Comparative Metabolomics Analysis of Four Pineapple (Ananas comosus L. Merr) Varieties with Different Fruit Quality
Abstract
1. Introduction
2. Results
2.1. Fruit Phenotyping of the Four Representative Pineapple Varieties
2.2. Metabolic Profiling Analysis of Four Pineapple Varieties
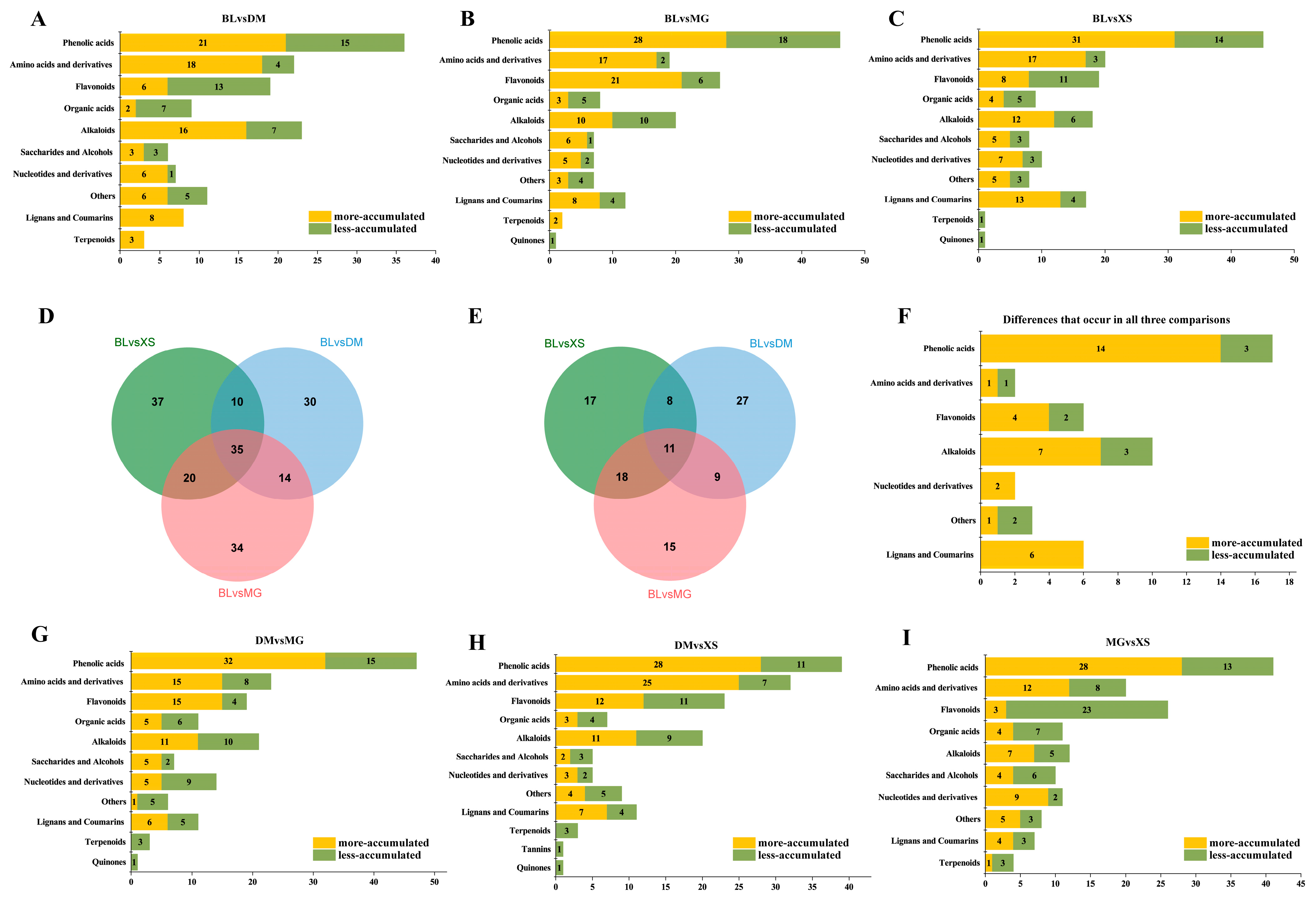
2.3. Identification of Differentially Accumulated Metabolites (DAMs)
2.4. Differences in the Metabolic Profiles of the Three Introduced Varieties Compared to BL
2.5. Differences in the Metabolic Profiles Between the Three Introduced Varieties
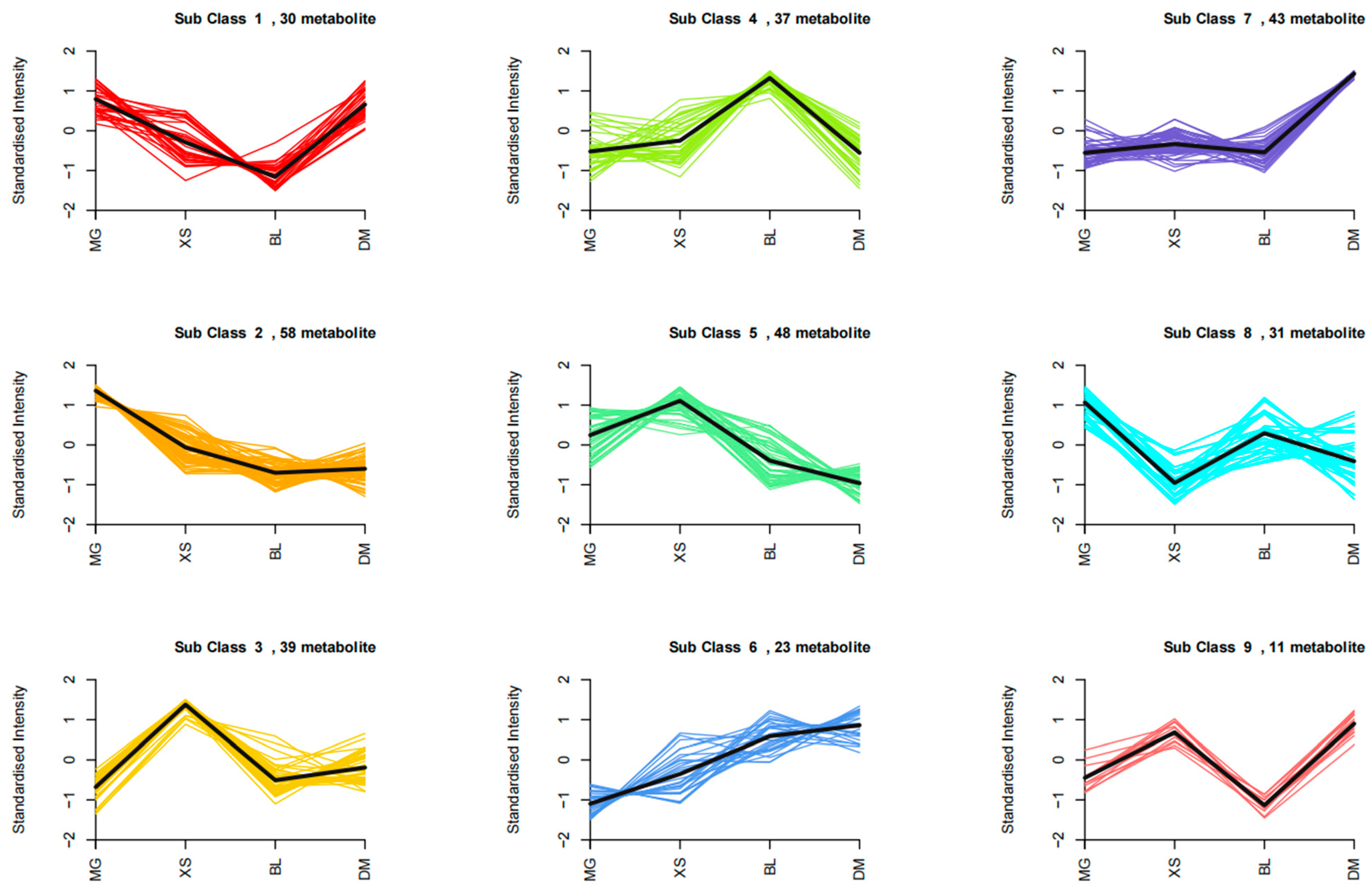
2.6. K-Means Analysis of Variety-Specific Metabolite Profiling Detection
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Measurements of Physicochemical Indices
4.3. Metabolite Extraction and Detection
4.4. Metabolomic Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ming, R.; VanBuren, R.; Wai, C.M.; Tang, H.; Schatz, M.C.; Bowers, J.E.; Lyons, E.; Wang, M.-L.; Chen, J.; Biggers, E. The pineapple genome and the evolution of CAM photosynthesis. Nat. Genet. 2015, 47, 1435–1442. [Google Scholar] [CrossRef]
- Li, D.; Jing, M.; Dai, X.; Chen, Z.; Ma, C.; Chen, J. Current status of pineapple breeding, industrial development, and genetics in China. Euphytica 2022, 218, 85. [Google Scholar] [CrossRef]
- Fakher, B.; Ashraf, M.A.; Wang, L.; Wang, X.; Zheng, P.; Aslam, M.; Qin, Y. Pineapple SWEET10 is a glucose transporter. Hortic. Res. 2023, 10, uhad175. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wu, Q.; Liu, S.; Sun, G. Comparative Analysis of Variety Characteristics of Tainong Series Pineapple. Chin. J. Trop. Crops 2016, 37, 2050–2055. [Google Scholar]
- Lv, R.; Zou, H.; Chen, X.; Li, W.; Bai, R.; Lin, C. Performance of Tainong Pineapple Varieties Introduced to Hainan. Chin. J. Trop. Agric. 2021, 41, 66–70. [Google Scholar]
- Hu, X.; Zhang, M.; Pang, S.; Wu, Z.; Zhan, S.; Li, K. Comparative Experiment on Introduction of New Taiwan Pineapple Varieties in Zhanjiang Agricultural Reclamation. Chin. J. Trop. Agric. 2024, 41–47. [Google Scholar]
- Zheng, B.; Zhao, Q.; Wu, H.; Wang, S.; Zou, M. A comparative metabolomics analysis of guava (Psidium guajava L.) fruit with different colors. ACS Food Sci. Technol. 2020, 1, 96–106. [Google Scholar] [CrossRef]
- Lai, T.; Shuai, L.; Han, D.; Lai, Z.; Du, X.; Guo, X.; Hu, W.; Wu, Z.; Luo, T. Comparative metabolomics reveals differences in primary and secondary metabolites between “Shixia” and “Chuliang” longan (Dimocarpus longan Lour.) pulp. Food Sci. Nutr. 2021, 9, 5785–5799. [Google Scholar] [CrossRef]
- Hu, F.; Bi, X.; Fu, X.; Li, Y.; Li, G.; Li, Y.; Liu, D.; Yang, Y.; Shi, R.; Dong, W. Comparative Metabolome Profiles and Antioxidant Potential of Four Coffea arabica L. Varieties Differing in Fruit Color. Diversity 2023, 15, 724. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lee, S.; Park, S.M.; Yun, S.H.; Gab, H.-S.; Kim, S.S.; Kim, H.-J. Comparative metabolomics analysis of citrus varieties. Foods 2021, 10, 2826. [Google Scholar] [CrossRef]
- Ikram, M.M.M.; Mizuno, R.; Putri, S.P.; Fukusaki, E. Comparative metabolomics and sensory evaluation of pineapple (Ananas comosus) reveal the importance of ripening stage compared to cultivar. J. Biosci. Bioeng. 2021, 132, 592–598. [Google Scholar] [CrossRef]
- Hong, K.; Chen, L.; Gu, H.; Zhang, X.; Chen, J.; Nile, S.H.; Hu, M.; Gong, D.; Song, K.; Hou, X. Novel insight into the relationship between metabolic profile and fatty acid accumulation altering cellular lipid content in pineapple fruits at different stages of maturity. J. Agric. Food Chem. 2021, 69, 8578–8589. [Google Scholar] [CrossRef]
- Ikram, M.M.M.; Ridwani, S.; Putri, S.P.; Fukusaki, E. GC-MS based metabolite profiling to monitor ripening-specific metabolites in pineapple (Ananas comosus). Metabolites 2020, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yao, Y.; Chen, X.; Wu, J.; Wu, Q.; Liu, S.; Guo, A.; Zhang, X. Metabolomic and transcriptomic analyses reveal the mechanism of sweet-acidic taste formation during pineapple fruit development. Front. Plant Sci. 2022, 13, 971506. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Zhang, X.; Liu, J.; Yao, Q.; Li, Y.; Hou, X.; Liu, S.; Qiu, X.; Yang, Y.; Chen, L. Integration of Metabolomics and Transcriptomics to Explore Dynamic Alterations in Fruit Color and Quality in ‘Comte de Paris’ Pineapples during Ripening Processes. Int. J. Mol. Sci. 2023, 24, 16384. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yao, Y.; Zeng, H.; Zhang, X. Integrated Metabolome and transcriptome analysis reveals a potential mechanism for water accumulation mediated translucency in pineapple (Ananas comosus (L.) Merr.) fruit. Int. J. Mol. Sci. 2023, 24, 7199. [Google Scholar] [CrossRef]
- Luengwilai, K.; Beckles, D.M.; Roessner, U.; Dias, D.A.; Lui, V.; Siriphanich, J. Identification of physiological changes and key metabolites coincident with postharvest internal browning of pineapple (Ananas comosus L.) fruit. Postharvest Biol. Technol. 2018, 137, 56–65. [Google Scholar] [CrossRef]
- Lin, W.; Liu, S.; Xiao, X.; Sun, W.; Lu, X.; Gao, Y.; He, J.; Zhu, Z.; Wu, Q.; Zhang, X. Integrative Analysis of Metabolome and Transcriptome Provides Insights into the Mechanism of Flower Induction in Pineapple (Ananas comosus (L.) Merr.) by Ethephon. Int. J. Mol. Sci. 2023, 24, 17133. [Google Scholar] [CrossRef]
- Azizan, A.; Lee, A.X.; Abdul Hamid, N.A.; Maulidiani, M.; Mediani, A.; Abdul Ghafar, S.Z.; Zolkeflee, N.K.Z.; Abas, F. Potentially bioactive metabolites from pineapple waste extracts and their antioxidant and α-glucosidase inhibitory activities by 1H NMR. Foods 2020, 9, 173. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, H.; Zhang, X. Integrative transcriptomic and metabolomic analysis of D-leaf of seven pineapple varieties differing in NPK% contents. BMC Plant Biol. 2021, 21, 550. [Google Scholar] [CrossRef]
- Liu, C.-h.; He, H.; He, X.-g.; Chen, X.; Liu, K.; Shao, X.-h.; Lai, D.; Qin, J.; Zhuang, Q.-l.; Kuang, S.-z. Physiological and Metabolitic Mechanisms of Different Pineapple Cultivars Responding to Low Temperature Stress. Biotechnol. Bull. 2023, 39, 219. [Google Scholar]
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant potential overviews of secondary metabolites (polyphenols) in fruits. Int. J. Food Sci. 2020, 2020, 9081686. [Google Scholar] [CrossRef] [PubMed]
- Harnly, J.M.; Doherty, R.F.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Bhagwat, S.; Gebhardt, S. Flavonoid content of US fruits, vegetables, and nuts. J. Agric. Food Chem. 2006, 54, 9966–9977. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-H.; Sun, D.-Q.; Wu, Q.-S.; Liu, S.-H.; Sun, G.-M. Physico-chemical properties, antioxidant activity and mineral contents of pineapple genotypes grown in China. Molecules 2014, 19, 8518–8532. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Neuhaus, H.E.; Cheng, J.; Bie, Z. Contributions of sugar transporters to crop yield and fruit quality. J. Exp. Bot. 2022, 73, 2275–2289. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Eprintsev, A.T. Organic acids: The pools of fixed carbon involved in redox regulation and energy balance in higher plants. Front. Plant Sci. 2016, 7, 1042. [Google Scholar] [CrossRef]
- Maoz, I.; Lewinsohn, E.; Gonda, I. Amino acids metabolism as a source for aroma volatiles biosynthesis. Curr. Opin. Plant Biol. 2022, 67, 102221. [Google Scholar] [CrossRef]
- Gonda, I.; Bar, E.; Portnoy, V.; Lev, S.; Burger, J.; Schaffer, A.A.; Tadmor, Y.; Gepstein, S.; Giovannoni, J.J.; Katzir, N. Branched-chain and aromatic amino acid catabolism into aroma volatiles in Cucumis melo L. fruit. J. Exp. Bot. 2010, 61, 1111–1123. [Google Scholar] [CrossRef]
- Veberic, R.; Colaric, M.; Stampar, F. Phenolic acids and flavonoids of fig fruit (Ficus carica L.) in the northern Mediterranean region. Food Chem. 2008, 106, 153–157. [Google Scholar] [CrossRef]
- Albert, N.W.; Iorizzo, M.; Mengist, M.F.; Montanari, S.; Zalapa, J.; Maule, A.; Edger, P.P.; Yocca, A.E.; Platts, A.E.; Pucker, B. Vaccinium as a comparative system for understanding of complex flavonoid accumulation profiles and regulation in fruit. Plant Physiol. 2023, 192, 1696–1710. [Google Scholar] [CrossRef]
- Haminiuk, C.W.; Maciel, G.M.; Plata-Oviedo, M.S.; Peralta, R.M. Phenolic compounds in fruits—an overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Roaa, M. A review article: The importance of the major groups of plants secondary metabolism phenols, alkaloids, and terpenes. Int. J. Res. Appl. Sci. Biotechnol. (IJRASB) 2020, 7, 354–358. [Google Scholar]
- Robe, K.; Izquierdo, E.; Vignols, F.; Rouached, H.; Dubos, C. The coumarins: Secondary metabolites playing a primary role in plant nutrition and health. Trends Plant Sci. 2021, 26, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Ražná, K.; Nôžková, J.; Vargaová, A.; Harenčár, Ľ.; Bjelková, M. Biological functions of lignans in plants. Agriculture 2021, 67, 155–165. [Google Scholar] [CrossRef]
- Liao, G.; Xu, Q.; Allan, A.C.; Xu, X. L-Ascorbic acid metabolism and regulation in fruit crops. Plant Physiol. 2023, 192, 1684–1695. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Zahid, Z.; Mubarik, M.S.; Javed, R.; Siddiqui, M.H.; Hasanuzzaman, M. Jasmonic acid: A key frontier in conferring abiotic stress tolerance in plants. Plant Cell Rep. 2021, 40, 1513–1541. [Google Scholar] [CrossRef]
- Liu, C.-H.; Liu, Y. Fruit quality and differentially expressed genes of winter-harvested pineapple in response to elevated temperature over a short postharvest period. Postharvest Biol. Technol. 2017, 130, 21–27. [Google Scholar] [CrossRef]
- Liu, H.; Jin, Y.; Huang, L.; Miao, C.; Tang, J.; Zhang, H.; Yin, H.; Lu, X.; Li, N.; Dai, S. Transcriptomics and metabolomics reveal the underlying mechanism of drought treatment on anthocyanin accumulation in postharvest blood orange fruit. BMC Plant Biol. 2024, 24, 160. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, L.; Chen, J.; Zhang, N.; Zhou, W.; Song, Y. Altitudinal variation of dragon fruit metabolite profiles as revealed by UPLC-MS/MS-based widely targeted metabolomics analysis. BMC Plant Biol. 2024, 24, 344. [Google Scholar] [CrossRef]
- Kolde, R.; Kolde, M.R. Package ‘pheatmap’. R Package 2015, 1, 790. [Google Scholar]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef]
- Wickham, H.; Wickham, H. Getting Started with ggplot2. Ggplot2: Elegant Graph. Data Anal. 2016, 11–31. [Google Scholar]
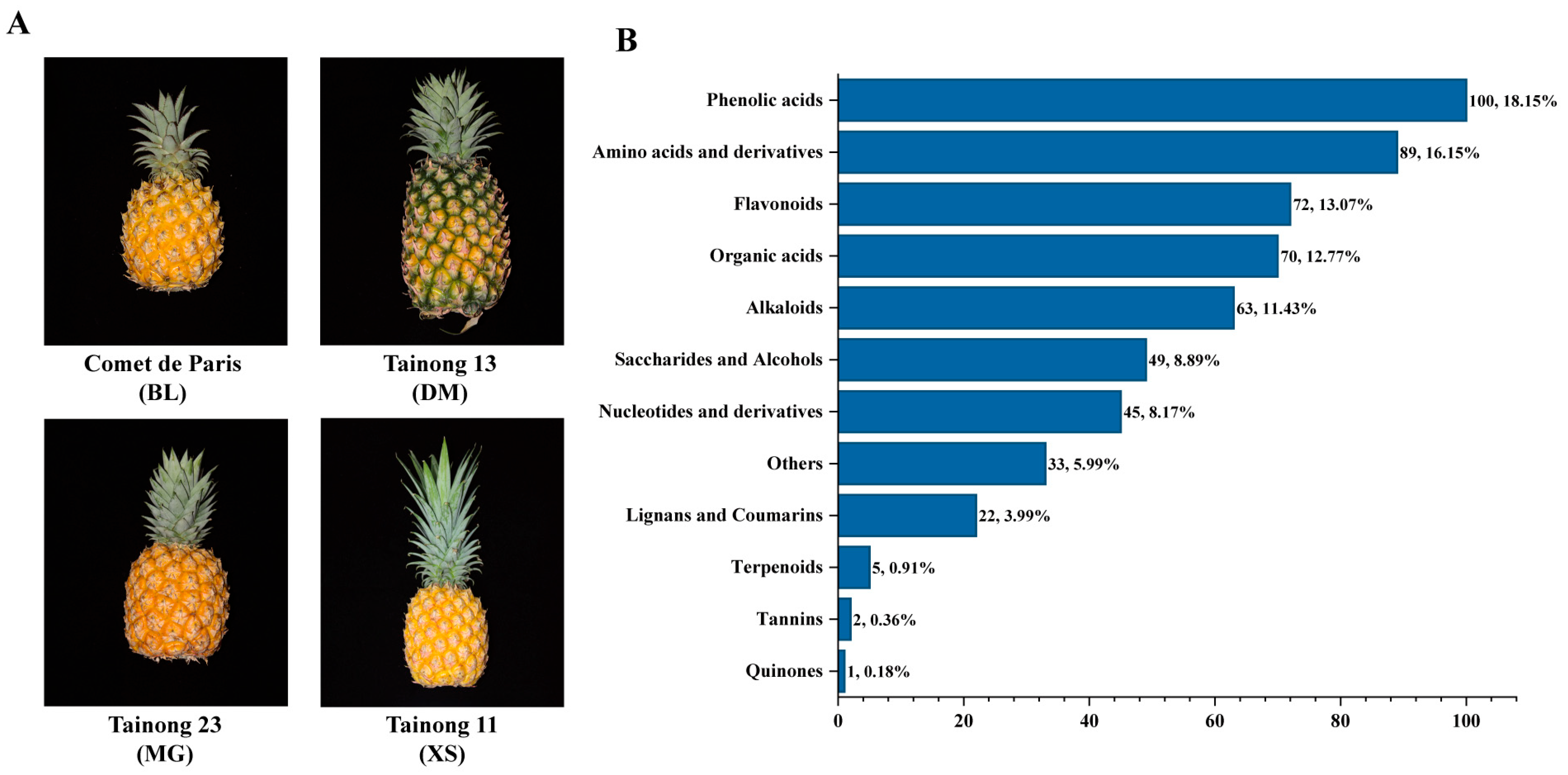


| Cultivar | Soluble Solids Content (%) | Titratable Acid Content (%) | Average Fruit Weight (g) | Diameter (mm) | Length (mm) |
|---|---|---|---|---|---|
| BL | 17.8 ± 1.8 | 0.61 ± 0.07 | 1043.00 ± 94.92 | 99.33 ± 4.62 | 136.25 ± 8.92 |
| MG | 17.9 ± 1.4 | 0.56 ± 0.06 | 1002.00 ± 165.52 | 110.46 ± 4.62 ** | 113.72 ± 9.79 ** |
| XS | 17.2 ± 1.8 | 0.60 ± 0.07 | 1015.35 ± 137.14 | 98.90 ± 5.50 | 132.98 ± 11.29 |
| DM | 18.1 ± 1.2 | 0.53 ± 0.12 | 1409.04 ± 145.58 ** | 111.51 ± 4.24 ** | 151.61±19.54 * |
| Class | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Saccharides and alcohols | 0 | 4 | 4 | 1 | 1 | 0 | 2 | 3 | 0 | 15 |
| Organic acids | 0 | 2 | 3 | 3 | 1 | 7 | 1 | 5 | 0 | 22 |
| Amino acids and derivatives | 6 | 9 | 6 | 2 | 16 | 0 | 10 | 2 | 3 | 54 |
| Nucleotides and derivatives | 0 | 3 | 5 | 3 | 3 | 3 | 2 | 1 | 2 | 22 |
| Phenolic acids | 7 | 19 | 14 | 7 | 10 | 5 | 8 | 4 | 2 | 76 |
| Flavonoids | 8 | 9 | 0 | 10 | 6 | 2 | 4 | 12 | 0 | 51 |
| Alkaloids | 3 | 8 | 1 | 8 | 6 | 0 | 10 | 2 | 1 | 39 |
| Lignans and coumarins | 2 | 4 | 4 | 0 | 3 | 3 | 1 | 0 | 1 | 18 |
| Terpenoids | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 4 |
| Quinones | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Tannins | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Others | 2 | 0 | 2 | 3 | 2 | 1 | 3 | 2 | 2 | 17 |
| 30 | 58 | 39 | 37 | 48 | 23 | 43 | 31 | 11 | 320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, P.; Wu, J.; Li, D.; Xie, S.; Cai, X.; Xiao, Q.; Wang, J.; Yao, Q.; Chen, S.; Liu, R.; et al. Comparative Metabolomics Analysis of Four Pineapple (Ananas comosus L. Merr) Varieties with Different Fruit Quality. Plants 2025, 14, 2400. https://doi.org/10.3390/plants14152400
Zheng P, Wu J, Li D, Xie S, Cai X, Xiao Q, Wang J, Yao Q, Chen S, Liu R, et al. Comparative Metabolomics Analysis of Four Pineapple (Ananas comosus L. Merr) Varieties with Different Fruit Quality. Plants. 2025; 14(15):2400. https://doi.org/10.3390/plants14152400
Chicago/Turabian StyleZheng, Ping, Jiahao Wu, Denglin Li, Shiyu Xie, Xinkai Cai, Qiang Xiao, Jing Wang, Qinglong Yao, Shengzhen Chen, Ruoyu Liu, and et al. 2025. "Comparative Metabolomics Analysis of Four Pineapple (Ananas comosus L. Merr) Varieties with Different Fruit Quality" Plants 14, no. 15: 2400. https://doi.org/10.3390/plants14152400
APA StyleZheng, P., Wu, J., Li, D., Xie, S., Cai, X., Xiao, Q., Wang, J., Yao, Q., Chen, S., Liu, R., Liang, Y., Zhang, Y., Deng, B., Qin, Y., & Wang, X. (2025). Comparative Metabolomics Analysis of Four Pineapple (Ananas comosus L. Merr) Varieties with Different Fruit Quality. Plants, 14(15), 2400. https://doi.org/10.3390/plants14152400







