Drought–Rewatering Cycles: Impact on Non-Structural Carbohydrates and C:N:P Stoichiometry in Pinus yunnanensis Seedlings
Abstract
1. Introduction
2. Results and Analysis
2.1. Effects of Repeated Drought–Rewatering on NSC Content and Carbon, Nitrogen, and Phosphorus Stoichiometry of Pinus yunnanensis Seedlings
2.2. Effects of Repeated Drought–Rewatering on NSC and Its Components in Pinus yunnanensis Seedlings
2.3. Effects of Repeated Drought–Rewatering on C, N, and P Contents and Their Stoichiometric Ratios in Pinus yunnanensis Seedlings
2.4. Phenotypic Plasticity Index Analysis of Various Indexes in Repeated Drought–Rewatering Pinus yunnanensis Seedlings
2.5. Correlation Analysis of Various Indexes of Pinus yunnanensis Seedlings After Repeated Drought–Rewatering
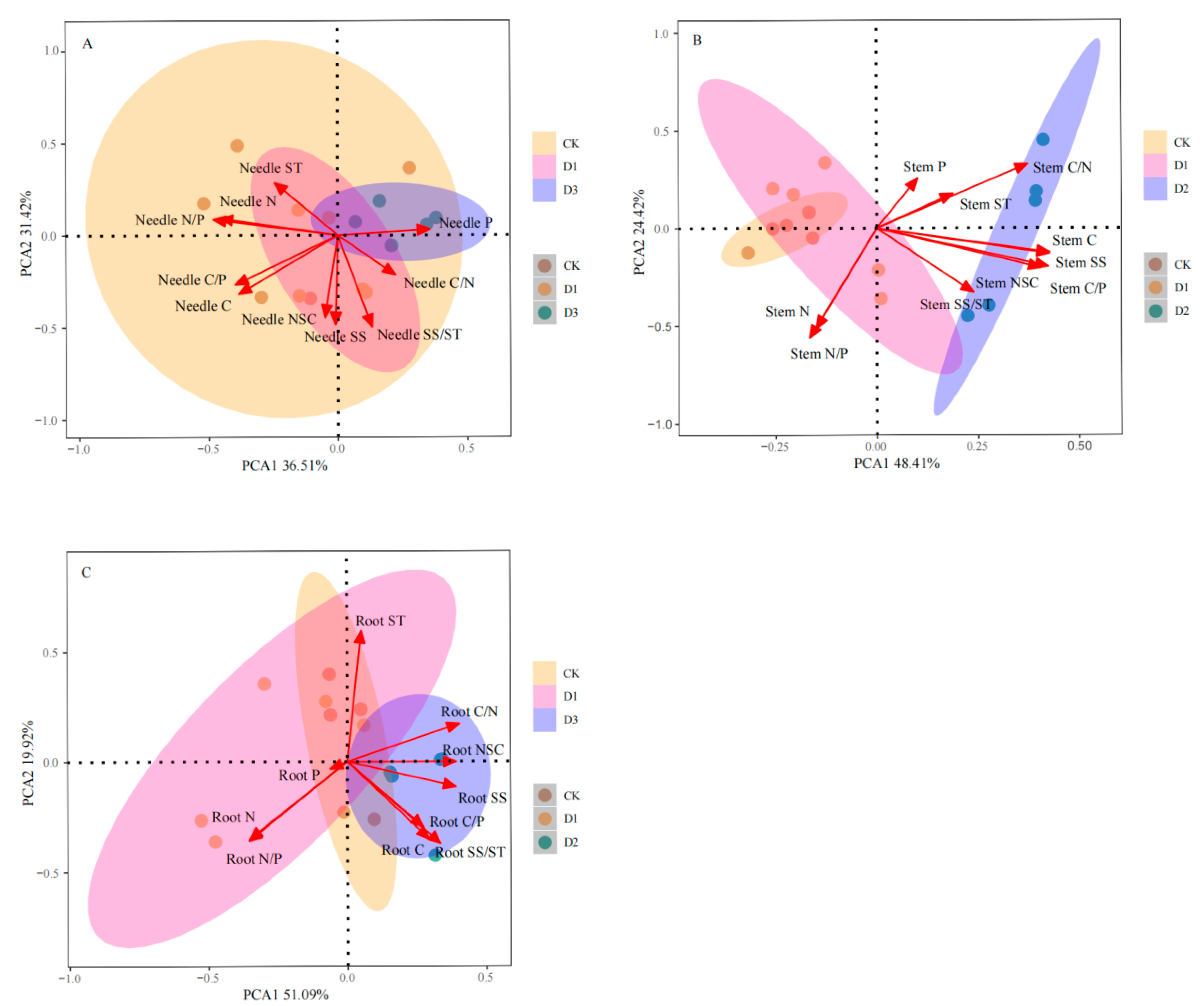
2.6. Principal Component Analysis of Various Indexes of Pinus yunnanensis Seedlings After Repeated Drought–Rewatering
3. Discussion
3.1. Effects of Repeated Drought–Rewatering on NSC of Pinus yunnanensis Seedlings
3.2. Effects of Repeated Drought–Rewatering on C, N, and P Stoichiometric Characteristics of Pinus yunnanensis Seedlings
3.3. Main Strategies for Pinus yunnanensis Seedlings to Adapt to Repeated Drought–Rewatering
4. Material and Methods
4.1. Summary of the Study Area
4.2. Experimental Materials
4.3. Experimental Design
4.4. Measurement of Indicators
4.5. Data Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahmede, C.; Iacovacci, J.; Arenas, A.; Bianconi, G. New perspective on spring vegetation phenology and global climate change based on Tibetan Plateau tree-ring data. Proc. Natl. Acad. Sci. USA 2017, 114, 6966–6971. [Google Scholar]
- Zhang, Z.; Cao, B.; Gao, S.; Xu, K. Grafting improves tomato drought tolerance through enhancing photosynthetic capacity and reducing ROS accumulation. Protoplasma 2019, 256, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Forzieri, G.; Feyen, L.; Russo, S.; Vousdoukas, M.; Alfieri, L.; Outten, S.; Migliavacca, M.; Bianchi, A.; Rojas, R.; Cid, A. Multi-hazard assessment in Europe under climate change. Clim. Change 2016, 137, 105–119. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Quiring, S.M.; Peña-Gallardo, M.; Yuan, S.; Domínguez-Castro, F. A review of environmental droughts: Increased risk under global warming? Earth-Sci. Rev. 2020, 201, 102953. [Google Scholar] [CrossRef]
- Rubin, R.L.; van Groenigen, K.J.; Hungate, B.A. Plant growth promoting rhizobacteria are more effective under drought: A meta-analysis. Plant Soil 2017, 416, 309–323. [Google Scholar] [CrossRef]
- Adams, H.D.; Zeppel, M.J.; Anderegg, W.R.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Guan, C.; Zhang, J.; He, C.; Yin, C.; Meng, P. Drought effects on tree growth, water use efficiency, vulnerability and canopy health of Quercus variabilis-Robinia pseudoacacia mixed plantation. Front. Plant Sci. 2022, 13, 1018405. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, C.; Gao, L.; Ying, L. Coordination between non-structure carbohydrates and hydraulic function under different drought duration, intensity, and post-drought recovery. Environ. Exp. Bot. 2023, 215, 105485. [Google Scholar] [CrossRef]
- Ruehr, N.K.; Grote, R.; Mayr, S.; Arneth, A. Beyond the extreme: Recovery of carbon and water relations in woody plants following heat and drought stress. Tree Physiol. 2019, 39, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Skelton, R.P.; Brodribb, T.J.; McAdam, S.A.M.; Mitchell, P.J. Gas exchange recovery following natural drought is rapid unless limited by loss of leaf hydraulic conductance: Evidence from an evergreen woodland. New Phytol. 2017, 215, 1399–1412. [Google Scholar] [CrossRef] [PubMed]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef]
- Kozlowski, T. Carbohydrate sources and sinks in woody plants. Bot. Rev. 1992, 58, 107–222. [Google Scholar] [CrossRef]
- Koch, K. Carbohydrate-modulated gene expression in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 509–540. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S.I.; Schulze, E.; Mooney, H.A. The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- Jacquet, J.S.; Bosc, A.; O’Grady, A.; Jacte, H. Combined effects of defoliation and water stress on pine growth and non-structural carbohydrates. Adv. Eng. Softw. 2014, 34, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Quentin, A.G.; Pinkard, E.A.; Ryan, M.G. Non-structural carbohydrates in woody plants compared among laboratories. Tree Physiol. 2015, 35, 1146–1165. [Google Scholar] [CrossRef]
- Wang, K.; Lu, S.; Liu, C.; Kang, H.Z.; Lyu, L.Y.; Jiao, X.L. Effects of soil water content on non—Structural carbohydrates and growth of Pinus sylvestris var. Mongolica seedlings. Chin. J. Ecol. 2023, 42, 617–625. [Google Scholar]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The elemental stoichiometry of aquatic and terrestrial ecosystems and its relationships with organismic lifestyle and ecosystem structure and function: A review and perspectives. Biogeochem 2012, 111, 1–39. [Google Scholar] [CrossRef]
- Tian, D.; Reich, P.B.; Chen, H.Y.; Xiang, Y.; Luo, Y.; Shen, Y.; Meng, C.; Han, W.; Niu, S. Global changes alter plant multi-element stoichiometric coupling. New Phytol. 2019, 221, 807–817. [Google Scholar] [CrossRef]
- Güsewell, S.N. P Ratios in Terrestrial Plants: Variation and Functional Significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Sainju, U.M.; Lenssen, A.W.; Ghimire, R.P. Root biomass, root/shoot ratio, and soil water content under perennial grasses with different nitrogen rates. Field Crops Res. 2017, 210, 183–191. [Google Scholar] [CrossRef]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C:N:P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Zhou, C.; Gu, X.; Li, J.; Su, X.; Chen, S.; Tang, J.R.; Chen, L.; Cai, N.H.; Xu, Y.L. Physiological characteristics and transcriptomic responses of Pinus yunnanensis lateral branching to different shading environments. Plants 2024, 13, 1588. [Google Scholar] [CrossRef] [PubMed]
- Stone, R. Severe Drought Puts Spotlight on Chinese Dams. Science 2010, 327, 1311. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.Y.; Li, Z.S.; Gao, C.J.; Li, S.F.; Huang, X.B.; Lang, X.D.; Su, J.R. Radial growth response of Pinus yunnanensis to rising temperature and drought on the Yunnan Plateau, southwestern China. For. Ecol. Manag. 2020, 474, 118357. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Wang, L.N.; Liu, Y.X. Effects of drought on non-structural carbohydrates and C, N, and P stoichiometric characteristics of P. yunnanensis seedlings. J. For. Res. 2024, 35, 94–106. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Huang, Z.J.; Wang, Z.N.; Chen, Y.F.; Wen, Z.M.; Liu, B.; Mulualem, T. Responses of leaf morphology, NSCs contentsand C:N:P stoichiometry of Cunninghamia lanceolata and Schima superba to shading. BMC Plant Biol. 2020, 20, 354. [Google Scholar]
- Mcdowell, N.G. Mechanisms Linking Drought, Hydraulics, Carbon Metabolism, and Vegetation Mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef]
- Silva, E.N.; Ferreira-Silva, S.L.; Viégas, R.A.; Silveira, J.A. The role of organic and inorganic solutes in the osmotic adjustment of drought-stressed Jatropha curcas plants. Environ. Exp. Bot. 2010, 69, 279–285. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Yi, J.; Yang, Y.; Lei, C.; Gong, M. Transcriptome Response to Drought, Rehydration and Re-Dehydration in Potato. Int. J. Mol. Sci. 2019, 21, 159. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Li, Y.; Jin, Z.; Luo, G.; Chen, C.; Shan, F. Effects of Shading on Photosynthetic Characteristics and Non-structural Carbohydrate Content of Heptacodium miconioides Seedlings. Bull. Bot. Res. 2022, 42, 10. [Google Scholar]
- Piper, F.I. Drought induces opposite changes in the concentration of non-structural carbohydrates of two evergreen Nothofagus species of differential drought resistance. Ann. For. Sci. 2011, 68, 415–424. [Google Scholar] [CrossRef]
- Zhao, N. Response of Carbon and Carbohydrate Characteristics of Liquidambar Formosana and Schima Superba Seedlings to Drought Rehydration; Nanchang Institute of Technology: Nanchang, China, 2019. [Google Scholar]
- Chen, R.; Han, L.; Zhao, Y.H. Response of plant element traits to soil arsenic stress and its implications for vegetation restoration in a post-mining area. Ecol. Indic. 2023, 146, 109931. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, S.; Shi, S.; Qi, M.; Liu, X.; Wang, H.; Wang, Y.; Jiang, C. Effects of different management approaches on the stoichiometric characteristics of soil C, N, and P in a mature Chinese fir plantation. Sci. Total Environ. 2020, 723, 137868. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y. Effects of Drought Stress on Carbon, Nitrogen and Phosphorus Stoichiometry of Robinia pseudoacacia Seedlings. Master’s Thesis, Northwest A&F University, Xianyang, China, 2015. [Google Scholar]
- Poorter, L.; Bongers, F. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 2006, 87, 1733–1743. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, Y.; Wu, J.; Li, C. Adaptations of Pinus yunnanensis Seedlings to Simulated Light Patches: Growth Dynamics and C:N:P Stoichiometry. Forests 2025, 16, 517. [Google Scholar] [CrossRef]
- Chen, W.B.; Uoyang, Z.Y.; Uoyang, S.L.; He, Y.J.; Liu, Q. Research progress on effects of low phosphorus stress on plant growth and physiological characteristics. Hunan For. Sci. Technol. 2020, 47, 132–136. [Google Scholar]
- Kai, W.; Lei, H.; Wang, Z.Y.; Lyu, L.Y.; Song, L.N. C, N and P distribution and stoichiometry characteristics of Caragana microphylla seedlings to drought stress. For. Res. 2019, 32, 47–56. [Google Scholar]
- Liu, C.; Wu, J.; Gu, J.; Duan, H. Growth and C:N:P Stoichiometry of Pinus yunnanensis Seedlings in Response to Drought and Rewatering. Forests 2024, 15, 2175. [Google Scholar] [CrossRef]
- Ågren, G.I. The C:N:P stoichiometry of autotrophs: Theory and observations. Ecol. Lett. 2004, 7, 185–191. [Google Scholar] [CrossRef]
- Klausmeier, C.A.; Litchman, E.; Daufresne, T.; Levin, S.A. Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature 2004, 429, 171–174. [Google Scholar] [CrossRef]
- Bradshaw, A.D. Unravelling phenotypic plasticity why should we bother. New Phytol. 2006, 170, 644–648. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Wang, L.N.; Wu, J.W.; Li, S.M. Non-structural carbohydrate and biomass characteristics of Pinus yunnanensis seedlings under continuous drought stress. Sci. Silvae Sin. 2024, 60, 71–85. [Google Scholar]
- Chen, X.Y.; Luo, Y.P. Effect of water stress and rewatering on the grain leaf ratio of winter wheat. Agric. Res. Arid Areas 2001, 1, 66–71. [Google Scholar]
- Liu, Y.; Sun, J.; Dai, C.; Du, G.; Shi, R.; Wu, J. Physiological and Biochemical Adaptations to Repeated Drought-Rehydration Cycles in Ochroma lagopus Swartz: Implications for Growth and Stress Resilience. Plants 2025, 14, 1636. [Google Scholar] [CrossRef] [PubMed]
- Shidan, B. Soil Chemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
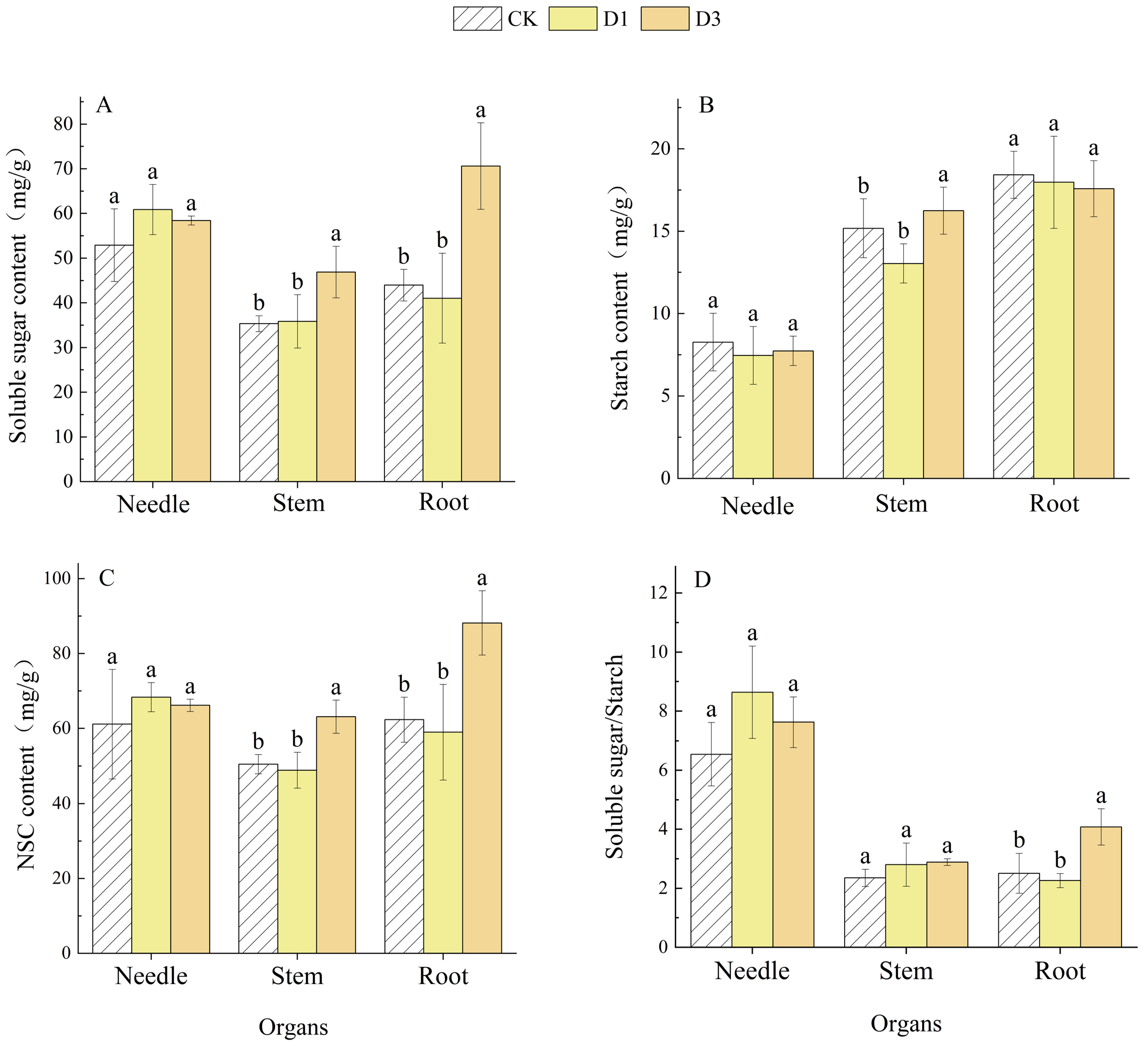
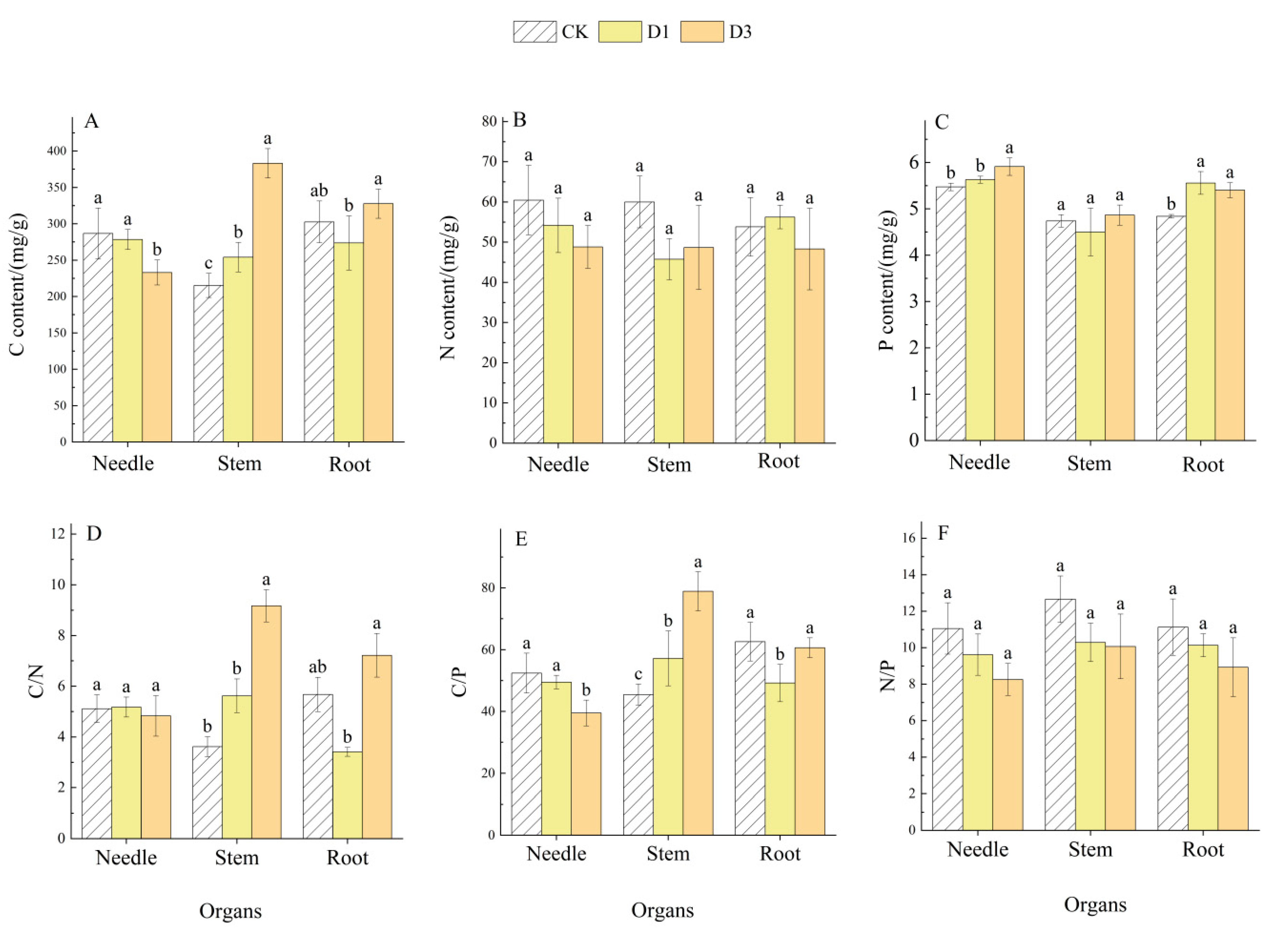
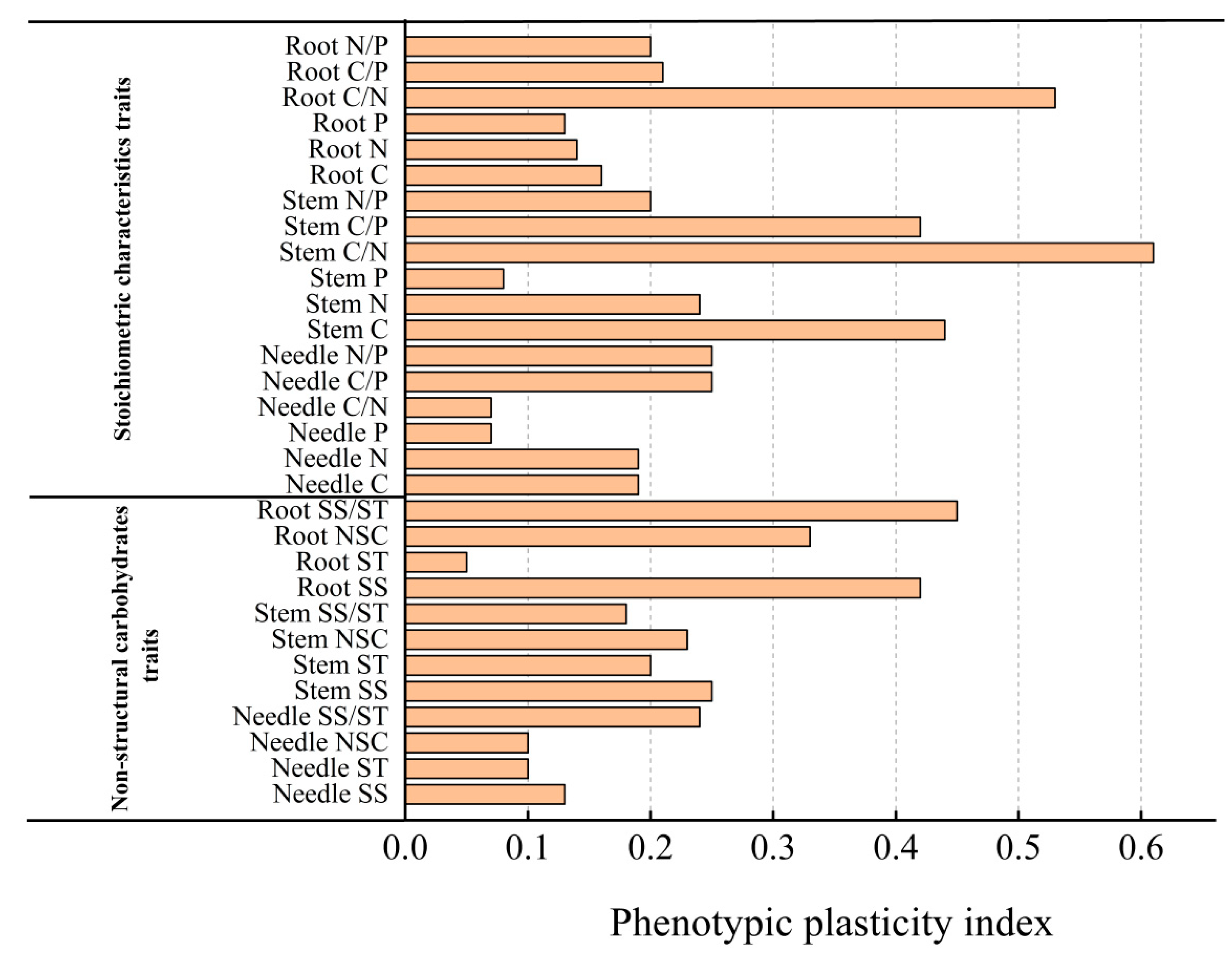
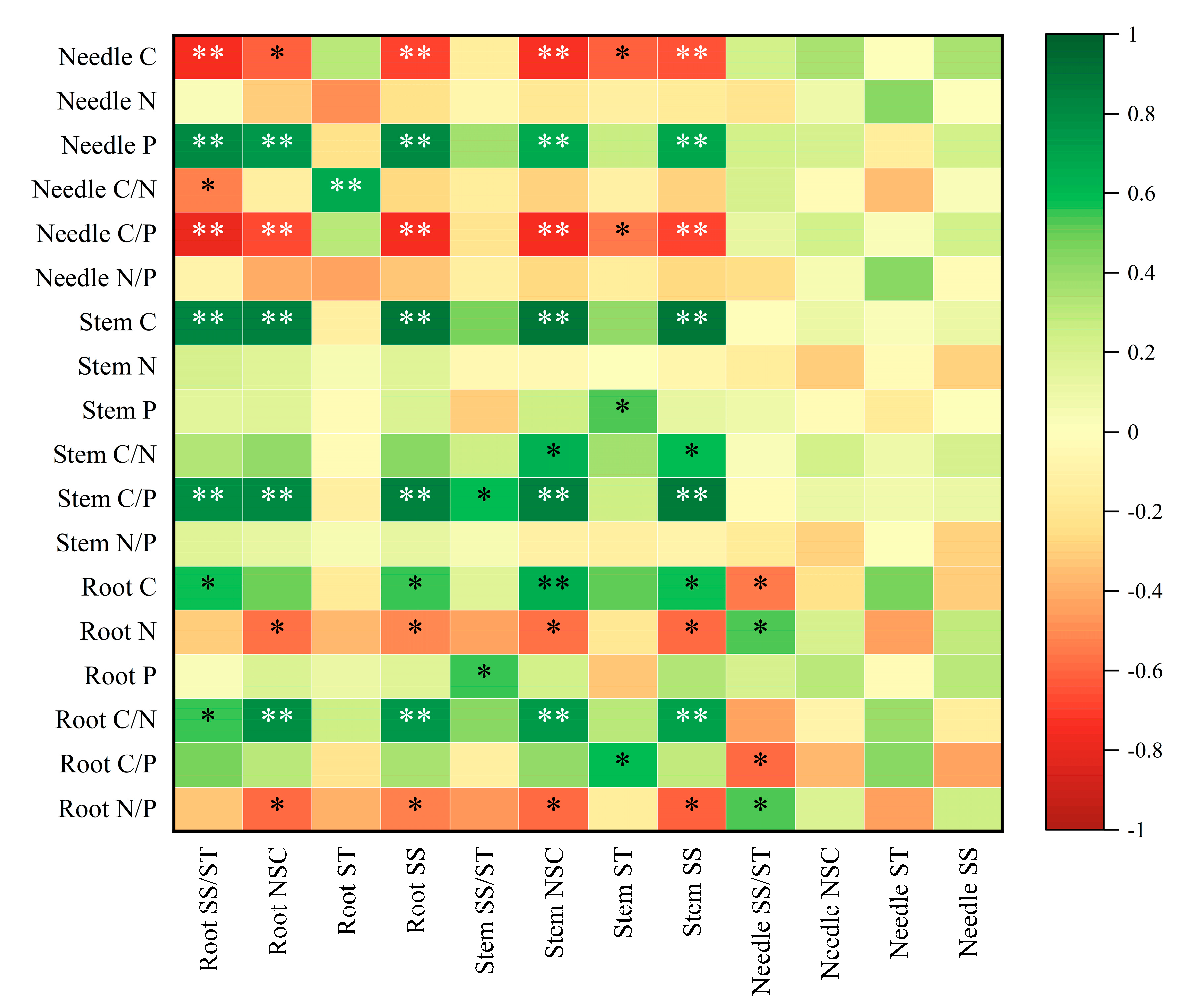
| Indicators | Organs | Number of Droughts | Organs × Number of Droughts | |||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| Soluble sugar | 24.076 ** | 0.000 | 17.724 ** | 0.000 | 6.388 ** | 0.001 |
| Starch | 86.752 ** | 0.000 | 1.259 | 0.296 | 0.978 | 0.432 |
| NSC | 16.411 ** | 0.000 | 16.827 ** | 0.000 | 5.467 ** | 0.002 |
| Soluble sugar/starch | 76.106 ** | 0.000 | 2.991 | 0.063 | 2.077 | 0.104 |
| C | 7.762 ** | 0.002 | 17.659 ** | 0.000 | 29.811 ** | 0.000 |
| N | 2.323 | 0.113 | 2.376 | 0.107 | 3.072 * | 0.028 |
| P | 68.042 ** | 0.000 | 10.222 ** | 0.000 | 5.773 ** | 0.001 |
| C/N | 1.620 | 0.212 | 9.339 ** | 0.001 | 5.538 ** | 0.001 |
| C/P | 23.650 ** | 0.000 | 8.051 ** | 0.001 | 26.874 ** | 0.000 |
| N/P | 2.159 | 0.130 | 2.523 | 0.094 | 2.509 | 0.059 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Liu, Y.; Li, Z.; Chen, J.; Wu, J. Drought–Rewatering Cycles: Impact on Non-Structural Carbohydrates and C:N:P Stoichiometry in Pinus yunnanensis Seedlings. Plants 2025, 14, 2448. https://doi.org/10.3390/plants14152448
Zhu W, Liu Y, Li Z, Chen J, Wu J. Drought–Rewatering Cycles: Impact on Non-Structural Carbohydrates and C:N:P Stoichiometry in Pinus yunnanensis Seedlings. Plants. 2025; 14(15):2448. https://doi.org/10.3390/plants14152448
Chicago/Turabian StyleZhu, Weisong, Yuanxi Liu, Zhiqi Li, Jialan Chen, and Junwen Wu. 2025. "Drought–Rewatering Cycles: Impact on Non-Structural Carbohydrates and C:N:P Stoichiometry in Pinus yunnanensis Seedlings" Plants 14, no. 15: 2448. https://doi.org/10.3390/plants14152448
APA StyleZhu, W., Liu, Y., Li, Z., Chen, J., & Wu, J. (2025). Drought–Rewatering Cycles: Impact on Non-Structural Carbohydrates and C:N:P Stoichiometry in Pinus yunnanensis Seedlings. Plants, 14(15), 2448. https://doi.org/10.3390/plants14152448





