Projecting Extinction Risk and Assessing Conservation Effectiveness for Three Threatened Relict Ferns in the Western Mediterranean Basin
Abstract
1. Introduction
2. Results
2.1. Correlation Between Climate and Demographic Data
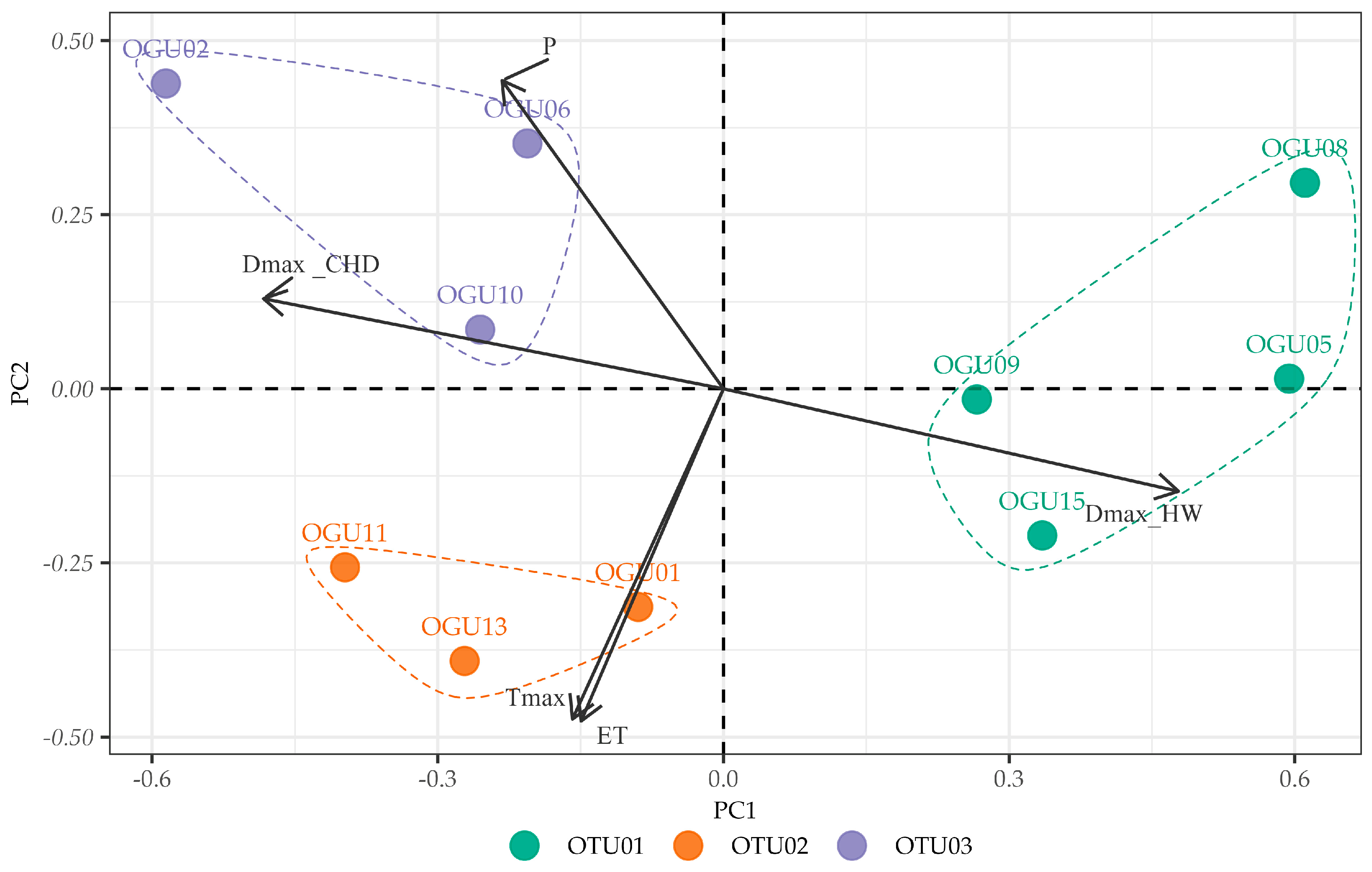
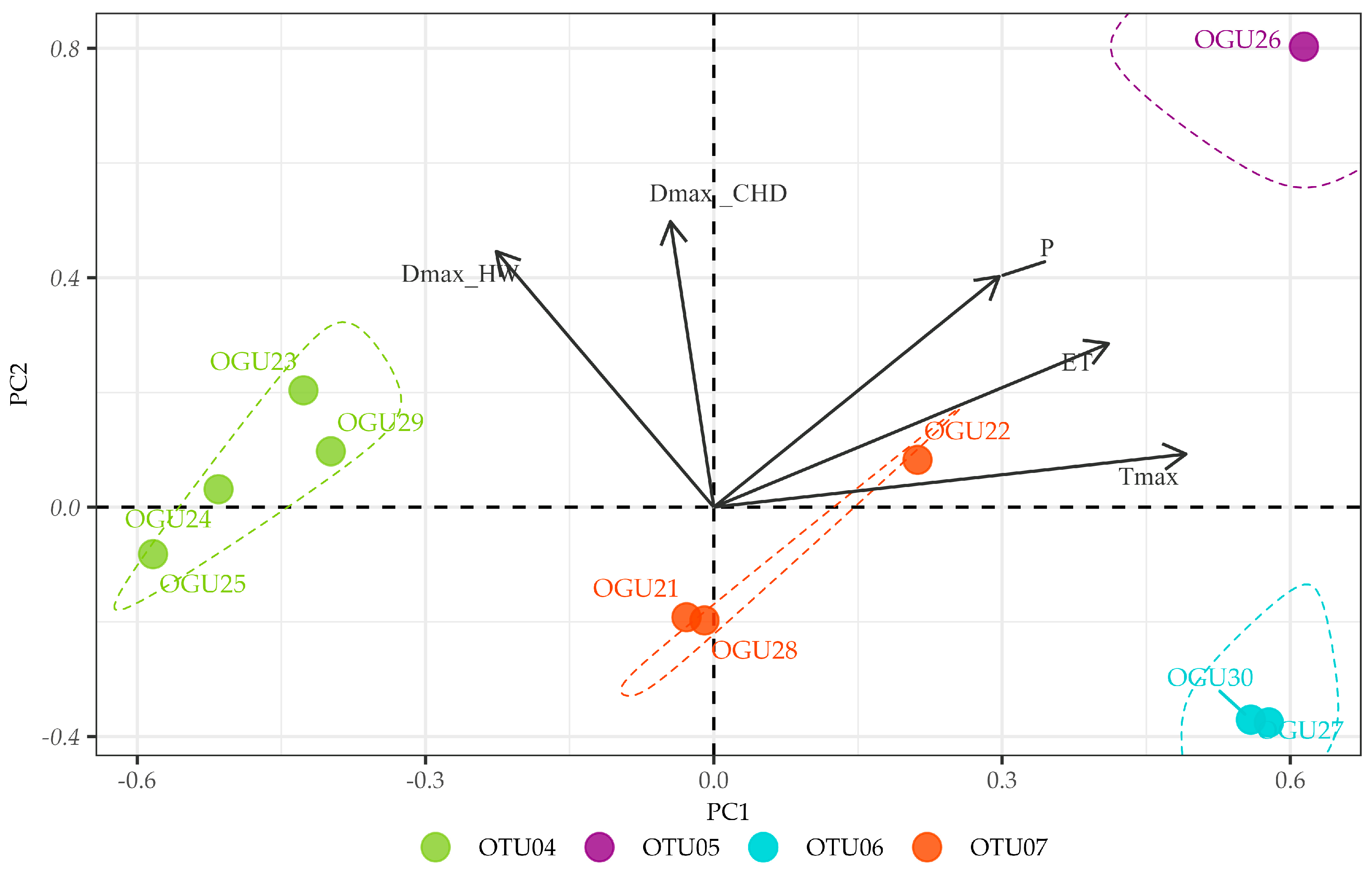
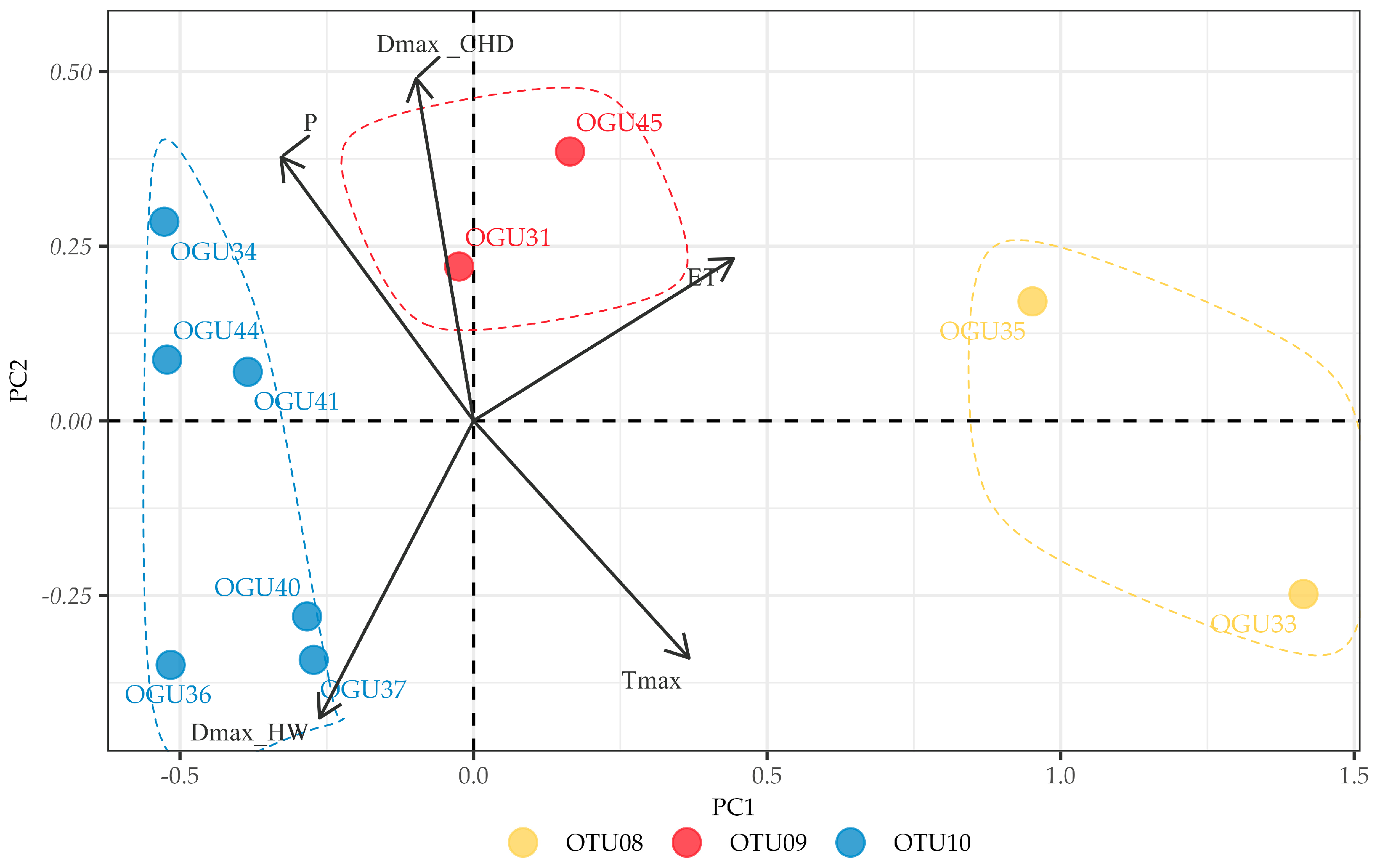
2.2. Clustering in Operational Territorial Units (OTUs)
2.3. Principal Component Analysis of Climate–Abundance Relationships
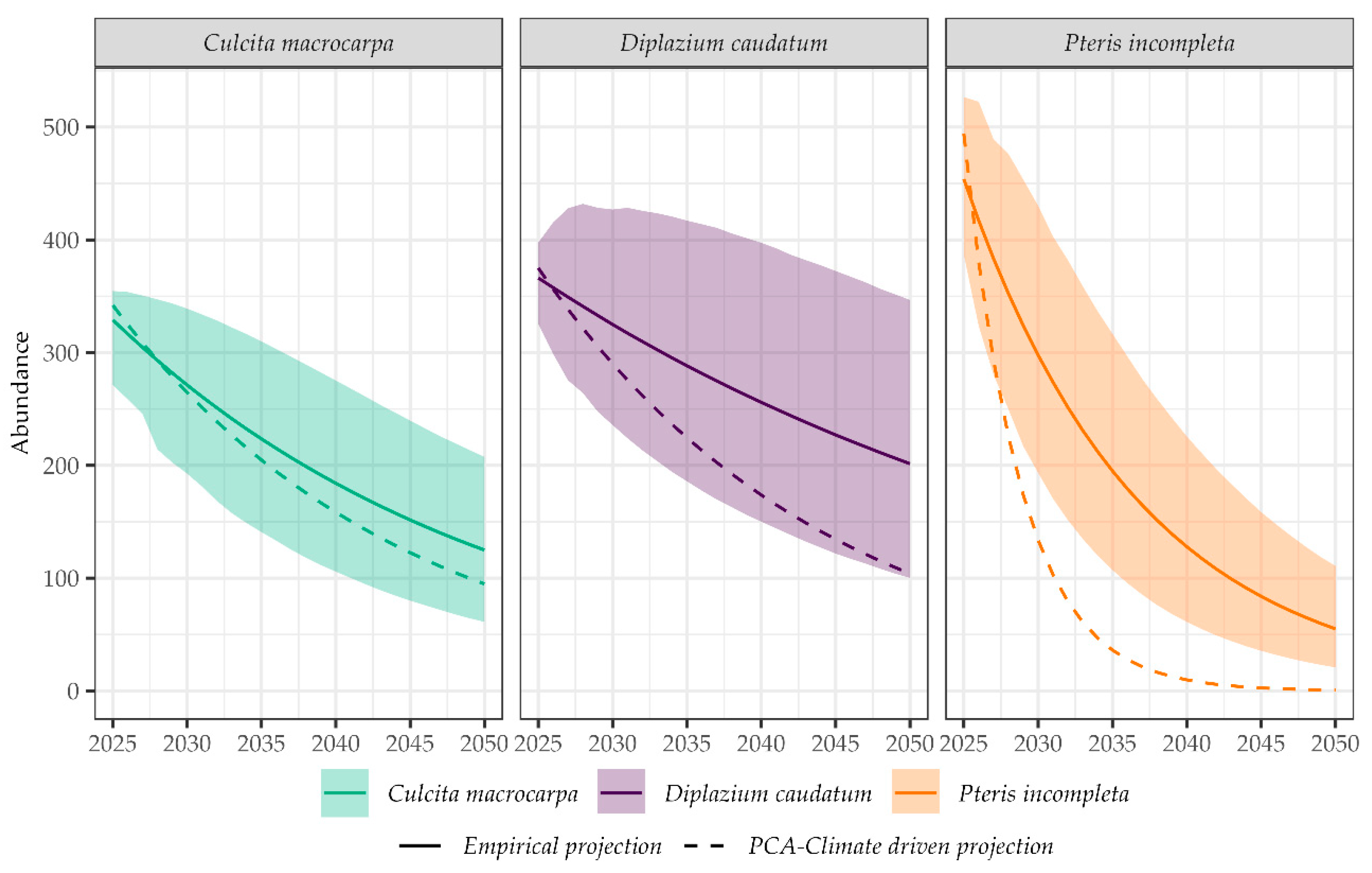
2.4. Deterministic Population Projection Based on PCA-Derived Predictive Algorithm
2.5. Comparison of Population Projections Derived from Empirical and Climate-Driven Models
3. Discussion
- (1)
- Incorporating PCA-derived climatic variable weights into the population projection algorithm constitutes a multivariate approach to link environmental variability with demographic trends in relict fern populations. This framework allows for the simultaneous consideration of key climatic drivers and their combined influence on population trajectories. However, this methodology has inherent limitations. The model’s linear and additive structure may oversimplify complex biological processes that are often non-linear and influenced by threshold effects, feedback mechanisms, or interactions among climatic and ecological factors. Furthermore, it assumes that the principal components captured by PC1 and PC2 fully represent the climatic drivers of population change, potentially neglecting other important abiotic or biotic influences. Critical demographic processes such as reproduction, mortality, dispersal, and genetic variability are also absent from this model, limiting its ability to fully capture population viability.
- (2)
- The deterministic survival projection model, grounded in empirical annual rates derived from a decade of census data, offers a practical tool for projecting population trends. By capturing the net effects of demographic and environmental factors observed during the study period, the model reflects the integrated trajectory of population change. However, its assumption of temporal constancy in demographic conditions, given that the bootstrap simulations are based solely on past observed rates, limits its reliability under dynamic scenarios, such as those induced by climate change, land-use transformations, or unforeseen ecological disturbances. The model does not account for demographic stochasticity, density dependence, interannual variability, or the impact of rare but consequential events like extreme droughts or disease outbreaks, all of which can be particularly influential in small, isolated populations.
- (3)
- The divergence between observed and modeled population abundances has been used as indicator of conservation effectiveness. While this approach offers valuable preliminary insights, it is important to acknowledge that such differences may also arise from a range of other ecological and demographic dynamics not fully captured in the current modeling framework. These include natural population variability and external environmental factors that are inherently challenging to parameterize comprehensively. Additionally, model–data mismatch is an expected outcome when dealing with complex ecological systems. As such, while the observed–predicted discrepancies can help identify potential conservation outcomes, they should be interpreted within the broader context of model assumptions, data limitations, and ecological variability.
4. Materials and Methods
4.1. Study Area
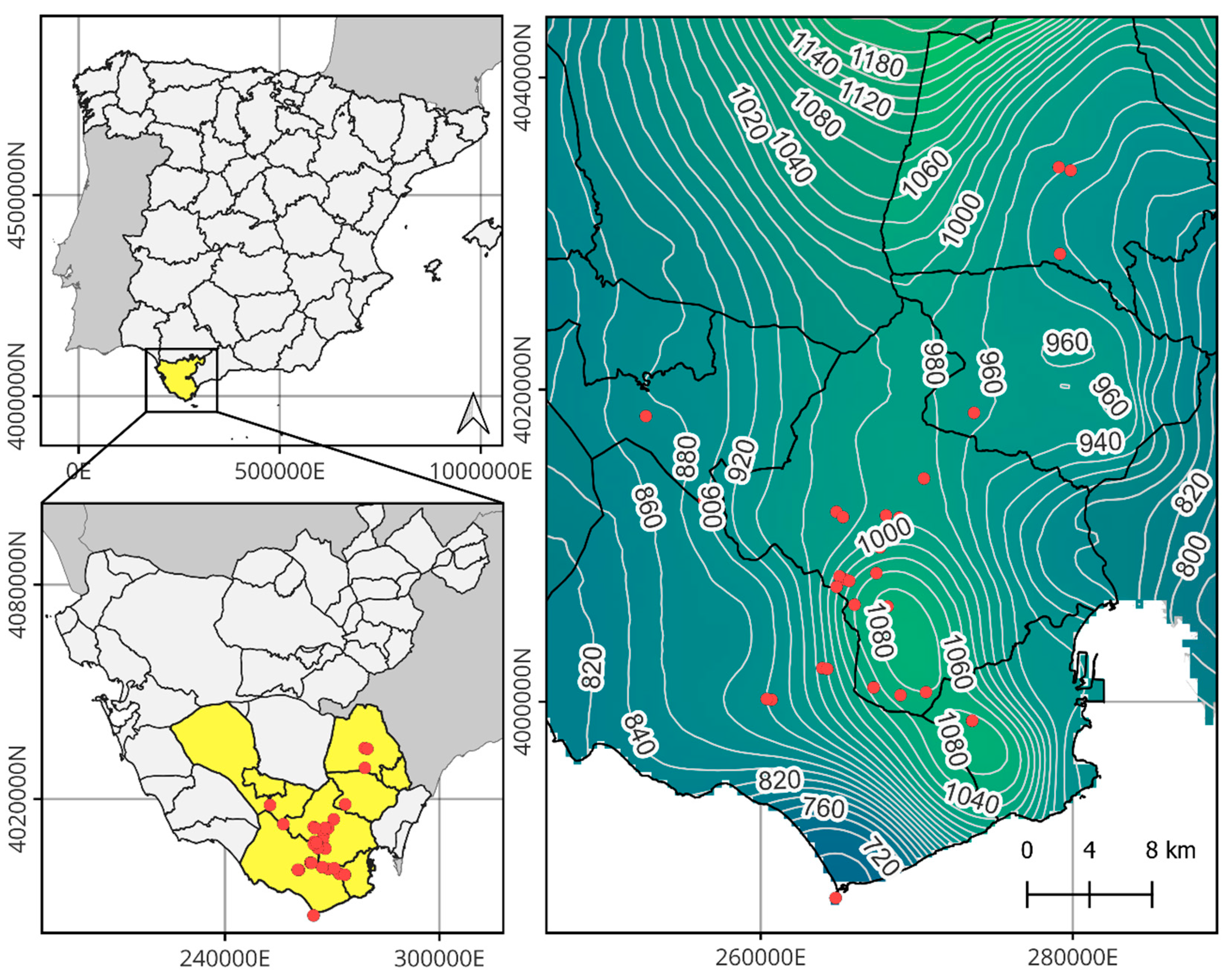
4.2. Ecology of the Studied Species and Key Threats
- (1)
- Habitat degradation. The conversion of native laurel and mixed forests to monoculture plantations, particularly Eucalyptus spp., leads to soil desiccation and loss of shaded, humid niches. Wildfires, especially prevalent in mainland Portugal, further threaten C. macrocarpa, already classified as Critically Endangered in that region. Similarly, D. caudatum and P. incompleta are highly sensitive to the degradation of hygrophilous forest ravines and streambanks where they occur.
- (2)
- Hydrological alterations, such as water extraction, river channeling, and wetland desiccation, directly impact the moisture-dependent habitats of these species. Contamination from agricultural runoff, livestock waste, and untreated urban or industrial effluents further degrades water quality, altering edaphic and microclimatic conditions essential for fern survival.
- (3)
- Land-use changes, including deforestation, land clearing for agriculture, and infrastructure development (e.g., roads, firebreaks), fragment habitats and reduce the extent of suitable environments. Overgrazing contributes to trampling, herbivory, and soil nitrification, which in turn promotes colonization by competitive native or invasive species, displacing native ferns.
- (4)
- Recreational pressures, such as unregulated tourism, hiking, and the construction of leisure infrastructure, can result in physical disturbance to sensitive fern populations, especially when located near trails or accessible forested areas.
- (5)
- Population isolation and small population sizes exacerbate genetic erosion and vulnerability to stochastic events, including droughts, landslides, and fires. The scattered and fragmented nature of existing populations limits gene flow and reduces resilience.
- (6)
- Biotic threats, such as invasive plant species, increase competition for light, space, and moisture. Although specific pathogens or pests have not yet been documented in these species, their potential impact remains a concern, particularly under changing environmental conditions.
- (7)
- Climate change emerges as a major overarching threat. Projections under the RCP 4.5 scenario [73] indicate a sustained increase in mean annual maximum temperatures and a decline in annual precipitation (Figure 10), leading to higher evapotranspiration rates and, consequently, reduced water availability (Figure 11). These changes are particularly detrimental during the most sensitive stages of the fern life cycle, spore germination, gametophyte growth, and fertilization, where moisture is essential for success.

4.3. Methods
4.3.1. Structured Abundance Data of Fern Populations
4.3.2. Climate Data Description
4.3.3. Correlation Analysis and Grouping in Operational Territorial Units (OTUs)
4.3.4. Deterministic Population Projection Based on PCA-Derived Predictive Algorithm
4.3.5. Deterministic Population Projection Based on Empirical Annual Change Rates
4.3.6. Comparison and Validation of Projection Methods
- Retrospective model validation: By comparing modeled versus observed data during the last decade (2016–2022), we assessed the degree to which each model could reproduce historical population trajectories. Positive deviations (observed > expected) may suggest additional buffering factors (e.g., microhabitat protection, successful management), while negative deviations (observed < expected) might indicate unmodeled stressors or limited conservation effectiveness.
- Model performance by species: This analysis allowed identification of which species were better captured by the modeling framework.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ovaskainen, O.; Meerson, B. Stochastic Models of Population Extinction. Trends Ecol. Evol. 2010, 25, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Purvis, A.; Jones, K.E.; Mace, G.M. Extinction. BioEssays 2000, 22, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Mace, G.M.; Lande, R. Assessing Extinction Threats: Toward a Reevaluation of IUCN Threatened Species Categories. Conserv. Biol. 1991, 5, 148–157. [Google Scholar] [CrossRef]
- Caughley, G. Directions in Conservation Biology. J. Anim. Ecol. 1994, 63, 215–244. [Google Scholar] [CrossRef]
- Frankham, R. Genetics and Extinction. Biol. Conserv. 2005, 126, 131–140. [Google Scholar] [CrossRef]
- Brook, B.W.; Sodhi, N.S.; Bradshaw, C.J.A. Synergies among Extinction Drivers under Global Change. Trends Ecol. Evol. 2008, 23, 453–460. [Google Scholar] [CrossRef]
- Betts, J.; Young, R.P.; Hilton-Taylor, C.; Hoffmann, M.; Rodríguez, J.P.; Stuart, S.N.; Milner-Gulland, E.J. A Framework for Evaluating the Impact of the IUCN Red List of Threatened Species. Conserv. Biol. 2020, 34, 632–643. [Google Scholar] [CrossRef]
- Ripa, J.; Lundberg, P. The Route to Extinction in Variable Environments. Oikos 2000, 90, 89–96. [Google Scholar] [CrossRef]
- Laakso, J.; Kaitala, V.; Ranta, E. Non-Linear Biological Responses to Environmental Noise Affect Population Extinction Risk. Oikos 2004, 104, 142–148. [Google Scholar] [CrossRef]
- Fagan, W.F.; Holmes, E.E. Quantifying Extinction Vortex. Ecol. Lett. 2006, 9, 51–60. [Google Scholar] [CrossRef]
- Exposito-Alonso, M. Understanding Local Plant Extinctions before It Is Too Late: Bridging Evolutionary Genomics with Global Ecology. New Phytol. 2023, 237, 2005–2011. [Google Scholar] [CrossRef]
- Hautphenne, S.; Latouche, G. Markovian Trees Subject to Catastrophes: Transient Features and Extinction Probability. Stoch. Models 2011, 27, 569–590. [Google Scholar] [CrossRef]
- Khasin, M.; Meerson, B.; Sasorov, P.V. Time-Resolved Extinction Rates of Stochastic Populations. Phys. Rev. E 2010, 81, 031126. [Google Scholar] [CrossRef]
- Smits, P.; Finnegan, S. How Predictable Is Extinction? Forecasting Species Survival at Million-Year Timescales. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20190392. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, F. Un Análisis Integrado de la Respuesta de las Especies al Cambio Climático: Biogeografía y Ecología de Árboles Relictos en el Mediterráneo. Ecosistemas 2011, 20, 177–184. Available online: https://www.revistaecosistemas.net/index.php/ecosistemas/article/view/641 (accessed on 1 May 2025).
- Orihuela-Rivero, R.; Morente-López, J.; Reyes-Betancort, J.A.; Schaefer, H.; Valido, A.; Menezes de Sequeira, M.; Romeiras, M.M.; Góis-Marques, C.A.; Salas-Pascual, M.; Vanderpoorten, A.; et al. Geographic and Biological Drivers Shape Anthropogenic Extinctions in the Macaronesian Vascular Flora. Glob. Change Biol. 2025, 31, e70072. [Google Scholar] [CrossRef] [PubMed]
- Taillie, P.J.; McCleery, R.A. Climate Relict Vulnerable to Extinction from Multiple Climate-Driven Threats. Divers. Distrib. 2021, 27, 2124–2135. [Google Scholar] [CrossRef]
- Hampe, A.; Jump, A.S. Climate Relicts: Past, Present, Future. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 313–333. [Google Scholar] [CrossRef]
- Grandcolas, P.; Nattier, R.; Trewick, S. Relict Species: A Relict Concept? Trends Ecol. Evol. 2014, 29, 655–663. [Google Scholar] [CrossRef]
- Vanderpoorten, A.; Rumsey, F.J.; Carine, M.A. Does Macaronesia Exist? Conflicting Signal in the Bryophyte and Pteridophyte Floras. Am. J. Bot. 2007, 94, 625–639. [Google Scholar] [CrossRef]
- Sunding, P. Origins of the Macaronesian flora. In Plants and Islands; Bramwell, D., Ed.; Academic Press: London, UK, 1979; pp. 13–40. [Google Scholar]
- Engler, A. Versuch einer Eintwicklungsgeschichte, Insbesondere der Florengebiete seit der Tertiarperiode; Die extra-tropischen Gebiete der nordlischen Hemisphare; Engelmann: Leipzig, Germany, 1879. [Google Scholar]
- Gibby, M. Palaeoendemism and evolution in Macaronesian Dryopteris. In Plants and Islands; Academic Press: London, UK, 1979; pp. 347–358. [Google Scholar]
- Manton, I.; Lovis, J.D.; Vida, G.; Gibby, M. Cytology of the fern flora of Madeira. Bull. Br. Museum. Nat. Hist. Bot. 1986, 15, 123–161. [Google Scholar]
- Pichi-Sermolli, R.E.G.; España, L.; Salvo, A.E. El valor biogeográfico de la pteridoflora ibérica. Lazaroa 1988, 10, 187–205. [Google Scholar]
- Salvo-Tierra, A.E.; Garcia-Verdugo, J.C. Biogeografía numérica en pteridología. Taxonomía, biogeografía y conservación de pteridófitos. In Sociedad Historia Natural Baleares; Rita, J., Ed.; IME: Palma de Mallorca, Spain, 1990; pp. 115–150. [Google Scholar]
- Kondraskov, P.; Schütz, N.; Schüßler, C.; de Sequeira, M.M.; Guerra, A.S.; Caujapé-Castells, J.; Jaén-Molina, R.; Marrero-Rodríguez, Á.; Koch, M.A.; Linder, P.; et al. Biogeography of Mediterranean Hotspot Biodiversity: Re-Evaluating the “Tertiary Relict” Hypothesis of Macaronesian Laurel Forests. PLoS ONE 2015, 10, e0132091. [Google Scholar] [CrossRef] [PubMed]
- Florencio, M.; Patiño, J.; Nogué, S.; Traveset, A.; Borges, P.A.V.; Schaefer, H.; Amorim, I.R.; Arnedo, M.; Ávila, S.P.; Cardoso, P.; et al. Macaronesia as a Fruitful Arena for Ecology, Evolution, and Conservation Biology. Front. Ecol. Evol. 2021, 9, 718169. [Google Scholar] [CrossRef]
- Schuler, S.; Picazo-Aragonés, J.; Rumsey, F.J.; Romero-García, A.T.; Suárez-Santiago, V.N. Macaronesia Acts as a Museum of Genetic Diversity of Relict Ferns: The Case of Diplazium caudatum (Athyriaceae). Plants 2021, 10, 2425. [Google Scholar] [CrossRef]
- Vigalondo, B.; Patiño, J.; Draper, I.; Mazimpaka, V.; Shevock, J.R.; Losada-Lima, A.; González-Mancebo, J.M.; Garilleti, R.; Lara, F. The Long Journey of Orthotrichum shevockii (Orthotrichaceae, Bryopsida): From California to Macaronesia. PLoS ONE 2019, 14, e0211017. [Google Scholar] [CrossRef]
- Bell, P.R. El ciclo de vida de las criptógamas. Acta Bot. Malacit. 1991, 16, 5–16. [Google Scholar] [CrossRef]
- Tryon, R.; Tryon, A. Ferns and Allied Plants; Springer: New York, NY, USA, 1982. [Google Scholar] [CrossRef]
- Given, D.R. Changing Aspects of Endemism and Endangerment in Pteridophyta. J. Biogeogr. 1993, 20, 293–302. [Google Scholar] [CrossRef]
- Qian, H.; Kessler, M.; Zhang, J.; Jin, Y.; Jiang, M. Global Patterns and Climatic Determinants of Phylogenetic Structure of Regional Fern Floras. New Phytol. 2023, 239, 415–428. [Google Scholar] [CrossRef]
- Qian, H.; Kessler, M.; Zhang, J.; Jin, Y.; Jiang, M. Evolutionary Causes of Global Patterns of Species Richness in Regional Fern Floras across the World. J. Biogeogr. 2024, 51, 1429–1437. [Google Scholar] [CrossRef]
- Salvo-Tierra, A.E.; Escámez, A.M. Influencia del Estrecho de Gibraltar en el relictualismo de la flora pteridofítica de sus zonas adyacentes: Análisis pteridogeográfico. In Actas del Congreso Internacional” El Estrecho de Gibraltar”; UNED-Universidad Nacional de Educación a Distancia: Ceuta, Spain, 1987; pp. 433–446. [Google Scholar] [CrossRef]
- Oreskes, N. The Scientific Consensus on Climate Change. Science 2004, 306, 1686. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting Species Distribution: Offering More than Simple Habitat Models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Red de Seguimiento del Cambio Global (RSCG). Observar de Cerca el Cambio Global en los Parques Nacionales Españoles; Organismo Autónomo Parques Nacionales, Ministerio de Agricultura y Pesca, Alimentación y Medio Ambiente: Madrid, Spain, 2017. [Google Scholar]
- Aurelle, D.; Thomas, S.; Albert, C.; Bally, M.; Bondeau, A.; Boudouresque, C.-F.; Cahill, A.E.; Carlotti, F.; Chenuil, A.; Cramer, W.; et al. Biodiversity, Climate Change, and Adaptation in the Mediterranean. Ecosphere 2022, 13, e3915. [Google Scholar] [CrossRef]
- Salvo-Tierra, A.E. Guía de helechos de la Península Ibérica y Baleares. In Pirámide; Madrid, Spain, 1990; pp. 188–190. ISBN 10:8436805488. [Google Scholar]
- Hidalgo-Triana, N.; Solakis, A.; Casimiro-Soriguer, F.; Choe, H.; Navarro, T.; Pérez-Latorre, A.V.; Thorne, J.H. The High Climate Vulnerability of Western Mediterranean Forests. Sci. Total Environ. 2023, 895, 164983. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, G.; Dong, W. Global Species Diversity Patterns of Polypodiaceae under Future Climate Changes. Plants 2025, 14, 711. [Google Scholar] [CrossRef]
- de Gasper, A.L.; Grittz, G.S.; Russi, C.H.; Schwartz, C.E.; Rodrigues, A.V. Expected Impacts of Climate Change on Tree Ferns Distribution and Diversity Patterns in Subtropical Atlantic Forest. Perspect. Ecol. Conserv. 2021, 19, 369–378. [Google Scholar] [CrossRef]
- Wang, C.-J.; Wan, J.-Z.; Zhang, Z.-X.; Zhang, G.-M. Identifying Appropriate Protected Areas for Endangered Fern Species under Climate Change. SpringerPlus 2016, 5, 904. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Hu, X.; Cheng, Q.; Ali, S.; Ni, J. Distribution Patterns of Fern Species Richness along Elevations the Tibetan Plateau in China: Regional Differences and Effects of Climate Change Variables. Front. Plant Sci. 2023, 14, 1178603. [Google Scholar] [CrossRef] [PubMed]
- Hollenbeck, E.C.; Sax, D.F. Experimental Evidence of Climate Change Extinction Risk in Neotropical Montane Epiphytes. Nat. Commun. 2024, 15, 6045. [Google Scholar] [CrossRef]
- Potter, K.A.; Arthur Woods, H.; Pincebourde, S. Microclimatic Challenges in Global Change Biology. Glob. Chang. Biol. 2013, 19, 2932–2939. [Google Scholar] [CrossRef]
- Morris, W.F.; Ehrlén, J.; Dahlgren, J.P.; Loomis, A.K.; Louthan, A.M. Biotic and Anthropogenic Forces Rival Climatic/Abiotic Factors in Determining Global Plant Population Growth and Fitness. Proc. Natl. Acad. Sci. USA 2020, 117, 1107–1112. [Google Scholar] [CrossRef]
- Dallas, T.A.; Hastings, A. Habitat Suitability Estimated by Niche Models Is Largely Unrelated to Species Abundance. Glob. Ecol. Biogeogr. 2018, 27, 1448–1456. [Google Scholar] [CrossRef]
- Reif, J.; Hořák, D.; Sedláček, O.; Riegert, J.; Pešata, M.; Hrázský, Z.; Janeček, Š.; Storch, D. Unusual Abundance–Range Size Relationship in an Afromontane Bird Community: The Effect of Geographical Isolation? J. Biogeogr. 2006, 33, 1959–1968. [Google Scholar] [CrossRef]
- Lande, R.; Engen, S.; Saether, B.E. Stochastic Population Dynamics in Ecology and Conservation; Oxford University Press: Oxford, UK, 2003. [Google Scholar] [CrossRef]
- Bucharová, A.; Münzbergová, Z.; Tájek, P. Population Biology of Two Rare Fern Species: Long Life and Long-Lasting Stability. Am. J. Bot. 2010, 97, 1260–1271. [Google Scholar] [CrossRef]
- McCain, C.; Szewczyk, T.; Bracy Knight, K. Population Variability Complicates the Accurate Detection of Climate Change Responses. Glob. Chang. Biol. 2016, 22, 2081–2093. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, C.P.; Urban, M.C.; Bridle, J.R. Coarse Climate Change Projections for Species Living in a Fine-Scaled World. Glob. Chang. Biol. 2017, 23, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Díez Garretas, B.; Salvo Tierra, A.E. Ensayo biogeográfico de los pteridófitos de las Sierras de Algeciras. An. Jardín Botánico Madr. 1980, 37, 455–462. [Google Scholar]
- Chazarra-Bernabé, A.; Flórez García, E.; Peraza Sánchez, B.; Tohá Rebull, T.; Lorenzo Mariño, B.; Criado, E.; Moreno-Garcia, J.V.; Romero-Fresneda, R.; Botey, M.R. Mapas Climáticos de España (1981–2010) y ETo (1996–2016); Área de Climatología y Aplicaciones Operativas; Agencia Estatal de Meteorología (AEMET): Madrid, Spain, 2018; NIPO: 014-18-004-2. [Google Scholar] [CrossRef]
- Rivas-Martínez, S.; Rivas Sáenz, S.; Penas, Á. Worldwide bioclimatic classification system. Glob. Geobot. 2011, 1, 1–634. [Google Scholar] [CrossRef]
- Rivas Martínez, S. Mapa de series, geoseries y geopermaseries de vegetación de España: [Memoria del mapa de vegetación potencial de España]. Parte I. [Salvador Rivas Martínez y colaboradores]. Itinera Geobotánica 2007, 17, 5–436. [Google Scholar]
- Moreno-Saiz, J.C.; Iriondo Alegría, F.J.M.; Martínez García, J.; Rodríguez, M.; Mendías, C.S. (Eds.) Atlas y Libro Rojo de la Flora Vascular Amenazada de España; Adenda 2017; Ministerio para la Transición Ecológica-Sociedad Española de Biología de la Conservación de Plantas: Madrid, Spain, 2017; 220p. [Google Scholar]
- Latorre, A.V.P.; de Mera, A.G.; Cabezudo, B. La vegetación caracterizada por Rhododendron ponticum L. en Andalucía (España). Una complicada historia nomenclatural para una realidad fitocenológica. Acta Bot. Malacit. 2000, 25, 198–205. [Google Scholar] [CrossRef][Green Version]
- Red de Información Ambiental de la junta de Andalucía (REDIAM). Precipitación Anual en Andalucía (Serie consolidada). Consejería de Agricultura, Ganadería, Pesca y Desarrollo Sostenible. Junta de Andalucía. 2022. Available online: https://portalrediam.cica.es/geonetwork/srv/api/records/3e0f0b4b-b858-40b0-a92c-1b2b9a365702 (accessed on 1 May 2025).
- PPG, I. A community-derived classification for extant lycophytes and ferns. J. Syst. Evol. 2016, 54, 563–603. [Google Scholar] [CrossRef]
- Carta, A.; Bedini, G.; Peruzzi, L. A Deep Dive into the Ancestral Chromosome Number and Genome Size of Flowering Plants. New Phytol. 2020, 228, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-G.; Wang, A.-H.; Bai, C.-K.; Jin, D.-M.; Nie, L.-Y.; Harris, A.; Che, L.; Wang, J.-J.; Li, S.-Y.; Xu, L.; et al. Genome Size Evolution of the Extant Lycophytes and Ferns. Plant Divers. 2022, 44, 141–152. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, F.P.; Gonzatti, F.; Bahima, I.A.; de Souza-Chies, T.T.; Kaltchuk-Santos, E. A Global Review of Chromosome Number and Genome Size for the Filmy Ferns Family (Hymenophyllaceae, Polypodiopsida). Rodriguésia 2024, 75, e00552023. [Google Scholar] [CrossRef]
- Nowak, H.; Kustatscher, E.; Roghi, G.; Van Konijnenburg-van Cittert, J.H.A. In Situ Spores of Marattialean Ferns from the Triassic in Central and Northern Europe. Rev. Palaeobot. Palynol. 2023, 308, 104785. [Google Scholar] [CrossRef]
- Lončarević, N.; Liu, U.; Stefanaki, A.; Carapeto, A.; Ensslin, A.; Meade, C.; Metzing, D.; Peci, D.; Fantinato, E.; Colling, G.; et al. Database of European Vascular Plants Red Lists as a Contribution to More Coherent Plant Conservation. Sci. Data 2024, 11, 1138. [Google Scholar] [CrossRef]
- Suárez-Santiago, V.N.; Provan, J.; Romero-García, A.T.; Ben-Menni Schuler, S. Genetic Diversity and Phylogeography of the Relict Tree Fern Culcita Macrocarpa: Influence of Clonality and Breeding System on Genetic Variation. Plants 2024, 13, 1587. [Google Scholar] [CrossRef]
- Bento Elias, R.; Christenhusz, M.J.M.; Dyer, R.A.; García Criado, M.; Ivanenko, Y.; Ivanova, D.; Lansdown, R.V.; Molina, J.A.; Nieto, A.; Rouhan, G.; et al. European Red List of Lycopods and Ferns; IUCN: Gland, Switzerland, 2017; ISBN 978-2-8317-1855-2. [Google Scholar]
- Junta de Andalucía. Plan for the Recovery and Conservation of Ferns in Andalusia. In Order of May 20, 2015, by which the Action Programs of the Recovery and Conservation Plans for Catalogued Species of Andalusia are Approved; Ministry of Sustainability and Environment. Available online: https://www.juntadeandalucia.es/medioambiente/portal/areas-tematicas/biodiversidad-y-vegetacion/flora-protegida/conservacion-y-recuperacion-de-especies-de-flora-amenazada/helechos (accessed on 1 May 2025).
- Christenhusz, M.; Bento Elias, R.; Dyer, R.; Ivanenko, Y.; Rouhan, G.; Rumsey, F.; Väre, H. Culcita Macrocarpa. The IUCN Red List of Threatened Species 2017: E.T162293A85426759. 2017. Available online: https://doi.org/10.2305/IUCN.UK.2017-2.RLTS.T162293A85426759.en (accessed on 11 June 2025).
- AdapteCCa Miteco. AdapteCCa.es-Visor de Escenarios de Cambio Climático. Ministerio para la Transición Ecológica y el Reto Demográfico—Miteco. 2024. Available online: https://escenarios.adaptecca.es/ (accessed on 1 June 2024).
- Agencia Estatal de Meteorología (AEMET). Datos Climatológicos Diarios: San Roque—Cádiz (6056X) [Datos Horarios, Resúmenes Diarios de la Red de Estaciones Automaticas]. 2025. Available online: https://www.aemet.es/es/datos_abiertos/AEMET_OpenData (accessed on 1 March 2025).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Rue, H.; Martino, S.; Chopin, N. Approximate Bayesian Inference for Latent Gaussian Models by Using Integrated Nested Laplace Approximations. J. R. Stat. Soc. Ser. B Stat. Methodol. 2009, 71, 319–392. [Google Scholar] [CrossRef]
- Banerjee, S.; Carlin, B.P.; Gelfand, A.E. Hierarchical Modeling and Analysis for Spatial Data, 2nd ed.; Chapman and Hall/CRC: New York, NY, USA, 2014; ISBN 978-0-429-13717-4. [Google Scholar]
- Simpson, D.; Rue, H.; Riebler, A.; Martins, T.G.; Sørbye, S.H. Penalising Model Component Complexity: A Principled, Practical Approach to Constructing Priors. Stat. Sci. 2017, 32, 1–28. [Google Scholar] [CrossRef]

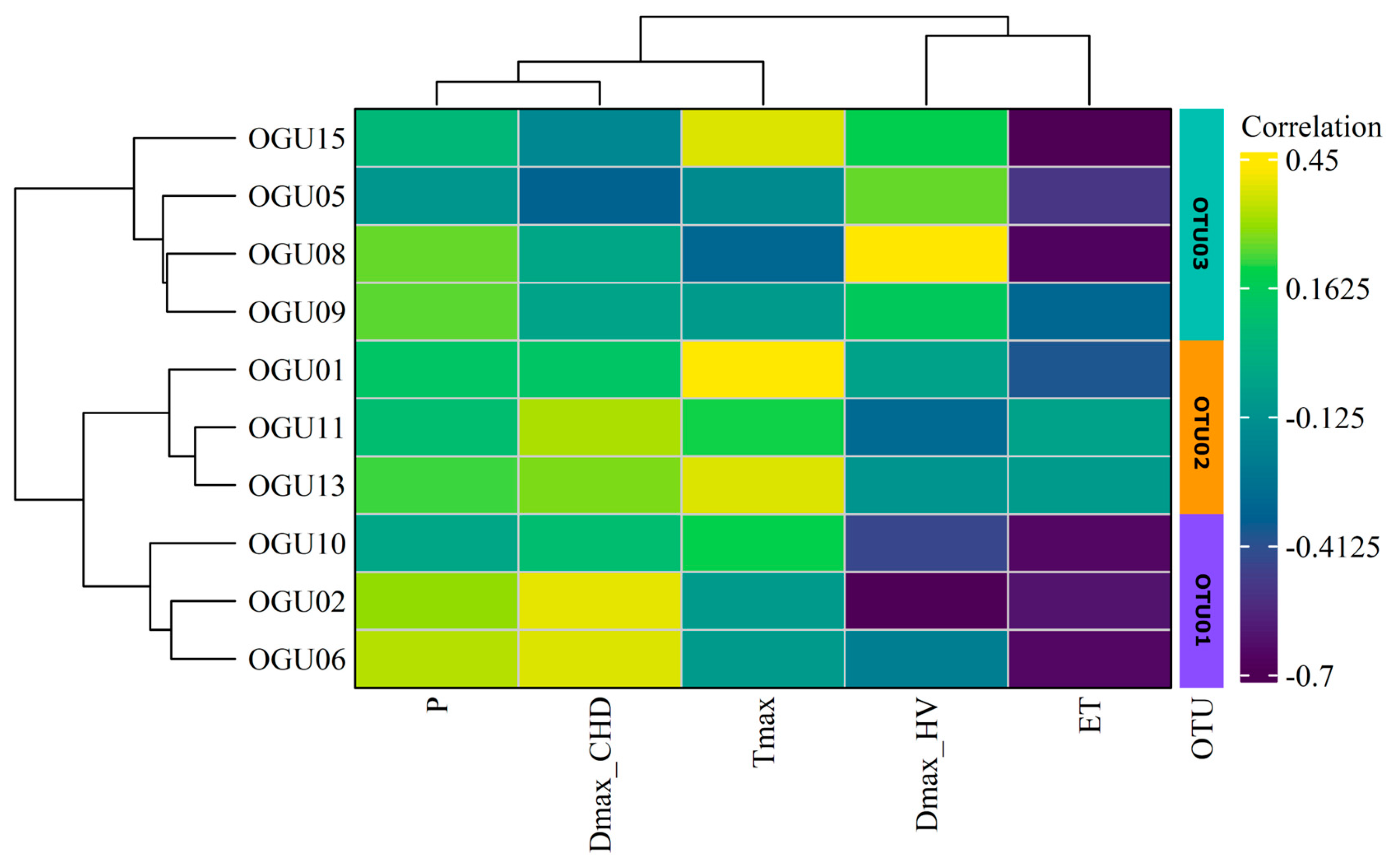
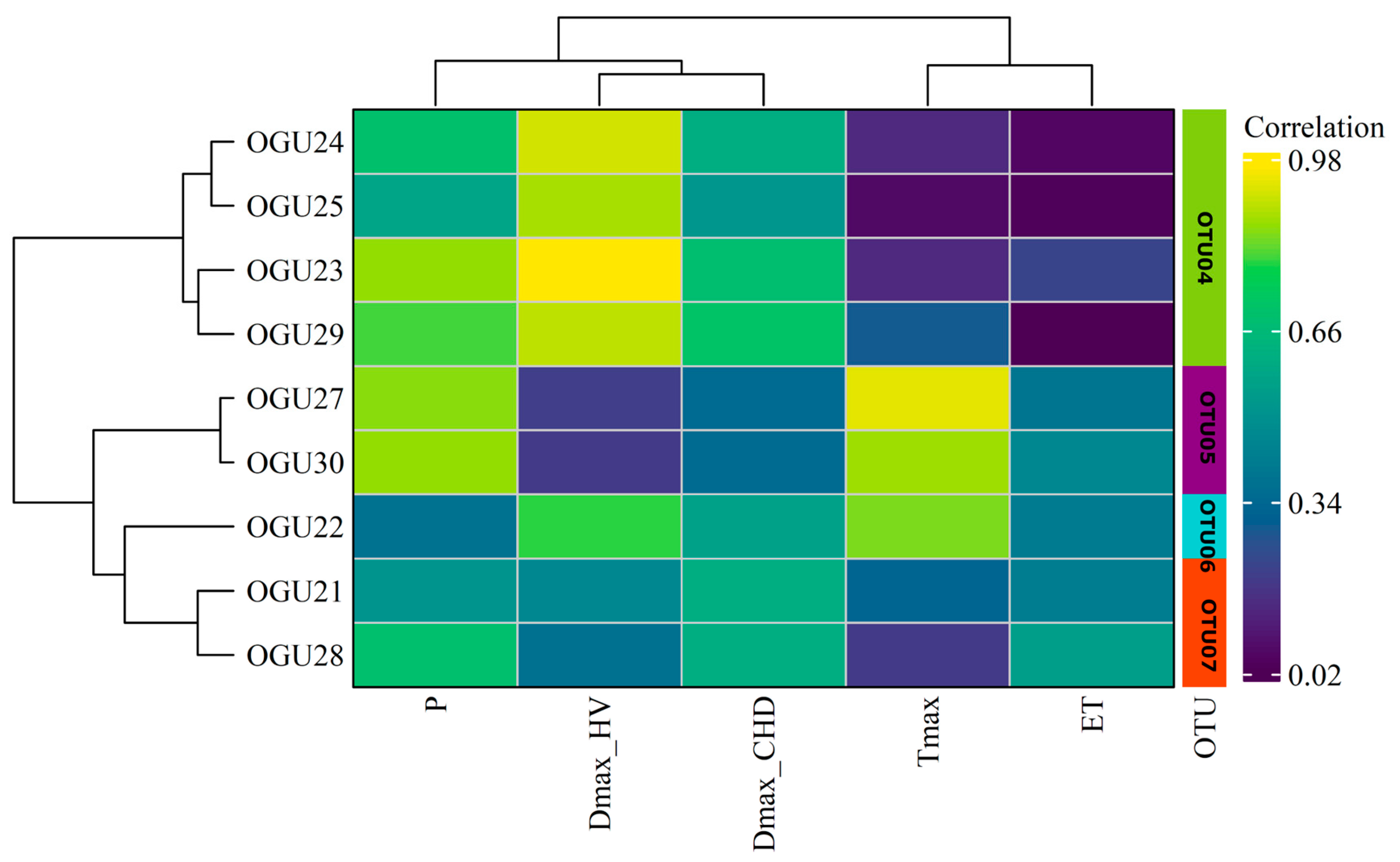


| Population | Tmax | P | DmaxHW | DmaxCHD | ET |
|---|---|---|---|---|---|
| Culcita macrocarpa | |||||
| OGU01 | 0.44 | 0.13 | −0.05 | 0.13 | −0.39 |
| OGU02 | −0.08 | 0.30 | −0.69 | 0.40 | −0.62 |
| OGU05 | −0.16 | −0.10 | 0.26 | −0.34 | −0.51 |
| OGU06 | −0.08 | 0.34 | −0.22 | 0.39 | −0.66 |
| OGU08 | −0.32 | 0.26 | 0.45 | −0.03 | −0.67 |
| OGU09 | −0.08 | 0.25 | 0.16 | −0.04 | −0.32 |
| OGU10 | 0.20 | −0.03 | −0.45 | 0.09 | −0.66 |
| OGU11 | 0.21 | 0.09 | −0.31 | 0.33 | −0.05 |
| OGU13 | 0.39 | 0.23 | −0.11 | 0.28 | −0.08 |
| OGU15 | 0.39 | 0.05 | 0.19 | −0.17 | −0.70 |
| Pteris incompleta | |||||
| OGU21 | 0.33 | 0.51 | 0.46 | 0.61 | 0.42 |
| OGU22 | 0.84 | 0.38 | 0.79 | 0.56 | 0.41 |
| OGU23 | 0.14 | 0.86 | 0.98 | 0.68 | 0.22 |
| OGU24 | 0.14 | 0.69 | 0.92 | 0.61 | 0.05 |
| OGU25 | 0.06 | 0.58 | 0.88 | 0.52 | 0.03 |
| OGU27 | 0.94 | 0.85 | 0.20 | 0.35 | 0.39 |
| OGU28 | 0.19 | 0.69 | 0.37 | 0.61 | 0.55 |
| OGU29 | 0.29 | 0.80 | 0.90 | 0.71 | 0.02 |
| OGU30 | 0.87 | 0.86 | 0.19 | 0.35 | 0.46 |
| Diplazium caudatum | |||||
| OGU31 | −0.27 | 0.09 | −0.35 | 0.34 | −0.42 |
| OGU33 | 0.53 | −0.39 | −0.16 | −0.22 | 0.71 |
| OGU34 | −0.69 | 0.47 | 0.19 | 0.28 | −0.51 |
| OGU35 | 0.42 | −0.02 | −0.46 | 0.24 | 0.33 |
| OGU36 | −0.24 | 0.26 | 0.25 | −0.03 | −0.88 |
| OGU37 | 0.21 | 0.37 | 0.02 | 0.08 | −0.84 |
| OGU40 | −0.22 | 0.05 | 0.09 | 0.03 | −0.72 |
| OGU41 | −0.19 | 0.37 | −0.06 | 0.34 | −0.72 |
| OGU44 | −0.33 | 0.38 | −0.05 | 0.34 | −0.81 |
| OGU45 | −0.05 | 0.49 | −0.14 | 0.39 | −0.07 |
| spp. | Operational Territorial Unit | Operational Geographical Unit | Toponym | Variables Explaining Similarities in Climate–Abundance Relationships |
|---|---|---|---|---|
| Culcita macrocarpa | OTU01 | OGU05 | Garganta de la Vegueta | DmaxHW |
| OGU08 | Canuto de la Leña | |||
| OGU09 | Canuto 6.7 Carril arriba | |||
| OGU15 | Laja del Pinalejo | |||
| OTU02 | OGU01 | Linde Comares-Las Corzas | P, Tmax, DmaxCHD | |
| OGU11 | Arroyo y Albinas del Viguetón | |||
| OGU13 | Garganta del Niño | |||
| OTU03 | OGU02 | Canuto 6.4 Arriba Carril | ET, DmaxHW | |
| OGU06 | Canuto 6.7 Carril abajo | |||
| OGU10 | Juan de Sevilla | |||
| Pteris incompleta | OTU04 | OGU23 | Albinas del Pino | ET, Tmax |
| OGU24 | Arroyo de Pepe Ayala 3 | |||
| OGU25 | Arroyo y Albinas del Pinillo | |||
| OGU29 | Chorreras del Alto Mariscal | |||
| OTU05 | OGU26 | Arroyo del Pino | P, Tmax, DmaxCHD, DmaxHW | |
| OTU06 | OGU27 | Arroyo de Huerto Campano | DmaxHW | |
| OGU30 | Garganta del Rayo | |||
| OTU07 | OGU21 | Junta afluentes 6.1-6.4 | DmaxCHD | |
| OGU22 | Arroyo Pepe Ayala 1 | |||
| OGU28 | Albina y Alto Mariscal | |||
| Diplazium caudatum | OTU08 | OGU33 | Comares | ET, Tmax |
| OGU35 | Pedregoso | |||
| OTU09 | OGU31 | Pedregoso | DmaxCHD | |
| OGU45 | Ojén | |||
| OTU10 | OGU34 | Pedregoso | ET | |
| OGU36 | Pedregoso | |||
| OGU37 | Ojén | |||
| OGU40 | Pedregoso | |||
| OGU41 | Pedregoso | |||
| OGU44 | Pedregoso |
| Species | Principal Component | % Variance Explained | Variable | Loading PC1 | Loading PC2 |
|---|---|---|---|---|---|
| Culcita macrocarpa | 1 | 52.32% | Tmax | 0.25 | 0.73 |
| P | 0.10 | −0.20 | |||
| DmaxHW | −0.79 | 0.24 | |||
| DmaxCHD | 0.52 | −0.14 | |||
| ET | 0.18 | 0.58 | |||
| 2 | 25.11% | Tmax | 0.25 | 0.73 | |
| P | 0.10 | −0.20 | |||
| DmaxHW | −0.79 | 0.24 | |||
| DmaxCHD | 0.52 | −0.14 | |||
| ET | 0.18 | 0.58 | |||
| Pteris incompleta | 1 | 54.19% | Tmax | 0.76 | 0.15 |
| P | 0.13 | 0.18 | |||
| DmaxHW | −0.39 | 0.75 | |||
| DmaxCHD | −0.04 | 0.50 | |||
| ET | 0.51 | 0.36 | |||
| 2 | 28.68% | Tmax | 0.76 | 0.15 | |
| P | 0.13 | 0.18 | |||
| DmaxHW | −0.39 | 0.75 | |||
| DmaxCHD | −0.04 | 0.50 | |||
| ET | 0.51 | 0.36 | |||
| Diplazium caudatum | 1 | 57.41% | Tmax | −0.46 | 0.06 |
| P | 0.53 | 0.19 | |||
| DmaxHW | 0.37 | −0.66 | |||
| DmaxCHD | 0.35 | 0.71 | |||
| ET | −0.50 | 0.15 | |||
| 2 | 25.17% | Tmax | −0.46 | 0.06 | |
| P | 0.53 | 0.19 | |||
| DmaxHW | 0.37 | −0.66 | |||
| DmaxCHD | 0.35 | 0.71 | |||
| ET | −0.50 | 0.15 |
| 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |
|---|---|---|---|---|---|---|---|
| Culcita macrocarpa | |||||||
| Observed | 380 | 359 | 375 | 373 | 377 | 363 | 360 |
| Modeled | 370 | 349 | 345 | 370 | 373 | 370 | 352 |
| Difference (O–M) | 10 | 10 | 30 | 3 | 4 | −7 | 8 |
| Pteris incompleta | |||||||
| Observed | 312 | 456 | 535 | 613 | 582 | 547 | 494 |
| Modeled | 300 | 302 | 471 | 549 | 612 | 583 | 549 |
| Difference (O–M) | 12 | 154 | 64 | 64 | −30 | −36 | −55 |
| Diplazium caudatum | |||||||
| Observed | 233 | 397 | 405 | 438 | 421 | 379 | 366 |
| Modeled | 224 | 221 | 391 | 398 | 440 | 417 | 357 |
| Difference (O–M) | 9 | 176 | 14 | 40 | −19 | −38 | 9 |
| Species | MAE | RMSE | MBE | MAPE |
|---|---|---|---|---|
| Culcita macrocarpa | 10.29 | 13.54 | 8.29 | 2.78 |
| Pteris incompleta | 59.29 | 73.50 | 24.71 | 11.8 |
| Diplazium caudatum | 43.57 | 70.4 | 27.32 | 11.1 |
| Species | MAE | RMSE | MBE | MAPE (%) | |
|---|---|---|---|---|---|
| Culcita macrocarpa | 46.63 ± 26.67 [11.24–116.34] | 56.7 ± 32.69 [15.79–130.46] | 40.14 ± 35.92 [19.25–116.28] | 12.95 ± 8.18 [3.26–32.22] | |
| Pteris incompleta | 188.68 ± 12.60 [162.64–211.64] | 212.82 ± 13.64 [184.42–237.58] | 188.63 ± 12.65 [162.45– 211.64] | 47.92 ± 3.31 [41.11–53.97] | |
| Diplazium caudatum | 282.25 ± 30.18 [210.52–323.44] | 326.79 ± 32.48 [248.71–371.38] | 281.38 ± 31.08 [207.54–323.44] | 52.84 ± 5.88 [38.95–60.82] | |
| Family | General Distribution | Ratio nº spp. | Nº crom. | Spores (m, t) | Category of Threat | |
|---|---|---|---|---|---|---|
| Diplazium caudatum (Cav.) Jermy | Athyriaceae (Polypodiales) | Aljibic Sector and Macaronesian Region | 2/350 | 82 | m | D23: EN. LRFA: CR. LRFE: CR. |
| Culcita macrocarpa C.Presl | Culcitaceae (Cyatheales) | Aljiblical Sector, Cantabro-Atlantic Subprovince and Macaronesian Region. | 1/002 | 136 | t | D23: EN. LRFA: CR. LRFE: EN. |
| Pteris incompleta Cav. | Pteridaceae (Polypodiales) | Aljibic sector, Tingitana Peninsula and Macaronesian Region. | 3/250 | 58 | t | D23: EN. LRFA: CR LRFE: VU. |
| Variable | Definition | Units | Ecological Roles in Ferns |
|---|---|---|---|
| Tmax | Yearly maximum monthly mean air temperature measured at 2 m above ground | °C | Paleomediterranean ferns require high and stable temperatures that resemble the subtropical climatic conditions that prevailed in their habitats in the past [15,69]. |
| P | Annual cumulative precipitation | mm | Essential for completing the life cycle (sporophyte and gametophyte phases); all three species depend on high atmospheric and soil moisture [41]. |
| DmaxHW | Maximum duration of heat waves per year (≥5 consecutive days above the 90th percentile) | days | Potentially critical for fern populations; ferns are especially vulnerable to prolonged heat and dryness [69], but microclimatic conditions in canutos may buffer this effect [15]. |
| DmaxCHD | Maximum number of consecutive humid days per year (days with daily precipitation >1 mm) | days | Essential for reproductive processes. Consecutive humid periods facilitate spore germination and gametophyte development [15,69,70] |
| ET | Potential evapotranspiration, estimated by the Thornthwaite method (k = 0.69) | mm/month | Key indicator of drought stress; high values reflect greater atmospheric demand for water, leading to negative effects on ferns due to their strong dependence on moisture [41]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvo-Tierra, Á.E.; Pereña-Ortiz, J.F.; Ruiz-Valero, Á. Projecting Extinction Risk and Assessing Conservation Effectiveness for Three Threatened Relict Ferns in the Western Mediterranean Basin. Plants 2025, 14, 2380. https://doi.org/10.3390/plants14152380
Salvo-Tierra ÁE, Pereña-Ortiz JF, Ruiz-Valero Á. Projecting Extinction Risk and Assessing Conservation Effectiveness for Three Threatened Relict Ferns in the Western Mediterranean Basin. Plants. 2025; 14(15):2380. https://doi.org/10.3390/plants14152380
Chicago/Turabian StyleSalvo-Tierra, Ángel Enrique, Jaime Francisco Pereña-Ortiz, and Ángel Ruiz-Valero. 2025. "Projecting Extinction Risk and Assessing Conservation Effectiveness for Three Threatened Relict Ferns in the Western Mediterranean Basin" Plants 14, no. 15: 2380. https://doi.org/10.3390/plants14152380
APA StyleSalvo-Tierra, Á. E., Pereña-Ortiz, J. F., & Ruiz-Valero, Á. (2025). Projecting Extinction Risk and Assessing Conservation Effectiveness for Three Threatened Relict Ferns in the Western Mediterranean Basin. Plants, 14(15), 2380. https://doi.org/10.3390/plants14152380








