Establishment of Agrobacterium-Mediated Transient Transformation System in Sunflower
Abstract
1. Introduction
2. Results
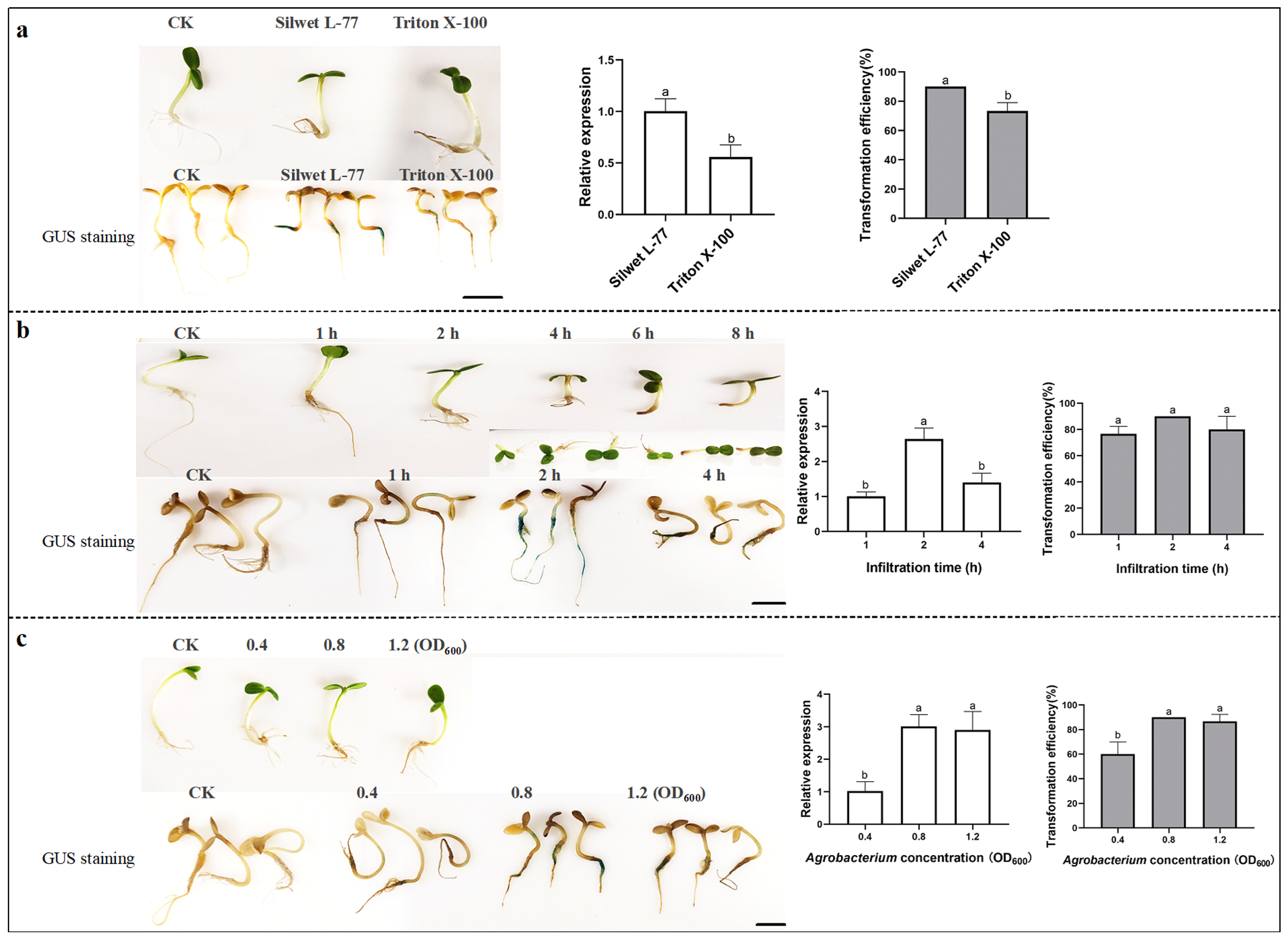
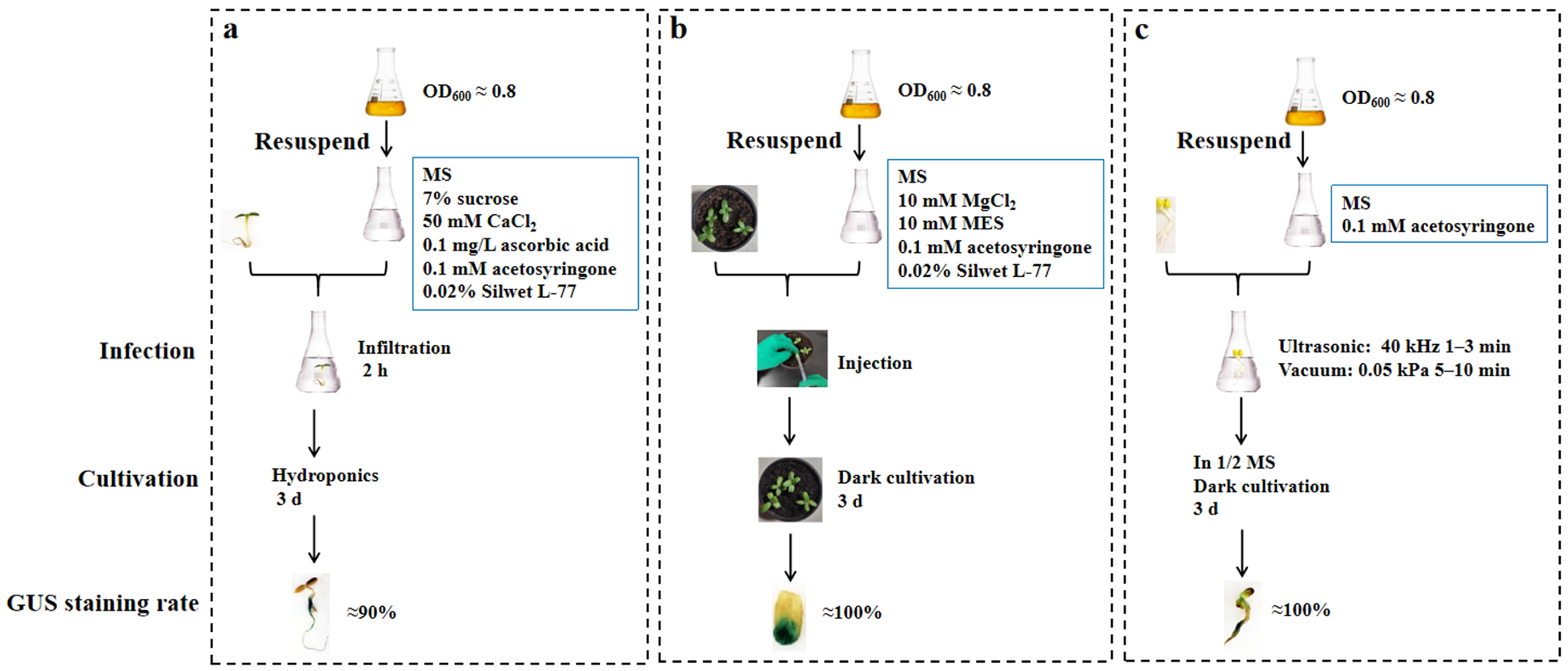
2.1. Optimization for Transient Transformation with the Infiltration Method in Sunflower
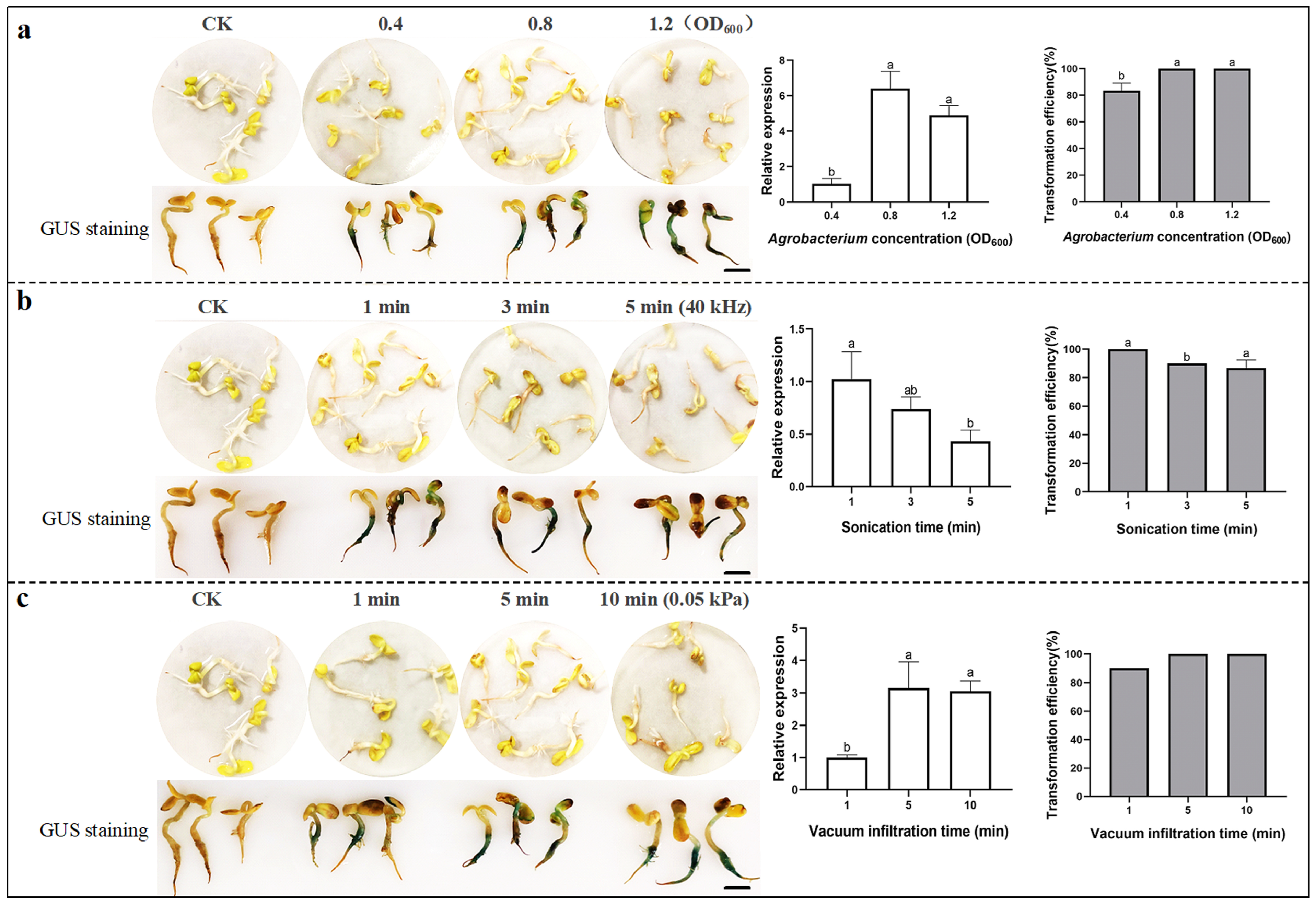
2.2. Optimization for Transient Transformation with the Injection in Sunflower
2.3. Optimization for Transient Transformation Using Ultrasonic-Vacuum in Sunflower
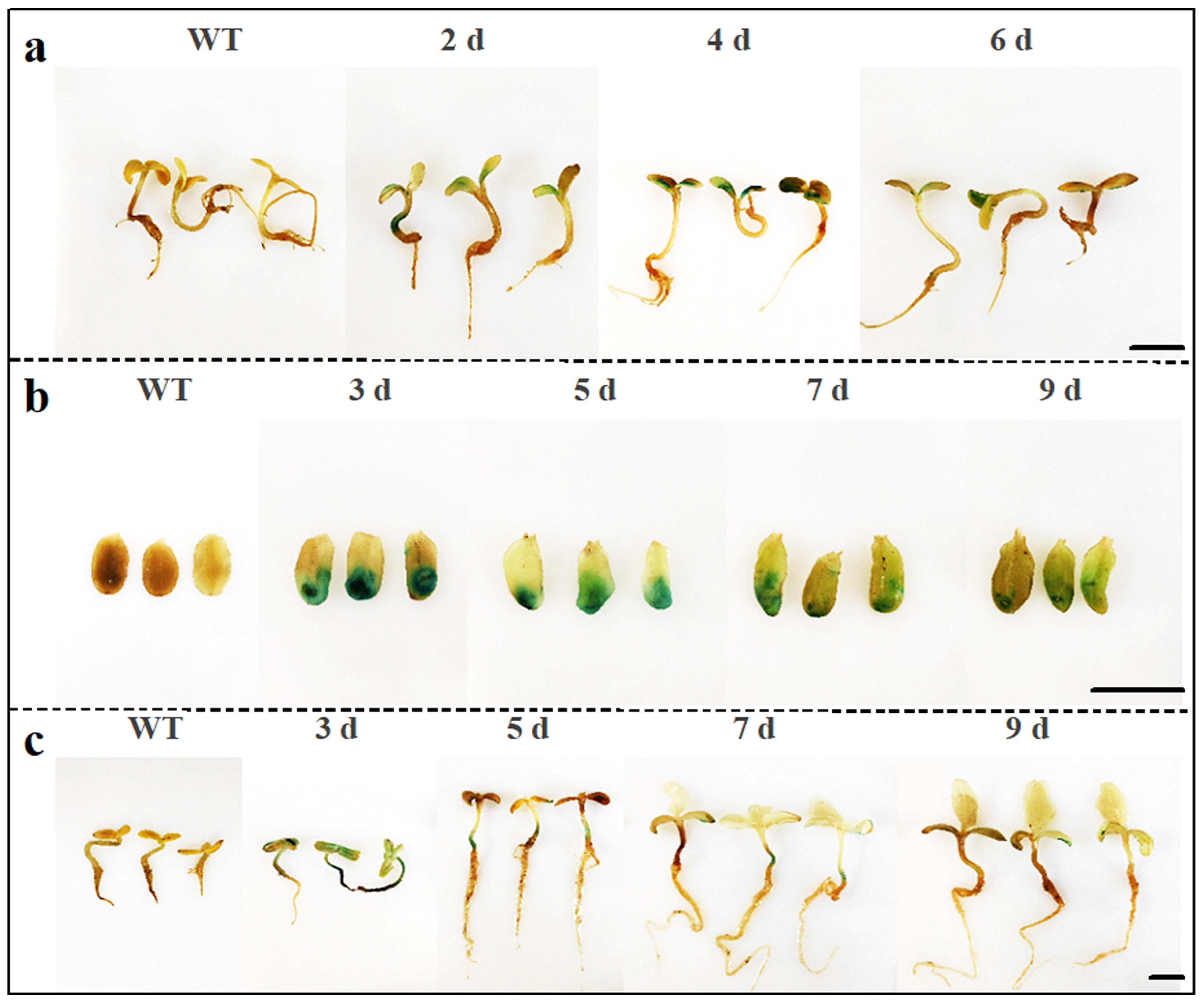
2.4. Duration of Gene Expression in the Sunflower Transient Transformation System Using Three Methods
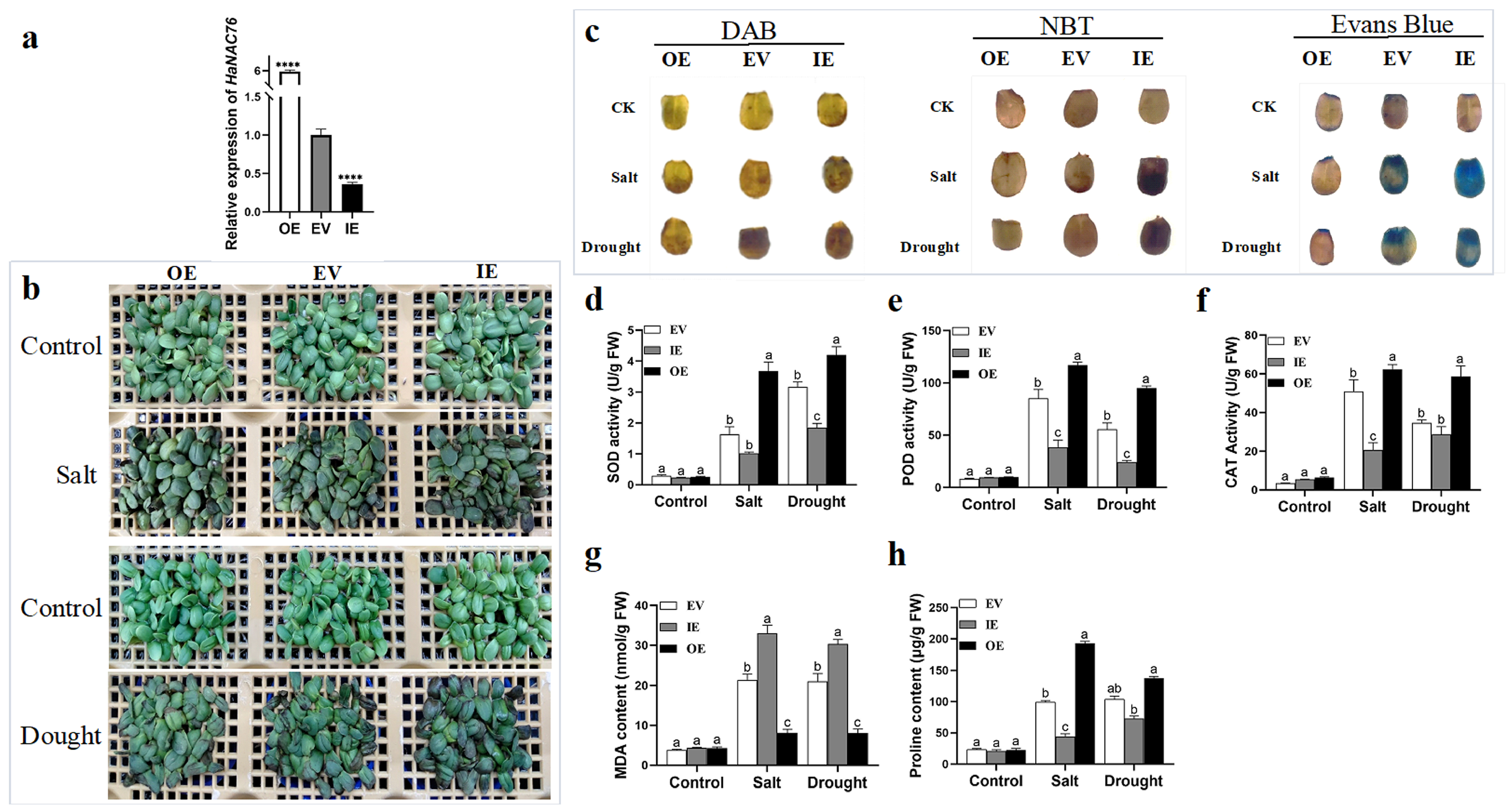
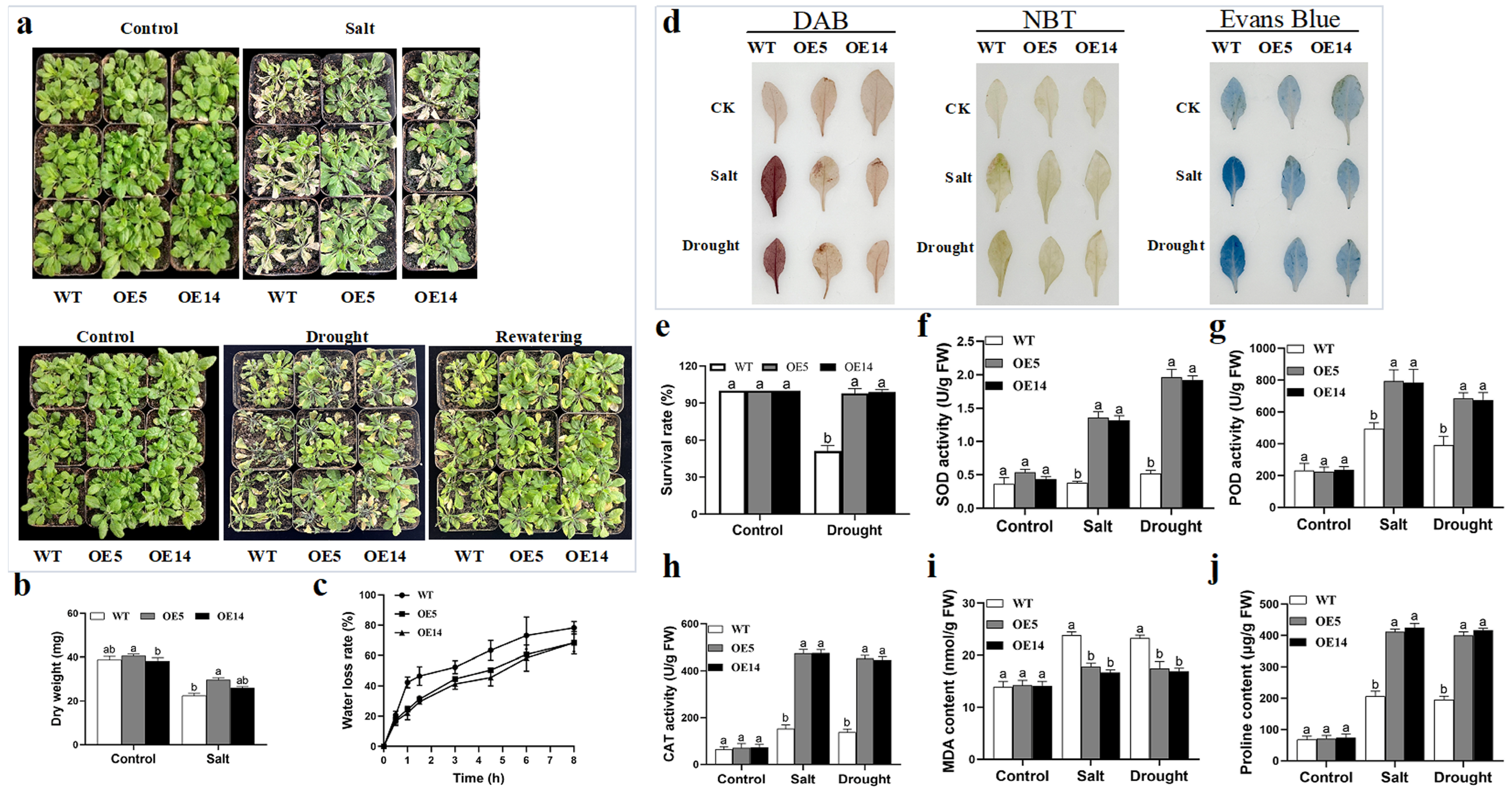
2.5. Functional Characterization of a Candidate Gene, HaNAC76 in Salt Tolerance and Drought Resistance by Agrobacterium-Mediated Transient Infiltration in Sunflower and Stable Genetic Transformation in Arabidopsis
3. Discussion
4. Materials and Methods
4.1. Preparation of Experimental Materials and Treatment of Salt and Drought Stresses
4.2. Agrobacterium-Mediated Transient Transformation in Sunflower
4.2.1. Infiltration
4.2.2. Cotyledonary Injection
4.2.3. Ultrasonic-Vacuum Method
4.3. Detection of Transient Transformation Efficiency and Gene Expression Duration
4.4. β-Glucuronidase (GUS) Staining
4.5. Construction of Recombinant Plasmids
4.6. qRT-PCR Experiment
4.7. Analysis of Physiological Indicators
4.8. Data Analysis and Graph Construction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Temme, A.A.; Kerr, K.L.; Masalia, R.R.; Burke, J.M.; Donovan, L.A. Key traits and genes associate with salinity tolerance independent from vigor in cultivated sunflower. Plant Physiol. 2020, 184, 865–880. [Google Scholar] [CrossRef]
- Kumar, A.P.K. Genetics, Genomics and Breeding of Sunflower; Excelic Press LLC: Lewes, Delaware, 2019. [Google Scholar]
- Badouin, H.; Gouzy, J.; Grassa, C.J.; Murat, F.; Staton, E.; Cottret, L.; Lelandais-Brière, C.; Owens, G.L.; Carrère, S.; Mayjonade, B.; et al. The sunflower genome provides insights into oil metabolism, flowering and asterid evolution. Nature 2017, 546, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Su, W.B.; Xu, M.Y.; Radani, Y.; Yang, L.M. Technological development and application of plant genetic transformation. Int. J. Mol. Sci. 2023, 24, 10646. [Google Scholar] [CrossRef] [PubMed]
- Darqui, F.S.; Radonic, L.M.; Beracochea, V.C.; Hopp, H.E.; Bilbao, L.M. Peculiarities of the transformation of asteraceae family species: The cases of sunflower and lettuce. Front. Plant Sci. 2021, 12, 767459. [Google Scholar] [CrossRef]
- Singareddy, V.; Sheri, V.R.; Muddanuru, T.; Tatineni, R.; Jain, R.K.; Sankaraneni, C.R.; Kodeboyina, V.S.; Mulpuri, S. Genetic engineering of Sunflower (Helianthus annuus L.) for resistance to necrosis disease through deployment of the TSV coat protein gene. Plant Cell Tissue Organ Cult. 2018, 135, 263–277. [Google Scholar] [CrossRef]
- Xiao, J.J.; Zhang, R.X.; Khan, A.; ul Haq, S.; Gai, W.X.; Gong, Z.H. CaFtsH06, A novel filamentous thermosensitive protease gene, is involved in heat, salt, and drought stress tolerance of pepper (Capsicum annuum L.). Int. J. Mol. Sci. 2021, 22, 6953. [Google Scholar] [CrossRef]
- Chen, J.J.; Li, J.R.; Huang, Y.H.; Li, Y.; Su, C.F.; Zeng, X.F. EuPIP1;2, a plasma membrane aquaporin gene from Eucommia ulmoides, enhances drought and salt tolerance in transgenic tobacco. Agronomy 2022, 12, 615. [Google Scholar] [CrossRef]
- Li, M.X.; Wu, Q.Q.; Guo, F.Q.; Ouyang, Y.Z.; Ao, D.Y.; You, S.J.; Liu, Y.Y. A versatile, rapid Agrobacterium-mediated transient expression system for functional genomics studies in cannabis seedling. Planta 2024, 260, 18. [Google Scholar] [CrossRef]
- Tyurin, A.A.; Suhorukova, A.V.; Kabardaeva, K.V.; Goldenkova-Pavlova, I.V. Transient gene expression is an effective experimental tool for the research into the fine mechanisms of plant gene function: Advantages, limitations, and solutions. Plants 2020, 9, 1187–1206. [Google Scholar] [CrossRef]
- Tsubasa, S. Analysis of the intracellular localization of transiently expressed and fluorescently labeled copper-containing amine oxidases, diamine oxidase and N-methylputrescine oxidase in tobacco, using an Agrobacterium infiltration protocol. Methods Mol. Biol. 2018, 1694, 215–223. [Google Scholar]
- Leissing, F.; Reinstdler, A.; Thieron, H.; Panstrega, R. Gene gun-mediated transient gene expression for functional studies in plant immunity. Methods Mol. Biol. 2022, 2523, 63–77. [Google Scholar] [PubMed]
- Wang, Y.; Zhang, Y.; Dong, Y.; Li, D.L.; Shi, S.L.; Li, S.H.; Li, L.Z.; He, Y.J.; Li, J.Y.; Chen, H.Y.; et al. A highly efficient mesophyll protoplast isolation and PEG-mediated transient expression system in eggplant. Sci. Hortic. 2022, 304, 111303. [Google Scholar] [CrossRef]
- Gou, Y.J.; Li, Y.L.; Bi, P.P.; Wang, D.J.; Feng, J.Y. Optimization of the protoplast transient expression system for gene functional studies in Strawberry (Fragaria vesca). Plant Cell Tissue Organ Cult. 2020, 141, 41–53. [Google Scholar] [CrossRef]
- Ren, R.; Gao, J.; Lu, C.Q.; Wei, Y.L.; Jin, J.P.; Wong, S.M.; Zhu, G.F.; Yang, F.X. Highly efficient protoplast isolation and transient expression system for functional characterization of flowering related genes in cymbidium orchids. Int. J. Mol. Sci. 2020, 21, 2264. [Google Scholar] [CrossRef]
- Zottini, M.; Barizza, E.; Costa, A.; Formentin, E.; Ruberti, C.; Carimi, F.; Schiavo, F.L. Agroinfiltration of grapevine leaves for fast transient assays of gene expression and for long-term production of stable transformed cells. Plant Cell Rep. 2008, 27, 845–853. [Google Scholar] [CrossRef]
- Tsuda, K.; Qi, Y.P.; Nguyen, L.V.; Bethke, G.; Tsuda, Y.; Glazebrook, J.; Katagiri, F. An efficient Agrobacterium-mediated transient transformation of Arabidopsis. Plant J. 2012, 69, 713–719. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Fu, J.; Wang, G.; Wang, J.; Liu, Y. Transcriptome analysis of maize immature embryos reveals the roles of cysteine in improving Agrobacterium infection efficiency. Front. Plant Sci. 2017, 8, 1778. [Google Scholar]
- Li, L.; Gu, H.; Yue, Y.; Yang, X.; Wang, L. Advances in transient transformation systems of woody plants. Mol. Plant Breed. 2020, 18, 7784–7794. [Google Scholar]
- Lao, X.A.; Jin, P.; Yang, R.R.; Liang, Y.Q.; Zhang, D.Y.; Zeng, Y.L.; Li, X.S. Establishment of Agrobacterium-mediated transient transformation system in desert legume Eremosparton songoricum (Litv.) Vass. Int. J. Mol. Sci. 2024, 25, 11934. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Hu, R.; Yang, L.; Zuo, Z.J. Establishment of a transient transformation protocol in Cinnamomum camphora. Forests 2023, 14, 1872. [Google Scholar] [CrossRef]
- Yasmin, A.; Debener, T. Transient gene expression in rose petals via Agrobacterium infiltration. Plant Cell Tissue Organ Cult. 2010, 102, 245–250. [Google Scholar] [CrossRef]
- Lü, Y.D.; Zhang, M.L.; Wu, T.; Zhong, Y. The infiltration efficiency of Agrobacterium-mediated transient transformation in four apple cultivars. Sci. Hortic. 2019, 256, 108597. [Google Scholar] [CrossRef]
- Zhong, S.W.; Dong, B.; Zhou, J.; Miao, Y.F.; Yang, L.Y.; Wang, Y.G.; Xiao, Z.; Fang, Q.; Wan, Q.Q.; Zhao, H.B. Highly efficient transient gene expression of three tissues in Osmanthus fragrans mediated by Agrobacterium tumefaciens. Sci. Hortic. 2023, 310, 111725. [Google Scholar] [CrossRef]
- Zhang, K.; He, J.J.; Liu, L.; Xie, R.D.; Qiu, L.; Li, X.C.; Yuan, W.J.; Chen, K.; Yin, Y.T.; Kyaw, M.M.M.; et al. A convenient, rapid and efficient method for establishing transgenic lines of Brassica napus. Plant Methods. 2020, 16, 43. [Google Scholar] [CrossRef]
- Yang, Y.F.; Chen, Z.C.; Zhao, J.N.; Zheng, G.S.; Wang, F.; Li, S.F.; Ren, X.R.; Li, J.B. Establishment and validation of an efficient Agrobacterium Tumefaciens-mediated transient transformation system for Salix Psammophila. Int. J. Mol. Sci. 2024, 25, 12934. [Google Scholar] [CrossRef]
- Wu, S.L.; Yang, X.B.; Liu, L.Q.; Jiang, T.; Wu, H.; Su, C.; Qian, Y.H.; Jiao, F. Agrobacterium-mediated transient MaFT expression in mulberry (Morus alba L.) leaves. Biosci. Biotechnol. Biochem. 2015, 79, 1266–1271. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, G.F.; Meng, X.N.; Li, Y.B.; Wang, Y.C. A versatile Agrobacterium-mediated transient gene expression system for herbaceous plants and trees. Biochem. Genet. 2012, 50, 761–769. [Google Scholar] [CrossRef]
- Xian, B.; Xi, Z.Q.; Ren, C.X.; Yan, J.; Chen, J.; Pei, J. The establishment of transient expression systems and their application for gene function analysis of favonoid biosynthesis in Carthamus tinctorius L. BMC Plant Biol. 2023, 23, 186. [Google Scholar] [CrossRef]
- Liu, K.; Yang, Q.; Yang, T.R.; Wu, Y.; Wang, G.X.; Yang, F.Y.; Wang, R.G.; Lin, X.F.; Li, G.J. Development of Agrobacterium-mediated transient expression system in Caragana intermedia and characterization of CiDREB1C in stress response. BMC Plant Biol. 2019, 19, 237. [Google Scholar] [CrossRef]
- Liao, J.J.; Xie, L.; Shi, H.W.; Cui, S.R.; Lan, F.S.; Luo, Z.L.; Ma, X.J. Development of an efficient transient expression system for Siraitia grosvenorii fruit and functional characterization of two NADPH-cytochrome P450 reductases. Phytochemistry 2021, 189, 112824. [Google Scholar] [CrossRef]
- Li, S.; Jing, X.L.; Tan, Q.P.; Wen, B.B.; Fu, X.l.; Li, D.M.; Chen, X.d.; Xiao, W.; Li, L. The NAC transcription factor MdNAC29 negatively regulates drought tolerance in apple. Front. Plant Sci. 2023, 14, 1173107. [Google Scholar] [CrossRef]
- Yang, C.F.; Huang, Y.Z.; Lv, P.Y.; Antwi-Boasiako, A.; Begum, N.; Zhao, T.J.; Zhao, J.M. NAC transcription factor GmNAC12 improved drought stress tolerance in Soybean. Int. J. Mol. Sci. 2022, 23, 12029. [Google Scholar] [CrossRef]
- Kim, M.J.; Baek, K.; Park, C.M. Optimization of conditions for transient Agrobacterium-mediated gene expression assays in Arabidopsis. Plant Cell Rep. 2009, 28, 1159–1167. [Google Scholar] [CrossRef]
- Jelly, N.S.; Valat, L.; Walter, B.; Maillot, P. Transient expression assays in grapevine: A step towards genetic improvement. Plant Biotechnol. J. 2014, 12, 1231–1245. [Google Scholar] [CrossRef]
- Wang, S.; Ku, S.S.; Ye, X.; He, C.; Kwon, S.Y.; Choi, P.S. Current status of genetic transformation technology developed in Cucumber (Cucumis Sativus L.). J. Integr. Agric. 2015, 14, 469–482. [Google Scholar] [CrossRef]
- Ebrahimzadegan, R.; Maroufi, A. In vitro regeneration and Agrobacterium-mediated genetic transformation of Dragon’s Head Plant (Lallemantia iberica). Sci. Rep. 2022, 12, 1784. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Zhen, C.; Xu, W.J.; Wang, C.; Cheng, Y.X. Simple, rapid and efficient transformation of genotype nisqually-1: A basic tool for the first sequenced model tree. Sci. Rep. 2017, 7, 2638. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Sun, Q.; Ma, C.N.; Wei, M.M.; Wang, C.K.; Zhao, Y.W.; Wang, W.Y.; Hu, D.G. MdWRKY31-MdNAC7 regulatory network: Orchestrating fruit softening by modulating cell wall-modifying enzyme MdXTH2 in response to ethylene signalling. Plant Biotechnol. J. 2024, 22, 3244–3261. [Google Scholar] [CrossRef]
- Lin, R.; Song, J.N.; Tang, M.J.; Wang, L.Y.; Yu, J.Q.; Zhou, Y.H. CALMODULIN6 negatively regulates cold tolerance by attenuating ICE1-dependent stress responses in tomato. Plant Physiol. 2023, 193, 2105–2121. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.K.; Rai, N.P.; Kumar, S.; Yadav, A.; Rathaur, S.; Singh, M. Effects of explant age, germination medium, pre-culture parameters, inoculation medium, pH, washing medium, and selection regime on Agrobacterium-mediated transformation of tomato. Vitr. Cell. Dev. Biol. 2012, 48, 565–578. [Google Scholar] [CrossRef]
- Mazumdar, P.; Basu, A.; Paul, A.; Mahanta, C.; Sahoo, L. Age and orientation of the cotyledonary leaf explants determine the efficiency of de novo plant regeneration and Agrobacterium tumefaciens-mediated transformation in Jatropha curcas L. S. Afr. J. Bot. 2010, 76, 337–344. [Google Scholar] [CrossRef]
- Li, H.P.; Li, K.; Guo, Y.T.; Guo, J.G.; Miao, K.T.; Botella, J.R.; Song, C.P.; Miao, Y.C. A transient transformation system for gene characterization in upland cotton (Gossypium hirsutum). Plant Methods 2018, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.X.; Kang, X.N.; Ge, J.Y.; Fei, R.W.; Duan, S.Y.; Sun, X.M. An efficient Agrobacterium-mediated transient transformation system and its application in gene function elucidation in Paeonia lactiflora Pall. Front. Plant Sci. 2022, 13, 999433. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.W.; Ma, J.J.; Liu, H.M.; Guo, Y.T.; Li, W.; Niu, S.H. An efficient system for Agrobacterium-mediated transient transformation in Pinus tabuliformis. Plant Methods 2020, 16, 52. [Google Scholar] [CrossRef]
- Li, Y.P.; Chen, T.T.; Wang, W.; Liu, H.; Yan, X.; Wu-Zhang, K.Y.; Qin, W.; Xie, L.H.; Zhang, Y.J.; Peng, B.W.; et al. A high-efciency Agrobacterium-mediated transient expression system in the leaves of Artemisia annua L. Plant Methods 2021, 17, 106. [Google Scholar] [CrossRef]
- Chen, X.J.; He, S.T.; Jiang, L.N.; Li, X.Z.; Guo, W.L.; Chen, B.H.; Zhou, J.G.; Skliar, V. An efficient transient transformation system for gene function studies in pumpkin (Cucurbita moschata D.). Sci. Hortic. 2021, 282, 110028. [Google Scholar] [CrossRef]
- Wu, Y.J.; Leng, Y.R.; Jing, S.L.; Zheng, C.P.; Lang, C.J.; Yang, L.P. Using vacuum infection method to transiently expression of GUS gene in Medicago sativa L. by vacuum infiltration. Mol. Plant Breed. 2021, 20, 859–864. [Google Scholar]
- Lacroix, B.; Citovsky, V. The roles of bacterial and host plant factors in Agrobacterium-mediated genetic transformation. Int. J. Dev. Biol. 2013, 57, 467–481. [Google Scholar] [CrossRef]
- Sharma, R.; Liang, Y.; Lee, Y.; Pidatala, V.R.; Mortimer, J.C.; Scheller, H.V. Agrobacterium-mediated transient transformation of sorghum leaves for accelerating functional genomics and genome editing studies. BMC Res. Notes 2020, 13, 116. [Google Scholar] [CrossRef]
- Sun, S.L.; Han, X.; Jin, R.X.; Jiao, J.B.; Wang, J.W.; Niu, S.Y.; Yang, Z.Y.; Wu, D.; Wang, Y.C. Generation of CRISPR-edited birch plants without DNA integration using Agrobacterium- mediated transformation technology. Plant Sci. 2024, 342, 112029. [Google Scholar] [CrossRef]
- Liu, C.Y.; Hu, J.; Fan, W.; Zhu, P.P.; Cao, B.N.; Zheng, S.; Xia, Z.Q.; Zhu, Y.X.; Zhao, A.C. Heterotrimeric G-protein γ subunits regulate ABA signaling in response to drought through interacting with PP2Cs and SnRK2s in mulberry (Morus alba L.). Plant Physiol. Biochem. 2021, 161, 210–221. [Google Scholar] [CrossRef]
- Zhou, L.M.; Huang, Y.Z.; Wang, Q.; Guo, D.J. AaHY5 ChIP-seq based on transient expression system reveals the role of AaWRKY14 in artemisinin biosynthetic gene regulation. Plant Physiol. Biochem. 2021, 168, 321–328. [Google Scholar] [CrossRef]
- Lei, X.J.; Fang, J.R.; Lv, J.X.; Li, Z.Y.; Liu, Z.Y.; Wang, Y.C.; Wang, C.; Gao, C.Q. Overexpression of ThSCL32 confers salt stress tolerance by enhancing ThPHD3 gene expression in Tamarix hispida. Tree Physiol. 2023, 43, 1444–1453. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Wang, L.; Huang, Q.; Jiang, R.; Li, W.; Hou, X.; Tan, Z.; Lei, Z.; Li, Q.; Zeng, Y. Establishment of Agrobacterium-Mediated Transient Transformation System in Sunflower. Plants 2025, 14, 2412. https://doi.org/10.3390/plants14152412
Chen F, Wang L, Huang Q, Jiang R, Li W, Hou X, Tan Z, Lei Z, Li Q, Zeng Y. Establishment of Agrobacterium-Mediated Transient Transformation System in Sunflower. Plants. 2025; 14(15):2412. https://doi.org/10.3390/plants14152412
Chicago/Turabian StyleChen, Fangyuan, Lai Wang, Qixiu Huang, Run Jiang, Wenhui Li, Xianfei Hou, Zihan Tan, Zhonghua Lei, Qiang Li, and Youling Zeng. 2025. "Establishment of Agrobacterium-Mediated Transient Transformation System in Sunflower" Plants 14, no. 15: 2412. https://doi.org/10.3390/plants14152412
APA StyleChen, F., Wang, L., Huang, Q., Jiang, R., Li, W., Hou, X., Tan, Z., Lei, Z., Li, Q., & Zeng, Y. (2025). Establishment of Agrobacterium-Mediated Transient Transformation System in Sunflower. Plants, 14(15), 2412. https://doi.org/10.3390/plants14152412





