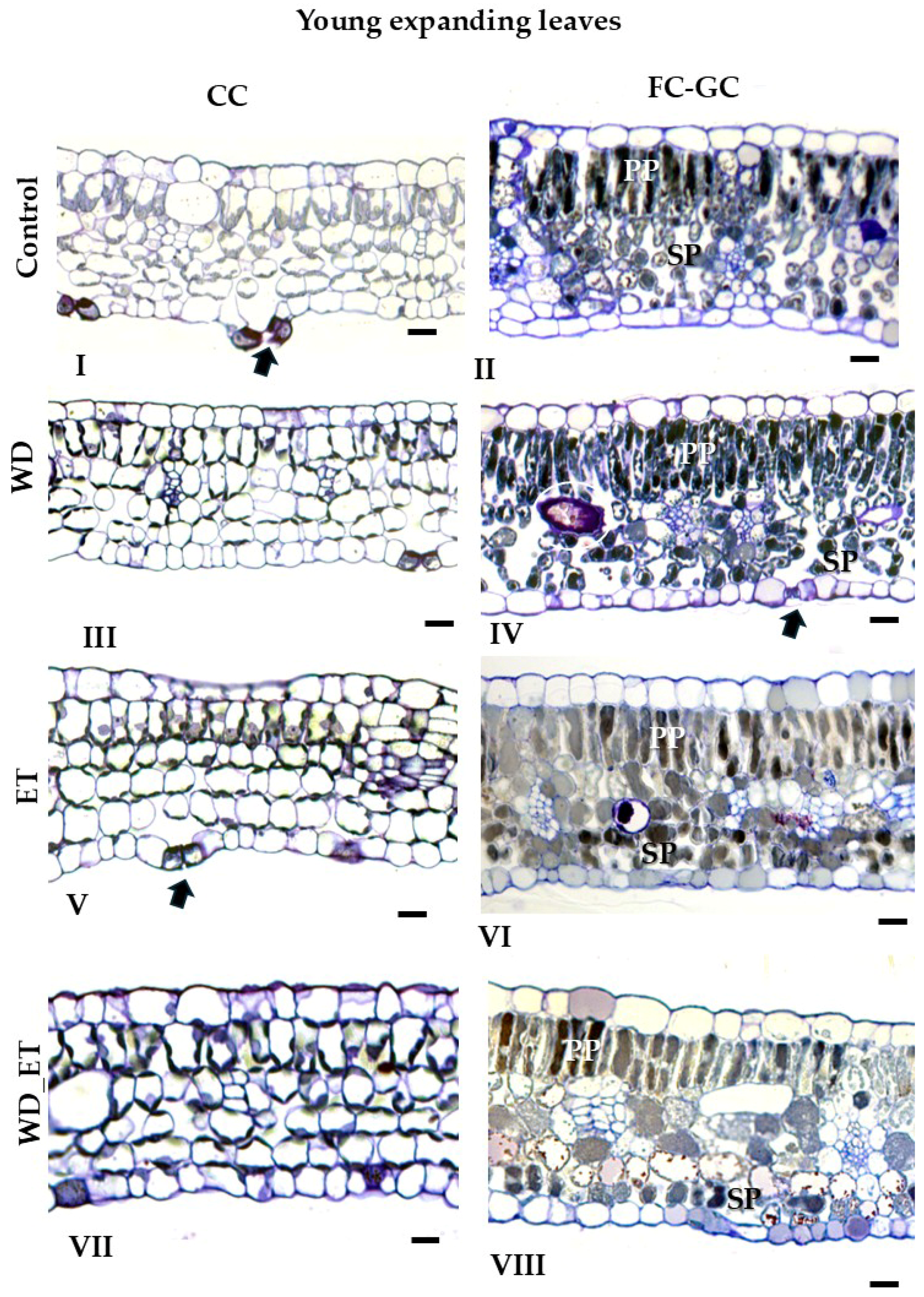
| Young Expanding Leaves | ||||
|---|---|---|---|---|
| Controlled Conditions (CCs) | ||||
| Area | Thickness | Thickness | Thickness | |
| Treatment | Intercellular (μm2) | Palisade Parenchyma (μm) | Spongy Parenchyma (μm) | Leaf (μm) |
| Control | 298.02 a ± 171.11 | 18.67 a ± 0.73 | 40.50 a ± 4.95 | 76.92 a ± 4.52 |
| WD | 173.05 b ± 59.86 | 17.84 a ± 2.35 | 39.25 a ± 1.93 | 77.54 a ± 2.30 |
| ET | 54.93 c ± 26.15 | 16.37 bc ± 1.40 | 35.37 b ± 1.66 | 74.60 a ± 3.15 |
| WD_ET | 83.94 bc ± 23.23 | 16.37 bc ± 1.22 | 34.88 b ± 2.59 | 72.59 a ± 1.88 |
| Field Conditions (FCs)–Greenhouse Conditions (GCs) | ||||
| Control | 169.11 a ± 30.54 | 20.09 a ± 3.53 | 40.28 a ± 6.49 | 76.09 a ± 8.02 |
| WD | 172.90 a ± 83.33 | 20.02 a ± 2.62 | 37.33 a ± 4.74 | 72.38 a ± 8.31 |
| ET | 57.04 c ± 22.94 | 21.79 a ± 0.88 | 35.54 a ± 1.96 | 70.80 a ± 2.39 |
| WD_ET | 72.55 b ± 23.56 | 20.93 a ± 2.96 | 37.52 a ± 6.78 | 75.78 a ± 9.71 |
| Fully Expanded Leaves | ||||
|---|---|---|---|---|
| Controlled Conditions (CCs) | ||||
| Area | Thickness | Thickness | Thickness | |
| Treatment | Intercellular (μm2) | Palisade Parenchyma (μm) | Spongy Parenchyma (μm) | Leaf (μm) |
| Control | 256.67 cb ± 101.12 | 18.84 a ± 1.65 | 42.17 ab ± 5.65 | 80.97 bc ± 5.76 |
| WD | 416.81 ac ± 82.44 | 18.27 a ± 1.31 | 47.88 b ± 6.80 | 87.84 b ± 6.53 |
| ET | 443.74 a ± 72.60 | 17.21 a ± 2.50 | 56.55 a ± 4.90 | 96.48 a ± 6.54 |
| WD_ET | 183.53 bcd ± 66.12 | 14.10 bc ± 4.34 | 35.35 c ± 9.80 | 70.69 cd ± 4.58 |
| Field Conditions (FCs)–Greenhouse Conditions (GCs) | ||||
| Control | 258.71 a ± 93.62 | 29.61 a ± 2.07 | 39.47 c ± 2.65 | 86.92 bc ± 2.20 |
| WD | 283.40 a ± 92.83 | 30.40 a ± 2.58 | 47.62 b ± 5.94 | 92.44 b ± 10.05 |
| ET | 201.76 ab ± 90.89 | 26.62 a ± 3.82 | 62.81 a ± 2.50 | 101.92 a ± 4.17 |
| WD_ET | 126.24 b ± 56.99 | 22.80 a ± 2.58 | 39.14 c ± 2.90 | 79.22 bc ± 3.72 |
| Young Expanding Leaves Under Controlled Conditions (CCs) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Area Intercellular (μm2) | Thickness Palisade Parenchyma (μm) | Thickness Spongy Parenchyma (μm) | Thickness Leaf (μm) | Phenols (mg g−1) | Sugar (mg g−1) | Starch (mg g−1) | SLA (cm2 g−1) | |
| C | 0.78 | 0.11 | 0.31 | 0.17 | 0.44 | 0.81 | 0.74 | 0.22 |
| WD | 0.75 | 0.34 | 0.15 | 0.16 | 0.52 | 0.93 | 0.58 | 0.36 |
| ET | 0.76 | 0.24 | 0.15 | 0.14 | 0.53 | 0.74 | 0.65 | 0.54 |
| WD_ET | 0.69 | 0.24 | 0.21 | 0.14 | 0.48 | 0.71 | 0.64 | 0.38 |
| Young expanding leaves under Field Conditions (FC)-Greenhouse Conditions (GC) | ||||||||
| C | 0.95 | 0.38 | 0.37 | 0.26 | 0.59 | 0.37 | 0.66 | 0.38 |
| WD | 0.75 | 0.30 | 0.31 | 0.27 | 0.68 | 0.31 | 0.24 | 0.76 |
| ET | 0.79 | 0.21 | 0.16 | 0.20 | 0.69 | 0.33 | 0.49 | 0.77 |
| WD_ET | 0.89 | 0.28 | 0.44 | 0.32 | 0.56 | 0.53 | 0.53 | 0.81 |
| Fully expanded leaves under Controlled Conditions (CC) | ||||||||
| C | 0.70 | 0.24 | 0.33 | 0.19 | 0.22 | 0.67 | 0.71 | 0.34 |
| WD | 0.76 | 0.21 | 0.37 | 0.20 | 0.20 | 0.62 | 0.64 | 0.21 |
| ET | 0.83 | 0.39 | 0.24 | 0.22 | 0.33 | 0.63 | 0.78 | 0.61 |
| WD_ET | 0.72 | 0.60 | 0.62 | 0.64 | 0.24 | 0.72 | 0.68 | 0.51 |
| Fully expanded leaves under Field Conditions (FCs)–Greenhouse Conditions (GCs) | ||||||||
| C | 0.72 | 0.22 | 0.23 | 0.07 | 0.46 | 0.21 | 0.53 | 0.57 |
| WD | 0.85 | 0.28 | 0.28 | 0.23 | 0.25 | 0.25 | 0.66 | 0.73 |
| ET | 0.80 | 0.35 | 0.27 | 0.21 | 0.25 | 0.26 | 0.61 | 0.71 |
| WD_ET | 0.73 | 0.32 | 0.21 | 0.16 | 0.32 | 0.14 | 0.71 | 0.36 |
| Variable | Intercept Coef | Intercept p-Value | Leaf DS Coef | Leaf DS S.E. | Leaf DS p-Value | Group Variance (Treatment) |
|---|---|---|---|---|---|---|
| Phenols | 0.394 | 0.001 | 0.011 | 0.034 | 0.742 | 0.000 |
| Sugars | 74.714 | 0.001 | −9.827 | 7.624 | 0.198 | 0.062 |
| Starch | 62.175 | 0.001 | −11.175 | 1.927 | 0.001 | 62.532 |
| Intercellular Space | 271.190 | 0.001 | −139.589 | 18.334 | 0.001 | 3416.514 |
| Palisade Parenchyma | 22.213 | 0.001 | −3.223 | 0.672 | 0.001 | 1.786 |
| Spongy Parenchyma | 43.022 | 0.001 | −5.438 | 0.942 | 0.001 | 5.984 |
| Leaf Thickness | 83.814 | 0.001 | −9.226 | 1.323 | 0.001 | 9.507 |
| SLA | 101.351 | 0.001 | 15.284 | 12.028 | 0.204 | 596.286 |
| Dependent Variable | Treatment WD Leaf DS (p-Value) | Treatment ET Leaf DS (p-Value) | Treatment WD_ET Leaf DS (p-Value) |
|---|---|---|---|
| Leaf Thickness | 0.031 | 0.090 | 0.063 |
| Palisade Parenchyma | 0.668 | 0.097 | 0.001 |
| Spongy Parenchyma | 0.001 | 0.001 | 0.803 |
| Intercellular Space | 0.024 | 0.001 | 0.874 |
| SLA | 0.003 | 0.076 | 0.001 |
| Phenols | 0.113 | 0.082 | 0.024 |
| Sugars | 0.055 | 0.880 | 0.225 |
| Starch | 0.532 | 0.255 | 0.569 |
| Leaf Water Potential (Ψleaf, MPa) | ||
|---|---|---|
| Treatments | GC | FC |
| C (control) | −1.25 b ± 0.007 | −1.24 b ± 0.010 |
| WD (water deficit) | −1.58 c ± 0.003 | −1.60 d ± 0.005 |
| ET (elevated temperature) | −0.95 a ± 0.001 | −0.97 a ± 0.011 |
| WD_ET (water deficit and elevated temperature) | −1.53 c ± 0.009 | −1.49 c ± 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolyva, F.; Adamakis, I.-D.S.; Gkikas, D.; Meletiou-Christou, M.-S.; Rhizopoulou, S. Effects of Elevated Temperature and Water Deficiency on Functional Traits of Vitis vinifera L. cv. Assyrtiko Leaves. Plants 2025, 14, 2463. https://doi.org/10.3390/plants14162463
Kolyva F, Adamakis I-DS, Gkikas D, Meletiou-Christou M-S, Rhizopoulou S. Effects of Elevated Temperature and Water Deficiency on Functional Traits of Vitis vinifera L. cv. Assyrtiko Leaves. Plants. 2025; 14(16):2463. https://doi.org/10.3390/plants14162463
Chicago/Turabian StyleKolyva, Foteini, Ioannis-Dimosthenis S. Adamakis, Dimitrios Gkikas, Maria-Sonia Meletiou-Christou, and Sophia Rhizopoulou. 2025. "Effects of Elevated Temperature and Water Deficiency on Functional Traits of Vitis vinifera L. cv. Assyrtiko Leaves" Plants 14, no. 16: 2463. https://doi.org/10.3390/plants14162463
APA StyleKolyva, F., Adamakis, I.-D. S., Gkikas, D., Meletiou-Christou, M.-S., & Rhizopoulou, S. (2025). Effects of Elevated Temperature and Water Deficiency on Functional Traits of Vitis vinifera L. cv. Assyrtiko Leaves. Plants, 14(16), 2463. https://doi.org/10.3390/plants14162463


.png)




