Interacting Effects of Heat and Nanoplastics Affect Wheat (Triticum turgidum L.) Seedling Growth and Physiology
Abstract
1. Introduction
2. Results
2.1. Effect of Heat and Nanoplastics on Plant Growth Parameters and Photosynthetic Pigments Content
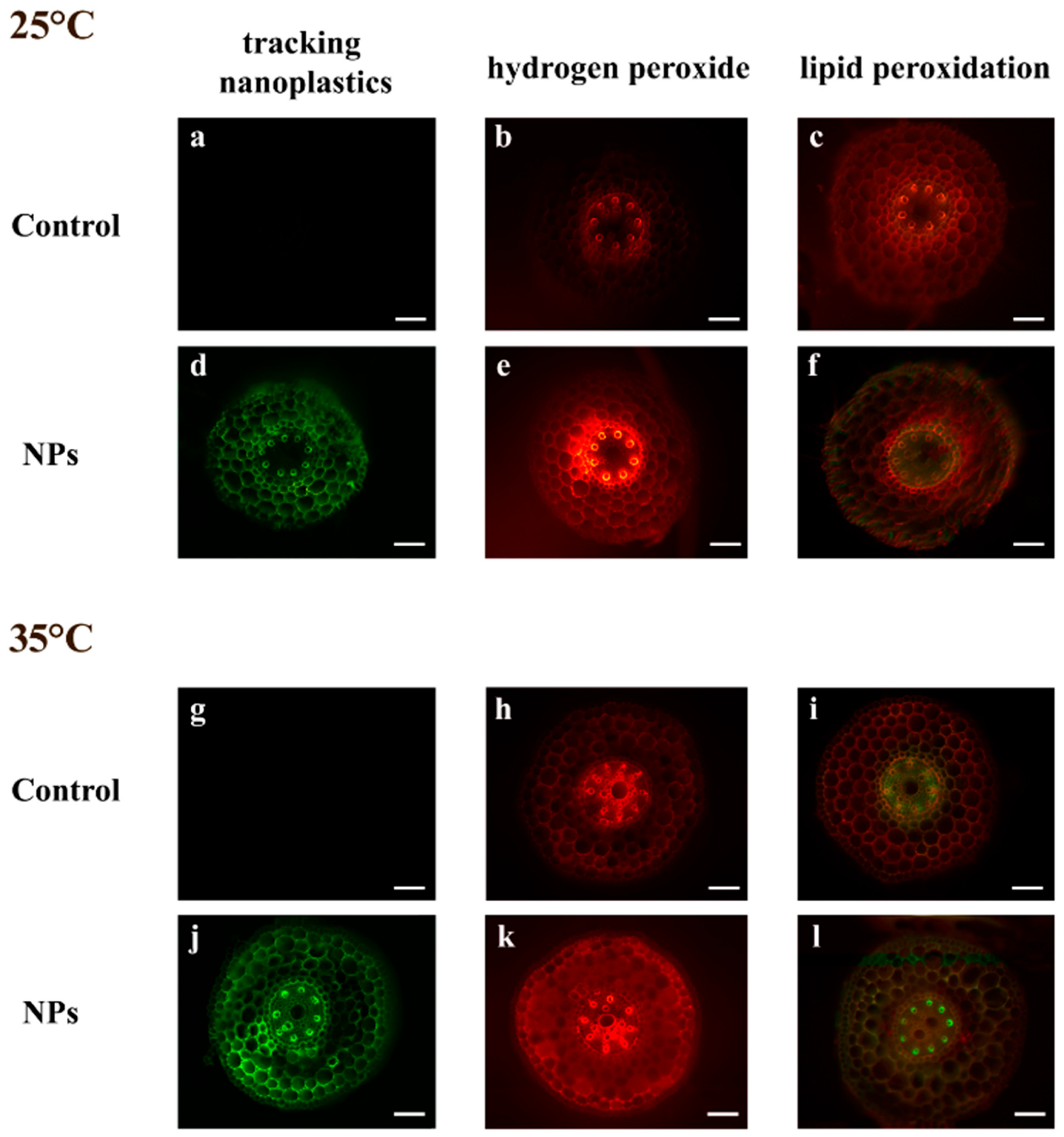
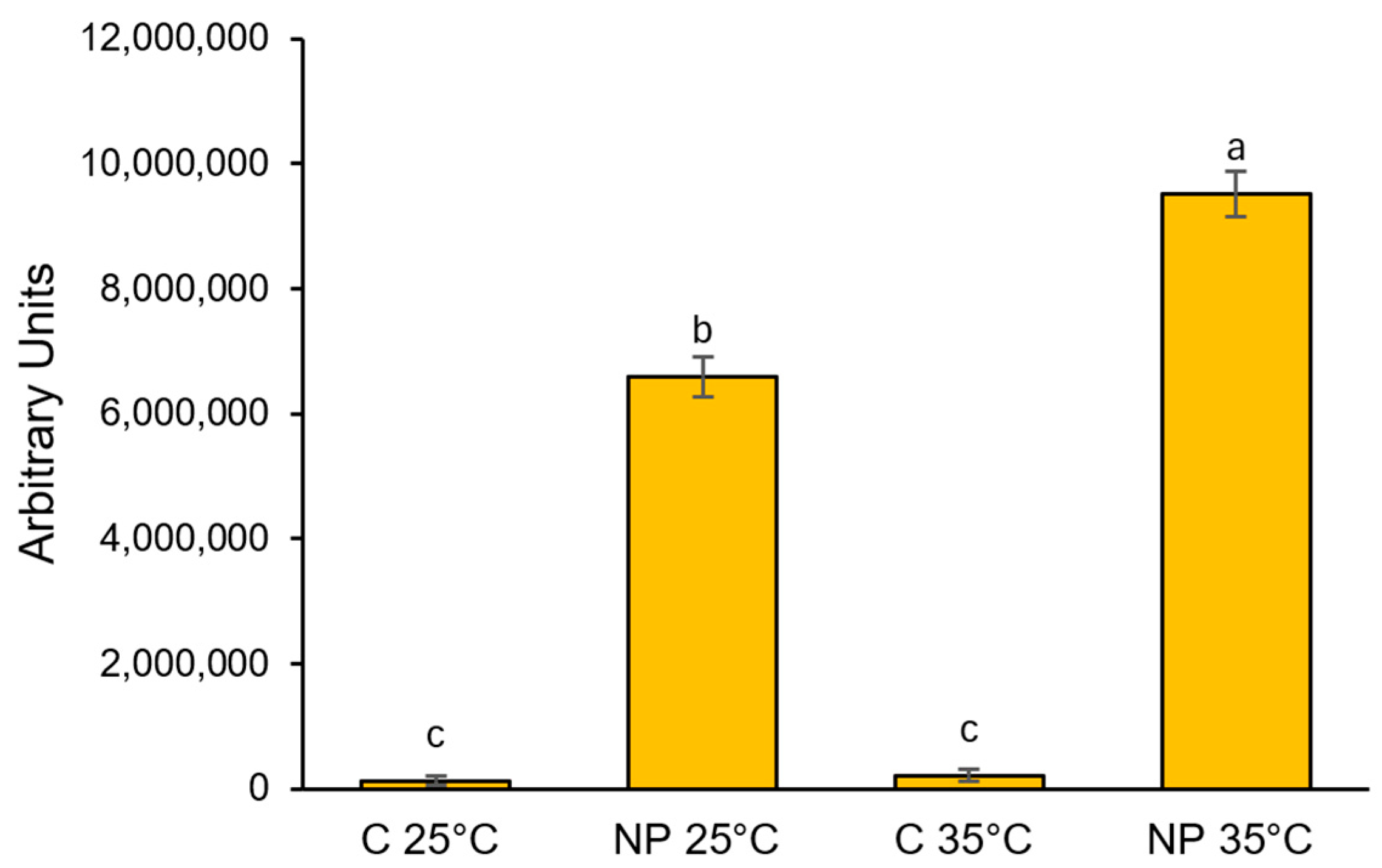
2.2. Tracking Nanoplastics Uptake
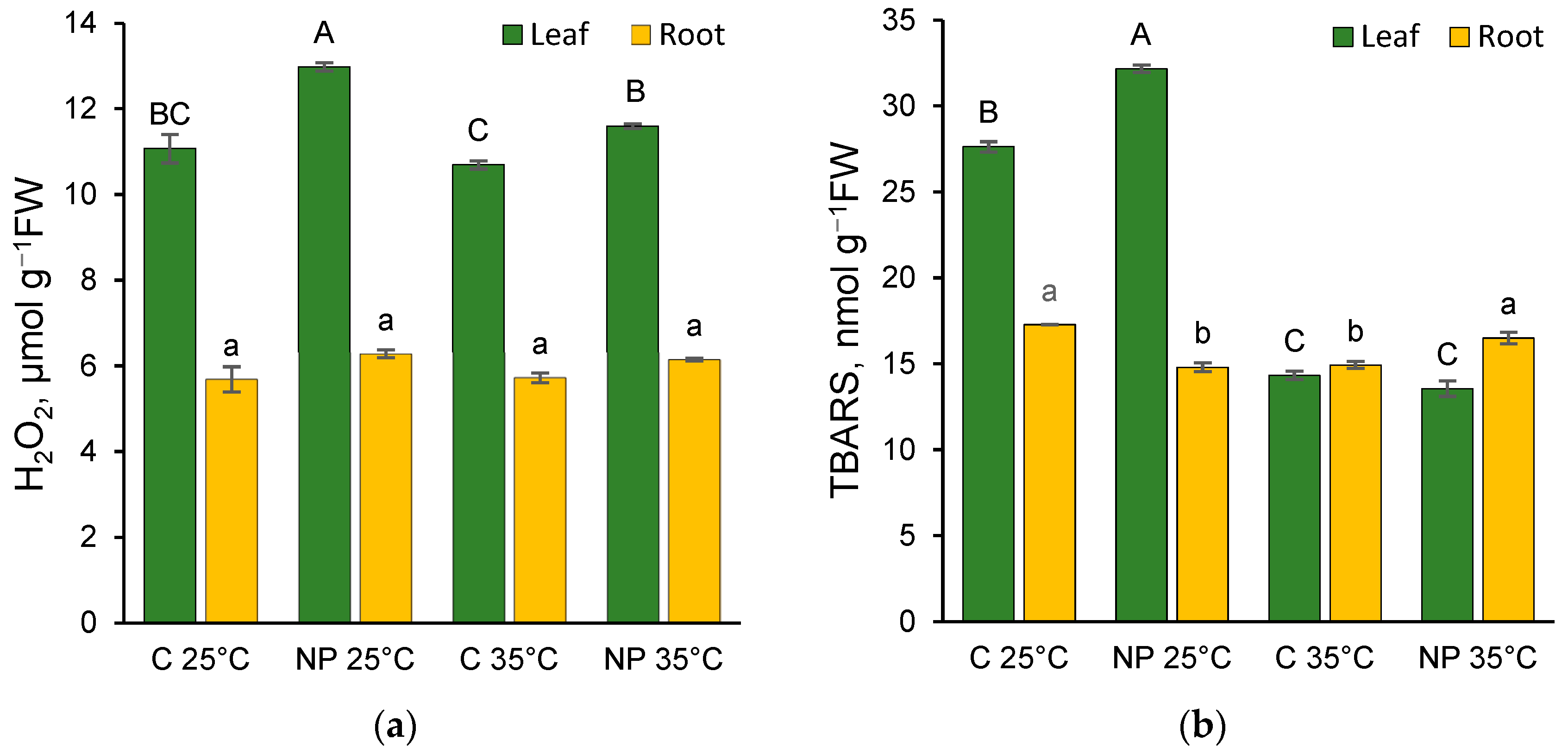
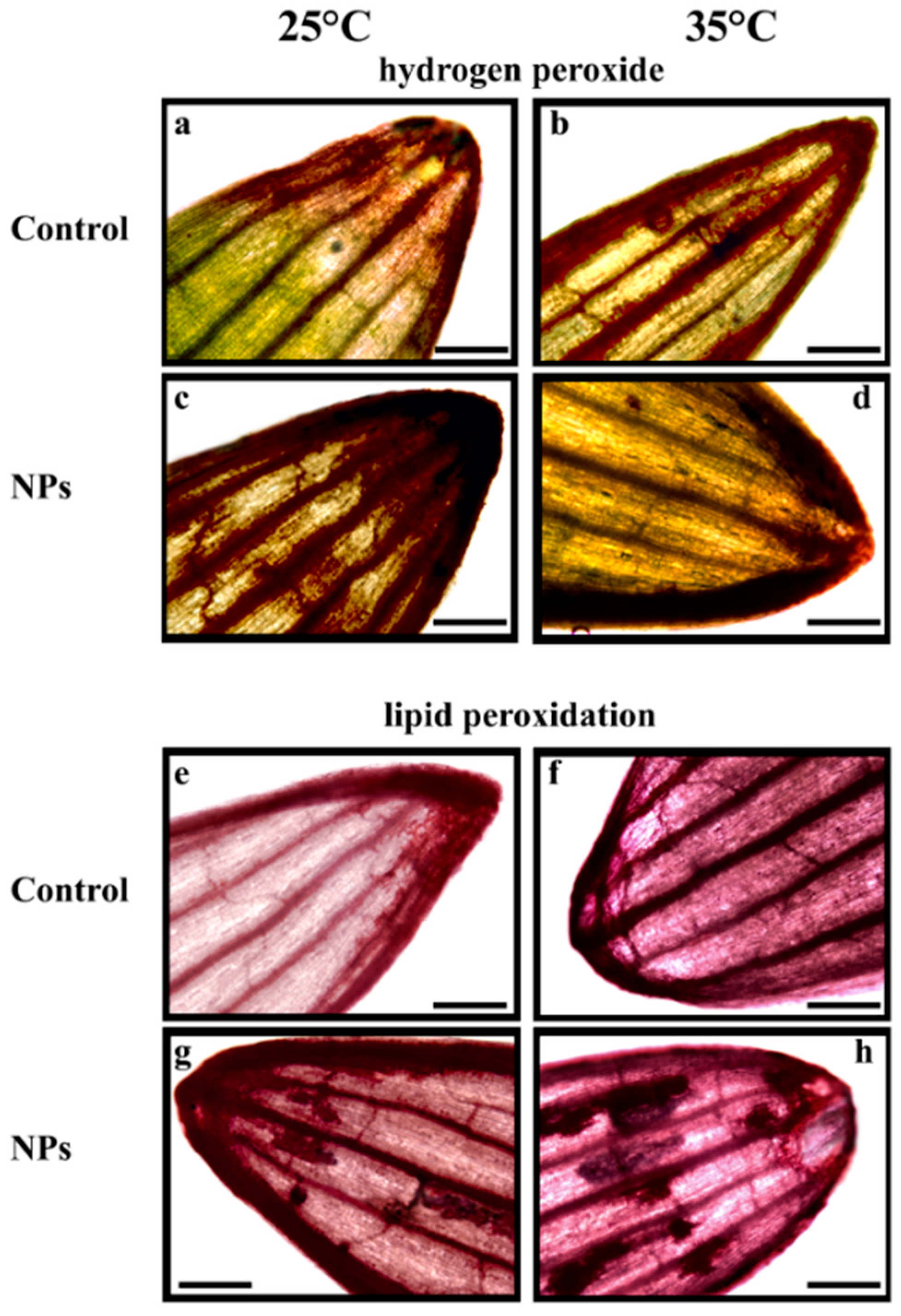
2.3. Effect of Heat and Nanoplastics on Oxidative Stress Markers
2.4. Effect of Heat and Nanoplastics on Proline Level
2.5. Effect of Heat and Nanoplastics on Soluble Protein Content and Enzymatic Activity
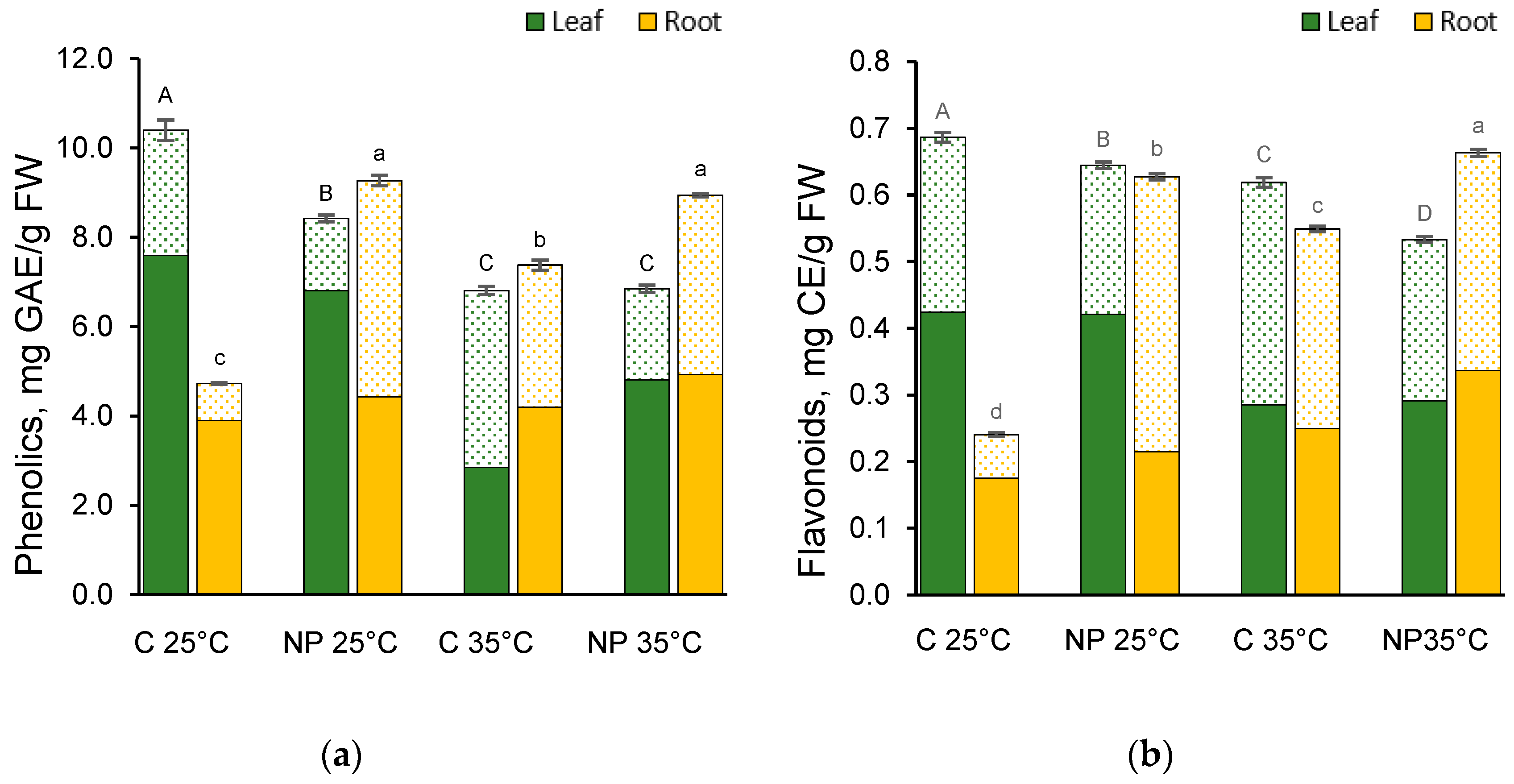
2.6. Effect of Heat and Nanoplastics on Polyphenols Content
3. Discussion
3.1. Nanoplastics Uptake
3.2. Effect of Heat and Nanoplastics on Plant Growth Parameters and Photosynthetic Pigments Content
3.3. Effects of Heat and Nanoplastics on Oxidative Stress Markers: Hydrogen Peroxide and TBARS Content
3.4. Effect of Heat and Nanoplastics on the Antioxidant Response
3.5. Effect of Heat and Nanoplastics on Polyphenols Content
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. In Situ Assessment of Nanoplastics Uptake, and Histochemical Evaluation of Oxidative Stress Markers
4.3. Extraction and Determination of Photosynthetic Pigments
4.4. Extraction and Determination of Oxidative Stress Markers: Hydrogen Peroxide and Thiobarbituric Acid Reactive Substances
4.5. Proline Extraction and Determination
4.6. Extraction and Determination of Antioxidant Enzyme Activities
4.7. Polyphenols Extraction and Quantification
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thompson, R.C.; Swan, S.H.; Moore, C.J.; vom Saal, F.S. Our plastic age. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1973–1976. [Google Scholar] [CrossRef]
- Dokl, M.; Copot, A.; Krajnc, D.; Van Fan, Y.; Vujanović, A.; Aviso, K.B.; Tan, R.R.; Kravanja, Z.; Čuček, L. Global projections of plastic use, end-of-life fate and potential changes in consumption, reduction, recycling and replacement with bioplastics to 2050. Sustain. Prod. Consum. 2024, 51, 498–518. [Google Scholar] [CrossRef]
- Bottega, S.; Fontanini, D.; Ruffini Castiglione, M.; Spanò, C. The impact of polystyrene nanoplastics on plants in the scenario of increasing temperatures: The case of Azolla filiculoides Lam. Plant Physiol. Biochem. 2024, 214, 108946. [Google Scholar] [CrossRef]
- Khan, A.R.; Ulhassan, Z.; Li, G.; Lou, J.; Iqbal, B.; Salam, A.; Azhar, W.; Batool, S.; Zhao, T.; Li, K.; et al. Micro/nanoplastics: Critical review of their impacts on plants, interactions with other contaminants (antibiotics, heavy metals, and polycyclic aromatic hydrocarbons), and management strategies. Sci. Total Environ. 2024, 912, 169420. [Google Scholar] [CrossRef]
- Zantis, L.J.; Borchi, C.; Vijver, M.G.; Peijnenburg, W.; Di Lonardo, S.; Bosker, T. Nano- and microplastics commonly cause adverse impacts on plants at environmentally relevant levels: A systematic review. Sci. Total Environ. 2023, 867, 161211. [Google Scholar] [CrossRef]
- Parthasarathi, T.; Firdous, S.; David, E.M.; Lesharadevi, K.; Djanaguiraman, M. Effects of high temperature on crops. In Advances in Plant Defense Mechanisms; Ngui Kimatu, J., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Sanpradit, P.; Byeon, E.; Lee, J.S.; Jeong, H.; Kim, H.S.; Peerakietkhajorn, S.; Lee, J.S. Combined effects of nanoplastics and elevated temperature in the freshwater water flea Daphnia magna. J. Hazard. Mater. 2024, 465, 133325. [Google Scholar] [CrossRef]
- Sulukan, E.; Baran, A.; Şenol, O.; Yildirim, S.; Mavi, A.; Ceyhun, H.A.; Toraman, E.; Ceyhun, S.B. The synergic toxicity of temperature increases and nanopolystrene on zebrafish brain implies that global warming may worsen the current risk based on plastic debris. Sci. Total Environ. 2022, 808, 152092. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y.; O’Brien, A.M.; Lins, T.F.; Rochman, C.M.; Sinton, D. Biological responses to climate change and nanoplastics are altered in concert: Full-factor screening reveals effects of multiple stressors on primary producers. Environ. Sci. Technol. 2020, 54, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, S.; Wang, L.; Zhang, P.; Liu, T.; Li, X. Temperature fluctuation in soil alters the nanoplastic sensitivity in wheat. Sci. Total Environ. 2024, 929, 172626. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Feng, Y.X.; Yu, X.Z. Micro- and nano-plastics in hydroponic environment are critical for plants: A meta-analysis. Appl. Environ. Biotechnol. 2023, 8, 9–17. [Google Scholar] [CrossRef]
- Zytowski, E.; Mollavali, M.; Baldermann, S. Uptake and translocation of nanoplastics in mono and dicot vegetables. Plant Cell Environ. 2025, 48, 134–148. [Google Scholar] [CrossRef]
- Pradel, A.; Catrouillet, C.; Gigault, J. The environmental fate of nanoplastics: What we know and what we need to know about aggregation. NanoImpact 2023, 29, 100453. [Google Scholar] [CrossRef]
- Spanò, C.; Giorgetti, L.; Bottega, S.; Muccifora, S.; Ruffini Castiglione, M. Titanium dioxide nanoparticles enhance the detrimental effect of polystyrene nanoplastics on cell and plant physiology of Vicia lens (L.) Coss. & Germ. seedlings. Front. Plant Sci. 2024, 15, 391751. [Google Scholar] [CrossRef]
- Giorgetti, L.; Spanò, C.; Muccifora, S.; Bottega, S.; Barbieri, F.; Bellani, L.; Ruffini Castiglione, M. Exploring the interaction between polystyrene nanoplastics and Allium cepa during germination: Internalization in root cells, induction of toxicity and oxidative stress. Plant Physiol. Biochem. 2020, 149, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Xu, X.; Guo, L.; Jin, R.; Lu, Y. Uptake and transport of micro/nanoplastics in terrestrial plants: Detection, mechanisms, and influencing factors. Sci. Total Environ. 2024, 907, 168155. [Google Scholar] [CrossRef]
- Lian, J.; Wu, J.; Xiong, H.; Zeb, A.; Yang, T.; Su, X.; Su, L.; Weitao, L. Impact of polystyrene nanoplastics (PSNPs) on seed germination and seedling growth of wheat (Triticum aestivum L.). J. Hazard. Mater. 2020, 385, 121620. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, R.; Zhang, S.; Sun, Y.; Wang, F. Uptake and translocation of nano/microplastics by rice seedlings: Evidence from a hydroponic experiment. J. Hazard. Mater. 2022, 421, 126700. [Google Scholar] [CrossRef]
- Spanò, C.; Muccifora, S.; Ruffini Castiglione, M.; Bellani, L.; Bottega, S.; Giorgetti, L. Polystyrene nanoplastics affect seed germination, cell biology and physiology of rice seedlings in-short term treatments: Evidence of their internalization and translocation. Plant Physiol. Biochem. 2022, 172, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Jamil, A.; Ahmad, A.; Moeen-Ud-Din, M.; Zhang, Y.; Zhao, Y.; Chen, X.; Cui, X.; Tong, Y.; Liu, X. Unveiling the mechanism of micro-and-nano plastic phytotoxicity on terrestrial plants: A comprehensive review of omics approaches. Environ. Int. 2025, 195, 109257. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Wang, L.; Gao, J.; Jiang, Y.; Adeel, M.; Hou, D. Nanoplastic–plant interaction and implications for soil health. Soil Use Manag. 2022, 39, 13–42. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Li, R.; Zhou, Q.; Peijnenburg, W.J.G.M.; Yin, N.; Yang, J.; Tu, C.; Zhang, Y. Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat. Sustain. 2020, 3, 929–937. [Google Scholar] [CrossRef]
- Cano-Ramirez, D.L.; Carmona-Salazar, L.; Morales-Cedillo, F.; Ramírez-Salcedo, J.; Cahoon, E.B.; Gavilanes-Ruíz, M. Plasma membrane fluidity: An environment thermal detector in plants. Cells 2021, 10, 2778. [Google Scholar] [CrossRef]
- Sadok, W.; Lopez, J.R.; Smith, K.P. Transpiration increases under high-temperature stress: Potential mechanisms, trade-offs and prospects for crop resilience in a warming world. Plant Cell Environ. 2021, 44, 2102–2116. [Google Scholar] [CrossRef]
- Lee, C.H.; Fang, J.K.H. Effects of temperature and particle concentration on aggregation of nanoplastics in freshwater and seawater. Sci. Total Environ. 2022, 817, 152562. [Google Scholar] [CrossRef]
- Iqbal, M.; Raja, N.I.; Yasmeen, F.; Hussain, M.; Ejaz, M.; Shah, M.A. Impacts of heat stress on wheat: A critical review. Adv. Crop Sci. Technol. 2017, 5, 251. [Google Scholar] [CrossRef]
- Farhad, M.; Kumar, U.; Tomar, V.; Bhati, P.K.; Krishnan, J.N.; Kishowar-E-Mustarin; Barek, V.; Brestic, M.; Hossain, A. Heat stress in wheat: A global challenge to feed billions in the current era of the changing climate. Front. Sustain. Food Syst. 2023, 7, 1203721. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, F.; Zhou, K.; Lin, M.; Shi, L.; Mi, S.; Pan, H.; Yao, Q.; Zhao, X. Polyethylene nanoplastics, tebuconazole and cadmium affect soil-wheat system by altering rhizosphere microenvironment under single or combined exposure. J. Hazard. Mater. 2024, 480, 135843. [Google Scholar] [CrossRef] [PubMed]
- Mohi-Ud-Din, M.; Siddiqui, M.N.; Rohman, M.M.; Jagadish, S.V.K.; Ahmed, J.U.; Hassan, M.M.; Hossain, A.; Islam, T. Physiological and biochemical dissection reveals a trade-off between antioxidant capacity and heat tolerance in bread wheat (Triticum aestivum L.). Antioxidants 2021, 10, 351. [Google Scholar] [CrossRef]
- Bascuñán-Godoy, L.; Sanhueza, C.; Cuba, M.; Zuñiga, G.E.; Corcuera, L.J.; Bravo, L.A. Cold-acclimation limits low temperature induced photoinhibition by promoting a higher photochemical quantum yield and a more effective PSII restoration in darkness in the Antarctic rather than the Andean ecotype of Colobanthus quitensis Kunt Bartl (Cariophyllaceae). BMC Plant Biol. 2012, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, S.; Lasorella, C.; Dipierro, N.; Vita, F.; de Pinto, M.C. Redox signaling in plant heat stress response. Antioxidants 2023, 12, 605. [Google Scholar] [CrossRef]
- Ekner-Grzyb, A.; Duka, A.; Grzyb, T.; Lopes, I.; Chmielowska-Bąk, J. Plants oxidative response to nanoplastic. Front. Plant Sci. 2022, 13, 1027608. [Google Scholar] [CrossRef]
- Ruffini Castiglione, M.; Bottega, S.; Sorce, C.; Spanò, C. Effects of zinc oxide particles with different sizes on root development in Oryza sativa. Rice Sci. 2023, 30, 449–458. [Google Scholar] [CrossRef]
- Spanò, C.; Bottega, S. Durum wheat seedlings in saline conditions: Salt spray versus root-zone salinity. Estuar. Coast. Shelf Sci. 2016, 169, 173–181. [Google Scholar] [CrossRef]
- Mudgal, V.; Madaan, N.; Mudgal, A. Biochemical mechanisms of salt tolerance in plants: A review. Int. J. Bot. 2010, 6, 136–143. [Google Scholar] [CrossRef]
- Spormann, S.; Nadais, P.; Sousa, F.; Pinto, M.; Martins, M.; Sousa, B.; Fidalgo, F.; Soares, C. Accumulation of proline in plants under contaminated soils-are we on the same page? Antioxidants 2023, 12, 666. [Google Scholar] [CrossRef]
- Li, Z.; Li, R.; Li, Q.; Zhou, J.; Wang, G. Physiological response of cucumber (Cucumis sativus L.) leaves to polystyrene nanoplastics pollution. Chemosphere 2020, 255, 127041. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in abiotic stress response. Trends Plant Sci. 2004, 9, 244–253. [Google Scholar] [CrossRef]
- Levitt, J. Responses of Plants to Environmental Stresses, Vol. I; Academic Press: New York, NY, USA, 1980; pp. 347–370. [Google Scholar]
- Li, Z.; Li, Q.; Li, R.; Zhou, J.; Wang, G. The distribution and impact of polystyrene nanoplastics on cucumber plants. Environ. Sci. Pollut. Res. 2021, 28, 16042–16053. [Google Scholar] [CrossRef]
- Pandey, S.; Fartyal, D.; Agarwal, A.; Shukla, T.; James, D.; Kaul, T.; Negi, Y.K.; Arora, S.; Reddy, M.K. Abiotic stress tolerance in plants: Myriad roles of ascorbate peroxidase. Front. Plant Sci. 2017, 8, 581. [Google Scholar] [CrossRef]
- Dongsansuk, A.; Paethaisong, W.; Theerakulpisut, P. Membrane stability and antioxidant enzyme activity of rice seedlings in response to short-term high temperature treatments. Chil. J. Agric. Res. 2021, 81, 607–617. [Google Scholar] [CrossRef]
- Yemelyanov, V.V.; Lastochkin, V.V.; Prikaziuk, E.G.; Chirkova, T.V. Activities of catalase and peroxidase in wheat and rice plants under conditions of anoxia and post-anoxic aeration. Russ. J. Plant Physiol. 2022, 69, 117. [Google Scholar] [CrossRef]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef]
- Rao, M.J.; Zheng, B. The role of polyphenols in abiotic stress tolerance and their antioxidant properties to scavenge reactive oxygen species and free radicals. Antioxidants 2025, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, M.; Babawale, E.A.; Furtado, A.; Henry, R.J.; Eck, P.K.; Jones, P.J.H. Effects of genotype and temperature on accumulation of plant secondary metabolites in Canadian and Australian wheat grown under controlled environments. Sci. Rep. 2017, 7, 9133. [Google Scholar] [CrossRef]
- Ren, F.; Huang, J.; Yang, Y. Unveiling the impact of microplastics and nanoplastics on vascular plants: A cellular metabolomic and transcriptomic review. Ecotoxicol. Environ. Saf. 2024, 279, 116490. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Qiao, C.; Han, L.; Bi, Y.; Cao, M.; Wang, S.; Guo, L.; Pang, R.; Xie, H. Multi-omics analyses reveal the responses of wheat (Triticum aestivum L.) and rhizosphere bacterial community to nano(micro)plastics stress. J. Nanobiotechnol. 2024, 22, 507. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ruffini Castiglione, M.; Giorgetti, L.; Bellani, L.; Muccifora, S.; Bottega, S.; Spanò, C. Root responses to different types of TiO2 nanoparticles and bulk counterpart in plant model system Vicia faba L. Environ. Exp. Bot. 2016, 130, 11–21. [Google Scholar] [CrossRef]
- Spanò, C.; Bottega, S.; Sorce, C.; Bartoli, G.; Ruffini Castiglione, M. TiO2 nanoparticles may alleviate cadmium toxicity in co-treatment experiments on the model hydrophyte Azolla filiculoides. Environ. Sci. Pollut. Res. 2019, 26, 29872–29882. [Google Scholar] [CrossRef]
- Spanò, C.; Bottega, S.; Lorenzi, R.; Grilli, I. Ageing in embryos from wheat grains stored at different temperatures: Oxidative stress and antioxidant response. Funct. Plant Biol. 2011, 38, 624–631. [Google Scholar] [CrossRef]
- Spanò, C.; Bottega, S.; Ruffini Castiglione, M.; Pedranzani, H.E. Antioxidant response to cold stress in two oil plants of the genus Jatropha. Plant Soil Environ. 2017, 63, 271–276. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Spanò, C.; Bruno, M.; Bottega, S. Calystegia soldanella: Dune versus laboratory plants to highlight key adaptive physiological traits. Acta Physiol. Plant. 2013, 35, 1329–1336. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Arezki, O.; Boxus, P.; Kevers, C.; Gaspar, T. Changes in peroxidase activity, and level of phenolic compounds during light-induced plantlet regeneration from Eucalyptus camaldulensis Dhen. nodes in vitro. Plant Growth Regul. 2001, 33, 215–219. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Fontanini, D.; Bulleri, F.; Ravaglioli, C.; Capocchi, A. A comparison of methods for assessing the antioxidant expression in Posidonia oceanica (L.) Delile. Molecules 2025, 30, 1828. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
| Leaf Length | Proline | Protein | APX | POX | CAT | |
|---|---|---|---|---|---|---|
| C 25 °C | 172.78 ± 4.38 b | 4.08 ± 0.09 b | 3.01 ± 0.21 a | 2.09 ± 0.02 d | 1.87 ± 0.03 c | 4.58 ± 0.31 a |
| NP 25 °C | 191.12 ± 4.03 a | 3.98 ± 0.09 b | 3.23 ± 0.20 a | 2.58 ± 0.04 c | 1.96 ± 0.04 c | 2.09 ± 0.14 c |
| C 35 °C | 66.20 ± 1.89 d | 6.03 ± 0.25 a | 3.08 ± 0.09 a | 3.50 ± 0.05 a | 2.92 ± 0.06 a | 3.42 ± 0.23 b |
| NP 35 °C | 85.60 ± 1.86 c | 3.65 ± 0.21 b | 3.34 ± 0.19 a | 2.83 ± 0.05 b | 2.43 ± 0.08 b | 2.22 ± 0.08 c |
| Root Length | Proline | Protein | APX | POX | CAT | |
|---|---|---|---|---|---|---|
| C 25 °C | 83.78 ± 3.17 b | 4.64 ± 0.07 a | 1.72 ± 0.03 c | 4.78 ± 0.23 a | 5.35 ± 0.80 a | 3.51 ± 0.17 a |
| NP 25 °C | 97.52 ± 3.12 a | 2.92 ± 0.22 b | 2.06 ± 0.20 bc | 4.89 ± 0.07 a | 6.14 ± 0.18 a | 2.37 ± 0.18 b |
| C 35 °C | 20.26 ± 1.11 c | 3.22 ± 0.12 b | 2.79 ± 0.12 a | 3.22 ± 0.10 b | 5.51 ± 0.34 a | 2.45 ± 0.24 b |
| NP 35 °C | 22.46 ± 0.94 c | 3.09 ± 0.18 b | 2.54 ± 0.11 ab | 5.33 ± 0.27 a | 5.97 ± 0.44 a | 2.82 ± 0.17 ab |
| Total Chl | Carotenoids | Chla/Chlb | Carotenoids/Total Chl | |
|---|---|---|---|---|
| C 25 °C | 1.16 ± 0.23 a | 0.16 ± 0.03 ab | 2.71 ± 0.07 a | 0.14 ± 0.00 c |
| NP 25 °C | 1.52 ± 0.23 a | 0.22 ± 0.03 a | 2.65 ± 0.06 a | 0.15 ± 0.00 c |
| C 35 °C | 0.32 ± 0.04 b | 0.06 ± 0.01 c | 1.56 ± 0.10 b | 0.21 ± 0.02 b |
| NP 35 °C | 0.44 ± 0.05 b | 0.11 ± 0.01 bc | 2.03 ± 0.13 b | 0.27 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontanini, D.; Bottega, S.; Ruffini Castiglione, M.; Spanò, C. Interacting Effects of Heat and Nanoplastics Affect Wheat (Triticum turgidum L.) Seedling Growth and Physiology. Plants 2025, 14, 2426. https://doi.org/10.3390/plants14152426
Fontanini D, Bottega S, Ruffini Castiglione M, Spanò C. Interacting Effects of Heat and Nanoplastics Affect Wheat (Triticum turgidum L.) Seedling Growth and Physiology. Plants. 2025; 14(15):2426. https://doi.org/10.3390/plants14152426
Chicago/Turabian StyleFontanini, Debora, Stefania Bottega, Monica Ruffini Castiglione, and Carmelina Spanò. 2025. "Interacting Effects of Heat and Nanoplastics Affect Wheat (Triticum turgidum L.) Seedling Growth and Physiology" Plants 14, no. 15: 2426. https://doi.org/10.3390/plants14152426
APA StyleFontanini, D., Bottega, S., Ruffini Castiglione, M., & Spanò, C. (2025). Interacting Effects of Heat and Nanoplastics Affect Wheat (Triticum turgidum L.) Seedling Growth and Physiology. Plants, 14(15), 2426. https://doi.org/10.3390/plants14152426








