Influence of Various Fruit Preservation Methods on the Phenolic Composition and Antioxidant Activity of Prunus spinosa L. Fruit Extract
Abstract
1. Introduction
2. Results and Discussion
2.1. Morphological Description of Blackthorns
2.2. Determination of Water Content of Blackthorns
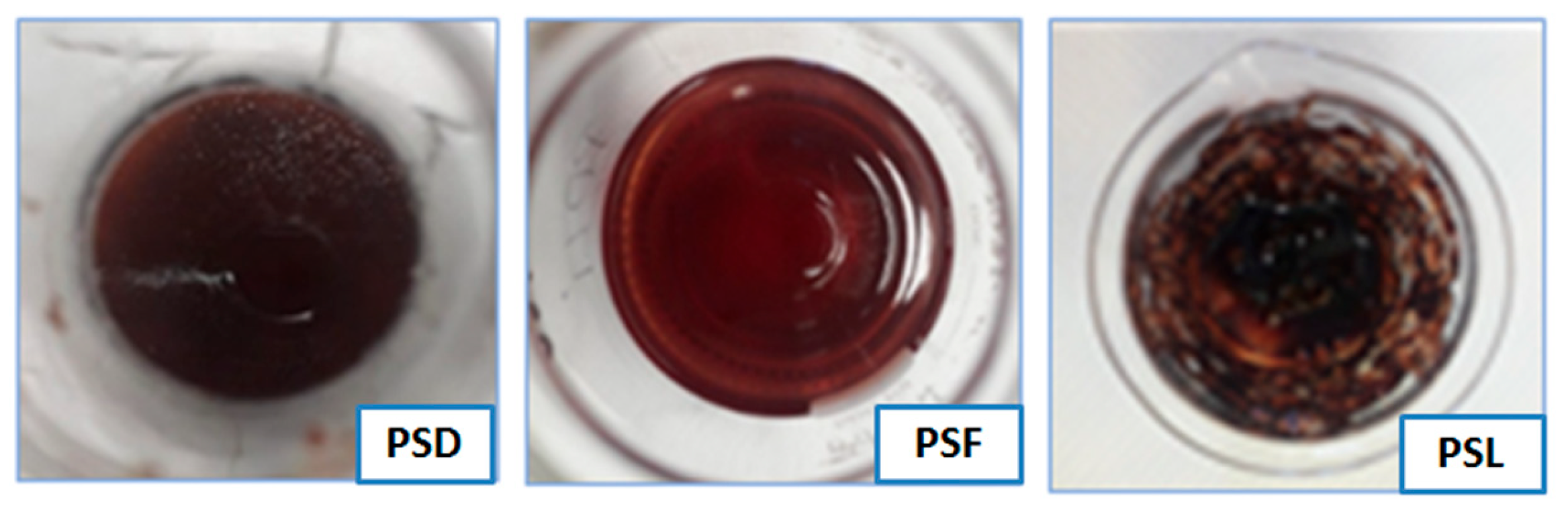
2.3. Extraction by Bio-Solvents of Air-Dried (PSD), Frozen (PSF), and Lyophilized (PSL) Blackthorns
2.4. Analysis of Blackthorn Extracts
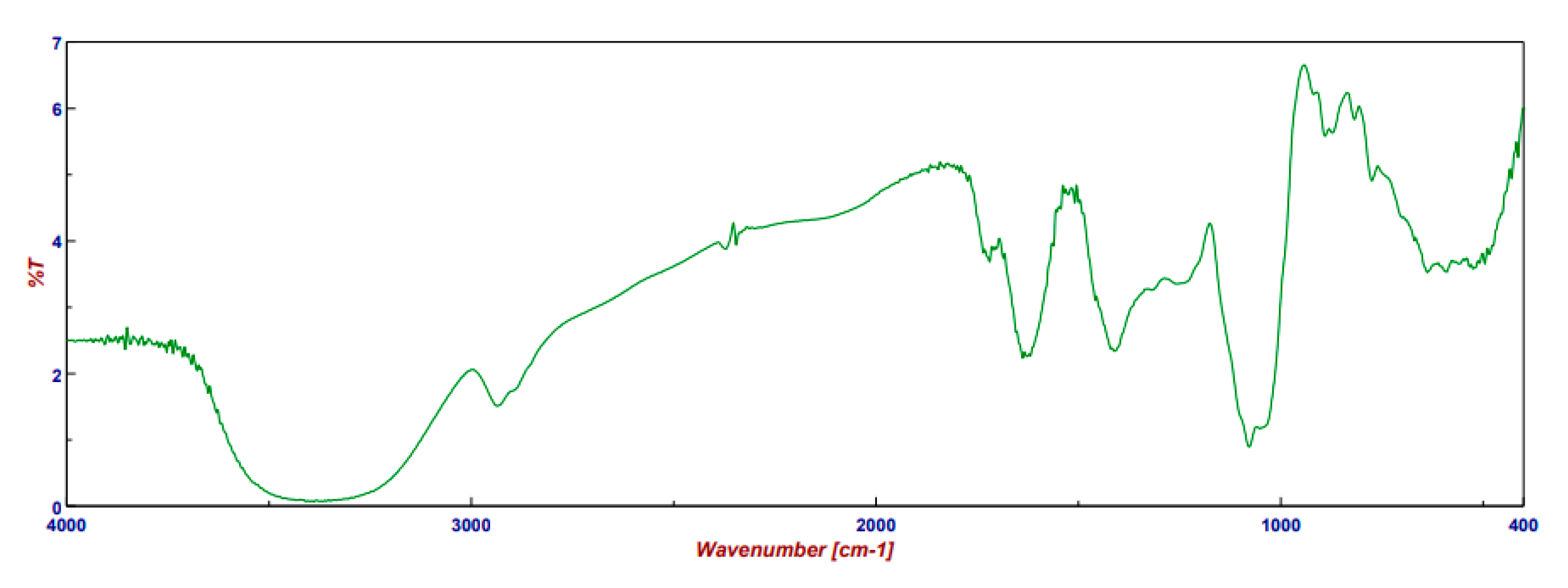
2.5. FT-IR of PSF Extract
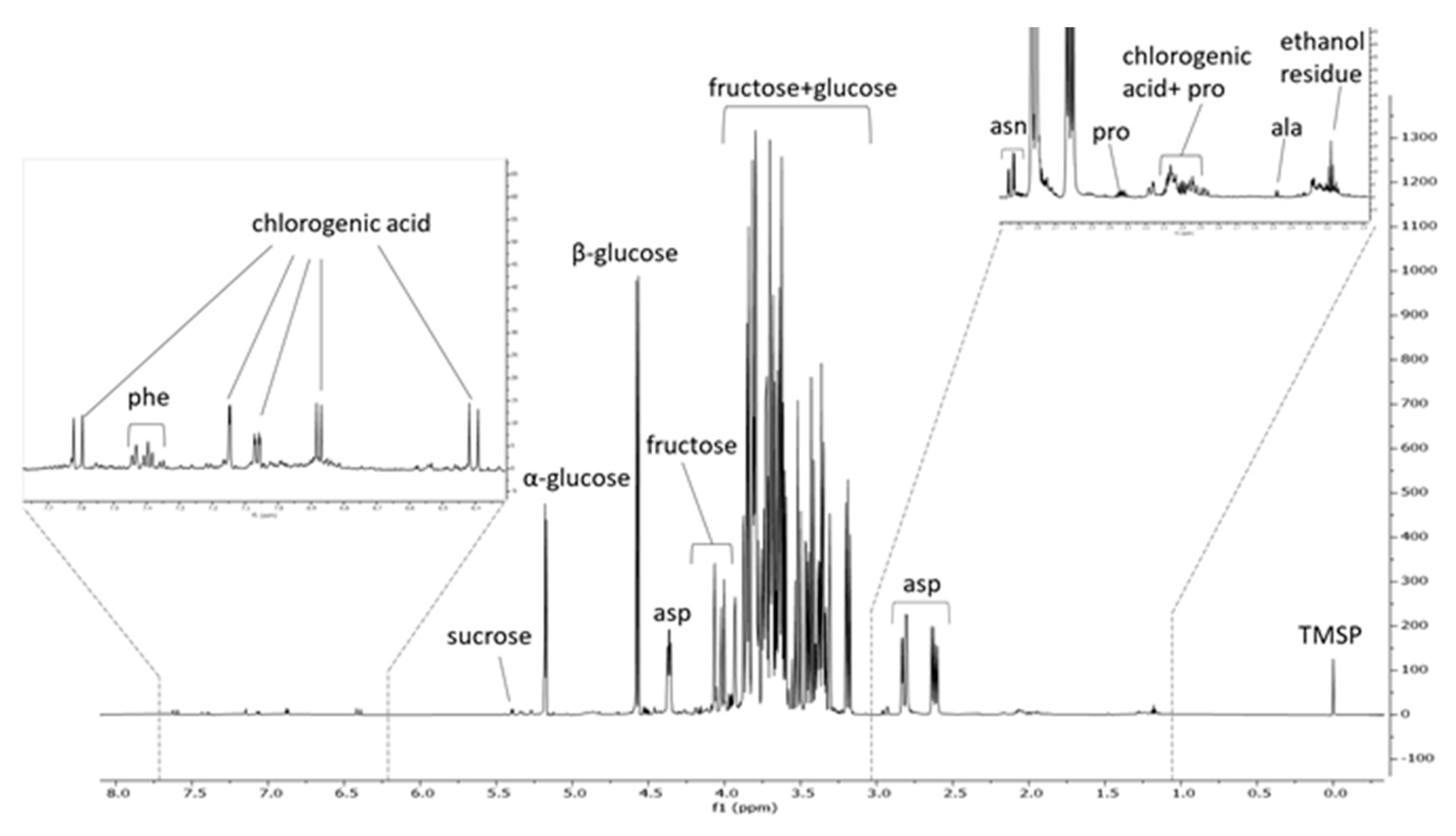
2.6. 1H-NMR Analysis of PSF Extract
2.7. HPLC-DAD-MS/MS Analysis of PSF Extract
2.8. Determination of Antioxidant Activity (AA%)
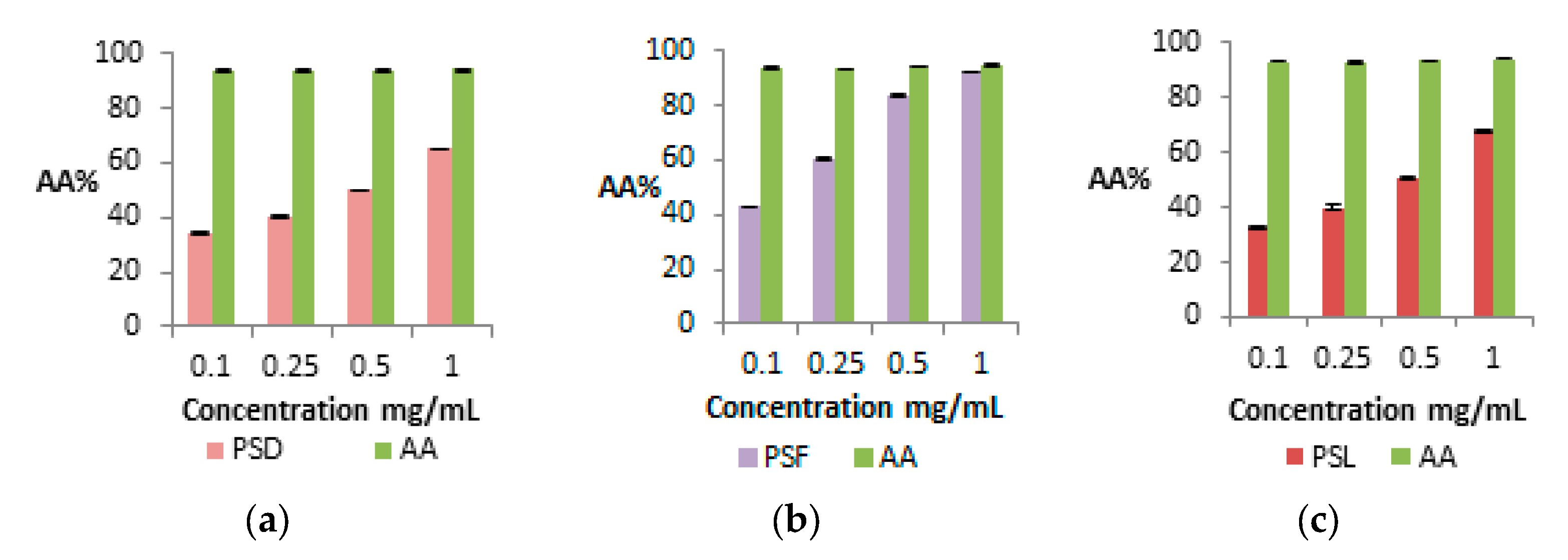
2.8.1. Determination of AA% by DPPH
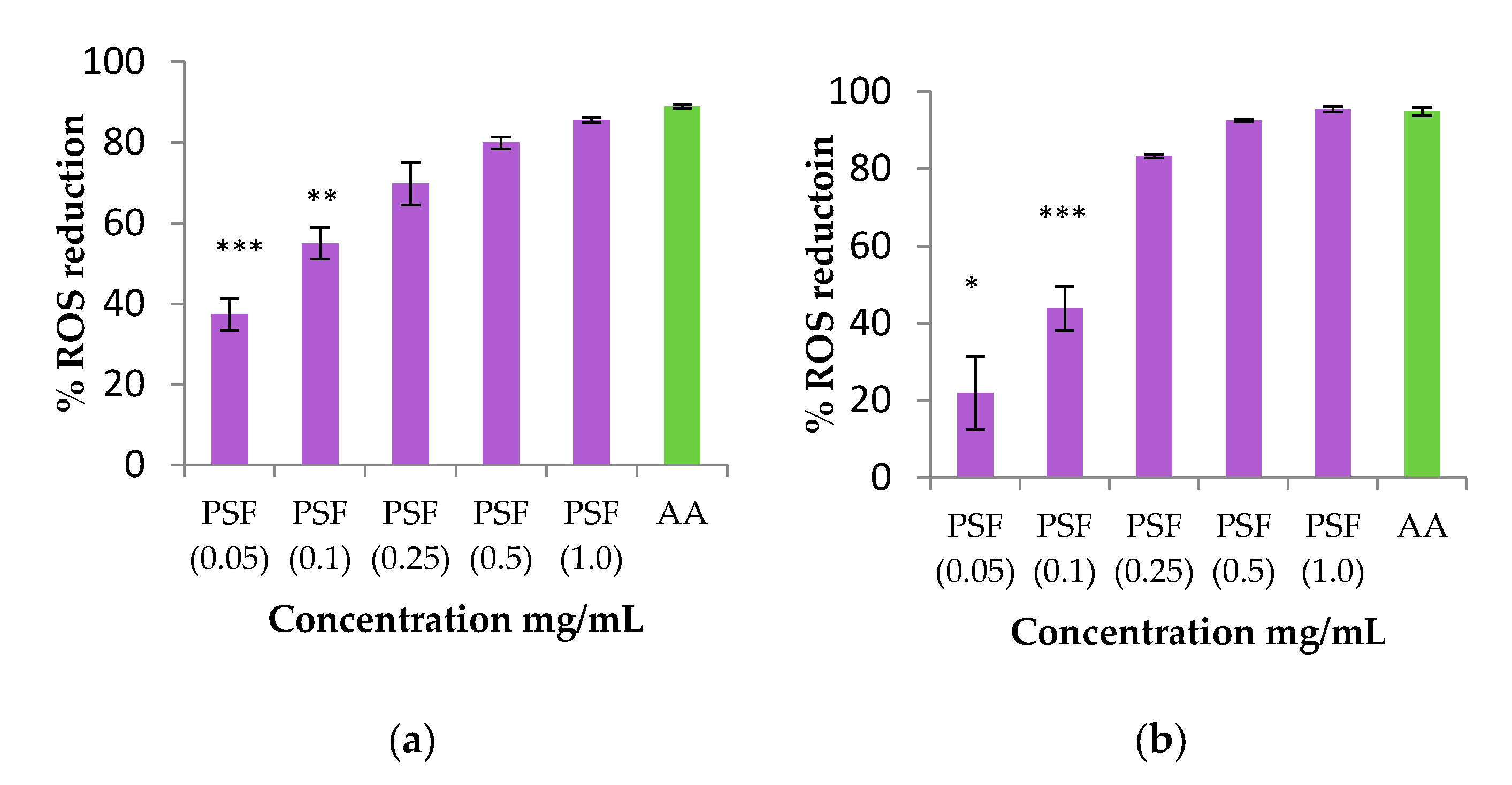
2.8.2. Antioxidant Activity by ROS Determination of PSF Extract
3. Materials and Methods
3.1. Chemicals
3.2. Plant Materials
3.3. Morphological Description
3.4. Determination of Water Content
3.5. Fruit Preservation Methods
3.6. Extraction of Blackthorns by Bio-Solvents
3.7. Analysis of Extracts
3.7.1. Determination of the pH
3.7.2. Determination of Total Phenolic Content (TPC)
3.7.3. Determination of Total Flavonoid Content (TFC)
3.8. FT-IR of PSF Extract
3.9. 1H-NMR of PSF Extract
3.10. HPLC-DAD-MS/MS of PSF Extract
3.11. Antioxidant Activity Determined by DPPH
3.12. Antioxidant Activity by Reduction Oxygen Species (ROS) Production Measurement
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talucder, M.S.A.; Ruba, U.B.; Robi, M.A.S. Potentiality of Neglected and Underutilized Species (NUS) as a Future Resilient Food: A Systematic Review. J. Agric. Food Res. 2024, 16, 101116. [Google Scholar] [CrossRef]
- Pozzo, L.; Russo, R.; Frassinetti, S.; Vizzarri, F.; Árvay, J.; Vornoli, A.; Casamassima, D.; Palazzo, M.; Della Croce, C.M.; Longo, V. Wild Italian Prunus spinosa L. Fruit Exerts In Vitro Antimicrobial Activity and Protects Against In Vitro and In Vivo Oxidative Stress. Foods 2019, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Cosmulescu, S.N.; Gavrila Calusaru, F. Morphologic Characteristics Variability in Prunus spinosa L. Shrubs Identified in Southern Area of Oltenia, Romania. Not. Sci. Biol. 2018, 10, 447–451. [Google Scholar] [CrossRef]
- Bei, M.F.; Apahidean, A.I.; Budău, R.; Rosan, C.A.; Popovici, R.; Memete, A.R.; Domocoș, D.; Vicas, S.I. An Overview of the Phytochemical Composition of Different Organs of Prunus spinosa L., Their Health Benefits and Application in Food Industry. Horticulturae 2023, 10, 29. [Google Scholar] [CrossRef]
- Chiocchio, I.; Marincich, L.; Mandrone, M.; Trincia, S.; Tarozzi, C.; Poli, F. Saving the Local Tradition: Ethnobotanical Survey on the Use of Plants in Bologna District (Italy). J. Ethnobiol. Ethnomed. 2024, 20, 33. [Google Scholar] [CrossRef]
- Magiera, A.; Czerwińska, M.E.; Owczarek, A.; Marchelak, A.; Granica, S.; Olszewska, M.A. Polyphenols and Maillard Reaction Products in Dried Prunus Spinosa Fruits: Quality Aspects and Contribution to Anti-Inflammatory and Antioxidant Activity in Human Immune Cells Ex Vivo. Molecules 2022, 27, 3302. [Google Scholar] [CrossRef]
- Negrean, O.-R.; Farcas, A.C.; Pop, O.L.; Socaci, S.A. Blackthorn—A Valuable Source of Phenolic Antioxidants with Potential Health Benefits. Molecules 2023, 28, 3456. [Google Scholar] [CrossRef]
- Gunes, R. A Study on Quality Properties of Blackthorn (Prunus spinosa L.) Fruit Powder Obtained by Different Drying Treatments. BIO Web Conf. 2024, 85, 01011. [Google Scholar] [CrossRef]
- Backes, E.; Leichtweis, M.G.; Pereira, C.; Carocho, M.; Barreira, J.C.M.; Kamal Genena, A.; José Baraldi, I.; Filomena Barreiro, M.; Barros, L.; Ferreira, I.C.F.R. Ficus carica L. and Prunus spinosa L. Extracts as New Anthocyanin-Based Food Colorants: A Thorough Study in Confectionery Products. Food Chem. 2020, 333, 127457. [Google Scholar] [CrossRef]
- Leichtweis, M.G.; Pereira, C.; Prieto, M.A.; Barreiro, M.F.; Baraldi, I.J.; Barros, L.; Ferreira, I.C.F.R. Ultrasound as a Rapid and Low-Cost Extraction Procedure to Obtain Anthocyanin-Based Colorants from Prunus spinosa L. Fruit Epicarp: Comparative Study with Conventional Heat-Based Extraction. Molecules 2019, 24, 573. [Google Scholar] [CrossRef]
- Capek, P.; Uhliariková, I. Antioxidant Active Polysaccharides Extracted with Oxalate from Wild Blackthorn Fruits (Prunus spinosa L.). Int. J. Mol. Sci. 2024, 25, 4519. [Google Scholar] [CrossRef]
- Nistor, O.V.; Milea, Ș.A.; Păcularu-Burada, B.; Andronoiu, D.G.; Râpeanu, G.; Stănciuc, N. Technologically Driven Approaches for the Integrative Use of Wild Blackthorn (Prunus spinosa L.) Fruits in Foods and Nutraceuticals. Antioxidants 2023, 12, 1637. [Google Scholar] [CrossRef] [PubMed]
- Karakas, N.; Okur, M.E.; Ozturk, I.; Ayla, S.; Karadağ, A.E.; Polat, D.Ç. Antioxidant Activity and Cytotoxic Effects of Prunus spinosa L. Fruit Extract on Various Cancer Cell Lines. Medeni. Med. J. 2019, 34, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Magiera, A.; Czerwińska, M.E.; Owczarek, A.; Marchelak, A.; Granica, S.; Olszewska, M.A. Polyphenol-Enriched Extracts of Prunus Spinosa Fruits: Anti-Inflammatory and Antioxidant Effects in Human Immune Cells Ex Vivo in Relation to Phytochemical Profile. Molecules 2022, 27, 1691. [Google Scholar] [CrossRef] [PubMed]
- Sikora, E.; Bieniek, M.I.; Borczak, B. Composition and antioxidant properties of fresh and frozen stored blackthorn fruits (Prunus spinosa L.). Acta Sci. Pol. Technol. Aliment. 2013, 12, 365–372. [Google Scholar]
- Gunes, R. In Vitro Gastrointestinal Digestion of Anthocyanins from Marshmallows Enriched with Blackthorn Fruit Powders Obtained by Convective Hot Air and Freeze Drying Treatments. Food Res. Int. 2025, 205, 116001. [Google Scholar] [CrossRef]
- Drăghici-Popa, A.-M.; Boscornea, A.C.; Brezoiu, A.-M.; Tomas, Ș.T.; Pârvulescu, O.C.; Stan, R. Effects of Extraction Process Factors on the Composition and Antioxidant Activity of Blackthorn (Prunus spinosa L.) Fruit Extracts. Antioxidants 2023, 12, 1897. [Google Scholar] [CrossRef]
- Kotsou, K.; Stoikou, M.; Athanasiadis, V.; Chatzimitakos, T.; Mantiniotou, M.; Sfougaris, A.I.; Lalas, S.I. Enhancing Antioxidant Properties of Prunus Spinosa Fruit Extracts via Extraction Optimization. Horticulturae 2023, 9, 942. [Google Scholar] [CrossRef]
- Oleińska, K.; Sugier, D.; Seczyk, L. The influence of selected preservation methods and storage time on the content of antioxidants. Agron. Sci. 2019, 74, 1. [Google Scholar] [CrossRef]
- Um, M.; Han, T.-H.; Lee, J.-W. Ultrasound-Assisted Extraction and Antioxidant Activity of Phenolic and Flavonoid Compounds and Ascorbic Acid from Rugosa Rose (Rosa rugosa Thunb.) Fruit. Food Sci. Biotechnol. 2017, 27, 375–382. [Google Scholar] [CrossRef]
- Pinacho, R.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D.; Calvo, M.I. Phenolic Compounds of Blackthorn (Prunus spinosa L.) and Influence of in Vitro Digestion on Their Antioxidant Capacity. J. Funct. Foods 2015, 19, 49–62. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Vicente-Zurdo, D.; Rosales-Conrado, N.; León-González, M.E. Unlocking the in Vitro Neuroprotection of Sloe Residues Phenolic Extracts by Bioanalytical and Chemometric Strategies. Food Chem. 2025, 463, 141208. [Google Scholar] [CrossRef]
- Bayram, Y. Optimizing the Extraction of Polyphenols from Prunus spinosa L. Fruit Using Response Surface Methodology and Production of Powders from Optimized Extracts by Foam Mat Drying. Food Meas. 2024, 18, 6673–6686. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Igual, M.; García-Herrera, P.; Cámara, R.M.; Martínez-Monzó, J.; García-Segovia, P.; Cámara, M. Bioactive Compounds in Rosehip (Rosa Canina) Powder with Encapsulating Agents. Molecules 2022, 27, 4737. [Google Scholar] [CrossRef]
- Kuru Berk, S.; Tas, A.; Orman, E.; Gundogdu, M.; Necas, T.; Ondrasek, I.; Karatas, N.; Ercisli, S. Agro-Morphological and Biochemical Characterization of Wild Prunus spinosa L. Subsp. Dasyphylla (Schur) Domin Genotypes Naturally Grown in Western Black Sea Region of Turkey. Agronomy 2020, 10, 1748. [Google Scholar] [CrossRef]
- Marčetić, M.; Samardžić, S.; Ilić, T.; Božić, D.D.; Vidović, B. Phenolic Composition, Antioxidant, Anti-Enzymatic, Antimicrobial and Prebiotic Properties of Prunus spinosa L. Fruits. Foods 2022, 11, 3289. [Google Scholar] [CrossRef]
- Andronie, L.; Holonec, L.; Pop, I.; Truta, A.M.; Odagiu, A.; Sălăgean, T.; Sobolu, R.; Coroian, A.; Balta, I.; Șuba, E.E. Antioxidant Capacity of Several Romanian Forest Fruits (Rosa canina L., Prunus spinosa L., Vaccium vitis-idaea L. and Cornus mas L.). Not. Bot. Horti Agrobo 2019, 47, 1178–1184. [Google Scholar] [CrossRef]
- Pancerz, M.; Ptaszek, A.; Sofińska, K.; Barbasz, J.; Szlachcic, P.; Kucharek, M.; Łukasiewicz, M. Colligative and Hydrodynamic Properties of Aqueous Solutions of Pectin from Cornelian Cherry and Commercial Apple Pectin. Food Hydrocoll. 2019, 89, 406–415. [Google Scholar] [CrossRef]
- Samborska, K.; Kamińska, P.; Jedlińska, A.; Matwijczuk, A.; Kamińska-Dwórznicka, A. Membrane Processing in the Sustainable Production of Low-Sugar Apple-Cranberry Cloudy Juice. Appl. Sci. 2018, 8, 1082. [Google Scholar] [CrossRef]
- Hager, T.J.; Howard, L.R.; Prior, R.L. Processing and Storage Effects on Monomeric Anthocyanins, Percent Polymeric Color, and Antioxidant Capacity of Processed Blackberry Products. J. Agric. Food Chem. 2008, 56, 689–695. [Google Scholar] [CrossRef]
- Remedio, L.N.; Parada Quinayá, C. Intelligent Packaging Systems with Anthocyanin: Influence of Different Polymers and Storage Conditions. Polymers 2024, 16, 2886. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Oxidants and Antioxidants Part A; Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Sallustio, V.; Chiocchio, I.; Mandrone, M.; Cirrincione, M.; Protti, M.; Farruggia, G.; Abruzzo, A.; Luppi, B.; Bigucci, F.; Mercolini, L.; et al. Extraction, Encapsulation into Lipid Vesicular Systems, and Biological Activity of Rosa canina L. Bioactive Compounds for Dermocosmetic Use. Molecules 2022, 27, 3025. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]


| Sample | Length (mm) | Width (mm) | Shape Index | Weight (g) |
|---|---|---|---|---|
| Blackthorn | 12.11 ± 0.88 | 11.98 ± 0.91 | 1.07 ± 0.03 | 1.30 ± 0.25 |
| Sample | PSD | PSF | PSL |
|---|---|---|---|
| Yield (g extract/g raw material) | 0.52 ± 0.08 | 0.51 ± 0.28 | 0.55 ± 0.14 |
| Type of Extract | TPC (mg GAE/gRM) * | TPC (mg GAE/gRM) * 24 Months | TFC (mg QE/gRM) ** | TFC (mg QE/gRM) ** 24 Months |
|---|---|---|---|---|
| PSD | 7.97 ± 0.04 A | 3.71 ± 0.03 A | 2.42 ± 0.16 A | 1.27 ± 0.08 A |
| PSF | 13.99 ± 0.04 B | 9.90 ± 0.19 B | 3.14 ± 0.15 B | 1.61 ± 0.07 B |
| PSL | 7.39 ± 0.08 C | 4.02 ± 0.06 C | 2.32 ± 0.03 A | 1.29 ± 0.02 A |
| Metabolite | δ | Multiplicity | μg/mg PSF Extract | Percentage |
|---|---|---|---|---|
| Alanine | 1.49 | d | 0.2 | 0.02 |
| Asparagine | 2.94 | dd | 3.8 | 0.4 |
| Aspartic acid | 2.64 | dd | 152.1 | 15.2 |
| Chlorogenic acid | 6.37 | d | 9.9 | 1 |
| Fructose | 3.98 | m | 150.2 | 15 |
| Phenylalanine | 7.37 | m | 1.3 | 0.1 |
| Proline | 2.35 | m | 2.6 | 0.3 |
| Sucrose | 5.4 | d | 10.8 | 1.1 |
| α glucose | 5.2 | d | 172.7 | 17.3 |
| β glucose | 4.6 | d | 288.3 | 28.8 |
| Phenolic Acid | Flavanol | Flavonol | Anthocyanin | Vitamin | |
|---|---|---|---|---|---|
| Gallic Acid (mg/g RM) | Catechin (mg/g RM) | Quercetin (mg/g RM) | Cyanidin (mg/g RM) | Ascorbic Acid (mg/g RM) | |
| PSF | 0.18 ± 0.07 | 5.96 ± 0.74 | 1.46 ± 0.22 | 1.77 ± 0.32 | 1.08 ± 0.34 |
| Sample | PSD | PSF | PSL |
|---|---|---|---|
| AA% | 65.02 ± 0.22 A | 91.78 ± 0.80 A | 68.39 ± 0.35 A |
| AA% 24 months | 37.79 ± 1.80 B | 81.57 ± 1.14 B | 29.79 ± 0.46 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sallustio, V.; Marto, J.; Gonçalves, L.M.; Mandrone, M.; Chiocchio, I.; Protti, M.; Mercolini, L.; Luppi, B.; Bigucci, F.; Abruzzo, A.; et al. Influence of Various Fruit Preservation Methods on the Phenolic Composition and Antioxidant Activity of Prunus spinosa L. Fruit Extract. Plants 2025, 14, 2454. https://doi.org/10.3390/plants14152454
Sallustio V, Marto J, Gonçalves LM, Mandrone M, Chiocchio I, Protti M, Mercolini L, Luppi B, Bigucci F, Abruzzo A, et al. Influence of Various Fruit Preservation Methods on the Phenolic Composition and Antioxidant Activity of Prunus spinosa L. Fruit Extract. Plants. 2025; 14(15):2454. https://doi.org/10.3390/plants14152454
Chicago/Turabian StyleSallustio, Valentina, Joana Marto, Lidia Maria Gonçalves, Manuela Mandrone, Ilaria Chiocchio, Michele Protti, Laura Mercolini, Barbara Luppi, Federica Bigucci, Angela Abruzzo, and et al. 2025. "Influence of Various Fruit Preservation Methods on the Phenolic Composition and Antioxidant Activity of Prunus spinosa L. Fruit Extract" Plants 14, no. 15: 2454. https://doi.org/10.3390/plants14152454
APA StyleSallustio, V., Marto, J., Gonçalves, L. M., Mandrone, M., Chiocchio, I., Protti, M., Mercolini, L., Luppi, B., Bigucci, F., Abruzzo, A., & Cerchiara, T. (2025). Influence of Various Fruit Preservation Methods on the Phenolic Composition and Antioxidant Activity of Prunus spinosa L. Fruit Extract. Plants, 14(15), 2454. https://doi.org/10.3390/plants14152454













