Morphology-Based Evaluation of Pollen Fertility and Storage Characteristics in Male Actinidia arguta Germplasm
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Procedures
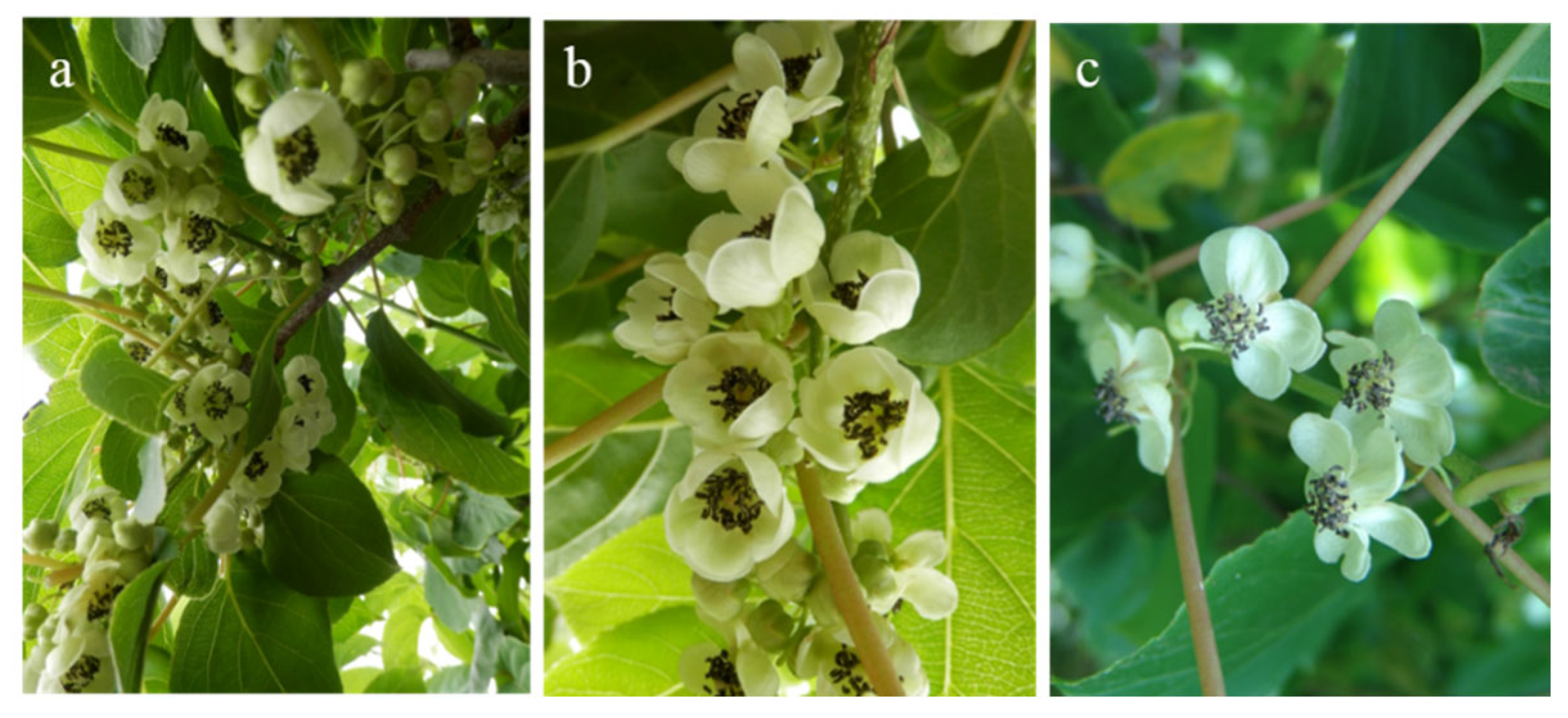
2.2.1. Flower Quantity Assessment and Collection
2.2.2. Anther Number and Pollen Quantity Measurement
2.2.3. Pollen Germination Rate Assay
2.2.4. Pollen Storage at Low and Ultra-Low Temperatures
2.2.5. Scanning Electron Microscopy (SEM)
2.2.6. Pollen Morphological Abnormality Analysis
2.2.7. Statistical Analysis
3. Results
3.1. Floral Characteristics and Pollen Germination Capacity of Different Germplasms
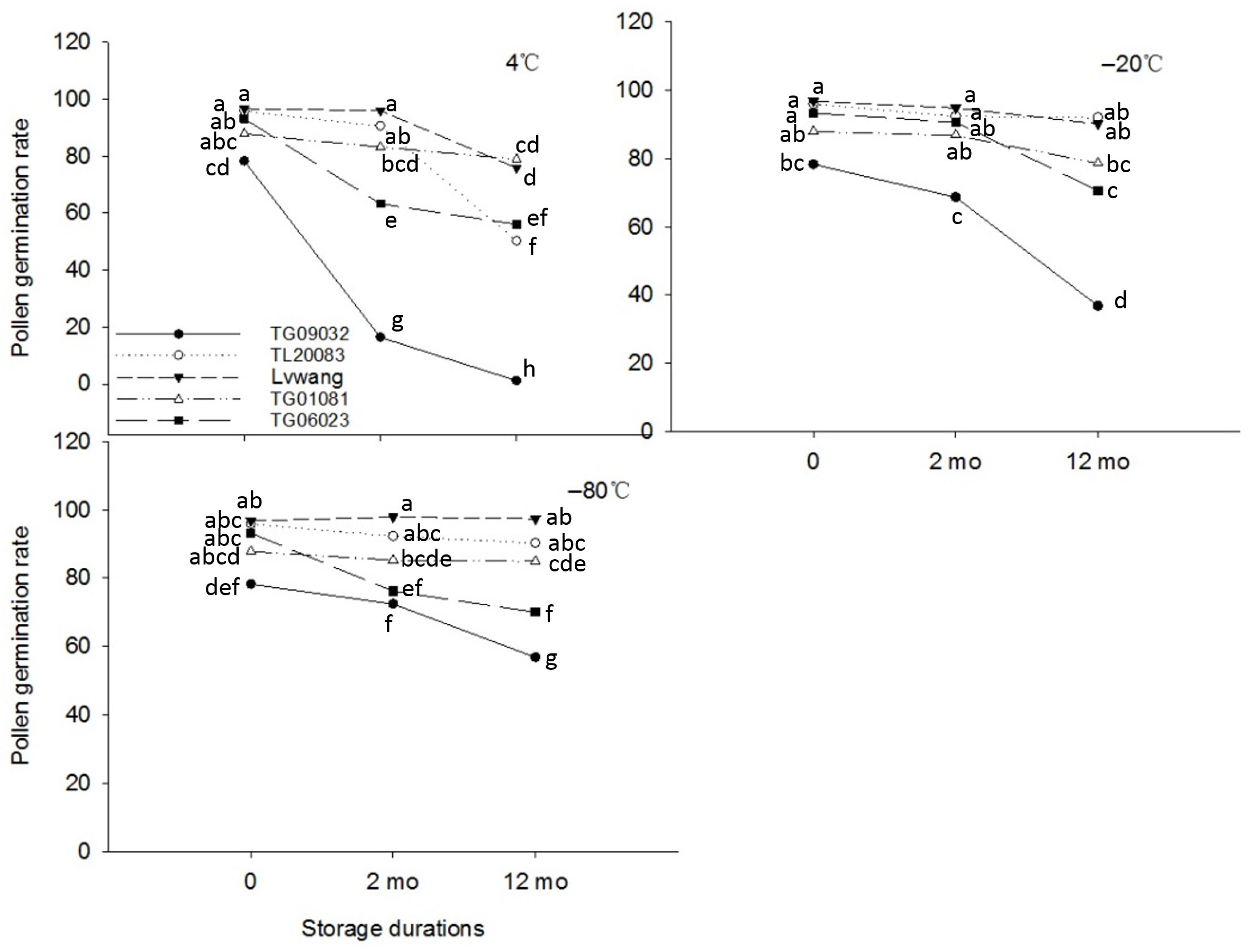
3.2. Pollen Storage at Low and Ultra-Low Temperatures
3.3. Pollen Morphological Characteristics of Different Germplasms
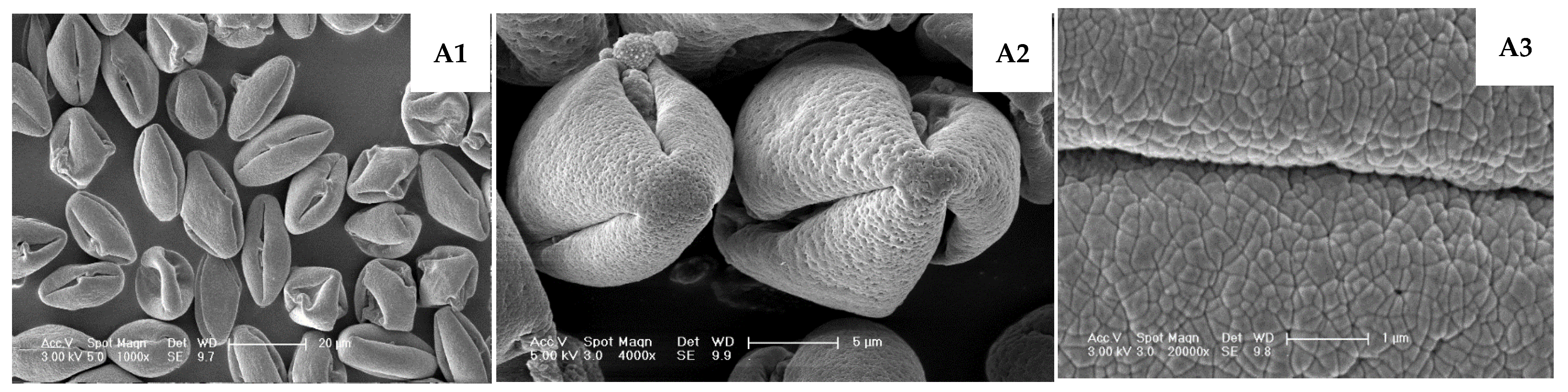

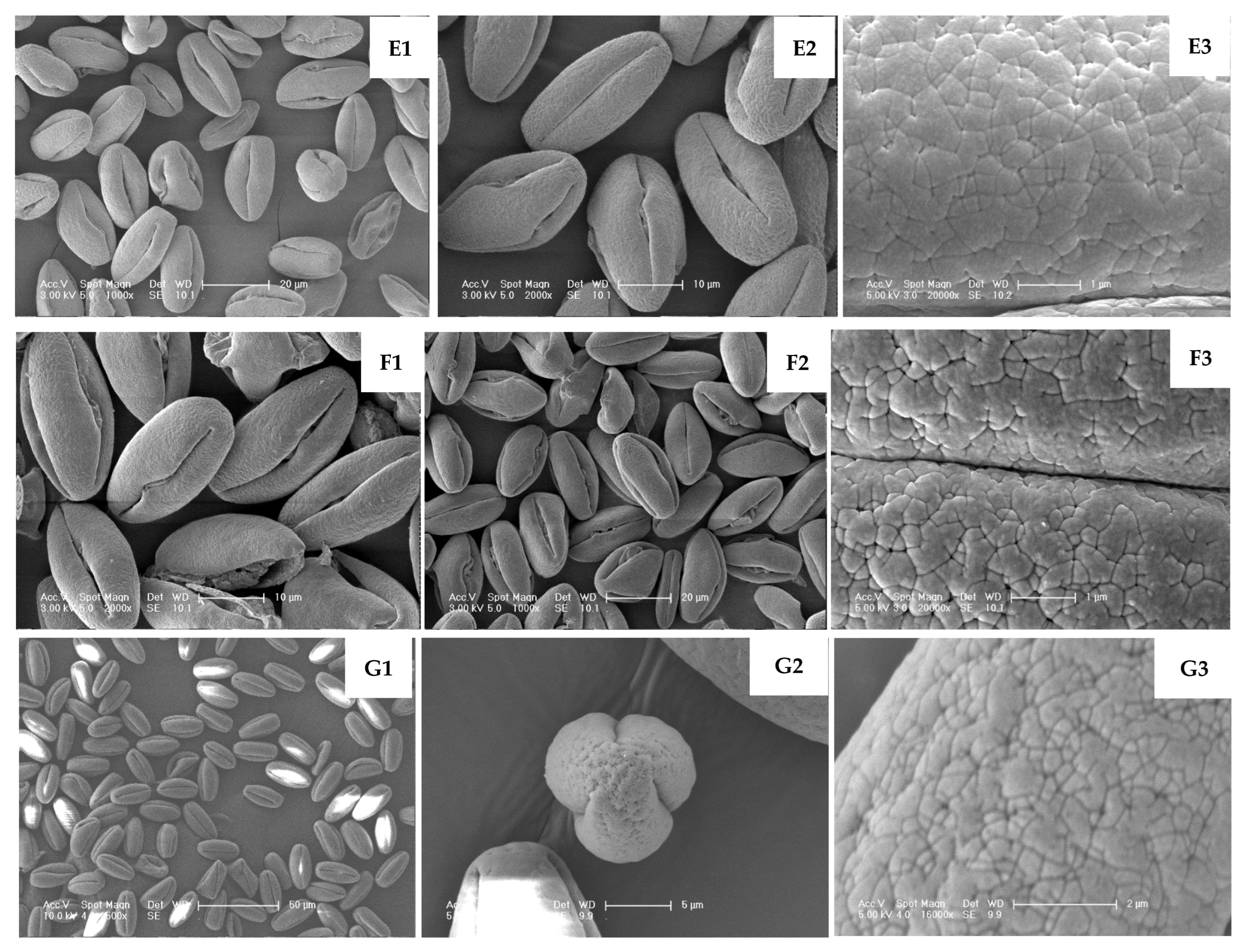

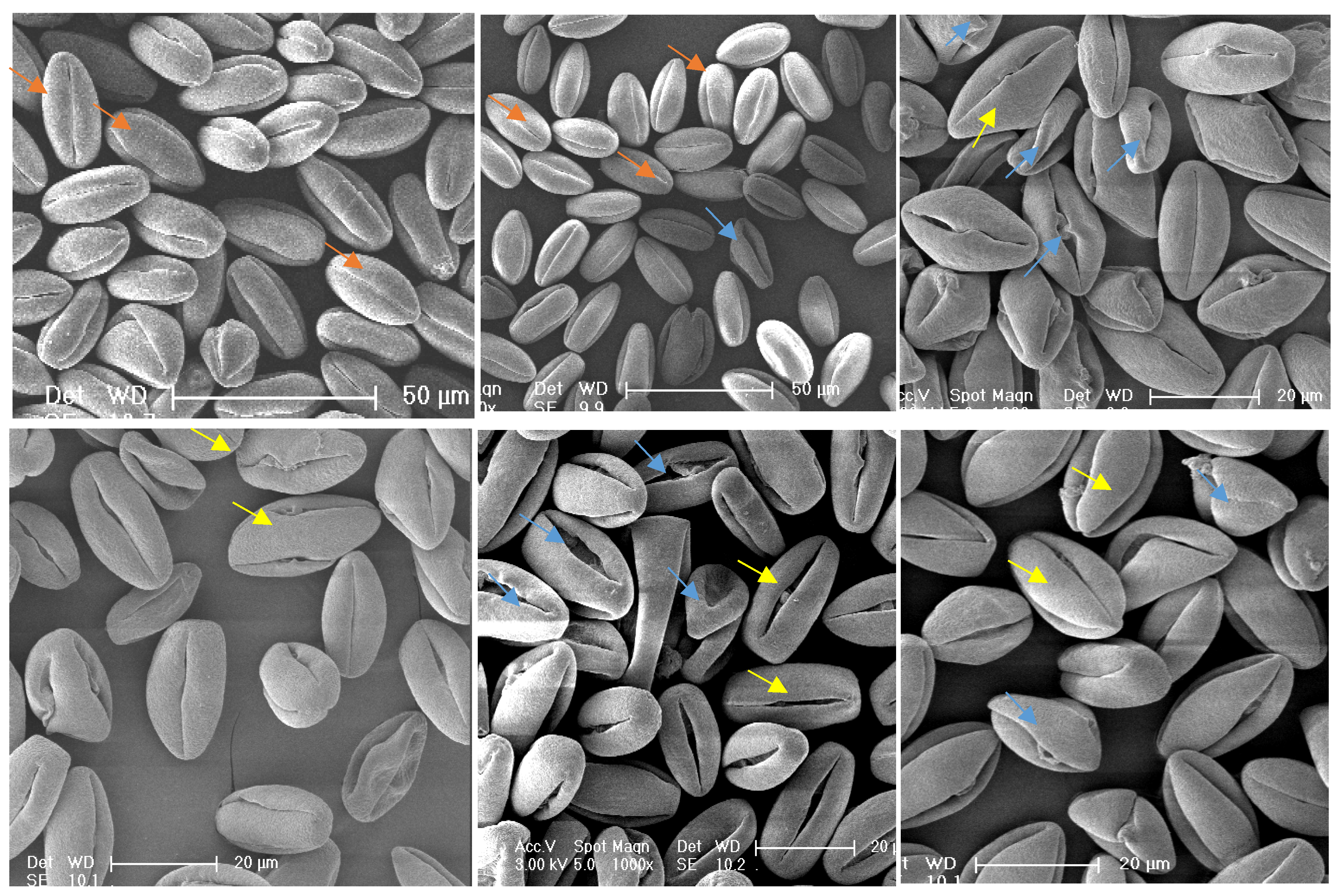
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Latocha, P.; Jankowski, P.; Radzanowska, J. Genotypic difference in postharvest characteristics of hardy kiwifruit (Actinidia arguta and its hybrids), as a new commercial crop Part I. Sensory profiling and physicochemical differences. Food Res. Int. 2011, 44, 1936–1945. [Google Scholar] [CrossRef]
- Okamoto, G.; Goto, S. Juice Constituents in Actinidia Arguta Fruits Produced in Shinjo, Okayama; Okayama University: Okayama, Japan, 2005; Volume 94, pp. 9–13. [Google Scholar]
- Boyd, L.; McNeilage, M.; MacRae, E.; Ferguson, A.; Beatson, R.; Martin, P.; Williams, M. Development and commercialization of ‘baby kiwi’(Actinidia arguta Planch.). V. Int. Symp. Kiwifruit 2002, 610, 81–86. [Google Scholar]
- Zhang, Z.; Lin, M.; Qi, Q. Research progress on Actinidia arguta. China Fruits 2024, 11, 21–26. [Google Scholar]
- Cai, Z.Y.; Dong, L.; Wang, H.Q.; Qiu, W.; Su, W.; Ren, H.; Liu, Y. Pollen viability, stigma receptivity and their effect on fruit set of passionfruit at different flower developmental stages. J. Fruit. Sci. 2023, 40, 969–977. [Google Scholar]
- Quinet, M.; Jacquemart, A. Troubles in pear pollination: Effects of collection and storage method on pollen viability and fruit production. Acta Oecologica 2020, 105, 103558. [Google Scholar] [CrossRef]
- Wang, S.; Zhong, M.; Liao, G.; Chen, L.; Xu, X. Comparison of pollen quantity and pollen viability of 41 male plants in Actinidia. Acta Agric. Univ. Jiangxiensis 2017, 39, 460–467. [Google Scholar]
- Shivanna, K.; Johri, B. The Angiosperm Pollen Structure and Function; Wiley Eastern Ltd.: New Delhi, India, 1985. [Google Scholar]
- Hanna, W.; Towill, L. Long term pollen storage. Plant Breed. Rev. 1995, 13, 179–207. [Google Scholar]
- Bomben, C.; Malossini, C.; Cipriani, G.; Testolin, R. Long term storage of kiwifruit pollen. Acta Hortic. 1999, 498, 105–110. [Google Scholar] [CrossRef]
- Abreu, I.; Oliveira, M. Fruit production in kiwifruit (Actinidia deliciosa) using preserved pollen. Aust. J. Agric. Res. 2004, 55, 565–569. [Google Scholar] [CrossRef]
- Naik, S.; Rana, P.; Rana, V. Pollen storage and use for enhancing fruit production in kiwifruit (Actinidia deliciosa A. Chev.). J. Appl. Hortic. 2013, 15, 128–132. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, B.; Ai, J.; Fan, S.; Yang, Y.; Wang, Z.; Song, H. Studies on pollen fertility of hardy kiwifruit (Actinidia arguta Planch.) two cultivars. Acta Bot. Boreali-Occident. Sin. 2017, 37, 909–914. [Google Scholar]
- Soares, T.; Silva, S.; Costa, M.; Santos-Serejo, J.; Souza, A.; Lino, L.; Souza, E.; Jesus, O. In vitro germination and viability of pollen grains of banana diploids Crop Breed. Appl. Biotechnol. 2008, 8, 111–118. [Google Scholar]
- Souza, E.; Souza, M.; Rossi, C.; Ledo, A. Martinelli Viability, storage and ultrastructure analysis of Aechmea bicolor (Bromeliaceae) pollen grains, an endemic species to the Atlantic Forest Euphytica. Euphytica 2015, 204, 13–28. [Google Scholar] [CrossRef]
- Souza, E.H.; Souza, F.V.; Rossi, M.L.; Packer, R.M.; Cruz-Barros, M.A.V.; Martinelli, A.P. Pollen morphology and viability in Bromeliaceae. An. Acad. Bras. Ciênc. 2017, 89, 3067–3082. [Google Scholar] [CrossRef]
- Santos, V.; Nievola, C.; Fidalgo, A.; Kanashiro, S.; Wanderley, M.; Gomes, E.; Luz, C. Floral morphology and pollen viability of an endangered and endemic Bromeliaceae species from the Atlantic Forest Grana. Grana 2021, 60, 327–346. [Google Scholar] [CrossRef]
- Langedijk, N.; Kaufmann, S.; Vos, E.; Ottiger, T. Evaluation of methods to assess the quality of cryopreserved Solanaceae pollen. Sci. Rep. 2023, 13, 7344. [Google Scholar] [CrossRef]
- Soares, T.; Jesus, O.; Souza, E.H.; Santos-Serejo, J.; Oliveira, E. Morphology and viability of pollen grains from passion fruit species (Passiflora spp.). Acta Bot. Bras. 2013, 27, 779–787. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Peter, J.; Shajitha, P.; Balaji, V.; Nisha, R.; Geetha, M.; Sivasamy, M. Development of in vitro pollen germination protocol for recalcitrant triticale pollen (X Triticosecale Wittmack). Cereal Res. Commun. 2023, 51, 189–196. [Google Scholar] [CrossRef]
- Kumari, M.; Prasad, A.; ur Rahman, L.; Mathur, A.K.; Mathur, A. In vitro germination, storage and microscopic studies of pollen grains of four Ocimum species. Ind. Crops Prod. 2022, 177, 114445. [Google Scholar] [CrossRef]
- Li, M.; Jiang, F.; Huang, L.; Wang, H.; Song, W.; Zhang, X.; Niu, L. Optimization of in vitro germination, viability tests and storage of Paeonia ostii pollen. Plants 2023, 12, 2460. [Google Scholar] [CrossRef] [PubMed]
- Althiab-Almasaud, R.; Teyssier, E.; Chervin, C.; Johnson, M.; Mollet, J. Pollen viability, longevity, and function in angiosperms: Key drivers and prospects for improvement. Plant Reprod. 2024, 37, 273–293. [Google Scholar] [CrossRef]
- Li, H.; Sun, Y.; Liu, Z.; Liu, G.; Chen, X. Study on Pollen Viability of Actinidia arguta. Liaoning For. Sci. Technol. 2018, 3, 32–33. [Google Scholar]
- Majeed, S.; Zafar, M.; Ahmad, M.; Kilic, O.; Sultana, S.; Raza, J.; Yaseen, G.; Gul, H.; Mir, S.; Jabeen, M. Pollen morphological investigations of family Cactaceae and its taxonomic implication by light microscopy and scanning electron microscopy. Microsc. Res. Tech. 2020, 83, 767–777. [Google Scholar] [CrossRef]
- Hayat, K.; Khan, W.M.; Khan, M.N.; Shah, S.N. Pollen morphological investigation of selected species of family Asteraceae from Pakistan by using light and scanning electron microscopy. Microsc. Res. Tech. 2023, 86, 1258–1273. [Google Scholar] [CrossRef]
- Mog, B.; Veena, G.L.; Adiga, J.D.; Hebbar, K.B.; Manjesh, G.N.; Eradasappa, E.; Mohana, G.S.; Thandaiman, V.; Vanitha, K.; Yadav, A.K. Pollen morphological study and temperature effect on the pollen germination of cashew (Anacardium occidentale L.) varieties. Sci. Hortic. 2023, 314, 111957. [Google Scholar] [CrossRef]
- Souza, S.; Oliveira, R.; Aona, L.; Souza, F.; Soares, T.; Rossi, M.; Souza, E. Pollen morphology and viability of Tillandsia (Bromeliaceae) species by light microscopy and scanning electron microscopy. Microsc. Res. Tech. 2021, 84, 441–459. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Ribeiro, E.; Luizi-Ponzo, A.; Faria, A. Ultrastructure and pollen morphology of Bromeliaceae species from the Atlantic Rainforest in Southeastern Brazil. An. Acad. Bras. Ciênc. 2016, 88, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, A.; Latocha, P.; Bieniasz, M. Effect of genetically diverse pollen on pollination, pollen tube overgrow, fruit set and morphology of kiwiberry (Actinidia arguta). Agronomy 2021, 11, 1814. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, J.; Wang, X.; Zhao, Y. Studies on pollen morphology, pollen vitality and preservation methods of Gleditsia sinensis Lam.(Fabaceae). Forests 2023, 14, 243. [Google Scholar] [CrossRef]
- Lukšić, K.; Zdunić, G.; Mucalo, A.; Marinov, L.; Ranković-Vasić, Z.; Ivanović, J.; Nikolić, D. Microstructure of Croatian Wild Grapevine (Vitis Vinifera Subsp Sylvestris Gmel Hegi) Pollen Grains Revealed by Scanning Electron Microscopy. Plants 2022, 11, 1479. [Google Scholar]
- Deng, Y.; Liang, L.; Sun, X.; Jia, X.; Gu, C.; Su, J. Ultrastructural abnormalities in pollen and anther wall development may lead to low pollen viability in jasmine (Jasminum sambac (L.) Aiton, Oleaceae). S. Afr. J. Bot. 2018, 114, 69–77. [Google Scholar] [CrossRef]
- Wan, R.X.; Ma, J.Q. Preliminary study on pollen abortion in Lemon. China Citrus 1987, 4, 6–7. [Google Scholar]
- Wang, D. Study on Pollen Fertility of ‘Jincheng’ Orange. China Citrus 1989, 4, 11–13. [Google Scholar]
| Germplasm ID | Visual Evaluation of Flower Abundance | Mean Anther Number | Pollen Grains per Anther | Pollen Germination Rate (%) |
|---|---|---|---|---|
| TT03021 | 2 | 40.2 hijk | 27,610 bcd | 4.33 k |
| TT04043 | 1 | 42.4 fghij | 16,190 ijkl | 1.56 k |
| TT05022 | 1 | 64.2 a | 16,625 ijkl | 17.62 j |
| TT06051 | 1 | 43.6 efghi | 24,445 cdefg | 35.21 gh |
| TT06023 | 3 | 44.6 defgh | 18,645 fghijkl | 26.44 i |
| TT07031 | 2 | 39.0 ijk | 14,375 jkl | 3.18 k |
| TT07033 | 1 | 36.0 k | 13,190 kl | 41.44 fg |
| TT07063 | 1 | 37.4 jk | 20,875 defghij | 7.13 k |
| TT08093 | 3 | 39.2 ijk | 42,250 a | 37.80 gh |
| TL21013 | 3 | 44.6 defgh | 20,250 efghijk | 45.58 f |
| TL20083 | 1 | 36.2 k | 28,065 bc | 95.85 a |
| TL18072 | 3 | 44.6 defgh | 25,875 bcde | 32.85 hi |
| TL17093 | 1 | 41.0 ghijk | 26,375 bcde | 18.04 j |
| Lvwang | 3 | 47.4 bcdef | 16,750 ijkl | 96.57 a |
| TL17061 | 3 | 42.2 ghij | 16,690 ijkl | 2.63 k |
| TL15012 | 1 | 41.0 ghijk | 12,875 l | 19.25 j |
| TL15022 | 3 | 40.0 hijk | 16,190 ijkl | 36.03 gh |
| TL14101 | 3 | 45.4 cdefg | 16,815 hijkl | 54.99 e |
| TL13091 | 3 | 47.6 bcde | 20,815 defghij | 63.90 e |
| TL12043 | 3 | 48.4 bcde | 24,315 cdefg | 17.65 j |
| TG02023 | 2 | 45.8 cdefg | 17,355 ghijkl | 65.98 d |
| TG01081 | 3 | 45.4 cdefg | 26,645 bcde | 87.84 b |
| TG04021 | 3 | 51.0 b | 22,500 cdefghi | 47.32 f |
| TG06011 | 3 | 39.8 hijk | 23,930 cdefgh | 58.25 e |
| TG06023 | 3 | 48.6 bcde | 20,750 defghij | 93.47 ab |
| TG06063 | 3 | 49.4 c | 32,315 b | 73.05 cd |
| TG06081 | 3 | 46.0 bcdefg | 14,750 jkl | 34.30 gh |
| TG07053 | 3 | 50.0 bc | 18,565 fghijkl | 37.66 gh |
| TG07031 | 3 | 44.0 efghi | 24,690 cdef | 40.56 fg |
| TG09032 | 3 | 48.4 bcde | 18,375 fghijkl | 78.48 c |
| Germplasm ID | Morphological Description | Pollen Size (Polar Axis × Equatorial Axis)/μm | P/E Ratio | Aperture Type | Aperture Length/μm | Aperture Width/μm | Abnormal Percentage/% | Slightly Abnormal Percentage/% | Figure |
|---|---|---|---|---|---|---|---|---|---|
| TT03021 | Prolate; very few normal; most grains shrunken/deformed with widened apertures and visible exudates | (25.46–30.67) × (13.48–16.21) | 1.85 | Tricolporoidate | 21.17–25.95 | 1.05–3.99 | 16.85 cdefg | 72.28 ab | Figure 5A1–A3 |
| TT04043 | Hyperprolate; few shrunken grains; apertures with exudates | (25.51–30.59) × (11.98–15.96) | 2.06 | Tricolporoidate (with exudate) | 22.29–28.07 | 1.81–2.94 | 10.09 efghijk | 18.56 mno | Figure 5B1–B3 |
| TT05022 | Prolate; few apertures fissured | (23.52–27.97) × (12.76–14.73) | 1.88 | Tricolporate | 19.22–26.06 | 1.19–3.48 | 15.7 cdefgh | 38.15 ghijk | |
| TT06051 | Hyperprolate; few slightly deformed | (25.27–31.78) × (11.70–16.11) | 2.19 | Tricolporoidate | 23.94–29.51 | 0.90–2.28 | 7.61 hijkl | 45.43 efghi | |
| TT06023 | Hyperprolate; few slightly deformed; few apertures fissured | (25.89–33.80) × (12.04–16.14) | 2.15 | Tricolporoidate | 22.65–30.10 | 1.73–3.04 | 8.11 ghijkl | 34.69 ghijk | |
| TT07031 | Prolate; few apertures fissured; no exudates | (24.85–30.17) × (13.08–19.60) | 1.93 | Tricolporoidate | 21.32–26.87 | 1.86–4.35 | 8.39 fghijkl | 30.83 ijklm | Figure 5C1–C3 |
| TT07033 | Hyperprolate; most apertures fissured; few deformed with exudates | (25.02–30.37) × (12.57–16.03) | 2.03 | Tricolporate | 21.00–27.09 | 2.39–4.25 | 17.74 cde | 35.24 ghijk | |
| TT07063 | Hyperprolate; very few shrunken | (22.19–30.92) × (10.27–13.78) | 2.21 | Tricolporoidate | 18.54–27.40 | 1.18–2.27 | 15.48 cdefghi | 34.13 hijkl | Figure 5D1–D3 |
| TT08093 | Prolate; most apertures fissured; few with exudates | (25.36–29.38) × (11.88–14.96) | 1.93 | Tricolporate (with exudate) | 20.62–24.59 | 1.66–4.89 | 17.25 cde | 75.92 a | |
| TL21013 | Prolate; few shortened and slightly deformed | (25.50–29.31) × (12.63–17.16) | 1.90 | Tricolporoidate | 22.69–26.54 | 0.97–3.23 | 7.5 hijkl | 44.15 efghi | |
| TL20083 | Prolate; relatively uniform; occasional deformations | (21.56–28.76) × (2.38–15.08) | 1.97 | Tricolporoidate | 18.47–25.76 | 1.14–3.79 | 0.71 l | 12.38 nop | Figure 5G1–G3 |
| TL18072 | Prolate; few apertures fissured | (22.79–30.12) × (12.30–15.15) | 1.97 | Tricolporoidate | 21.08–28.24 | 1.61–5.10 | 20.81 bc | 42.53 fghij | |
| TL17093 | Prolate; most deformed–shrunken/shortened; few apertures with exudates | (22.99–30.48) × (12.97–16.46) | 1.80 | Tricolporoidate (with exudate) | 17.04–26.08 | 1.51–4.10 | 17.89 cde | 54.31 cdef | Figure 5E1–E3 |
| Lvwang | Prolate; uniform | (26.40–28.11) × (12.89–19.84) | 1.90 | Tricolporoidate | 22.09–25.36 | 1.76–3.51 | 3.06 jkl | 0.00 p | Figure 5H1–H3 |
| TL17061 | Prolate; few deformed–shrunken/triangular-pyramidal | (23.72–27.53) × (11.57–16.34) | 1.87 | Tricolporoidate | 20.21–25.33 | 2.39–5.33 | 19.15 bcd | 40.12 fghijk | |
| TL15012 | Prolate; most apertures fissured; irregular surface | (23.24–30.54) × (13.19–16.19) | 1.81 | Tricolporate, Tricolporoidate | 20.91–26.31 | 2.23–6.05 | 17.09 cdef | 61.83 abcd | |
| TL15022 | Prolate; few apertures fissured | (25.23–29.75) × (12.57–15.42) | 1.99 | Tricolporoidate | 18.88–27.29 | 2.03–3.92 | 3.59 jkl | 58.52 bcde | |
| TL14101 | Prolate; most apertures fissured and twisted | (23.92–29.88) × (13.25–17.47) | 1.82 | Tricolporate, Tricolporoidate | 18.70–26.35 | 2.07–8.14 | 27.02 b | 63.05 abc | |
| TL13091 | Prolate; relatively uniform; occasional shrunken grains | (26.75–28.43) × (12.82–18.02) | 1.95 | Tricolporoidate | 22.91–27.70 | 2.06–5.26 | 20.21 bcd | 46.99 defgj | |
| TL12043 | Prolate; multiple fissured apertures with exudates | (26.47–29.96) × (14.21–17.82) | 1.81 | Tricolporate (with exudate) | 20.27–27.71 | 3.53–6.19 | 40.55 a | 49.4 cdefg | Figure 5F1–F3 |
| TG02023 | Hyperprolate; relatively uniform | (27.37–29.27) × (12.56–15.12) | 2.07 | Tricolporoidate | 21.61–25.91 | 1.40–2.65 | 0.63 l | 7.88 op | |
| TG01081 | Hyperprolate; relatively uniform | (25.42–28.29) × (11.93–14.29) | 2.04 | Tricolporoidate | 22.62–26.40 | 1.24–2.72 | 0.88 l | 6.29 op | Figure 5I1–I3 |
| TG04021 | Hyperprolate; very few normal; most grains shrunken/deformed with widened apertures and visible exudates | (24.00–29.17) × (10.75–14.54) | 2.16 | Tricolporoidate | 19.21–25.63 | 1.81–4.36 | 7.45 hijkl | 43.83 efghi | |
| TG06011 | Prolate; Generally uniform; Occasional deformed grains | (26.08–28.73) × (12.14–16.50) | 1.90 | Tricolporoidate | 21.64–26.24 | 1.47–3.77 | 6.74 ijkl | 19.37 lmno | |
| TG06023 | Hyperprolate; Minority slightly deformed | (25.05–29.03) × (12.08–14.30) | 2.01 | Tricolporoidate | 22.79–26.99 | 1.64–2.86 | 2.87 kl | 25.89 klmn | |
| TG06063 | Prolate; Relatively uniform; Minority slightly deformed | (23.72–28.07) × (11.55–14.39) | 1.99 | Tricolporoidate | 21.54–26.15 | 1.08–1.93 | 0.93 l | 2.69 p | |
| TG06081 | Prolate; Minority with fissured apertures; Pollen deformation present | (23.78–30.32) × (13.75–16.66) | 1.83 | Tricolporoidate | 21.48–27.64 | 2.36–5.25 | 11.62 defghijk | 31.28 ijklm | |
| TG07053 | Hyperprolate; 50% deformed grains (partial shrinkage or triangular-pyramidal transformation) | (23.42–28.96) × (11.59–14.51) | 2.01 | Tricolporoidate | 19.65–27.28 | 1.62–3.36 | 11.65 cdefghi | 28.64 jklm | |
| TG07030 | Hyperprolate; Isolated deformed grains | (26.27–30.92) × (11.95–14.81) | 2.12 | Tricolporoidate | 20.33–28.58 | 1.25–4.17 | 5.35 jkl | 40.73 fghijk | |
| TG09032 | Hyperprolate; Exceptionally uniform | (27.47–30.32) × (13.03–15.12) | 2.03 | Tricolporoidate | 22.73–27.32 | 1.71–3.42 | 0 l | 9.04 op | Figure 5J1–J3 |
| Visual Flower Abundance Rating | Mean Anther Number per Flower | Pollen Grains per Anther | Pollen Germination Rate | Normal Pollen Percentage | Abnormal Pollen Percentage | Slightly Abnormal Pollen Percentage | Polar Axis Length | Equatorial Axis Length | Aperture Length | Aperture Spacing | Polar/Equatorial Axis Ratio (P/E) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visual Flower Abundance Rating | 1 | |||||||||||
| Mean Anther Number per Flower | 0.25 | 1 | ||||||||||
| Pollen Grains per Anther | 0.17 | −0.12 | 1 | |||||||||
| Pollen Germination Rate | 0.35 | 0.16 | 0.18 | 1 | ||||||||
| Normal Pollen Percentage | 0.06 | 0.14 | −0.12 | 0.62 ** | 1 | |||||||
| Abnormal Pollen Percentage | −0.06 | 0.01 | 0.00 | −0.55 ** | −0.82 ** | 1 | ||||||
| Slightly Abnormal Pollen Percentage | −0.06 | −0.18 | 0.16 | −0.58 ** | −0.96 ** | 0.63 ** | 1 | |||||
| Polar Axis Length | −0.03 | −0.23 | −0.24 | −0.14 | 0.02 | −0.08 | 0.01 | 1 | ||||
| Equatorial Axis Length | 0.08 | −0.10 | −0.22 | −0.25 | −0.44 * | 0.49 ** | 0.37 * | 0.13 | 1 | |||
| Aperture Length | 0.11 | 0.04 | −0.27 | 0.05 | 0.30 | −0.31 | −0.27 | 0.73 ** | 0.03 | 1 | ||
| Aperture Spacing | 0.18 | −0.07 | −0.17 | −0.30 | −0.65 ** | 0.77 ** | 0.52 ** | −0.04 | 0.65 ** | −0.19 | 1 | |
| Polar/Equatorial Axis Ratio (P/E) | −0.09 | −0.05 | 0.05 | 0.12 | 0.40 * | −0.48 ** | −0.31 | 0.47 ** | −0.81 ** | 0.40 * | −0.61 ** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, H.; Fan, S.; Zhao, Y.; Xu, P.; Chen, X.; Li, J.; Yang, Y.; Wang, Y.; Wang, Y.; Li, C.; et al. Morphology-Based Evaluation of Pollen Fertility and Storage Characteristics in Male Actinidia arguta Germplasm. Plants 2025, 14, 2366. https://doi.org/10.3390/plants14152366
Qin H, Fan S, Zhao Y, Xu P, Chen X, Li J, Yang Y, Wang Y, Wang Y, Li C, et al. Morphology-Based Evaluation of Pollen Fertility and Storage Characteristics in Male Actinidia arguta Germplasm. Plants. 2025; 14(15):2366. https://doi.org/10.3390/plants14152366
Chicago/Turabian StyleQin, Hongyan, Shutian Fan, Ying Zhao, Peilei Xu, Xiuling Chen, Jiaqi Li, Yiming Yang, Yanli Wang, Yue Wang, Changyu Li, and et al. 2025. "Morphology-Based Evaluation of Pollen Fertility and Storage Characteristics in Male Actinidia arguta Germplasm" Plants 14, no. 15: 2366. https://doi.org/10.3390/plants14152366
APA StyleQin, H., Fan, S., Zhao, Y., Xu, P., Chen, X., Li, J., Yang, Y., Wang, Y., Wang, Y., Li, C., Liu, Y., Zhang, B., & Lu, W. (2025). Morphology-Based Evaluation of Pollen Fertility and Storage Characteristics in Male Actinidia arguta Germplasm. Plants, 14(15), 2366. https://doi.org/10.3390/plants14152366





