Regulatory Mechanisms of Tannins on the Decomposition Rate of Mixed Leaf Litter in Submerged Environments
Abstract
1. Introduction
2. Results and Analysis
2.1. Characteristics of Leaf Litter Decomposition Rates
2.1.1. Decomposition of Osmanthus fragrans
2.1.2. Decomposition of Canna glauca
2.1.3. Decomposition Characteristics of Mixed Leaf Litter
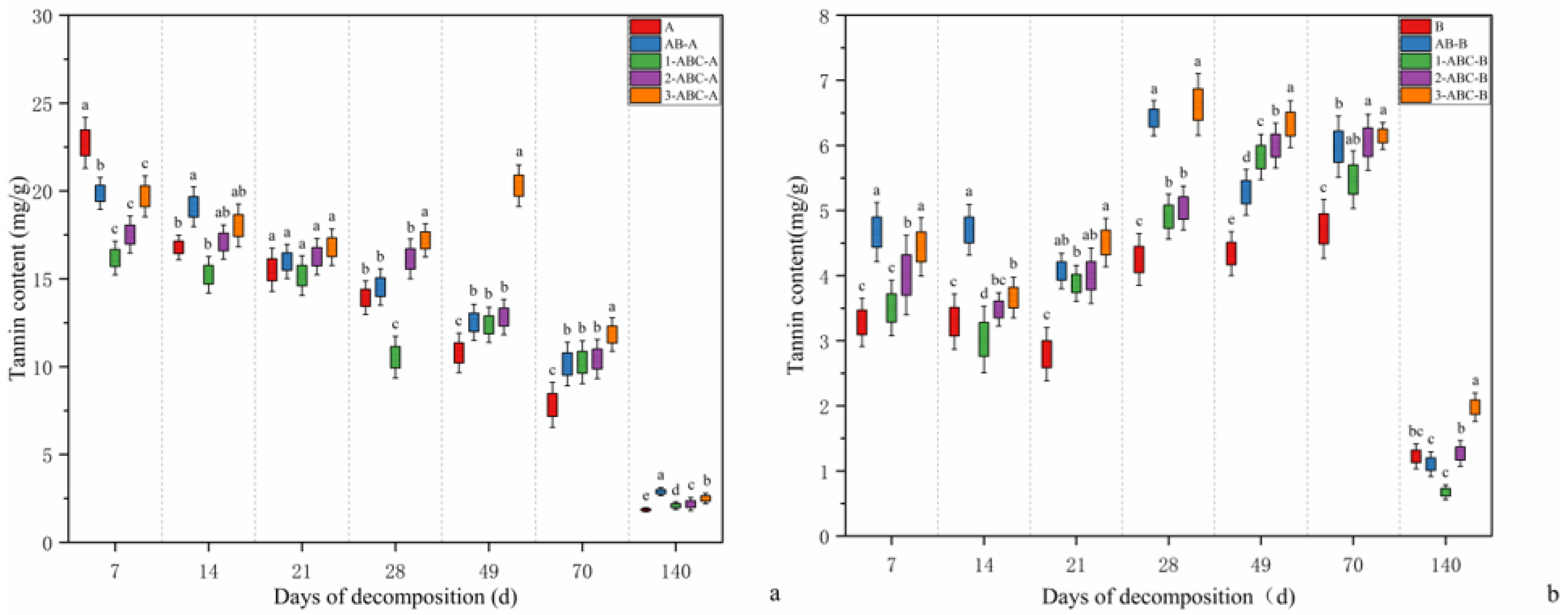
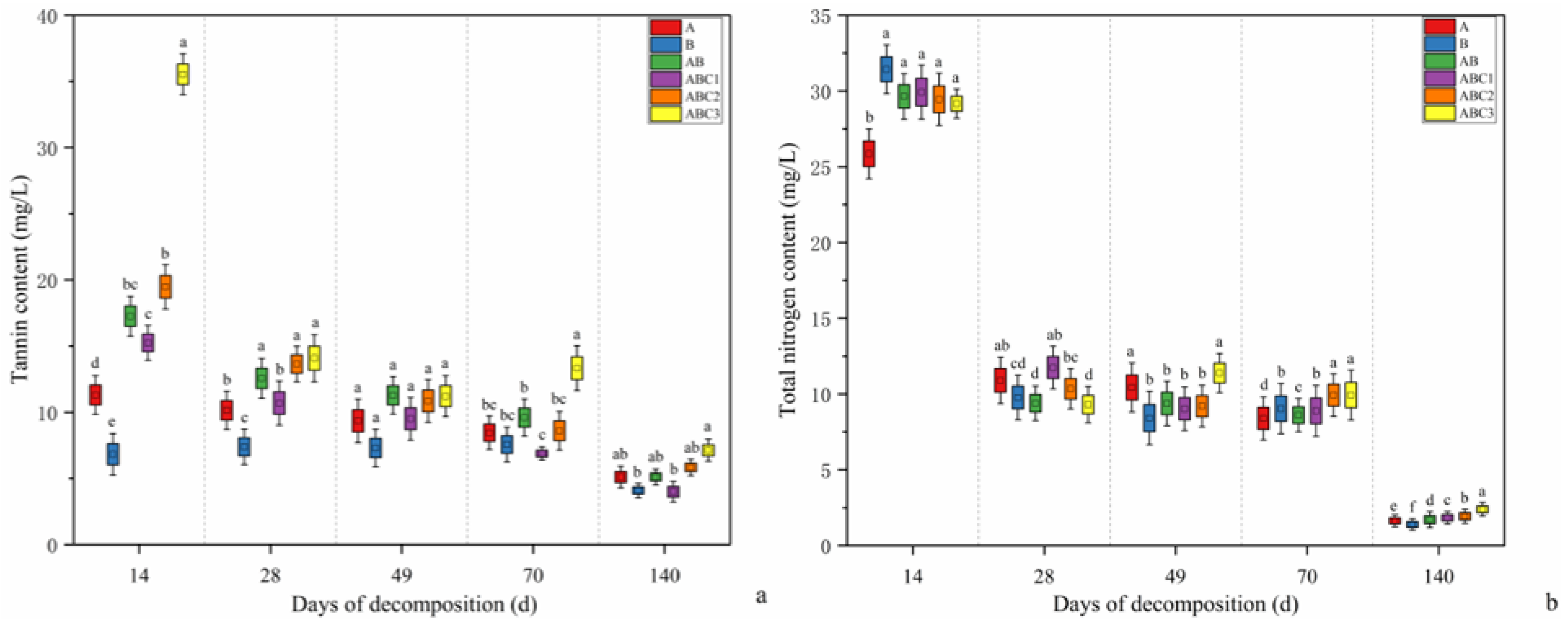
2.2. Changes in Tannin Content During Leaf Litter Decomposition
2.3. Changes in Total Nitrogen (TN) Content and the Nitrogen Accumulation Index (NAI) During Leaf Litter Decomposition
2.3.1. Osmanthus Fragrans TN Content and NAI Dynamics
2.3.2. Canna glauca TN Content and NAI Dynamics
2.4. Impact of Leaf Litter Decomposition on Water Quality
2.4.1. Changes in Tannin and TN Content in Water
2.4.2. Changes in the NH4+-N and NO3−-N Contents in Water
2.5. Bacterial Community Changes During Different Stages of Leaf Litter Decomposition Changes in Bacterial Diversity
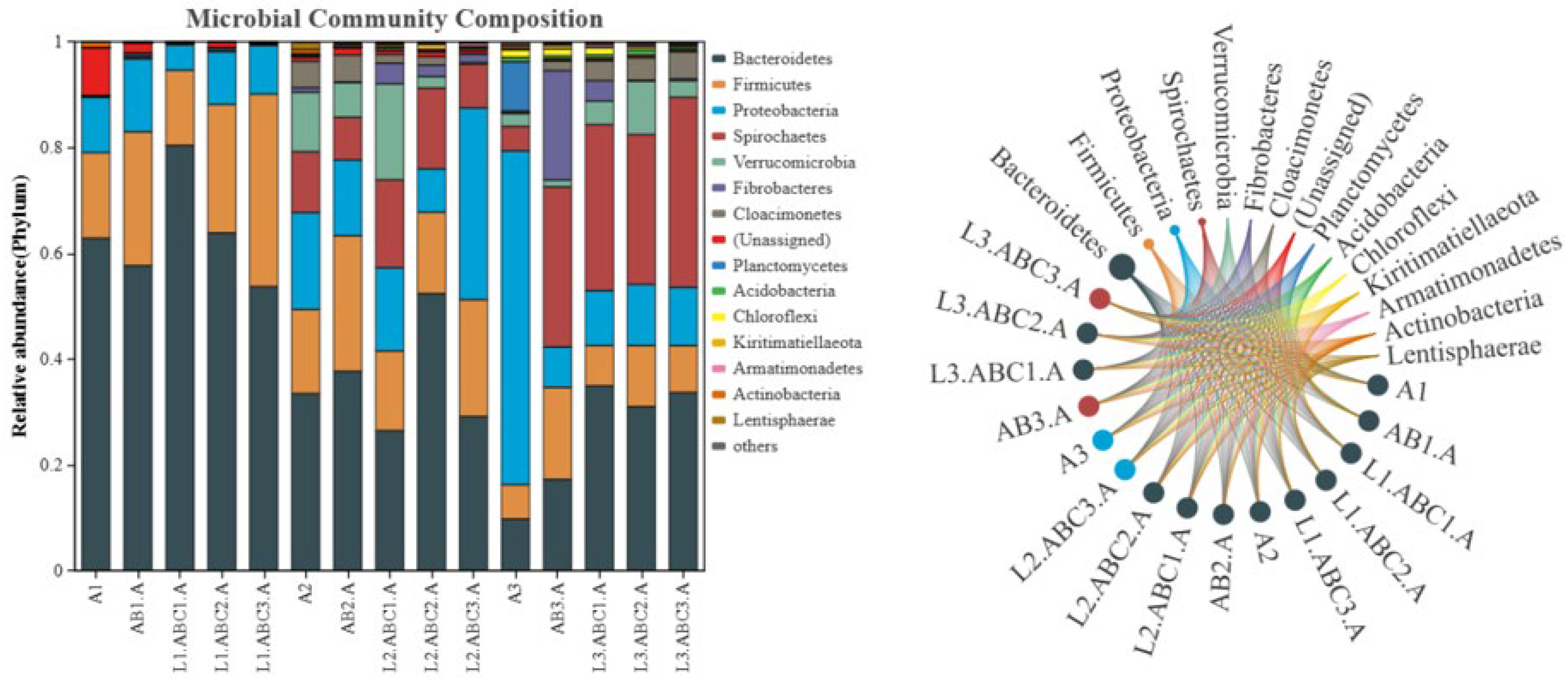
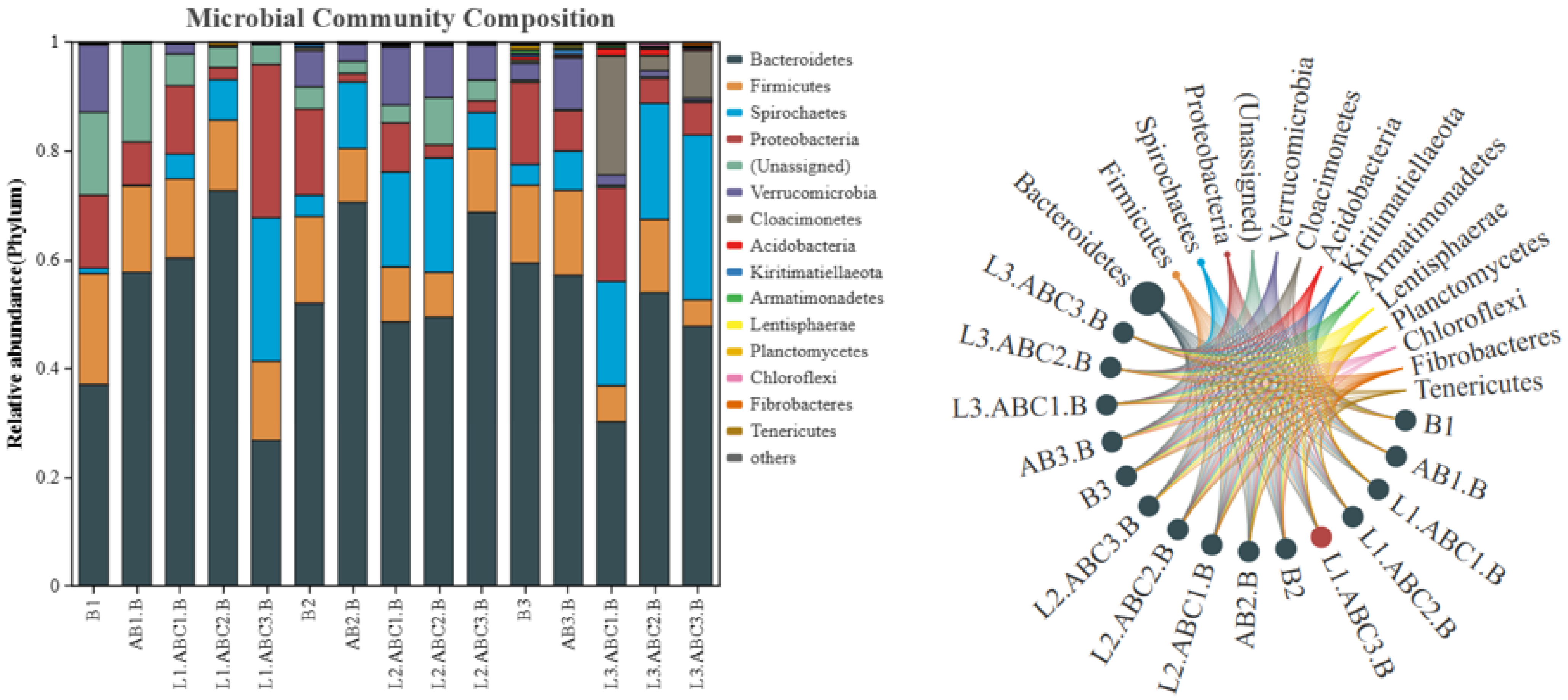
2.5.1. Bacterial Community Structure at the Phylum Level
2.5.2. Analysis of Bacterial Community Structure at the Genus Level
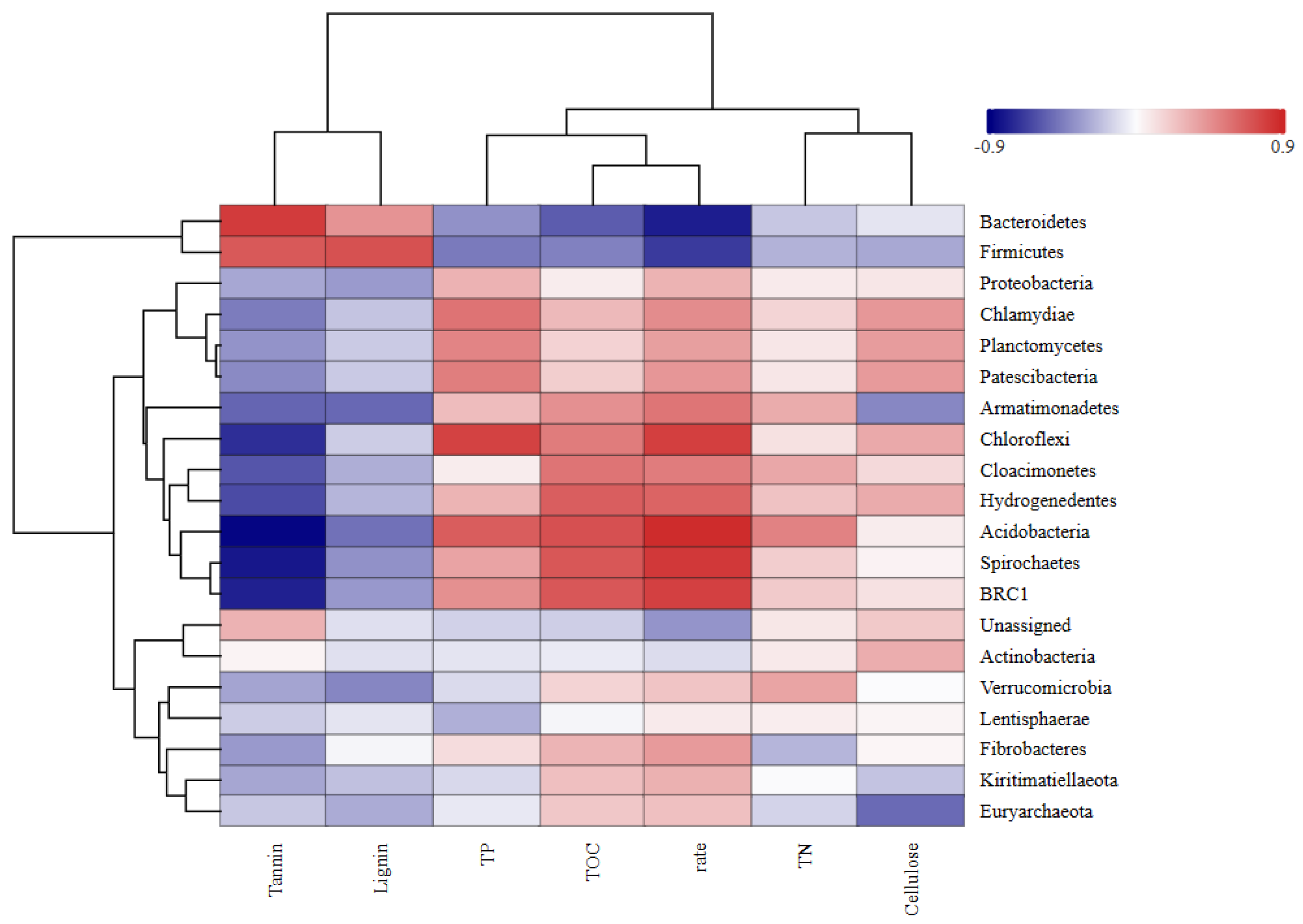
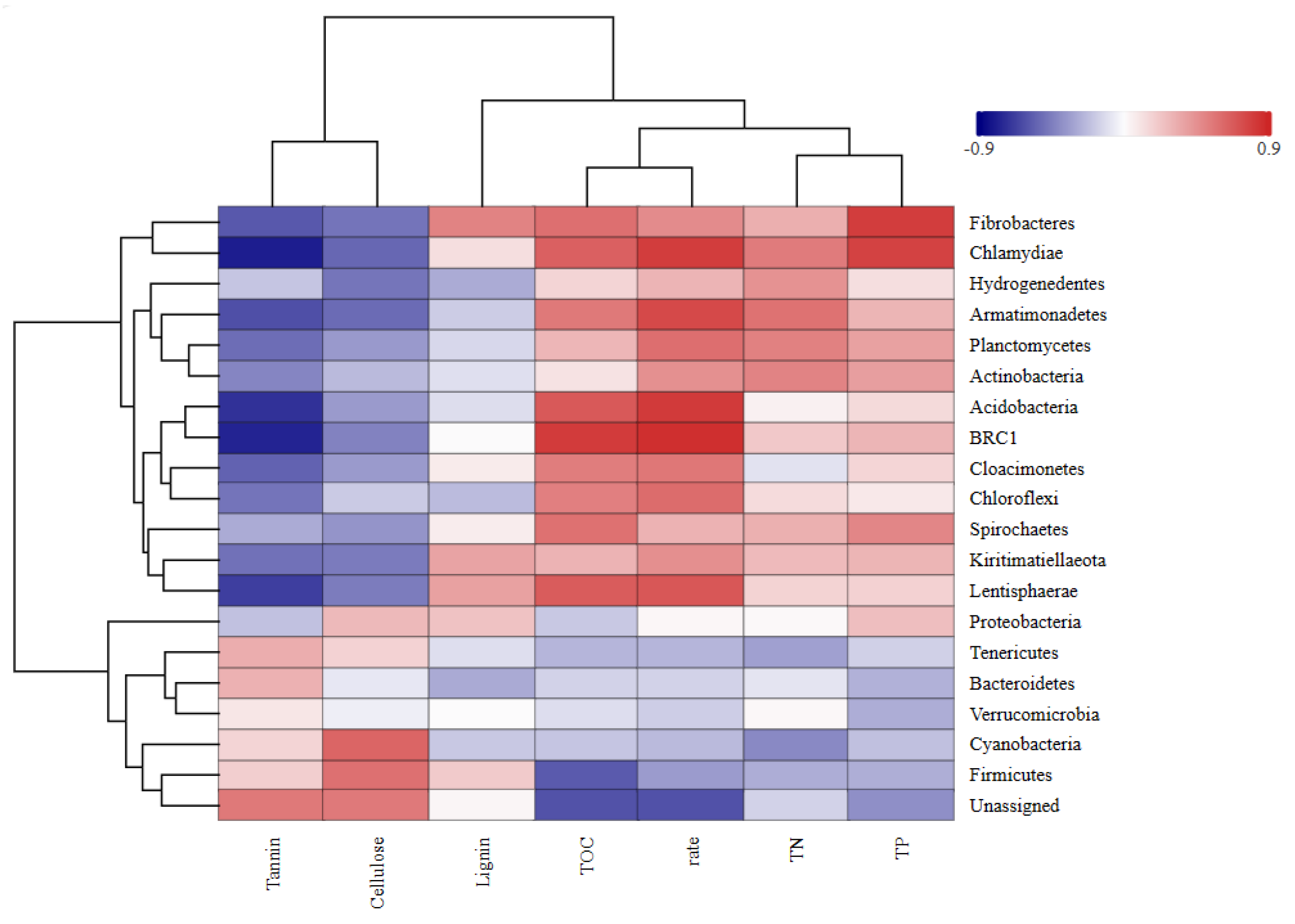
2.5.3. Correlations of Bacterial Community Structure with Leaf Litter Decomposition Rate and Tannin Content
3. Discussion
3.1. Tannins Exhibit Non-Linear Regulatory Effects on Leaf Litter Decomposition Rates
3.2. Inhibitory Effect of Tannin–Nitrogen Complexation on Leaf Litter Decomposition
3.3. Tannin-Driven Dynamics of Aquatic Nitrogen
3.4. Tannin-Mediated Regulation of Bacterial Community Succession
3.5. Ecological Management Implications
4. Materials and Methods
4.1. Plant Species
4.2. Experimental Design
4.3. Sample Collection and Analysis
4.4. DNA Extraction, Sequencing, and Bacterial Analysis of Leaf Litter
4.5. Data Processing and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abelho, M.; Descals, E. Litter movement pathways across terrestrial–aquatic ecosystem boundaries affect litter colonization and decomposition in streams. Funct. Ecol. 2019, 33, 1785–1797. [Google Scholar] [CrossRef]
- Tanentzap, A.J.; Kielstra, B.W.; Wilkinson, G.M.; Berggren, M.; Craig, N.; Del Giorgio, P.A.; Grey, J.; Gunn, J.M.; Jones, S.E.; Karlsson, J.; et al. Terrestrial support of lake food webs: Synthesis reveals controls over cross-ecosystem resource use. Sci. Adv. 2017, 3, e1601765. [Google Scholar] [CrossRef]
- Abelho, M. From Litterfall to Breakdown in Streams: A Review. Sci. World J. 2001, 1, 180292. [Google Scholar] [CrossRef]
- Scharnweber, K.; Syväranta, J.; Hilt, S.; Brauns, M.; Vanni, M.J.; Brothers, S.; Köhler, J.; Knežević-Jarić, J.; Mehner, T. Whole-lake experiments reveal the fate of terrestrial particulate organic carbon in benthic food webs of shallow lakes. Ecology 2014, 95, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Mehner, T.; Attermeyer, K.; Brauns, M.; Brothers, S.; Diekmann, J.; Gaedke, U.; Grossart, H.-P.; Köhler, J.; Lischke, B.; Meyer, N.; et al. Weak Response of Animal Allochthony and Production to Enhanced Supply of Terrestrial Leaf Litter in Nutrient-Rich Lakes. Ecosystems 2016, 19, 311–325. [Google Scholar] [CrossRef]
- Kritzberg, E.S.; Cole, J.J.; Pace, M.L.; Granéli, W.; Bade, D.L. Autochthonous versus allochthonous carbon sources of bacteria: Results from whole-lake13 C addition experiments. Limnol. Oceanogr. 2004, 49, 588–596. [Google Scholar] [CrossRef]
- Makkonen, M.; Berg, M.P.; Handa, I.T.; Hättenschwiler, S.; van Ruijven, J.; van Bodegom, P.M.; Aerts, R. Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol. Lett. 2012, 15, 1033–1041. [Google Scholar] [CrossRef]
- Tiegs, S.D.; Costello, D.M.; Isken, M.W.; Woodward, G.; McIntyre, P.B.; Gessner, M.O.; Chauvet, E.; Griffiths, N.A.; Flecker, A.S.; Acuña, V.; et al. Global patterns and drivers of ecosystem functioning in rivers and riparian zones. Sci. Adv. 2019, 5, eaav0486. [Google Scholar] [CrossRef]
- Cebrian, J. Role of first-order consumers in ecosystem carbon flow. Ecol. Lett. 2004, 7, 232–240. [Google Scholar] [CrossRef]
- Adair, E.C.; Parton, W.J.; Del Grosso, S.J.; Silver, W.L.; Harmon, M.E.; Hall, S.A.; Burke, I.C.; Hart, S.C. Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Glob. Change Biol. 2008, 14, 2636–2660. [Google Scholar] [CrossRef]
- Boyero, L.; Graça, M.A.S.; Tonin, A.M.; Pérez, J.; Swafford, A.J.; Ferreira, V.; Landeira-Dabarca, A.; Alexandrou, M.A.; Gessner, M.O.; McKie, B.G.; et al. Riparian plant litter quality increases with latitude. Sci. Rep. 2017, 7, 10562. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Hämmerle, I.; Fuchslueger, L.; Hofhansl, F.; Knoltsch, A.; Schnecker, J.; Takriti, M.; Watzka, M.; Wild, B.; et al. Adjustment of microbial nitrogen use efficiency to carbon: Nitrogen imbalances regulate soil nitrogen cycling. Nat. Commun. 2014, 5, 3694. [Google Scholar] [CrossRef]
- Gill, A.L.; Schilling, J.; Hobbie, S.E. Experimental nitrogen fertilisation globally accelerates, then slows decomposition of leaf litter. Ecol. Lett. 2021, 24, 802–811. [Google Scholar] [CrossRef]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Kowalska, G.; Baj, T.; Kowalski, R.; Hanif, M.A. Characteristics of Selected Silphium Species as Alternative Plants for Cultivation and Industry with Particular Emphasis on Research Conducted in Poland: A Review. Sustainability 2022, 14, 5092. [Google Scholar] [CrossRef]
- Naumann, H.D.; Tedeschi, L.O.; Zeller, W.E.; Huntley, N.F. The role of condensed tannins in ruminant animal production: Advances, limitations and future directions. Rev. Bras. De Zootec. 2017, 46, 929–949. [Google Scholar] [CrossRef]
- Chomel, M.; Guittonny-Larchevêque, M.; Fernandez, C.; Gallet, C.; DesRochers, A.; Paré, D.; Jackson, B.G.; Baldy, V. Plant secondary metabolites: A key driver of litter decomposition and soil nutrient cycling. J. Ecol. 2016, 104, 1527–1541. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Constabel, C.P. Tannins in plant–herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef]
- Kraus, T.E.C.; Dahlgren, R.A.; Zasoski, R.J. Tannins in nutrient dynamics of forest ecosystems—A review. Plant Soil 2003, 256, 41–66. [Google Scholar] [CrossRef]
- Schweitzer, J.A.; Bailey, J.K.; Hart, S.C.; Wimp, G.M.; Chapman, S.K.; Whitham, T.G. The interaction of plant genotype and herbivory decelerate leaf litter decomposition and alter nutrient dynamics. Oikos 2005, 110, 133–145. [Google Scholar] [CrossRef]
- Berg, B. Decomposition patterns for foliar litter—A theory for influencing factors. Soil Biol. Biochem. 2014, 78, 222–232. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Laanbroek, H.J. Tannins from senescent Rhizophora mangle mangrove leaves have a distinctive effect on prokaryotic and eukaryotic communities in a Distichlis spicata salt marsh soil. FEMS Microbiol. Ecol. 2020, 96, fiaa148. [Google Scholar] [CrossRef]
- Wurzburger, N.; Hendrick, R.L. Plant litter chemistry and mycorrhizal roots promote a nitrogen feedback in a temperate forest. J. Ecol. 2009, 97, 528–536. [Google Scholar] [CrossRef]
- Joanisse, G.D.; Bradley, R.L.; Preston, C.M.; Munson, A.D. Soil enzyme inhibition by condensed litter tannins may drive ecosystem structure and processes: The case of Kalmia angustifolia. New Phytol. 2007, 175, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Northup, R.R.; Yu, Z.; Dahlgren, R.A.; Vogt, K.A. Polyphenol control of nitrogen release from pine litter. Nature 1995, 377, 227–229. [Google Scholar] [CrossRef]
- Adamczyk, B.; Sieti, O.-M.; Biasi, C.; Heinonsalo, J. Interaction between tannins and fungal necromass stabilizes fungal residues in boreal forest soils. New Phytol. 2019, 223, 16–21. [Google Scholar] [CrossRef]
- Xie, J.; Lei, P.; Zhu, Y. Enhanced Foliar Litter Decomposition Rate of Pinus massoniana when Admixed with Broadleaf Species. Forests 2024, 15, 9. [Google Scholar] [CrossRef]
- Kagata, H.; Ohgushi, T. Non-additive effects of leaf litter and insect frass mixture on decomposition processes. Ecol. Res. 2012, 27, 69–75. [Google Scholar] [CrossRef]
- Santos, F.M.; Balieiro, F.d.C.; Fontes, M.A.; Chaer, G.M. Understanding the enhanced litter decomposition of mixed-species plantations of Eucalyptus and Acacia mangium. Plant Soil 2018, 423, 141–155. [Google Scholar] [CrossRef]
- Stoler, A.B.; Relyea, R.A. Reviewing the role of plant litter inputs to forested wetland ecosystems: Leafing through the literature. Ecol. Monogr. 2020, 90, e01400. [Google Scholar] [CrossRef]
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; McKie, B.G.; Bardgett, R.D.; Wall, D.H.; Hättenschwiler, S. Diversity meets decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef]
- Yue, K.; De Frenne, P.; Van Meerbeek, K.; Ferreira, V.; Fornara, D.A.; Wu, Q.; Ni, X.; Peng, Y.; Wang, D.; Heděnec, P.; et al. Litter quality and stream physicochemical properties drive global invertebrate effects on instream litter decomposition. Biol. Rev. 2022, 97, 2023–2038. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.; Zhang, W.; Zhang, Q.; Lu, D.; Zhang, Y.; Zheng, X.; Xu, S.; Wang, G.G. Litter decomposition and nutrient release from monospecific and mixed litters: Comparisons of litter quality, fauna and decomposition site effects. J. Ecol. 2022, 110, 1673–1686. [Google Scholar] [CrossRef]
- Maie, N.; Pisani, O.; JaffÉ, R. Mangrove tannins in aquatic ecosystems: Their fate and possible influence on dissolved organic carbon and nitrogen cycling. Limnol. Oceanogr. 2008, 53, 160–171. [Google Scholar] [CrossRef]
- Lang, T.; Ke, X.; Wei, J.; Hussain, M.; Li, M.; Gao, C.; Jiang, M.; Wang, Y.; Fu, Y.; Wu, K.; et al. Dynamics of tannin variations in mangrove leaf litter decomposition and their effects on environmental nitrogen and microbial activity. Sci. Total Environ. 2024, 908, 168150. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, B.; Tabibiazar, M.; Golchinfar, Z.; Mohammadifar, M.; Hamishehkar, H. A review of protein-phenolic acid interaction: Reaction mechanisms and applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 3539–3555. [Google Scholar] [CrossRef]
- Murúa, J.M.; Gaxiola, A. Variability in terrestrial litter decomposition can be explained by nutrient allocation strategies among soil decomposer communities. Funct. Ecol. 2023, 37, 1642–1652. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Vitousek, P.M. The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol. Evol. 2000, 15, 238–243. [Google Scholar] [CrossRef]
- Jalal, A.; de Oliveira Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; dos Reis, A.R. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef]
- Baldrian, P.; Valášková, V. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol. Rev. 2008, 32, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Adamczyk, B.; Kitunen, V.; Smolander, A. Polyphenol oxidase, tannase and proteolytic activity in relation to tannin concentration in the soil organic horizon under silver birch and Norway spruce. Soil Biol. Biochem. 2009, 41, 2085–2093. [Google Scholar] [CrossRef]
- Seidi, F.; Liu, Y.; Huang, Y.; Xiao, H.; Crespy, D. Chemistry of lignin and condensed tannins as aromatic biopolymers. Chem. Soc. Rev. 2025, 54, 3140–3232. [Google Scholar] [CrossRef]
- Liu, W.; Tian, R.; Peng, Z.; Yang, S.; Liu, X.; Yang, Y.; Zhang, W.; Liu, L. Nonlinear responses of the Vmax and Km of hydrolytic and polyphenol oxidative enzymes to nitrogen enrichment. Soil Biol. Biochem. 2020, 141, 107656. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Weedon, J.T. Decomposition trajectories of diverse litter types: A model selection analysis. Methods Ecol. Evol. 2014, 5, 173–182. [Google Scholar] [CrossRef]
- Palozzi, J.E.; Lindo, Z. Are leaf litter and microbes team players? Interpreting home-field advantage decomposition dynamics. Soil Biol. Biochem. 2018, 124, 189–198. [Google Scholar] [CrossRef]
- Field, J.A.; Lettinga, G. Toxicity of Tannic Compounds to Microorganisms. In Plant Polyphenols; Hemingway, R.W., Laks, P.E., Eds.; Springer: Boston, MA, USA, 1992; pp. 673–692. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P.; Cates, R.G.; Zou, J. Influence of balsam poplar tannin fractions on carbon and nitrogen dynamics in Alaskan taiga floodplain soils. Soil Biol. Biochem. 2001, 33, 1827–1839. [Google Scholar] [CrossRef]
- Parton, W.; Silver, W.L.; Burke, I.C.; Grassens, L.; Harmon, M.E.; Currie, W.S.; King, J.Y.; Adair, E.C.; Brandt, L.A.; Hart, S.C.; et al. Global-Scale Similarities in Nitrogen Release Patterns During Long-Term Decomposition. Science 2007, 315, 361–364. [Google Scholar] [CrossRef]
- Widdig, M.; Schleuss, P.-M.; Biederman, L.A.; Borer, E.T.; Crawley, M.J.; Kirkman, K.P.; Seabloom, E.W.; Wragg, P.D.; Spohn, M. Microbial carbon use efficiency in grassland soils subjected to nitrogen and phosphorus additions. Soil Biol. Biochem. 2020, 146, 107815. [Google Scholar] [CrossRef]
- Riaz, M.; Mian, I.A.; Cresser, M.S. Litter effects on ammonium dynamics in an acid soil under grassland. Geoderma 2010, 159, 198–208. [Google Scholar] [CrossRef]
- Quick, A.M.; Reeder, W.J.; Farrell, T.B.; Tonina, D.; Feris, K.P.; Benner, S.G. Nitrous oxide from streams and rivers: A review of primary biogeochemical pathways and environmental variables. Earth-Sci. Rev. 2019, 191, 224–262. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, S.; Li, S.; Zhang, L.; Wang, G.; Zhang, L.; Wang, J.; Li, Z. The cycle of nitrogen in river systems: Sources, transformation, and flux. Environ. Sci. Process. Impacts 2018, 20, 863–891. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Y.; Sun, R.; Hu, S.; Qiao, Z.; Wang, S.; Mi, X. NH4+-N/NO3−-N ratio controlling nitrogen transformation accompanied with NO2−-N accumulation in the oxic-anoxic transition zone. Environ. Res. 2020, 189, 109962. [Google Scholar] [CrossRef]
- Baldwin, D.S.; Mitchell, A.M. The effects of drying and re-flooding on the sediment and soil nutrient dynamics of lowland river–floodplain systems: A synthesis. Regul. Rivers Res. Manag. 2000, 16, 457–467. [Google Scholar] [CrossRef]
- Fierer, N.; Breitbart, M.; Nulton, J.; Salamon, P.; Lozupone, C.; Jones, R.; Robeson, M.; Edwards, R.A.; Felts, B.; Rayhawk, S.; et al. Metagenomic and Small-Subunit rRNA Analyses Reveal the Genetic Diversity of Bacteria, Archaea, Fungi, and Viruses in Soil. Appl. Environ. Microbiol. 2007, 73, 7059–7066. [Google Scholar] [CrossRef]
- Saini, N.; Anmol, A.; Kumar, S.; Wani, A.W.; Bakshi, M.; Dhiman, Z. Exploring phenolic compounds as natural stress alleviators in plants- a comprehensive review. Physiol. Mol. Plant Pathol. 2024, 133, 102383. [Google Scholar] [CrossRef]
- Wang, S.; Heal, K.V.; Zhang, Q.; Yu, Y.; Tigabu, M.; Huang, S.; Zhou, C. Soil microbial community, dissolved organic matter and nutrient cycling interactions change along an elevation gradient in subtropical China. J. Environ. Manag. 2023, 345, 118793. [Google Scholar] [CrossRef]
- Deng, Y.; Mou, T.; Wang, J.; Su, J.; Yan, Y.; Zhang, Y.-Q. Characterization of three rapidly growing novel Mycobacterium species with significant polycyclic aromatic hydrocarbon bioremediation potential. Front. Microbiol. 2023, 14, 1225746. [Google Scholar] [CrossRef]
- Koelschbach, J.S.; Mouttaki, H.; Pickl, C.; Heipieper, H.J.; Rachel, R.; Lawson, P.A.; Meckenstock, R.U. Rectinema cohabitans gen. Nov., sp. Nov., a rod-shaped spirochaete isolated from an anaerobic naphthalene-degrading enrichment culture. Int. J. Syst. Evol. Microbiol. 2017, 67, 1288–1295. [Google Scholar] [CrossRef]
- Brinkman, M.B.; Mcgill, M.A.; Pettersson, J.; Rogers, A.; Matejkova, P.; Smajs, D.; Weinstock, G.M.; Norris, S.J.; Palzkill, T. A Novel Treponema pallidum Antigen, TP0136, Is an Outer Membrane Protein That Binds Human Fibronectin. Infect. Immun. 2008, 76, 1848–1857. [Google Scholar] [CrossRef]
- Floudas, D.; Binder, M.; Riley, R.; Barry, K.; Blanchette, R.A.; Henrissat, B.; Martínez, A.T.; Otillar, R.; Spatafora, J.W.; Yadav, J.S.; et al. The Paleozoic Origin of Enzymatic Lignin Decomposition Reconstructed from 31 Fungal Genomes. Science 2012, 336, 1715–1719. [Google Scholar] [CrossRef] [PubMed]
- Wylensek, D.; Hitch, T.C.A.; Riedel, T.; Afrizal, A.; Kumar, N.; Wortmann, E.; Liu, T.; Devendran, S.; Lesker, T.R.; Hernández, S.B.; et al. A collection of bacterial isolates from the pig intestine reveals functional and taxonomic diversity. Nat. Commun. 2020, 11, 6389. [Google Scholar] [CrossRef] [PubMed]
- Buckel, W. Energy Conservation in Fermentations of Anaerobic Bacteria. Front. Microbiol. 2021, 12, 703525. [Google Scholar] [CrossRef] [PubMed]
- Buckel, W.; Thauer, R.K. Flavin-Based Electron Bifurcation, A New Mechanism of Biological Energy Coupling. Chem. Rev. 2018, 118, 3862–3886. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Haddix, M.L.; Ayres, E.; Steltzer, H.; Magrini-Bair, K.A.; Paul, E.A. Litter chemistry changes more rapidly when decomposed at home but converges during decomposition–transformation. Soil Biol. Biochem. 2013, 57, 311–319. [Google Scholar] [CrossRef]
- Kardel, M.; Taube, F.; Schulz, H.; Schütze, W.; Gierus, M. Different approaches to evaluate tannin content and structure of selected plant extracts—Review and new aspects. J. Appl. Bot. Food Qual. 2013, 86, 154–166. [Google Scholar] [CrossRef]
- Soest, P.J.V. Use of Detergents in the Analysis of Fibrous Feeds. II. A Rapid Method for the Determination of Fiber and Lignin. J. Assoc. Off. Agric. Chem. 1963, 46, 829–835. [Google Scholar] [CrossRef]
- Harmon, M.E.; Silver, W.L.; Fasth, B.; Chen, H.; Burke, I.C.; Parton, W.J.; Hart, S.C.; Currie, W.S.; LIDET. Long-term patterns of mass loss during the decomposition of leaf and fine root litter: An intersite comparison. Glob. Change Biol. 2009, 15, 1320–1338. [Google Scholar] [CrossRef]
- Olson, J.S. Energy Storage and the Balance of Producers and Decomposers in Ecological Systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Romero, L.M.; Smith Iii, T.J.; Fourqurean, J.W. Changes in mass and nutrient content of wood during decomposition in a south Florida mangrove forest. J. Ecol. 2005, 93, 618–631. [Google Scholar] [CrossRef]





| Specimen | R2 | K | Formula | t0.95 (d) |
|---|---|---|---|---|
| A | 0.911 | 0.003 | y = 7.868e−0.003t | 1521.19 |
| B | 0.991 | 0.009 | y = 8.624e−0.009t | 616.72 |
| AB-A | 0.909 | 0.003 | y = 7.892e−0.003t | 1670.42 |
| AB-B | 0.985 | 0.007 | y = 8.126e−0.007t | 741.95 |
| ABC1-A | 0.927 | 0.003 | y = 7.919e−0.003t | 1605.34 |
| ABC1-B | 0.980 | 0.008 | y = 8.469e−0.008t | 666.42 |
| ABC2-A | 0.938 | 0.003 | y = 7.930e−0.003t | 1730.53 |
| ABC2-B | 0.993 | 0.007 | y = 8.272e−0.007t | 741.68 |
| ABC3-A | 0.928 | 0.002 | y = 7.881e−0.002t | 2158.49 |
| ABC3-B | 0.968 | 0.007 | y = 8.559e−0.007t | 771.12 |
| Specimen | Tannin | TOC | TN | TP | Cellulose | Lignin | C/N | C/P |
|---|---|---|---|---|---|---|---|---|
| (mg/g) | ||||||||
| Osmanthus fragrans | 18.69 ± 0.14 b | 437.40 ± 6.9 a | 12.51 ± 0.02 c | 1.94 ± 0.6 c | 235.06 ± 4.56 a | 190.84 ± 2.61 a | 34.96 ± 2.12 a | 225.31 ± 3.64 a |
| Canna glauca | 12.20 ± 0.11 c | 381.90 ± 3.0 b | 14.63 ± 0.07 b | 2.22 ± 0.06 b | 143.89 ± 2.89 b | 112.01 ± 1.90 c | 26.10 ± 0.08 b | 172.24 ± 3.15 b |
| Myriophyllum verticillatum | 130.02 ± 0.15 a | 365.70 ± 2.2 c | 25.45 ± 0.57 a | 3.28 ± 0.04 a | 155.28 ± 3.70 b | 136.91 ± 2.67 b | 14.37 ± 0.24 c | 108.12 ± 2.02 c |
| Treatment | Leaf Litter Species and Weight per Bag |
|---|---|
| A | Osmanthus fragrans (10 g) |
| B | Canna glauca (10 g) |
| AB | A (10 g) + B (10 g) |
| ABC1 | A (10 g) + B (10 g) + C (3.85 g Myriophyllum verticillatum, containing 0.5 g tannins) |
| ABC2 | A (10 g) + B (10 g) + C (19.23 g Myriophyllum verticillatum, containing 2.5 g tannins) |
| ABC3 | A (10 g) + B (10 g) + C (34.61 g Myriophyllum verticillatum, containing 4.5 g tannins) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Tan, J.; Yang, G.; Huang, Y.; Deng, Y.; Huang, Y.; Yang, M.; Cao, J.; Wang, H. Regulatory Mechanisms of Tannins on the Decomposition Rate of Mixed Leaf Litter in Submerged Environments. Plants 2025, 14, 3064. https://doi.org/10.3390/plants14193064
Li L, Tan J, Yang G, Huang Y, Deng Y, Huang Y, Yang M, Cao J, Wang H. Regulatory Mechanisms of Tannins on the Decomposition Rate of Mixed Leaf Litter in Submerged Environments. Plants. 2025; 14(19):3064. https://doi.org/10.3390/plants14193064
Chicago/Turabian StyleLi, Lisha, Jiahao Tan, Gairen Yang, Yu Huang, Yusong Deng, Yuhan Huang, Mingxia Yang, Jizhao Cao, and Huili Wang. 2025. "Regulatory Mechanisms of Tannins on the Decomposition Rate of Mixed Leaf Litter in Submerged Environments" Plants 14, no. 19: 3064. https://doi.org/10.3390/plants14193064
APA StyleLi, L., Tan, J., Yang, G., Huang, Y., Deng, Y., Huang, Y., Yang, M., Cao, J., & Wang, H. (2025). Regulatory Mechanisms of Tannins on the Decomposition Rate of Mixed Leaf Litter in Submerged Environments. Plants, 14(19), 3064. https://doi.org/10.3390/plants14193064


.png)




