Role of Plant Growth Regulators in Adventitious Populus Tremula Root Development In Vitro
Abstract
1. Introduction
2. Results
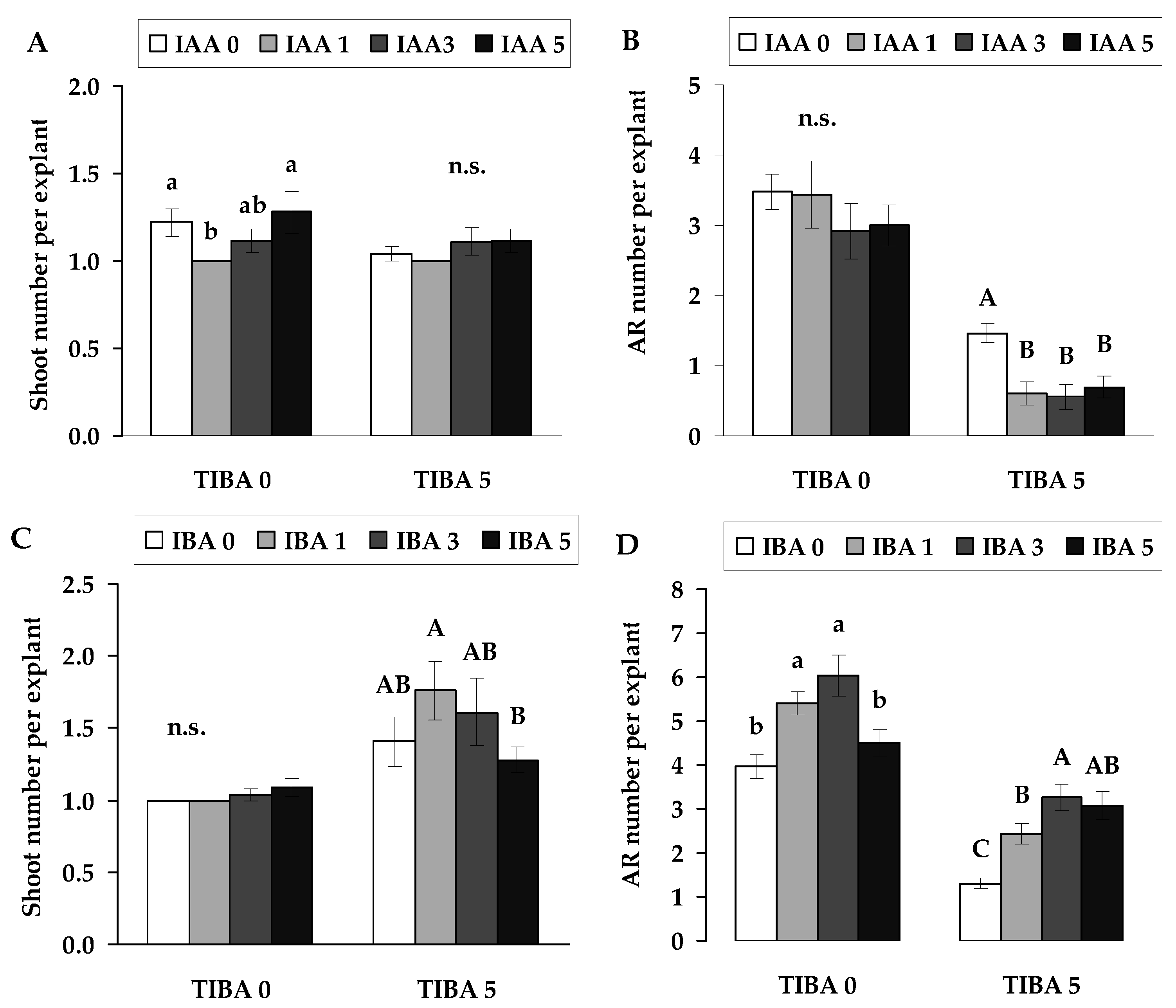
2.1. The Effect of Inhibition of Auxin Transport on Root Formation
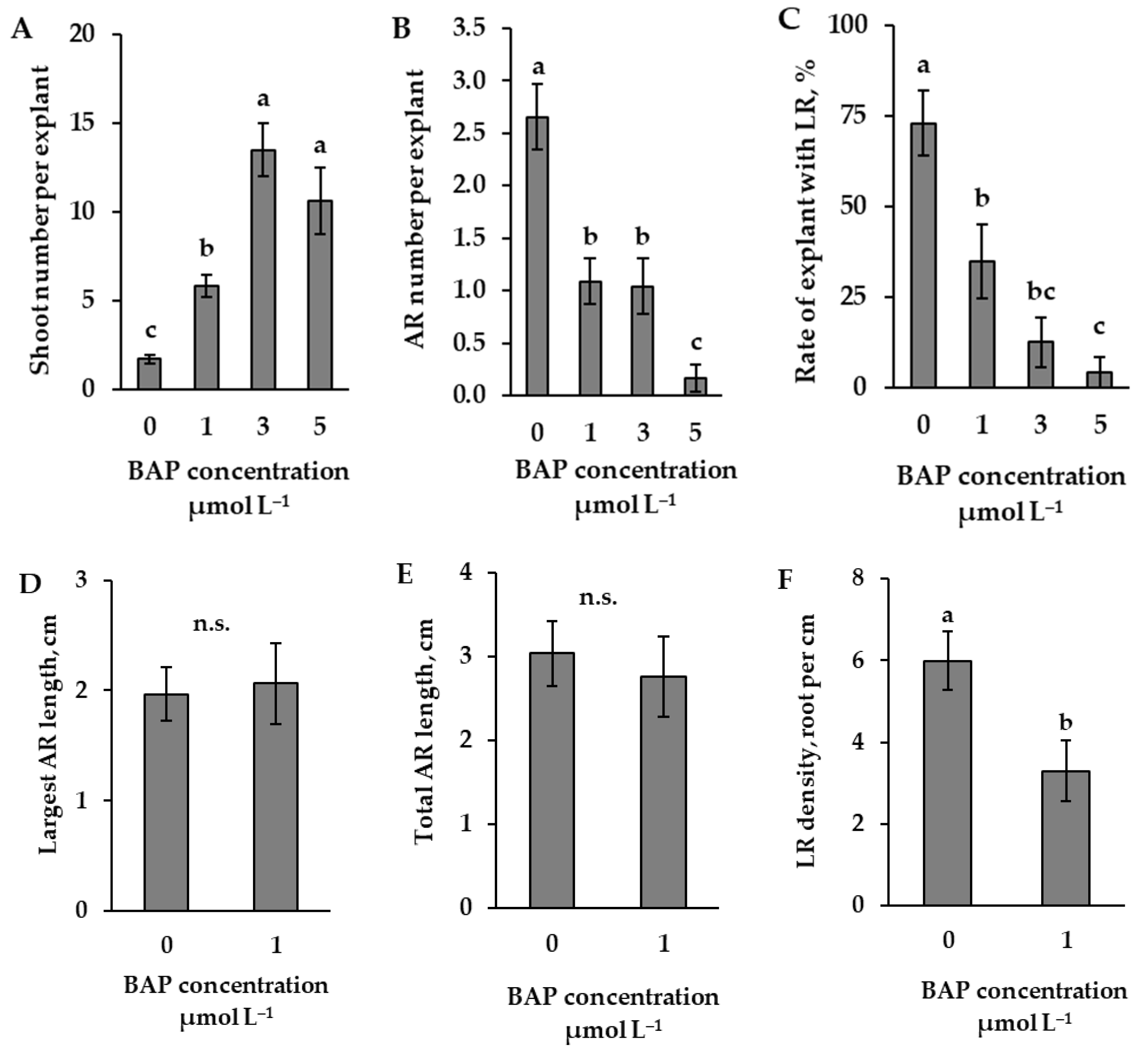
2.2. The Influence of Cytokinin, BAP, on Root Formation
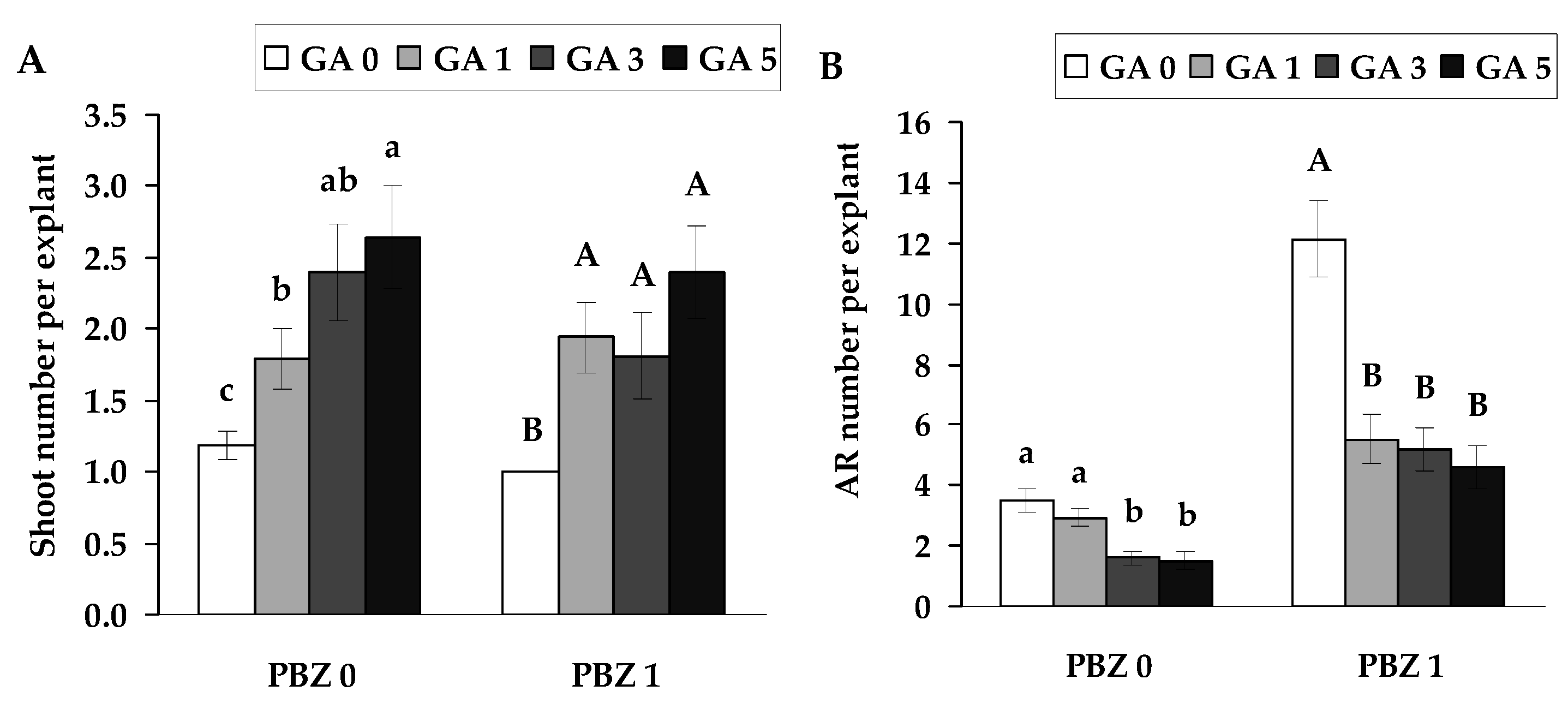
2.3. The Influence of GA on Root Formation
3. Discussion
4. Materials and Methods
4.1. Study Site and Experimental Material
4.2. Experiments on the Influence of Exogenously Applied PGR
4.3. Experimental Design and Data Analysis
4.4. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | Adventitious roots |
| BAP | 6-Benzylaminopurine |
| GA4+7 | Gibberellins A4 and A7 |
| IAA | Indole-3-acetic acid |
| IBA | Indole-3-butyric acid |
| LR | Lateral roots |
| PBZ | Paclobutrazol |
| TIBA | 2,3,5-Triiodobenzoic acid |
| WPM | Woody Plant Medium |
References
- Kusbach, A.; Šebesta, J.; Hruban, R.; Peška, P.; Rogers, P.C. Eurasian aspen (Populus tremula L.): Central Europe’s keystone species ‘hiding in plain sight’. PLoS ONE 2024, 19, e0301109. [Google Scholar] [CrossRef]
- Li, A.; Hou, Z. The complete chloroplast genome sequence of Populus tremula (Salicaceae). Mitochondrial DNA Part B 2020, 5, 2195–2196. [Google Scholar] [CrossRef]
- Robinson, K.M.; Schiffthaler, B.; Liu, H.; Rydman, S.M.; Rendón-Anaya, M.; Kalman, T.A.; Kumar, V.; Canovi, C.; Bernhardsson, C.; Delhomme, N.; et al. An improved chromosome-scale genome assembly and population genetics resource for Populus tremula. Physiol. Plant. 2024, 176, e14511. [Google Scholar] [CrossRef]
- Escamez, S.; Robinson, K.M.; Luomaranta, M.; Gandla, M.L.; Mähler, N.; Yassin, Z.; Grahn, T.; Scheepers, G.; Stener, L.G.; Jansson, S.; et al. Genetic markers and tree properties predicting wood biorefining potential in aspen (Populus tremula) bioenergy feedstock. Biotechnol. Biofuels Bioprod. 2023, 16, 65. [Google Scholar] [CrossRef]
- Kondratovičs, T.; Zeps, M.; Rupeika, D.; Zeltiņš, P.; Gailis, A.; Matisons, R. Morphological and physiological responses of hybrid aspen (Populus tremuloides Michx. × Populus tremula L.) clones to light in vitro. Plants 2022, 11, 2692. [Google Scholar] [CrossRef]
- Fracchia, F.; Mangeot-Peter, L.; Jacquot, L.; Martin, F.; Veneault-Fourrey, C.; Deveau, A. Colonization of naive roots from Populus tremula × alba involves successive waves of fungi and bacteria with different trophic abilities. Appl. Environ. Microbiol. 2021, 87, e02541-20. [Google Scholar] [CrossRef]
- Fracchia, F.; Guinet, F.; Engle, N.L.; Tschaplinski, T.J.; Veneault-Fourrey, C.; Deveau, A. Microbial colonisation rewires the composition and content of poplar root exudates, root and shoot metabolomes. Microbiome 2024, 12, 173. [Google Scholar] [CrossRef]
- Bernula, D.; Benkő, P.; Kaszler, N.; Domonkos, I.; Valkai, I.; Szőllősi, R.; Ferenc, G.; Ayaydin, F.; Fehér, A.; Gémes, K. Timely removal of exogenous cytokinin and the prevention of auxin transport from the shoot to the root affect the regeneration potential of Arabidopsis roots. Plant Cell Tissue Organ. Cult. 2020, 140, 327–339. [Google Scholar] [CrossRef]
- Ding, L.; Yang, Z.; Zheng, L.; Chen, Y.; Wei, J.; Wang, H. Exploring Transcriptional Regulatory Network During Direct De Novo Shoot Regeneration in Poplar. In Plant, Cell Environment; Wiley: Hoboken, NJ, USA, 2025. [Google Scholar] [CrossRef]
- Liu, S.; Strauss, S.; Adibi, M.; Mosca, G.; Yoshida, S.; Ioio, R.D.; Runions, A.; Andersen, T.G.; Grossmann, G.; Huijser, P.; et al. Cytokinin promotes growth cessation in the Arabidopsis root. Curr. Biol. 2022, 32, 1974–1985. [Google Scholar] [CrossRef]
- Márquez, G.; Alarcón, M.V.; Salguero, J. Cytokinin inhibits lateral root development at the earliest stages of lateral root primordium initiation in maize primary root. J. Plant Growth Regul. 2019, 38, 83–92. [Google Scholar] [CrossRef]
- Ramírez-Carvajal, G.A.; Morse, A.M.; Dervinis, C.; Davis, J.M. The cytokinin type-B response regulator PtRR13 is a negative regulator of adventitious root development in Populus. Plant Physiol. 2009, 150, 59–71. [Google Scholar] [CrossRef]
- Mandal, A. Micropropagation of Populus tremula L.: Condition for induction of shoots and roots. Scand. J. For. Res. 1989, 4, 285–293. [Google Scholar] [CrossRef]
- Peternel, Š.; Gabrovšek, K.; Gogala, N.; Regvar, M. In vitro propagation of European aspen (Populus tremula L.) from axillary buds via organogenesis. Sci. Hortic. 2009, 121, 109–112. [Google Scholar] [CrossRef]
- Huang, D.; Dai, W. Direct regeneration from in vitro leaf and petiole tissues of Populus tremula ‘Erecta’. Plant Cell Tiss. Organ. Cult. 2011, 107, 169–174. [Google Scholar] [CrossRef]
- Castro-Camba, R.; Sánchez, C.; Rico, S.; Vidal, N.; Covelo, P.; Cernadas, M.J.; Aldrey, A.; Vielba, J.M. A shift in auxin homeostasis is linked to the paclobutrazol-induced formation of adventitious roots in chestnut. J. Plant Growth Regul. 2025, 1–14. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Yang, Z.; Matsui, A.; Seki, M.; Li, S.; Yan, X.; Kohnen, M.V.; Gu, L.; Prasad, K.; et al. PtWOX11 acts as master regulator conducting the expression of key transcription factors to induce de novo shoot organogenesis in poplar. Plant Mol. Biol. 2018, 98, 389–406. [Google Scholar] [CrossRef]
- Darouez, H.; Werbrouck, S.P. In vitro rooting of poplar: Effects and metabolism of dichlorprop auxin ester prodrugs. Plants 2025, 14, 108. [Google Scholar] [CrossRef]
- Johnson, D.; Eckart, P.; Alsamadisi, N.; Noble, H.; Martin, C.; Spicer, R. Polar auxin transport is implicated in vessel differentiation and spatial patterning during secondary growth in Populus. Am. J. Bot. 2018, 105, 186–196. [Google Scholar] [CrossRef]
- Milhinhos, A.; Bollhöner, B.; Blazquez, M.A.; Novák, O.; Miguel, C.M.; Tuominen, H. ACAULIS5 is required for cytokinin accumulation and function during secondary growth of Populus trees. Front. Plant Sci. 2020, 11, 601858. [Google Scholar] [CrossRef]
- Chen, J.J.; Wang, L.Y.; Immanen, J.; Nieminen, K.; Spicer, R.; Helariutta, Y.; Zhang, J.; He, X.Q. Differential regulation of auxin and cytokinin during the secondary vascular tissue regeneration in Populus trees. New Phytol. 2019, 224, 188–201. [Google Scholar] [CrossRef]
- Dash, M.; Yordanov, Y.S.; Georgieva, T.; Kumari, S.; Wei, H.; Busov, V. A systems biology approach identifies new regulators of poplar root development under low nitrogen. Plant J. 2015, 84, 335–346. [Google Scholar] [CrossRef]
- Dash, M.; Yordanov, Y.S.; Georgieva, T.; Tschaplinski, T.J.; Yordanova, E.; Busov, V. Poplar Ptab ZIP 1-like enhances lateral root formation and biomass growth under drought stress. Plant J. 2017, 89, 692–705. [Google Scholar] [CrossRef]
- Mauriat, M.; Sandberg, L.G.; Moritz, T. Proper gibberellin localization in vascular tissue is required to control auxin-dependent leaf development and bud outgrowth in hybrid aspen. Plant J. 2011, 67, 805–816. [Google Scholar] [CrossRef]
- Deng, J.; Deng, X.; Yao, H.; Ji, S.; Dong, L. Gibberellins play an essential role in the bud growth of Petunia hybrida. Curr. Issues Mol. Biol. 2024, 46, 9906–9915. [Google Scholar] [CrossRef]
- Veerabagu, M.; van der Schoot, C.; Turečková, V.; Tarkowská, D.; Strnad, M.; Rinne, P.L. Light on perenniality: Para-dormancy is based on ABA-GA antagonism and endo-dormancy on the shutdown of GA biosynthesis. Plant Cell Environ. 2023, 46, 1785–1804. [Google Scholar] [CrossRef]
- Mauriat, M.; Petterle, A.; Bellini, C.; Moritz, T. Gibberellins inhibit adventitious rooting in hybrid aspen and Arabidopsis by affecting auxin transport. Plant J. 2014, 78, 372–384. [Google Scholar] [CrossRef]
- Li, J.; Xu, P.; Zhang, B.; Song, Y.; Wen, S.; Bai, Y.; Ji, L.; Lai, Y.; He, G.; Zhang, D. Paclobutrazol promotes root development of difficult-to-root plants by coordinating auxin and abscisic acid signaling pathways in Phoebe bournei. Int. J. Mol. Sci. 2023, 24, 3753. [Google Scholar] [CrossRef]
- El-Showk, S.; Ruonala, R.; Helariutta, Y. Crossing paths: Cytokinin signalling and crosstalk. Development 2013, 140, 1373–1383. [Google Scholar] [CrossRef]
- Hu, W.; Fagundez, S.; Katin-Grazzini, L.; Li, Y.; Li, W.; Chen, Y.; Wang, X.; Deng, Z.; Xie, S.; McAvoy, R.J.; et al. Endogenous auxin and its manipulation influence in vitro shoot organogenesis of citrus epicotyl explants. Hortic. Res. 2017, 4, 17071. [Google Scholar] [CrossRef]
- Ahuja, M.R. In vitro propagation of poplar and aspen. In Cell and Tissue Culture in Forestry. Forestry Sciences; Bonga, J.M., Durzan, D.J., Eds.; Springer: Dordrecht, The Netherlands, 1987; pp. 207–223. [Google Scholar]
- Sellmer, J.C.; Mccown, B.H.; Haissig, B.E. Shoot culture dynamics of six Populus clones. Tree Physiol. 1989, 5, 219–227. [Google Scholar] [CrossRef]
- De Almeida, M.R.; Aumond, M.; Da Costa, C.T.; Schwambach, J.; Ruedell, C.M.; Correa, L.R.; Fett-Neto, A.G. Environmental control of adventitious rooting in Eucalyptus and Populus cuttings. Trees 2017, 31, 1377–1390. [Google Scholar] [CrossRef]
- Ribeiro, Y.R.d.S.; Aragão, V.P.M.; Carrari-Santos, R.; Rodrigues de Sousa, K.; Ferreira Macedo, A.; Iochevet Segal Floh, E.; Silveira, V.; Santa-Catarina, C. 2,3,5-triiodobenzoic acid affects the in vitro propagation of Cedrela fissilis Vell. (Meliaceae) through alterations in endogenous polyamine and indol-3-acetic acid levels and the proteomic profile. Plant Cell Tiss. Organ. Cult. 2024, 25, 156–181. [Google Scholar] [CrossRef]
- Gou, J.; Strauss, S.H.; Tsai, C.J.; Fang, K.; Chen, Y.; Jiang, X.; Busova, V.B. Gibberellins regulate lateral root formation in Populus through interactions with auxin and other hormones. Plant Cell 2010, 22, 623–639. [Google Scholar] [CrossRef]
- Yordanov, Y.S.; Ma, C.; Yordanova, E.; Meilan, R.; Strauss, S.H.; Busov, V.B. BIG LEAF is a regulator of organ size and adventitious root formation in poplar. PLoS ONE 2017, 12, e0180527. [Google Scholar] [CrossRef]
- Baba, K.; Karlberg, A.; Schmidt, J.; Schrader, J.; Hvidsten, T.R.; Bako, L.; Bhalerao, R.P. Activity-dormancy transition in the cambial meristem involves stage-specific modulation of auxin response in hybrid aspen. Proc. Natl. Acad. Sci. USA 2011, 108, 3418–3423. [Google Scholar] [CrossRef]
- Nieminen, K.; Immanen, J.; Laxell, M.; Kauppinen, L.; Tarkowski, P.; Dolezal, K.; Tähtiharju, S.; Elo, A.; Decourteix, M.; Ljung, K.; et al. Cytokinin signaling regulates cambial development in poplar. Proc. Natl. Acad. Sci. USA 2008, 105, 20032–20037. [Google Scholar] [CrossRef]
- Shang, E.; Wei, K.; Lv, B.; Zhang, X.; Lin, X.; Ding, Z.; Leng, J.; Tian, H.; Ding, Z. VIK-Mediated auxin signaling regulates lateral root development in Arabidopsis. Adv. Sci. 2024, 11, e2402442. [Google Scholar] [CrossRef]
- Eliasson, L. Growth Regulators in Populus tremula I. Distribution of auxin and growth inhibitors. Physiol. Plant. 1969, 22, 1288–1301. [Google Scholar] [CrossRef]
- Eliasson, L. Growth Regulators in Populus tremula IV. Apical dominance and suckering in young plants. Physiol. Plant. 1971, 25, 263–267. [Google Scholar] [CrossRef]
- Tuominen, H.; Puech, L.; Fink, S.; Sundberg, B. A Radial concentration gradient of indole-3-acetic acid is related to secondary xylem development in hybrid aspen. Plant Physiol. 1997, 115, 577–585. [Google Scholar] [CrossRef]
- Bustillo-Avendaño, E.; Ibáñez, S.; Sanz, O.; Barros, J.A.S.; Gude, I.; Perianez-Rodriguez, J.; Micol, J.L.; Del Pozo, J.C.; Moreno-Risueno, M.A.; Pérez-Pérez, J.M. Regulation of Hormonal control, cell reprogramming, and patterning during de novo root organogenesis. Plant Physiol. 2018, 176, 1709–1727. [Google Scholar] [CrossRef] [PubMed]
- Abu-Abied, M.; Belausov, E.; Hagay, S.; Peremyslov, V.; Dolja, V.; Sadot, E. Myosin XI-K is involved in root organogenesis, polar auxin transport, and cell division. J. Exp. Bot. 2018, 69, 2869–2881. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.P.; Yang, R.H.; Wang, F.; Sun, L.N.; Song, X.S. Effect of auxins and associated metabolic changes on cuttings of hybrid aspen. Forest 2017, 8, 117. [Google Scholar] [CrossRef]
- Coleman, G.D.; Ernst, S.G. In vitro shoot regeneration of Populus deltoides: Effect of cytokinin and genotype. Plant Cell Rep. 1989, 8, 459–462. [Google Scholar] [CrossRef]
- Bishopp, A.; Help, H.; El-Showk, S.; Weijers, D.; Scheres, B.; Friml, J.; Benková, E.; Mähönen, A.P.; Helariutta, Y. A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Curr. Biol. 2011, 21, 917–926. [Google Scholar] [CrossRef]
- Eriksson, M.E.; Israelsson, M.; Olsson, O.; Moritz, T. Increased gibberellin biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nat. Biotechnol. 2000, 18, 784–788. [Google Scholar] [CrossRef]
- Allingham, R. The Effect of the Growth Retardant Paclobutrazol on the in Vitro Growth and Development of Betula and Populus species. Ph.D. Thesis, University of Central Lancashire, Preston, UK, 2005. [Google Scholar]
- Žiauka, J.; Kuusiene, S. Different inhibitors of the gibberellin biosynthesis pathway elicit various responses during in vitro culture of aspen (Populus tremula L.). Plant Cell Tiss. Organ. Cult. 2010, 102, 221–228. [Google Scholar] [CrossRef]
- McCown, B.H.; Lloyd, G. Woody plant medium (WPM)—A mineral nutrient formulation for microculture of woody plant species. Hort. Sci. 1981, 16, 453. [Google Scholar]
- Welch, B.L. The generalization of “student’s” problem when several different population variances are involved. Biometrika 1947, 34, 28–35. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaičiukynė, M.; Žiauka, J.; Černiauskas, V.; Varnagirytė-Kabašinskienė, I. Role of Plant Growth Regulators in Adventitious Populus Tremula Root Development In Vitro. Plants 2025, 14, 2427. https://doi.org/10.3390/plants14152427
Vaičiukynė M, Žiauka J, Černiauskas V, Varnagirytė-Kabašinskienė I. Role of Plant Growth Regulators in Adventitious Populus Tremula Root Development In Vitro. Plants. 2025; 14(15):2427. https://doi.org/10.3390/plants14152427
Chicago/Turabian StyleVaičiukynė, Miglė, Jonas Žiauka, Valentinas Černiauskas, and Iveta Varnagirytė-Kabašinskienė. 2025. "Role of Plant Growth Regulators in Adventitious Populus Tremula Root Development In Vitro" Plants 14, no. 15: 2427. https://doi.org/10.3390/plants14152427
APA StyleVaičiukynė, M., Žiauka, J., Černiauskas, V., & Varnagirytė-Kabašinskienė, I. (2025). Role of Plant Growth Regulators in Adventitious Populus Tremula Root Development In Vitro. Plants, 14(15), 2427. https://doi.org/10.3390/plants14152427





