Saussurea involucrata CML6 Enhances Freezing Tolerance by Activating Antioxidant Defense and the CBF-COR Pathway in Plants
Abstract
1. Introduction
2. Results
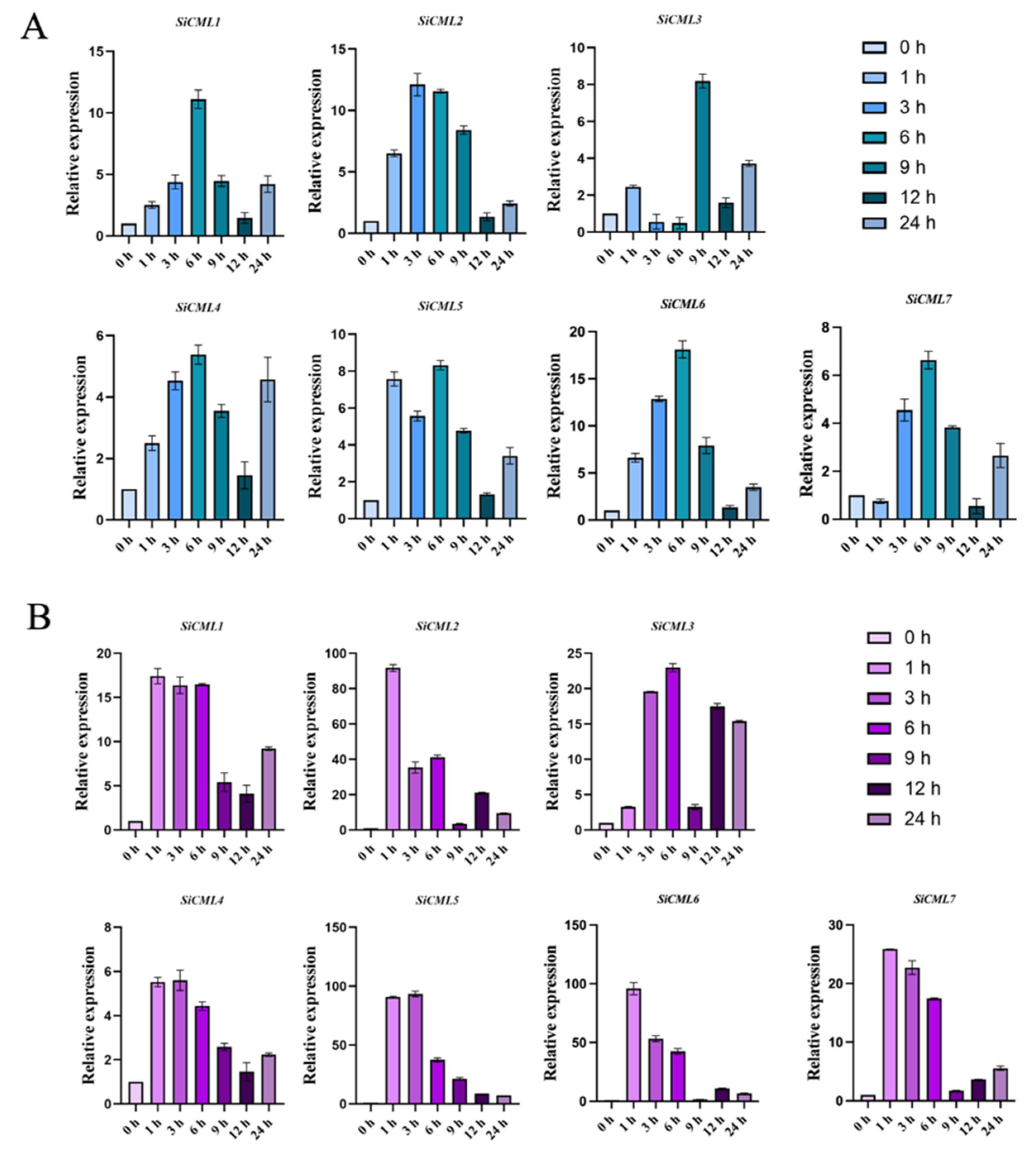
2.1. Expression Analysis of the CML Gene from Saussurea involucrata Under Low Temperature Stress
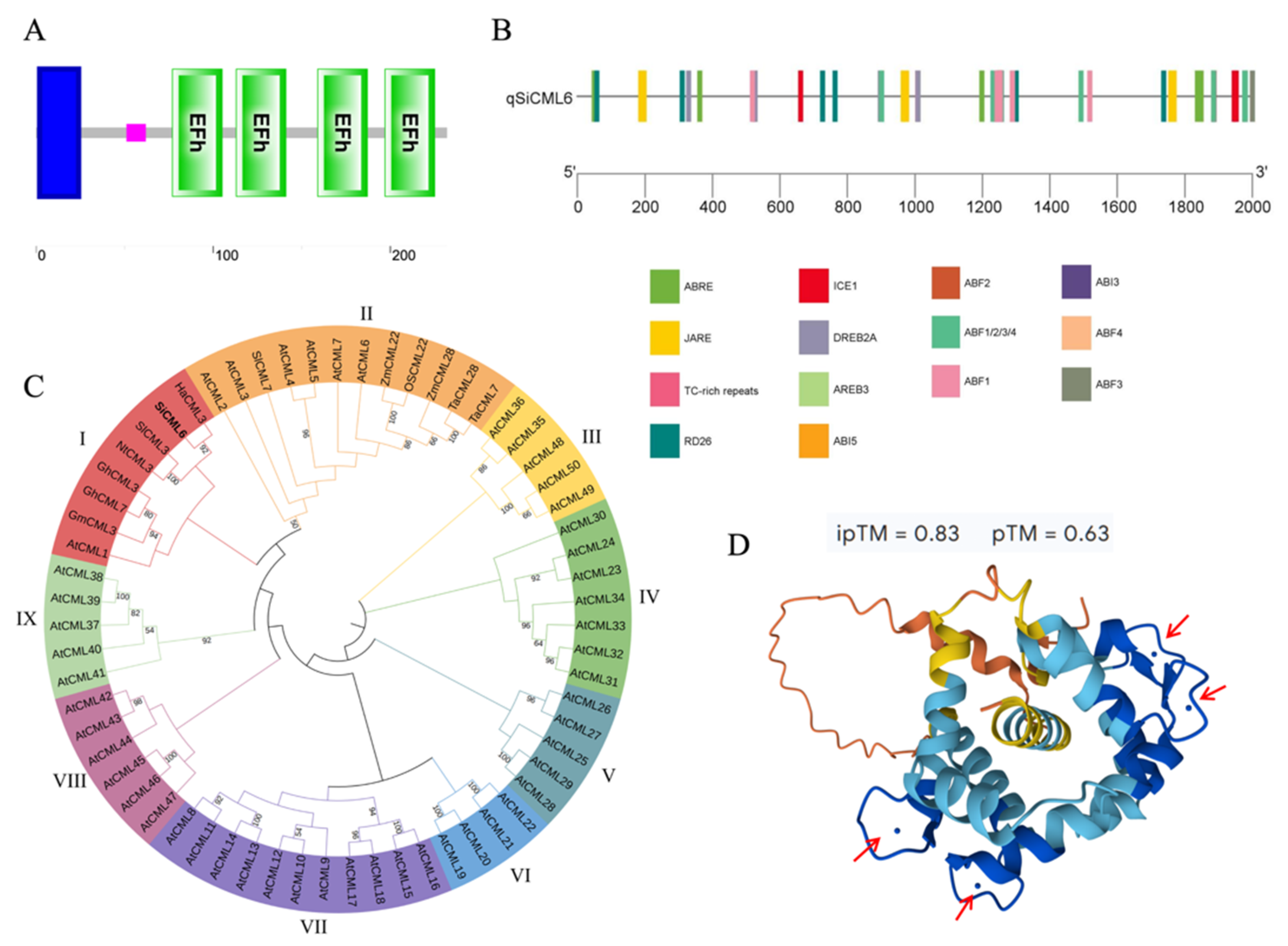
2.2. Bioinformatics Analysis of SiCML6
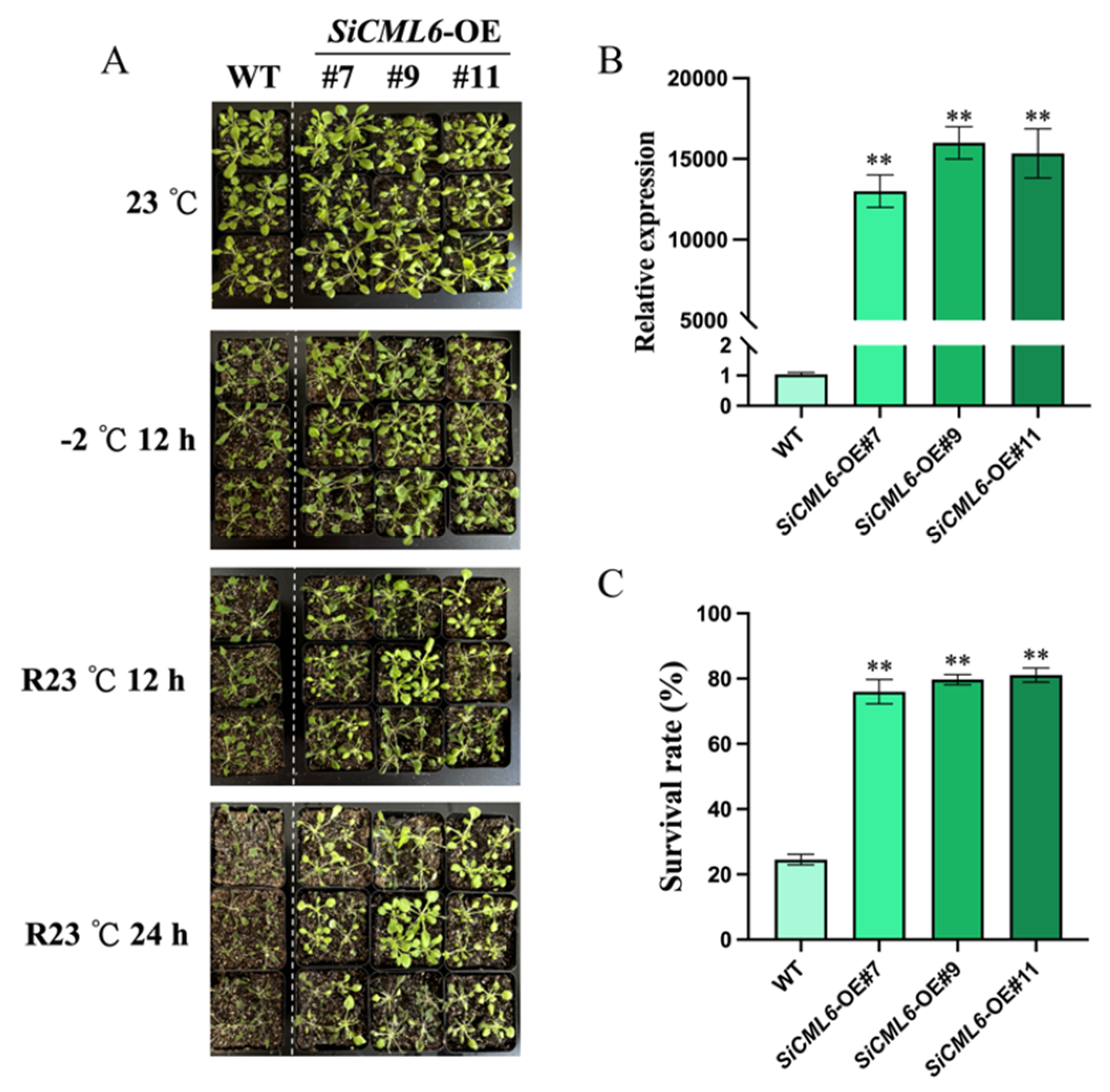
2.3. SiCML6 Increases the Survival Rate of Arabidopsis Under Low-Temperature Stress
2.4. Analysis of Physiological Indices Related to Cold Tolerance Assessment in SiCML6 Transgenic Arabidopsis
2.5. SiCML6 Regulates Downstream Genes of the Arabidopsis Low-Temperature Response Pathway
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Cultivation Conditions
4.2. Expression Profile Analysis of S. involucrata Calmodulin-like Protein Genes Under Low-Temperature Stress
4.3. Cloning and Sequence Analysis of SiCML6
4.4. Transformation of Arabidopsis Thaliana
4.5. Analysis of Cold Resistance in Transgenic Plants
4.6. Histochemical Staining
4.7. Determination of Physiological and Biochemical Indicators
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, M.; Luobu, Z.; Zhuoga, D.; Wei, X.; Tang, Y. Advances in Plant Response to Low-Temperature Stress. Plant Growth Regul. 2025, 105, 167–185. [Google Scholar] [CrossRef]
- Dodd, A.N.; Kudla, J.; Sanders, D. The Language of Calcium Signaling. Annu. Rev. Plant Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and Current Challenges in Calcium Signaling. New Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef]
- Bender, K.W.; Snedden, W.A. Calmodulin-Related Proteins Step Out from the Shadow of Their Namesake. Plant Physiol. 2013, 163, 486–495. [Google Scholar] [CrossRef]
- Zhu, X.; Dunand, C.; Snedden, W.; Galaud, J.-P. CaM and CML Emergence in the Green Lineage. Trends Plant Sci. 2015, 20, 483–489. [Google Scholar] [CrossRef]
- DeFalco, T.A.; Bender, K.W.; Snedden, W.A. Breaking the Code: Ca2+ Sensors in Plant Signalling. Biochem. J. 2010, 425, 27–40. [Google Scholar] [CrossRef]
- McCormack, E.; Braam, J. Calmodulins and Related Potential Calcium Sensors of Arabidopsis. New Phytol. 2003, 159, 585–598. [Google Scholar] [CrossRef]
- Magnan, F.; Ranty, B.; Charpenteau, M.; Sotta, B.; Galaud, J.; Aldon, D. Mutations in AtCML9, a Calmodulin-like Protein from Arabidopsis thaliana, Alter Plant Responses to Abiotic Stress and Abscisic Acid. Plant J. 2008, 56, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Liu, H.; Xing, Y.; Hussain, S.; Ouyang, B.; Zhang, Y.; Li, H.; Ye, Z. Overexpression of Calmodulin-like (ShCML44) Stress-Responsive Gene from Solanum Habrochaites Enhances Tolerance to Multiple Abiotic Stresses. Sci. Rep. 2016, 6, 31772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, T.; Liu, M.; Sun, W.; Zhang, W.-H. Calmodulin-like Gene MtCML40 Is Involved in Salt Tolerance by Regulating MtHKTs Transporters in Medicago truncatula. Environ. Exp. Bot. 2019, 157, 79–90. [Google Scholar] [CrossRef]
- Yang, S.; Xiong, X.; Arif, S.; Gao, L.; Zhao, L.; Shah, I.H.; Zhang, Y. A Calmodulin-like CmCML13 from Cucumis melo Improved Transgenic Arabidopsis Salt Tolerance through Reduced Shoot’s Na+, and Also Improved Drought Resistance. Plant Physiol. Biochem. 2020, 155, 271–283. [Google Scholar] [CrossRef]
- Jung, H.; Chung, P.J.; Park, S.; Redillas, M.C.F.R.; Kim, Y.S.; Suh, J.; Kim, J. Overexpression of OsERF48 Causes Regulation of OsCML16, a Calmodulin-like Protein Gene That Enhances Root Growth and Drought Tolerance. Plant Biotechnol. J. 2017, 15, 1295–1308. [Google Scholar] [CrossRef]
- Kalaipandian, S.; Xue, G.; Rae, A.L.; Glassop, D.; Bonnett, G.D.; McIntyre, L.C. Overexpression of TaCML20, a Calmodulin-like Gene, Enhances Water Soluble Carbohydrate Accumulation and Yield in Wheat. Physiol. Plant. 2019, 165, 790–799. [Google Scholar] [CrossRef]
- Scholz, S.S.; Reichelt, M.; Vadassery, J.; Mithöfer, A.; Scholz, S.S.; Reichelt, M.; Vadassery, J.; Mithöfer, A.; Scholz, S.S.; Reichelt, M.; et al. Calmodulin-like Protein CML37 Is a Positive Regulator of ABA during Drought Stress in Arabidopsis. Plant Signal. Behav. 2015, 10, e1011951. [Google Scholar] [CrossRef]
- Vadassery, J.; Reichelt, M.; Hause, B.; Gershenzon, J.; Boland, W.; Mithöfer, A. CML42-Mediated Calcium Signaling Coordinates Responses to Spodoptera Herbivory and Abiotic Stresses in Arabidopsis. Plant Physiol. 2012, 159, 1159–1175. [Google Scholar] [CrossRef]
- Kong, H.; Xia, W.; Hou, M.; Ruan, N.; Li, J.; Zhu, J. Cloning and Function Analysis of a Saussurea involucrata LEA4 Gene. Front. Plant Sci. 2022, 13, 957133. [Google Scholar] [CrossRef]
- Mu, J.; Fu, Y.; Liu, B.; Zhang, Y.; Wang, A.; Li, Y.; Zhu, J. SiFBA5, a Cold-Responsive Factor from Saussurea involucrata Promotes Cold Resilience and Biomass Increase in Transgenic Tomato Plants under Cold Stress. BMC Plant Biol. 2021, 21, 75. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, L.; Dong, G.; Xu, Z.; Li, G.; Liu, N.; Wang, A.; Zhu, J. A Novel Cold-Regulated Protein Isolated from Saussurea involucrata Confers Cold and Drought Tolerance in Transgenic Tobacco (Nicotiana tabacum). Plant Sci. 2019, 289, 110246. [Google Scholar] [CrossRef]
- Oliver, M.J.; Farrant, J.M.; Hilhorst, H.W.M.; Mundree, S.; Williams, B.; Bewley, J.D.; Oliver, M.J.; Farrant, J.M.; Hilhorst, H.W.M.; Mundree, S.; et al. Desiccation Tolerance: Avoiding Cellular Damage During Drying and Rehydration. Annu. Rev. Plant Biol. 2020, 71, 435–460. [Google Scholar] [CrossRef]
- Gu, Z.; Liu, T.; Ding, B.; Li, F.; Wang, Q.; Qian, S.; Ye, F.; Chen, T.; Yang, Y.; Wang, J.; et al. Two Lysin-Motif Receptor Kinases, Gh-LYK1 and Gh-LYK2, Contribute to Resistance against Verticillium wilt in Upland Cotton. Front. Plant Sci. 2017, 8, 2133. [Google Scholar] [CrossRef]
- Nakayama, S.; Kretsinger, R.H. Evolution of the EF-Hand Family of Proteins. Annu. Rev. Biophys. Biomol. Struct. 1994, 23, 473–507. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Aharon, G.S.; Sottosanto, J.B.; Blumwald, E. Vacuolar Na+/H+ Antiporter Cation Selectivity Is Regulated by Calmodulin from within the Vacuole in a Ca2+ and pH-Dependent Manner. Proc. Natl. Acad. Sci. USA 2005, 102, 16107–16112. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Li, J.; Yang, W.; Ci, J.; Ren, X.; Wang, W.; Wang, Y.; Jiang, L.; Yang, W. Identification and Expression Analysis Revealed Drought Stress-Responsive Calmodulin and Calmodulin-like Genes in Maize. J. Plant Interact. 2022, 17, 450–461. [Google Scholar] [CrossRef]
- Yip Delormel, T.; Boudsocq, M. Properties and Functions of Calcium-dependent Protein Kinases and Their Relatives in Arabidopsis thaliana. New Phytol. 2019, 224, 585–604. [Google Scholar] [CrossRef]
- Yu, Q.; Zheng, Q.; Shen, W.; Li, J.; Yao, W.; Xu, W. Grape CIPK18 Acts as a Positive Regulator of CBF Cold Signaling Pathway by Modulating ROS Homeostasis. Environ. Exp. Bot. 2022, 203, 105063. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, Salt, and Temperature Stress-Induced Metabolic Rearrangements and Regulatory Networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Tang, M.; Xu, C.; Cao, H.; Shi, Y.; Chen, J.; Chai, Y.; Li, Z. Tomato Calmodulin-like Protein SlCML37 Is a Calcium (Ca2+) Sensor That Interacts with Proteasome Maturation Factor SlUMP1 and Plays a Role in Tomato Fruit Chilling Stress Tolerance. J. Plant Physiol. 2021, 258–259, 153373. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Hou, Q.; Liu, Y.; Wang, J.; Deng, S. Isolation and Functional Characterization of a Salt-Responsive Calmodulin-Like Gene MpCML40 from Semi-Mangrove Millettia pinnata. Int. J. Mol. Sci. 2021, 22, 3475. [Google Scholar] [CrossRef]
- Chen, C.; Sun, X.; Duanmu, H.; Zhu, D.; Yu, Y.; Cao, L.; Liu, A.; Jia, B.; Xiao, J.; Zhu, Y. GsCML27, a Gene Encoding a Calcium-Binding Ef-Hand Protein from Glycine soja, Plays Differential Roles in Plant Responses to Bicarbonate, Salt and Osmotic Stresses. PLoS ONE 2015, 10, e0141888. [Google Scholar] [CrossRef]
- Yin, X.M.; Huang, L.F.; Zhang, X.; Wang, M.L.; Xu, G.Y.; Xia, X.J. OsCML4 Improves Drought Tolerance through Scavenging of Reactive Oxygen Species in Rice. J. Plant Biol. 2015, 58, 68–73. [Google Scholar] [CrossRef]
- Wu, X.; Qiao, Z.; Liu, H.; Acharya, B.R.; Li, C.; Zhang, W. CML20, an Arabidopsis Calmodulin-like Protein, Negatively Regulates Guard Cell ABA Signaling and Drought Stress Tolerance. Front. Plant Sci. 2017, 8, 824. [Google Scholar] [CrossRef]
- Miura, K.; Furumoto, T. Cold Signaling and Cold Response in Plants. Int. J. Mol. Sci. 2013, 14, 5312–5337. [Google Scholar] [CrossRef]
- Aleynova, O.A.; Kiselev, K.V.; Ogneva, Z.V.; Dubrovina, A.S. The Grapevine Calmodulin-Like Protein Gene CML21 Is Regulated by Alternative Splicing and Involved in Abiotic Stress Response. Int. J. Mol. Sci. 2020, 21, 7939. [Google Scholar] [CrossRef]
- Ding, H.; Qian, Y.; Fang, Y.; Ji, Y.; Sheng, J.; Ge, C. Characteristics of SlCML39, a Tomato Calmodulin-like Gene, and Its Negative Role in High Temperature Tolerance of Arabidopsis Thaliana during Germination and Seedling Growth. Int. J. Mol. Sci. 2021, 22, 11479. [Google Scholar] [CrossRef]
- Huang, C.; Ding, S.; Zhang, H.; Du, H.; An, L.; Huang, C.; Ding, S.; Zhang, H.; Du, H.; An, L. CIPK7 Is Involved in Cold Response by Interacting with CBL1 in Arabidopsis thaliana. Plant Sci. 2011, 181, 57–64. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Xia, W.; Mu, J.; Feng, Y.; Liu, R.; Yan, P.; Wang, A.; Lin, Z.; Guo, Y.; et al. De Novo Transcriptome Sequencing and the Hypothetical Cold Response Mode of Saussurea involucrata in Extreme Cold Environments. Int. J. Mol. Sci. 2017, 18, 1155. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 Improves Signal Peptide Predictions Using Deep Neural Networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Lescot, M. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “One for All, All for One” Bioinformatics Platform for Biological Big-Data Mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Henriques, R.; Lin, S.-S.; Niu, Q.-W.; Chua, N.-H. Agrobacterium-Mediated Transformation of Arabidopsis thaliana Using the Floral Dip Method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Liu, X.; Xin, H.; Wu, X.; Liu, R.; Li, J.; Zhu, J. Saussurea involucrata PIP2;7 Improves Photosynthesis and Drought Resistance by Decreasing the Stomatal Density and Increasing Intracellular Osmotic Pressure. Environ. Exp. Bot. 2021, 191, 104605. [Google Scholar] [CrossRef]
- Kong, X.; Pan, J.; Zhang, M.; Xing, X.; Zhou, Y.; Liu, Y.; Li, D.; Li, D. ZmMKK4, a Novel Group C Mitogen-activated Protein Kinase Kinase in Maize (Zea mays), Confers Salt and Cold Tolerance in Transgenic Arabidopsis. Plant Cell Environ. 2011, 34, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-L.; Wu, D.; Hui, M.; Wang, Y.; Han, X.; Yao, F.; Cao, X.; Li, Y.-H.; Li, H.; Wang, H. Screening of Cold Hardiness-Related Indexes and Establishment of a Comprehensive Evaluation Method for Grapevines (V. vinifera). Front. Plant Sci. 2022, 13, 1014330. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, M.; Kong, H.; Li, J.; Xia, W.; Zhu, J. Saussurea involucrata CML6 Enhances Freezing Tolerance by Activating Antioxidant Defense and the CBF-COR Pathway in Plants. Plants 2025, 14, 2360. https://doi.org/10.3390/plants14152360
Hou M, Kong H, Li J, Xia W, Zhu J. Saussurea involucrata CML6 Enhances Freezing Tolerance by Activating Antioxidant Defense and the CBF-COR Pathway in Plants. Plants. 2025; 14(15):2360. https://doi.org/10.3390/plants14152360
Chicago/Turabian StyleHou, Mengjuan, Hui Kong, Jin Li, Wenwen Xia, and Jianbo Zhu. 2025. "Saussurea involucrata CML6 Enhances Freezing Tolerance by Activating Antioxidant Defense and the CBF-COR Pathway in Plants" Plants 14, no. 15: 2360. https://doi.org/10.3390/plants14152360
APA StyleHou, M., Kong, H., Li, J., Xia, W., & Zhu, J. (2025). Saussurea involucrata CML6 Enhances Freezing Tolerance by Activating Antioxidant Defense and the CBF-COR Pathway in Plants. Plants, 14(15), 2360. https://doi.org/10.3390/plants14152360





