Ecophysiological Keys to the Success of a Native-Expansive Mediterranean Species in Threatened Coastal Dune Habitats
Abstract
1. Introduction
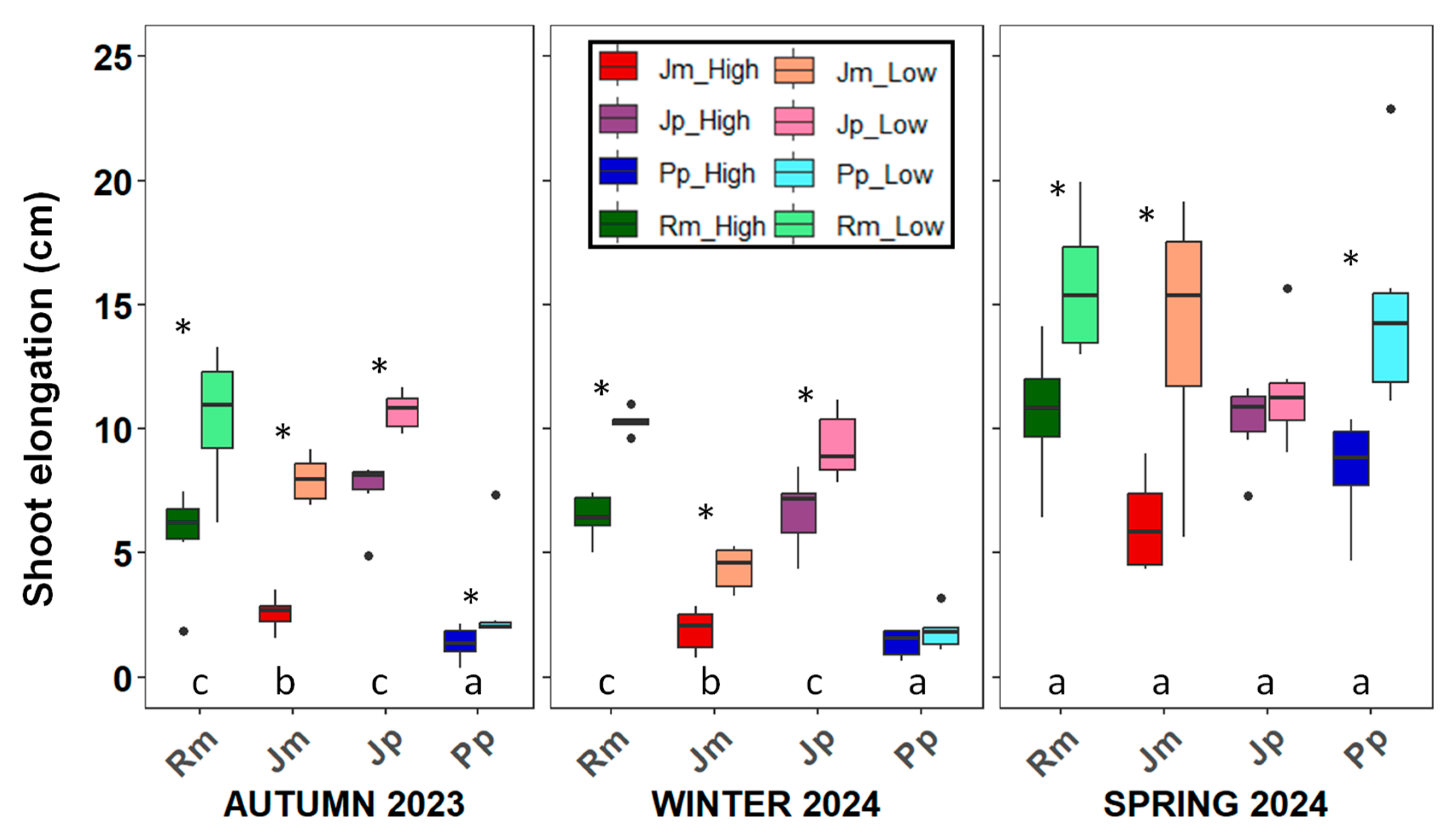
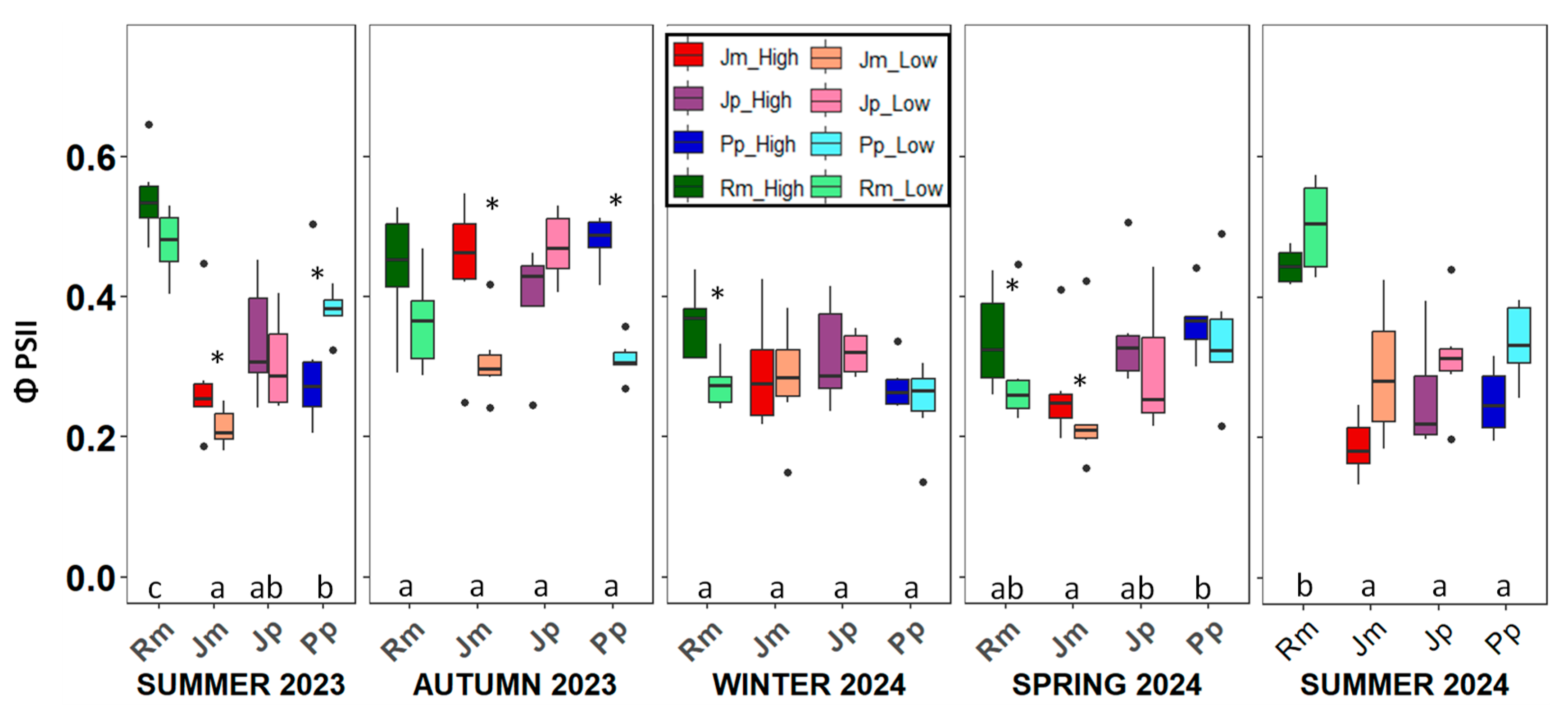
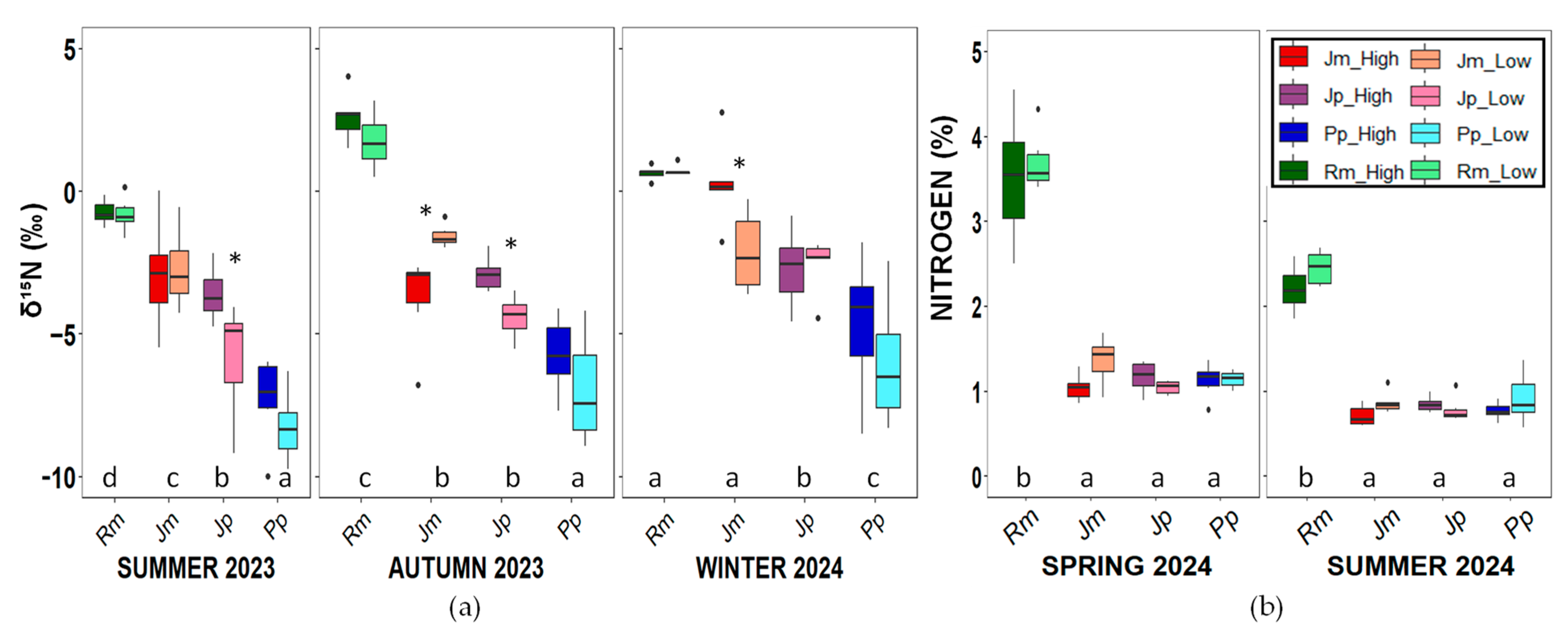
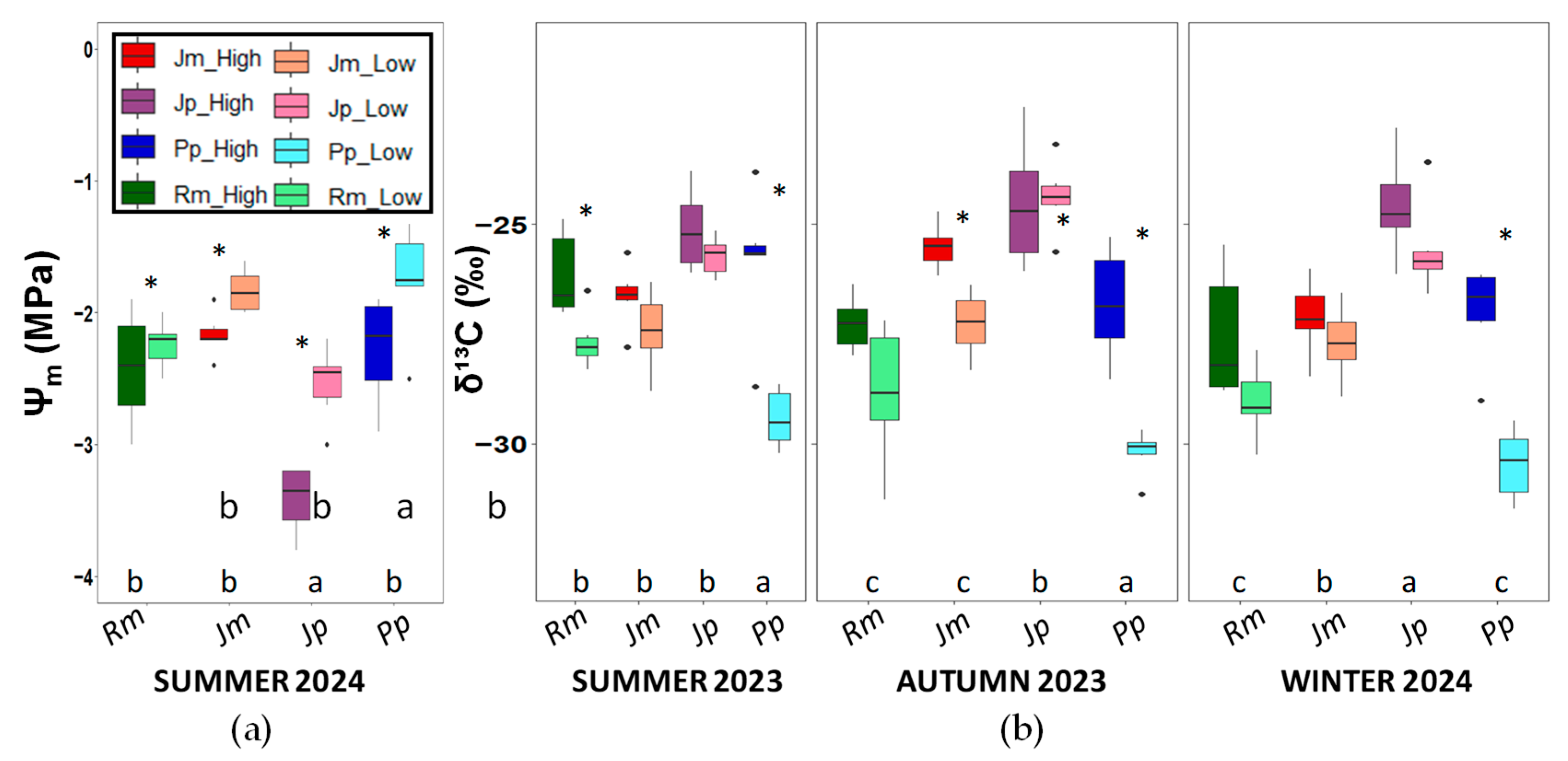
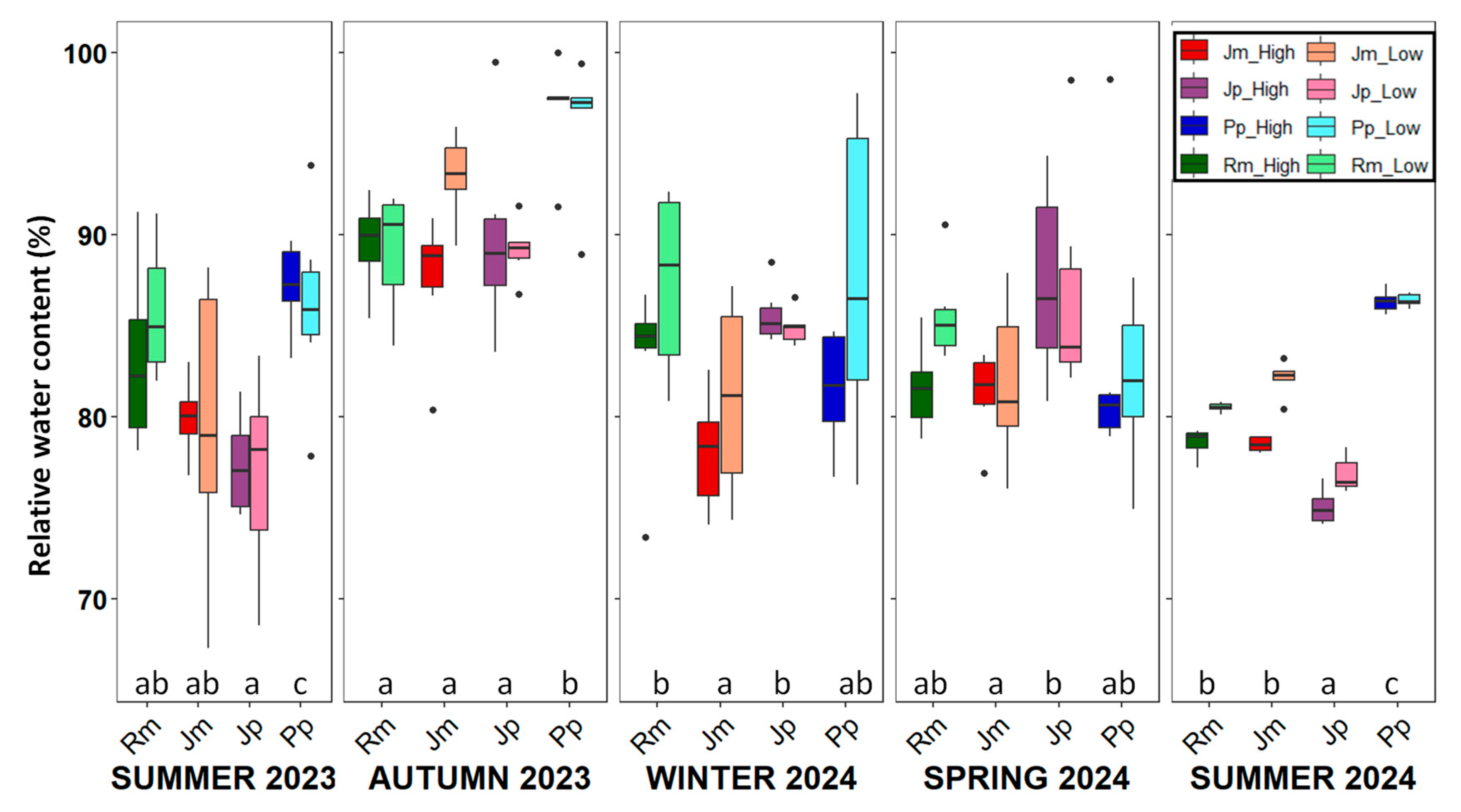
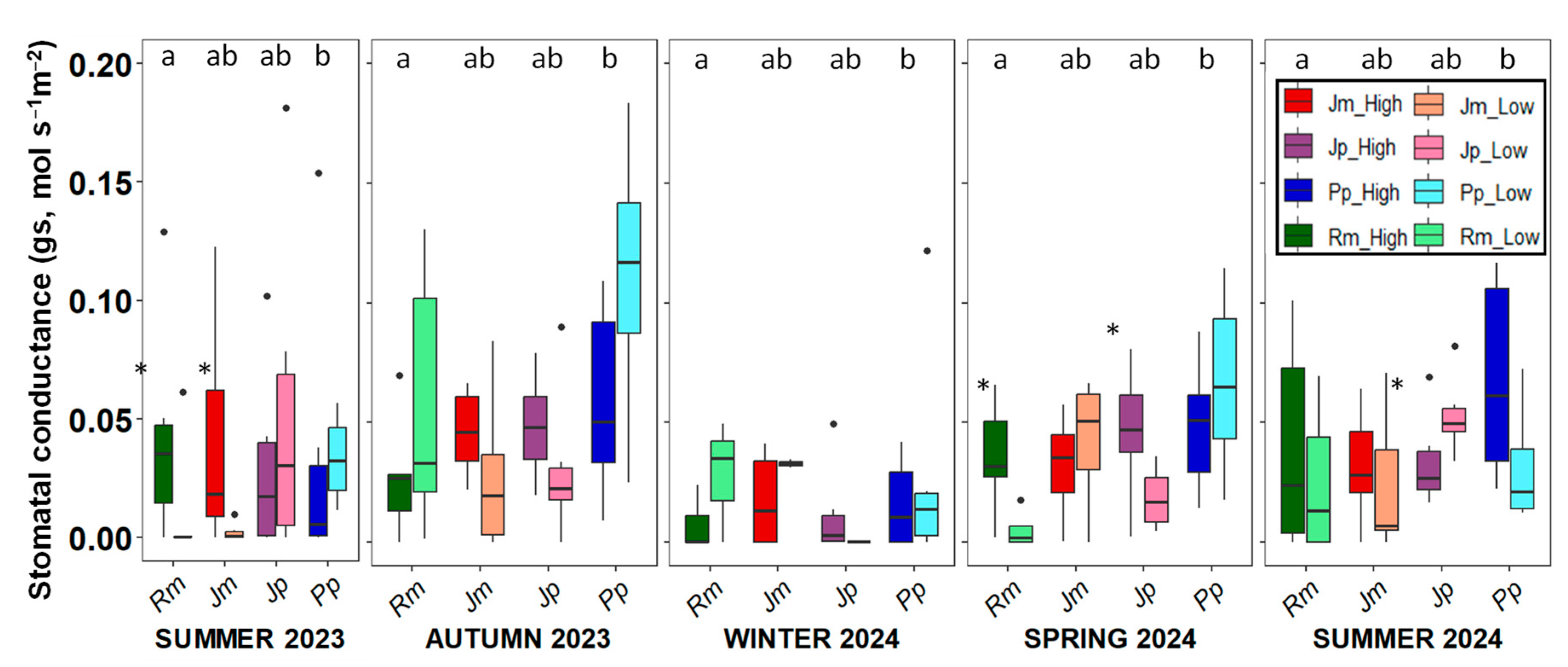
2. Results
3. Discussion
4. Materials and Methods
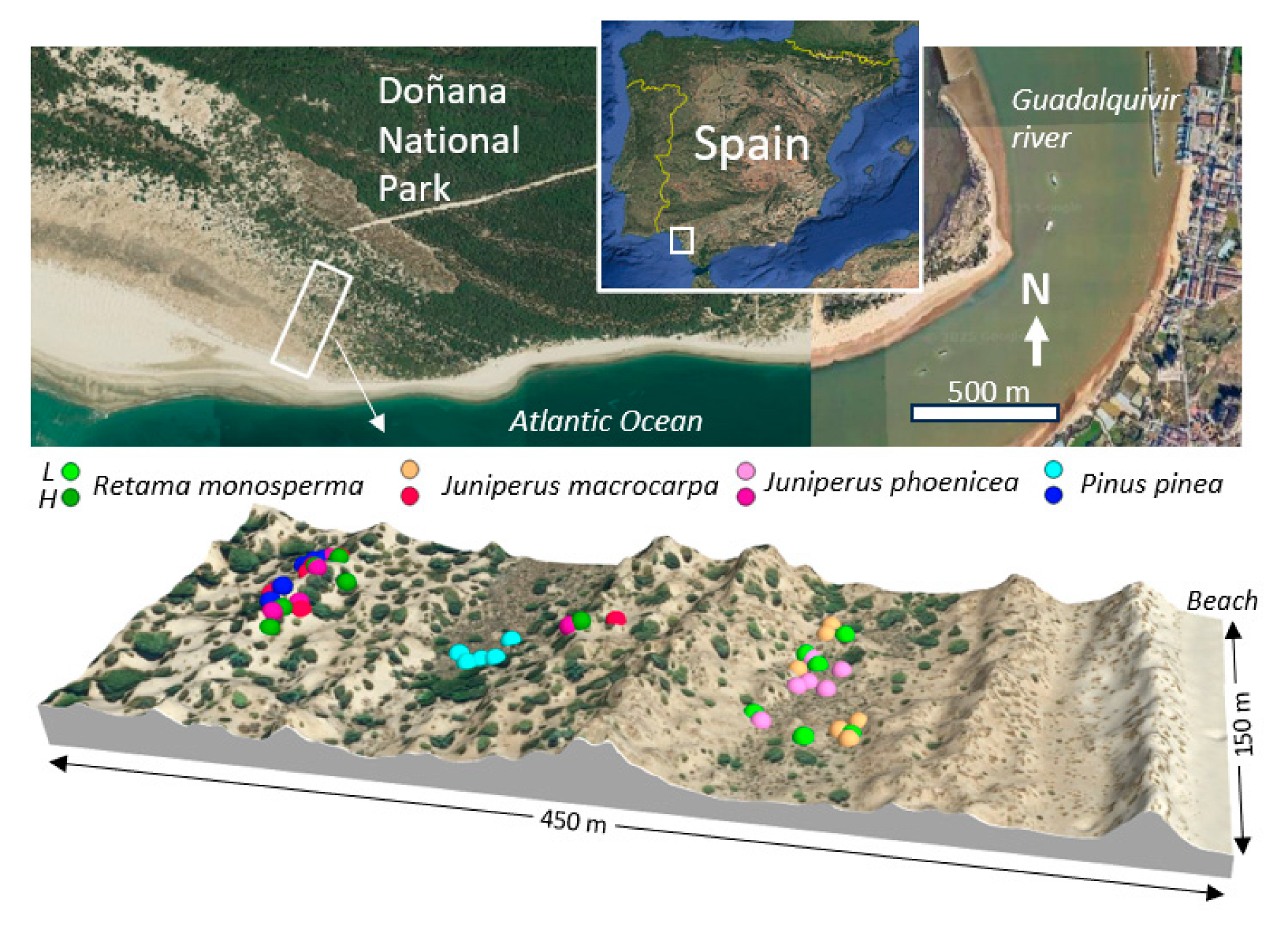
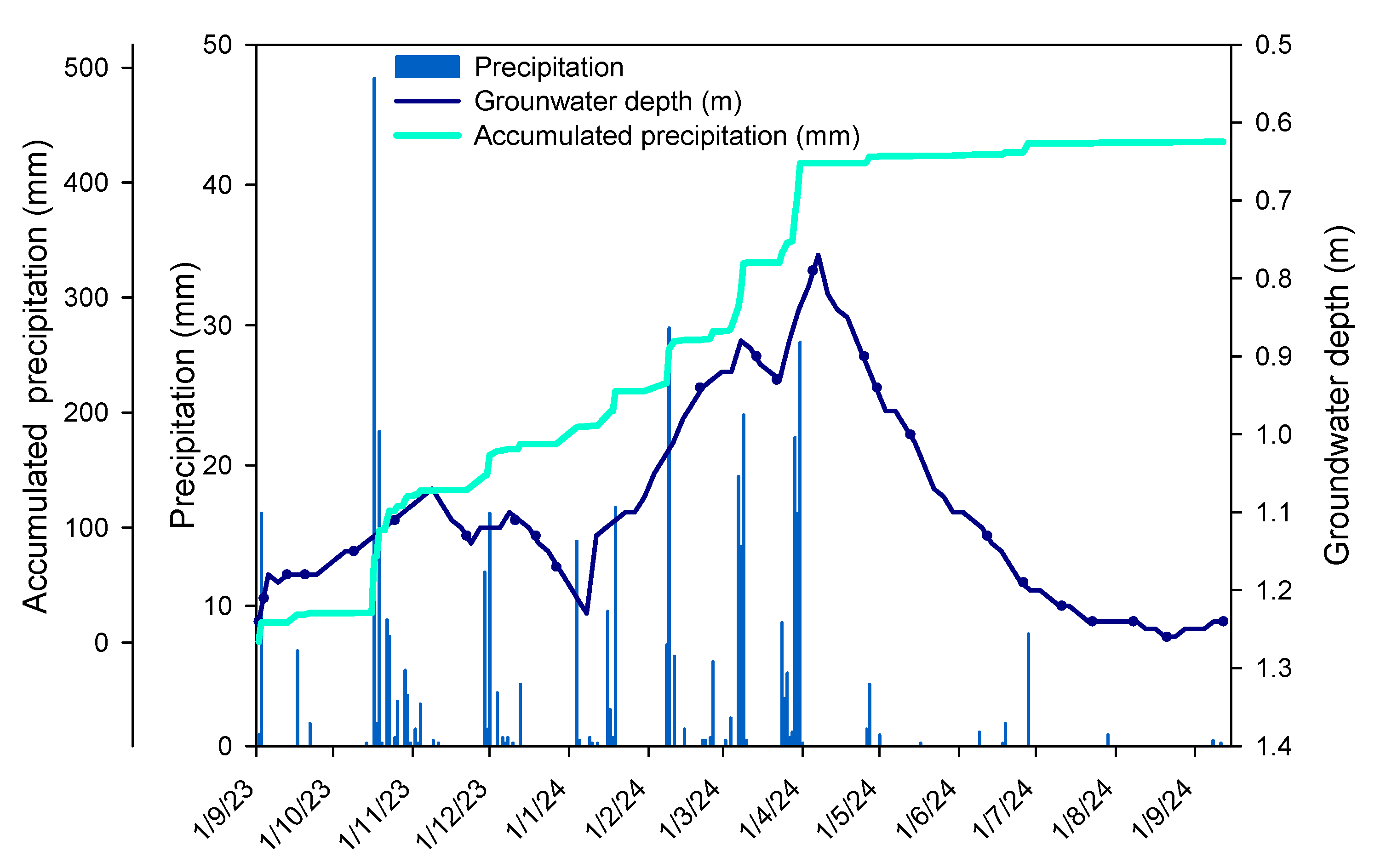
4.1. Study Site
4.2. Study Species
4.3. Methodology
4.3.1. Individual Size Measurements
4.3.2. Leaf Area Index (LAI, m2/m2)
4.3.3. Shoot Elongation and Brach Number
4.3.4. Photochemical Efficiency (ΦPSII) and Stomatal Conductance (gs)
4.3.5. Leaf and Cladode Isotopic Analysis and N and C Content
4.3.6. Midday Shoot Water Potential (Ψm)
4.3.7. Leaf Traits
- Relative water content (RWC, %): The leaf and stem water content was determined by weighing fresh leaves upon arrival at the laboratory (Mf). The leaves were then rehydrated in distilled water for 24 h at 5 °C to obtain their turgid mass (Mt) after gently blotting them dry. Finally, they were oven-dried at 70 °C for 48 h to obtain the dry mass (Md). RWC was calculated as follows:RWC% = (Mf − Md)/(Mt − Md) × 100
- Leaf dry matter content (LDMC, mg g−1): LDMC was calculated using the same samples as for RWC, as follows:LDMC = Md/Mt
- Leaf mass per area (LMA, g m−2): Leaf area was estimated using the mobile application Easy Leaf Area, which calculates the surface area by comparison with a known reference. The leaves were then oven-dried at 70 °C for 48 h and weighed; LMA was calculated as follows:LMA = Md/Leaf area
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RWC | Relative water content |
| Ψm | Midday shoot water potential |
| ΦPSII | Effective photochemical efficiency |
| gs | Stomatal conductance |
| LAI | Leaf area index |
| LMA | Leaf mass area |
| LDMC | Leaf dry matter content |
| %N | Total nitrogen content |
| %C | Total carbon content |
| δ15N | Nitrogen isotope signature (15N/14N) |
| δ13C | Carbon isotope signature (13C/12C) |
| WUEi | Long-term intrinsic water-use efficiency |
Appendix A
Results of the Statistical Analysis
| Within-Subject Effects | ||||||||||||
| Time | Time × Zone | Time × Species | T × Species × Z | |||||||||
| df | F | p | df | F | p | df | F | p | df | F | p | |
| RWC | 4 | 64.29 | <0.001 | 4 | 1.261 | 0.288 | 12 | 8.85 | <0.001 | 12.0 | 1.10 | 0.357 |
| LMA | 3.1 | 22.14 | <0.001 | 3.10 | 1.437 | 0.234 | 9.3 | 30.90 | <0.001 | 9.3 | 3.13 | 0.002 |
| LDMC | 8.2 | 341.21 | <0.001 | 2.73 | 6.309 | 0.001 | 8.2 | 41.91 | <0.001 | 8.2 | 6.50 | <0.0001 |
| Growth | 2.0 | 164.12 | <0.001 | 2 | 7.350 | 0.001 | 6 | 12.41 | <0.001 | 6.0 | 3.37 | 0.005 |
| LAI | 2.4 | 139.96 | <0.001 | 2.48 | 12.572 | <0.001 | 7.4 | 8.439 | <0.001 | 7.4 | 0.99 | 0.446 |
| ΦPSI | 2.6 | 19.94 | <0.001 | 2.64 | 8.630 | 0.005 | 7.9 | 9.007 | <0.001 | 7.9 | 5.51 | 0.016 |
| δ13C | 1.0 | 34.78 | <0.001 | 1.04 | 1.954 | 0.169 | 3.1 | 0.978 | 0.415 | 3.1 | 0.89 | 0.454 |
| δ15N | 3.0 | 259.08 | <0.001 | 3 | 0.750 | 0.524 | 9 | 26.57 | <0.001 | 9.0 | 5.46 | <0.0001 |
| Between-subject effects | ||||||||||||
| Species | Zone | Zone × Specie | ||||||||||
| df | F | p | df | F | p | df | F | p | ||||
| RWC | 3 | 10.72 | <0.001 | 1 | 4.20 | 0.047 | 3 | 0.97 | 0.414 | |||
| LMA | 3 | 213.63 | <0.001 | 1 | 4.84 | 0.034 | 3 | 0.16 | 0.922 | |||
| LDMC | 3 | 370.85 | <0.001 | 1 | 109.84 | <0.001 | 3 | 14.93 | <0.001 | |||
| Growth | 3 | 31.57 | <0.001 | 1 | 82.06 | <0.001 | 3 | 2.74 | 0.056 | |||
| LAI | 3 | 7.98 | <0.001 | 1 | 1.30 | 0.259 | 3 | 3.36 | 0.028 | |||
| Fv/Fm | 3 | 26.09 | <0.001 | 1 | 3.28 | 0.078 | 3 | 1.54 | 0.219 | |||
| δ13C | 3 | 10.64 | <0.001 | 1 | 1.86 | 0.179 | 3 | 2.55 | 0.069 | |||
| δ15N | 3 | 91.81 | <0.001 | 1 | 7.82 | 0.008 | 3 | 1.02 | 0.390 | |||
| Ψm | 3 | 20,73 | <0.001 | 1 | 23.24 | <0.001 | 3 | 4.905 | 0.005 | |||
| l | Zone | Species | Time | ||||||
|---|---|---|---|---|---|---|---|---|---|
| df | H | p | df | H | p | df | H | p | |
| % C | 1 | 4.76 | 0.029 | 3 | 5.92 | <0.116 | 1 | 0.87 | 0.35 |
| %N | 1 | 0.75 | 0.387 | 3 | 53.56 | <0.001 | 1 | 19.78 | <0.001 |
| gs | 1 | 0.61 | 0.433 | 3 | 10.72 | 0.013 | 1 | 24.10 | <0.001 |
| Species | Season | LAI ± sd | LMA ± sd | LDMC ± sd | |||
|---|---|---|---|---|---|---|---|
| Juniperus macrocarpa | SEPTEMBER-23 | 217 | ±22 | 461 | ±30.8 | ||
| APRIL-24 | 6.7 | ±2.1 | 163 | ±8 | 353 | ±26.4 | |
| DECEMBER-23 | 1.6 | ±0.5 | 429 | ±83 | 388 | ±80.5 | |
| FEBRUARY-24 | 2.9 | ±1.3 | 246 | ±17 | 470 | ±37.7 | |
| SEPTEMBER-24 | 2.1 | ±1.5 | 226 | ±30 | 465 | ±58.6 | |
| Pinus pinea | SEPTEMBER-23 | 236 | ±24 | 358 | ±19.2 | ||
| APRIL-24 | 3.4 | ±1.5 | 233 | ±53 | 237 | ±19.0 | |
| DECEMBER-23 | 0.9 | ±0.6 | 173 | ±34 | 232 | ±17.7 | |
| FEBRUARY-24 | 1.0 | ±0.7 | 188 | ±18 | 224 | ±14.8 | |
| SEPTEMBER-24 | 1.1 | 0.6 | 439 | ±84 | 443 | ±38.1 | |
| Retama monosperma | SEPTEMBER-23 | 458 | ±57 | 429 | ±33.5 | ||
| APRIL-24 | 5.1 | ±1.5 | 396 | ±47 | 395 | ±38.4 | |
| DECEMBER-23 | 2.1 | ±1.2 | 440 | ±58 | 411 | ±34.3 | |
| FEBRUARY-24 | 1.1 | ±1.0 | 405 | ±43 | 405 | ±35.5 | |
| SEPTEMBER-24 | 1.7 | 1.2 | 474 | ±78 | 544 | ±28.6 | |
| Juniperus phoenicia | SEPTEMBER-23 | 366 | ±29 | 483 | ±17.5 | ||
| APRIL-24 | 6.3 | ±1.8 | 349 | ±30 | 379 | ±18.2 | |
| DECEMBER-23 | 1.9 | ±1.1 | 292 | ±34 | 363 | ±18.7 | |
| FEBRUARY-24 | 2.3 | ±1.4 | 298 | ±37 | 369 | ±20.9 | |
| SEPTEMBER-24 | 2.5 | ±1.6 | 241 | ±22 | 383 | ±19.4 | |
| Variable | PC1 | PC2 |
|---|---|---|
| δ15N | 0.45 | −0.11 |
| WUEi | 0.35 | 0.134 |
| % N | 0.51 | 0.130 |
| % C | −0.33 | −0.151 |
| LAI | 0.18 | 0.81 |
| RWC | −0.43 | −0.15 |
| LMA | 0.77 | −0.34 |
| LDMC | 0.73 | −0.17 |
| Growth | 0.37 | 0.68 |
| ΦPSII | 0.29 | −0.49 |
| Ψm | −0.43 | 0.20 |
| gs | −0.29 | −0.13 |
Appendix B
Photographic Documentation of the Study Area and Species





References
- Bennett, J.A.; Riibak, K.; Kook, E.; Reier, Ü.; Tamme, R.; Guillermo Bueno, C.; Pärtel, M. Species Pools, Community Completeness and Invasion: Disentangling Diversity Effects on the Establishment of Native and Alien Species. Ecol. Lett. 2016, 19, 1496–1505. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M.; Rejmánek, M.; Webster, G.L.; Williamson, M.; Kirschner, J. Alien Plants in Checklists and Floras: Towards Better Communication between Taxonomists and Ecologists. TAXON 2004, 53, 131–143. [Google Scholar] [CrossRef]
- Diggins, C.A. Anthropogenically-Induced Range Expansion as an Invasion Front in Native Species: An Example in North American Flying Squirrels. Front. Ecol. Evol. 2023, 11, 1096244. [Google Scholar] [CrossRef]
- Kelbel, P.; Adamčíková, Z. Selected Invasive and Expansive Tree Species in Conditions of the Botanical Garden of PJ Šafárik University in Košice. Thaiszia J. Bot. 2011, 21, 141–152. [Google Scholar]
- Sholto-Douglas, C.; Shackleton, C.M.; Ruwanza, S.; Dold, T. The Effects of Expansive Shrubs on Plant Species Richness and Soils in Semi-Arid Communal Lands, South Africa. Land Degrad. Dev. 2017, 28, 2191–2206. [Google Scholar] [CrossRef]
- Axmanová, I.; Chytrý, K.; Boublík, K.; Chytry, M.; Dřevojan, P.; Ekrtová, E.; Fajmon, K.; Hájková, P.; Härtel, H.; Hejda, M.; et al. Catalogue of Expansive Plants of the Czech Republic. Preslia 2024, 96, 299–327. [Google Scholar] [CrossRef]
- Valéry, L.; Fritz, H.; Lefeuvre, J.-C.; Simberloff, D. Ecosystem-Level Consequences of Invasions by Native Species as a Way to Investigate Relationships between Evenness and Ecosystem Function. Biol. Invasions 2009, 11, 609–617. [Google Scholar] [CrossRef]
- Essl, F.; Bacher, S.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Katsanevakis, S.; Kowarik, I.; Kühn, I.; Pyšek, P.; Rabitsch, W.; et al. Which Taxa Are Alien? Criteria, Applications, and Uncertainties. BioScience 2018, 68, 496–509. [Google Scholar] [CrossRef]
- Bradley, B.A.; Laginhas, B.B.; Whitlock, R.; Allen, J.M.; Bates, A.E.; Bernatchez, G.; Diez, J.M.; Early, R.; Lenoir, J.; Vilà, M.; et al. Disentangling the Abundance–Impact Relationship for Invasive Species. Proc. Natl. Acad. Sci. USA 2019, 116, 9919–9924. [Google Scholar] [CrossRef]
- D’Antonio, C.M.; Vitousek, P.M. Biological Invasions by Exotic Grasses, the Grass/Fire Cycle, and Global Change. Annu. Rev. Ecol. Evol. Syst. 1992, 23, 63–87. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Pyšek, P. Extra-Regional Residence Time as a Correlate of Plant Invasiveness: European Archaeophytes in North America. Ecology 2009, 90, 2589–2597. [Google Scholar] [CrossRef]
- Arianoutsou, M.; Delipetrou, P.; Vilà, M.; Dimitrakopoulos, P.G.; Celesti-Grapow, L.; Wardell-Johnson, G.; Henderson, L.; Fuentes, N.; Ugarte-Mendes, E.; Rundel, P.W. Comparative Patterns of Plant Invasions in the Mediterranean Biome. PLoS ONE 2013, 8, e79174. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Fernández, J.B.; Martínez, M.L.; García-Franco, J.G.; Zunzunegui, M. The Impact on Plant Communities of an Invasive Alien Herb, Oenothera drummondii, Varies along the Beach-Coastal Dune Gradient. Flora 2019, 260, 151466. [Google Scholar] [CrossRef]
- Younsi, S.E.; Bouziane, Z. Plant Diversity in Mediterranean Coastal Dune Systems Subjected to Anthropogenic Disturbances. Biodivers. Res. Conserv. 2023, 72, 25–38. [Google Scholar] [CrossRef]
- Baker, C.M.; Diele, F.; Marangi, C.; Martiradonna, A.; Ragni, S. Optimal Spatiotemporal Effort Allocation for Invasive Species Removal Incorporating a Removal Handling Time and Budget. Nat. Resour. Model. 2018, 31, e12190. [Google Scholar] [CrossRef]
- Haile, M.; Birhane, E.; Rannestad, M.M.; Adaramola, M.S. Expansive Shrubs: Expansion Factors and Ecological Impacts in Northern Ethiopia. J. Nat. Conserv. 2021, 61, 125996. [Google Scholar] [CrossRef]
- Haile, M.; Semere, H.; Birhane, E.; Abraha, Z.; Rannestad, M.M.; Adaramola, M.S. Distribution of Expansive Shrubs under Climate Change Scenarios and Their Socio-Economic Impacts in a Dry Afromontane Forest. Trees For. People 2023, 13, 100414. [Google Scholar] [CrossRef]
- Essl, F.; Dullinger, S.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Katsanevakis, S.; Kühn, I.; Lenzner, B.; Pauchard, A.; Pyšek, P.; et al. A Conceptual Framework for Range-Expanding Species That Track Human-Induced Environmental Change. BioScience 2019, 69, 908–919. [Google Scholar] [CrossRef]
- Martínez, M.L.; Psuty, N.P.; Lubke, R.A. A Perspective on Coastal Dunes. In Coastal Dunes: Ecology and Conservation; Martínez, M.L., Psuty, N.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 3–10. ISBN 978-3-540-74002-5. [Google Scholar]
- Martínez, M.; Maun, M.; Psuty, N. The Fragility and Conservation of the World’s Coastal Dunes: Geomorphological, Ecological and Socioeconomic Perspectives; Springer: Berlin/Heidelberg, Germany, 2008; pp. 355–369. ISBN 978-3-540-74001-8. [Google Scholar]
- Kutiel, P. Conservation and Management of the Mediterranean Coastal Sand Dunes in Israel. J. Coast. Conserv. 2001, 7, 183–192. [Google Scholar] [CrossRef]
- Acosta, A.; Ercole, S.; Stanisci, A.; Pillar, V.D.P.; Blasi, C. Coastal Vegetation Zonation and Dune Morphology in Some Mediterranean Ecosystems. J. Coast. Res. 2007, 236, 1518–1524. [Google Scholar] [CrossRef]
- Kunstler, G.; Falster, D.; Coomes, D.A.; Hui, F.; Kooyman, R.M.; Laughlin, D.C.; Poorter, L.; Vanderwel, M.; Vieilledent, G.; Wright, S.J.; et al. Plant Functional Traits Have Globally Consistent Effects on Competition. Nature 2016, 529, 204–207. [Google Scholar] [CrossRef]
- García-Mora, M.R.; Gallego-Fernández, J.B.; García-Novo, F. Plant Functional Types in Coastal Foredunes in Relation to Environmental Stress and Disturbance. J. Veg. Sci. 1999, 10, 27–34. [Google Scholar] [CrossRef]
- García Novo, F.; Díaz, M.; Zunzunegui, M.; García-Mora, R.; Gallego-Fernández, J. Plant Functional Types in Coastal Dune Habitats; Springer: Berlin/Heidelberg, Germany, 2004; Volume 171, pp. 155–169. ISBN 978-3-540-40829-1. [Google Scholar]
- Everard, M.; Jones, L.; Watts, B. Have We Neglected the Societal Importance of Sand Dunes? An Ecosystem Services Perspective. Aquat. Conserv. Mar. Freshw. Ecosyst. 2010, 20, 476–487. [Google Scholar] [CrossRef]
- Kutiel, P.; Peled, Y.; Geffen, E. The Effect of Removing Shrub Cover on Annual Plants and Small Mammals in a Coastal Sand Dune Ecosystem. Biol. Conserv. 2000, 94, 235–242. [Google Scholar] [CrossRef]
- Martínez, M.; Hesp, P.; Gallego-Fernández, J. Coastal Dunes: Human Impact and Need for Restoration. In Coastal Dune Restoration; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–14. ISBN 978-3-642-33444-3. [Google Scholar]
- Maestre, F.T.; Callaway, R.M.; Valladares, F.; Lortie, C.J. Refining the Stress-gradient Hypothesis for Competition and Facilitation in Plant Communities. J. Ecol. 2009, 97, 199–205. [Google Scholar] [CrossRef]
- Conti, L.; de Bello, F.; Lepš, J.; Acosta, A.T.R.; Carboni, M. Environmental Gradients and Micro-Heterogeneity Shape Fine-Scale Plant Community Assembly on Coastal Dunes. J. Veg. Sci. 2017, 28, 762–773. [Google Scholar] [CrossRef]
- Bertness, M.D.; Callaway, R. Positive Interactions in Communities. Trends Ecol. Evol. 1994, 9, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Randall, J.M. Weed Alert! New Invasive Weeds in California; California Invasive Plant Council: Berkeley, CA, USA, 1997. [Google Scholar]
- Randall, R.P. The Introduced Flora of Australia and Its Weed Status; CRC for Australian Weed Management: Adelaide, Australia, 2007. [Google Scholar]
- Muñoz-Vallés, S.; Gallego-Fernández, J.B.; Dellafiore, C.; Cambrollé, J. Long-Term Spatio-Temporal Expansion of the Native-Invasive Retama monosperma on Coastal Dunes: Importance of Land-Use and Natural Dispersal Vectors. Flora-Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 259–267. [Google Scholar] [CrossRef][Green Version]
- Gallego-Fernández, J.B.; Muñoz-Valles, S.; Dellafiore, C.M. Spatio-Temporal Patterns of Colonization and Expansion of Retama monosperma on Developing Coastal Dunes. J. Coast. Conserv. 2015, 19, 577–587. [Google Scholar] [CrossRef]
- Esquivias, M.P.; Zunzunegui, M.; Díaz Barradas, M.C.; Álvarez-Cansino, L. Competitive Effect of a Native-Invasive Species on a Threatened Shrub in a Mediterranean Dune System. Oecologia 2015, 177, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Zunzunegui, M.; Esquivias, M.; Oppo; Gallego-Fernández, J. Interspecific Competition and Livestock Disturbance Control the Spatial Patterns of Two Coastal Dune Shrubs. Plant Soil 2012, 354, 299–309. [Google Scholar] [CrossRef]
- García-de-Lomas, J.; Fernández, L.; Martín, I.; Saavedra, C.; Rodríguez-Hiraldo, C.; Gallego-Fernández, J.B. Management of Coastal Dunes Affected by Shrub Encroachment: Are Rabbits an Ally or an Enemy of Restoration? J. Coast. Conserv. 2023, 27, 8. [Google Scholar] [CrossRef]
- Muñoz Vallés, S.; Gallego Fernández, J.B.; Dellafiore, C.; Cambrollé, J. Effects on Soil, Microclimate and Vegetation of the Native-Invasive Retama monosperma (L.) in Coastal Dunes. Plant Ecol. 2011, 212, 169–179. [Google Scholar] [CrossRef]
- Zunzunegui, M.; Esquivias, M.P.; Fernández-González, P.; Valera-Burgos, J.; Díaz Barradas, M.C.; Gallego-Fernández, J.B. Morpho-Physiological Response of Retama monosperma to Extreme Salinity Levels. Ecohydrology 2017, 10, e1871. [Google Scholar] [CrossRef]
- Zunzunegui, M.; Esquivias, M.P.; Álvarez-Cansino, L.; Gallego-Fernández, J.B. Seawater Spray as a Significant Nitrogen Source across Coastal Dune Vegetation Gradients. Estuar. Coast. Shelf Sci. 2024, 309, 108941. [Google Scholar] [CrossRef]
- Zunzunegui, M.; Díaz Barradas, M.C.; Ain-Lhout, F.; Alvarez-Cansino, L.; Esquivias, M.P.; García Novo, F. Seasonal Physiological Plasticity and Recovery Capacity after Summer Stress in Mediterranean Scrub Communities. Plant Ecol. 2011, 212, 127–142. [Google Scholar] [CrossRef]
- Werner, C.; Máguas, C. Carbon Isotope Discrimination as a Tracer of Functional Traits in a Mediterranean Macchia Plant Community. Funct. Plant Biol. 2010, 2010, 467–477. [Google Scholar] [CrossRef]
- Zunzunegui, M.; Esquivias, M.P.; Gallego-Fernández, J.B. Spatial and Seasonal Patterns of Water Use in Mediterranean Coastal Dune Vegetation. Plant Soil 2022, 477, 807–828. [Google Scholar] [CrossRef]
- Bar Kutiel, P.; Katz, O.; Ziso-Cohen, V.; Divinsky, I.; Katra, I. Water Availability in Sand Dunes and Its Implications for the Distribution of Artemisia monosperma. CATENA 2016, 137, 144–151. [Google Scholar] [CrossRef]
- Antunes, C.; Pereira, A.J.; Fernandes, P.; Ramos, M.; Ascensão, L.; Correia, O.; Máguas, C. Understanding Plant Drought Resistance in a Mediterranean Coastal Sand Dune Ecosystem: Differences between Native and Exotic Invasive Species. J. Plant Ecol. 2018, 11, 26–38. [Google Scholar] [CrossRef]
- Zunzunegui, M.; Ruiz-Valdepeñas, E.; Sert, M.A.; Díaz-Barradas, M.C.; Gallego-Fernández, J.B. Field Comparison of Ecophysiological Traits between an Invader and a Native Species in a Mediterranean Coastal Dune. Plant Physiol. Biochem. 2020, 146, 278–286. [Google Scholar] [CrossRef]
- Muñoz-Reinoso, J.C. Effects of Pine Plantations on Coastal Gradients and Vegetation Zonation in SW Spain. Estuar. Coast. Shelf Sci. 2021, 251, 107182. [Google Scholar] [CrossRef]
- Muñoz-Reinoso, J.C. Diversity of Maritime Juniper Woodlands. For. Ecol. Manag. 2004, 192, 267–276. [Google Scholar] [CrossRef]
- Zunzunegui, M.; Barradas, M.C.D.; Ain-Lhout, F.; Clavijo, A.; Novo, F.G. To Live or to Survive in Doñana Dunes: Adaptive Responses of Woody Species under a Mediterranean Climate. Plant Soil 2005, 273, 77–89. [Google Scholar] [CrossRef]
- Lopez-Iglesias, B.; Villar, R.; Poorter, L. Functional Traits Predict Drought Performance and Distribution of Mediterranean Woody Species. Acta Oecol. 2014, 56, 10–18. [Google Scholar] [CrossRef]
- Farquhar, G.; O’Leary, M.H.O.; Berry, J. On the Relationship between Carbon Isotope Discrimination and the Intercellular Carbon Dioxide Concentration in Leaves. Aust. J. Plant Physiol. 1982, 9, 121–137. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Hubick, K.T.; Condon, A.G.; Richards, R.A. Carbon Isotope Fractionation and Plant Water-Use Efficiency. In Stable Isotopes in Ecological Research; Rundel, P.W., Ehleringer, J.R., Nagy, K.A., Eds.; Springer: New York, NY, USA, 1989; pp. 21–40. [Google Scholar]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New Handbook for Standardised Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Carboni, M.; Santoro, R.; Acosta, A.T.R. Are Some Communities of the Coastal Dune Zonation More Susceptible to Alien Plant Invasion? J. Plant Ecol. 2010, 3, 139–147. [Google Scholar] [CrossRef]
- Pardini, E.A.; Vickstrom, K.E.; Knight, T.M. Early Successional Microhabitats Allow the Persistence of Endangered Plants in Coastal Sand Dunes. PLoS ONE 2015, 10, e0119567. [Google Scholar] [CrossRef] [PubMed]
- Zunzunegui, M.; Ain-Lhout, F.; Barradas, M.C.D.; Álvarez-Cansino, L.; Esquivias, M.P.; García Novo, F. Physiological, Morphological and Allocation Plasticity of a Semi-Deciduous Shrub. Acta Oecol. 2009, 35, 370–379. [Google Scholar] [CrossRef]
- Esquivias, M.P.; Zunzunegui, M.; Barradas, M.C.D.; Álvarez-Cansino, L. The Role of Water Use and Uptake on Two Mediterranean Shrubs’ Interaction in a Brackish Coastal Dune Ecosystem. Ecohydrology 2014, 7, 783–793. [Google Scholar] [CrossRef]
- Rascher, K.G.; Hellmann, C.; Máguas, C.; Werner, C. Community Scale 15N Isoscapes: Tracing the Spatial Impact of an Exotic N2-Fixing Invader. Ecol. Lett. 2012, 15, 484–491. [Google Scholar] [CrossRef]
- Dawson, T.E.; Mambelli, S.; Plamboeck, A.H.; Templer, P.H.; Tu, K.P. Stable Isotopes in Plant Ecology. Annu. Rev. Ecol. Syst. 2002, 33, 507–559. [Google Scholar] [CrossRef]
- Hesp, P.A. Ecological Processes and Plant Adaptations on Coastal Dunes. J. Arid Environ. 1991, 21, 165–191. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Macko, S.A.; Shugart, H.H. Interpretation of Nitrogen Isotope Signatures Using the NIFTE Model. Oecologia 1999, 120, 405–415. [Google Scholar] [CrossRef]
- Muñoz-Vallés, S.; Gallego-Fernández, J.B.; Cambrollé, J. The Role of the Expansion of Native-Invasive Plant Species in Coastal Dunes: The Case of Retama monosperma in SW Spain. Acta Oecol. 2014, 54, 82–89. [Google Scholar] [CrossRef]
- Jardeleza, M.-K.G.; Koch, J.B.; Pearse, I.S.; Ghalambor, C.K.; Hufbauer, R.A. The Roles of Phenotypic Plasticity and Adaptation in Morphology and Performance of an Invasive Species in a Novel Environment. Ecol. Entomol. 2022, 47, 25–37. [Google Scholar] [CrossRef]
- Antunes, C.; Chozas, S.; West, J.; Zunzunegui, M.; Diaz Barradas, M.C.; Vieira, S.; Máguas, C. Groundwater Drawdown Drives Ecophysiological Adjustments of Woody Vegetation in a Semi-Arid Coastal Ecosystem. Glob. Change Biol. 2018, 24, 4894–4908. [Google Scholar] [CrossRef] [PubMed]
- Zunzunegui, M.; Diaz Barradas, M.C.; Garcia Novo, F. Vegetation Fluctuation in Mediterranean Dune Ponds in Relation to Rainfall Variation and Water Extraction. Appl. Veg. Sci. 1998, 1, 151–160. [Google Scholar] [CrossRef]
- Serrano, L.; Esquivias-Segura, M.P.; Zunzunegui, M. Long-Term Hydrological Changes over a Seventeen-Year Period in Temporary Ponds of the Donana N. P. (SW Spain). Limnetica 2008, 28, 65–78. [Google Scholar] [CrossRef]
- Muñoz Vallés, S.; Gallego Fernández, J.B.; Cambrollé, J. The Biological Flora of Coastal Dunes and Wetlands: Retama monosperma (L.) Boiss. J. Coast. Res. 2013, 29, 1101–1110. [Google Scholar] [CrossRef]
- Cano, E.; Musarella, C.M.; Cano-Ortiz, A.; Piñar Fuentes, J.C.; Rodríguez Torres, A.; Del Río González, S.; Pinto Gomes, C.J.; Quinto-Canas, R.; Spampinato, G. Geobotanical Study of the Microforests of Juniperus oxycedrus Subsp. Badia in the Central and Southern Iberian Peninsula. Sustainability 2019, 11, 1111. [Google Scholar] [CrossRef]
- Cano Ortiz, A.; Spampinato, G.; Piñar Fuentes, J.C.; Pinto Gomes, C.J.; Quinto-Canas, R.; Cano, E. Taxonomy, Ecology and Distribution of Juniperus oxycedrus L. Group in the Mediterranean Basin Using Bioclimatic, Phytochemical and Morphometric Approaches, with Special Reference to the Iberian Peninsula. Forests 2021, 12, 703. [Google Scholar] [CrossRef]
- Farris, E.; Canopoli, L.; Cucca, E.; Landi, S.; Maccioni, A.; Filigheddu, R. Foxes Provide a Direct Dispersal Service to Phoenician junipers in Mediterranean Coastal Environments: Ecological and Evolutionary Implications. Plant Ecol. Evol. 2017, 150, 117–128. [Google Scholar] [CrossRef]
- Gianguzzi, L.; Ilardi, V.; Orazio, C.; Cusimano, D.; Pasquale, C.; Salvatore, R. Phytosociological Characterization of the Juniperus phoenicea L. Subsp. Turbinata (Guss.) Nyman Formations in the Italo-Tyrrhenian Province (Mediterranean Region). Plant Sociol. 2012, 49, 3–28. [Google Scholar] [CrossRef]
- Juan, R.; Díaz, J.; Fernández-González, I.; Diosdado, J. Seedling Emergence in the Endangered Juniperus oxycedrus Subsp. macrocarpa (Sm.) Ball in Southwest Spain. Acta Biol. Cracoviensia Ser. Bot. 2006, 48, 49–58. [Google Scholar]
- Muñoz-Reinoso, J. Juniperus oxycedrus Ssp. macrocarpa in SW Spain: Ecology and Conservation Problems. J. Coast. Conserv. 2003, 9, 113–122. [Google Scholar] [CrossRef]
- Granados Corona, M.; Martín Vicente, Á.; Fernández Alés, R.; García Novo, F. Etude diachronique d’un écosystème à longue échelle. La Pinède de Marismillas (Parc National de Doñana). Mix. Casa Velazquez 1984, 20, 393–418. [Google Scholar] [CrossRef]
- Porté, A.; Huard, F.; Dreyfus, P. Microclimate beneath Pine Plantation, Semi-Mature Pine Plantation and Mixed Broadleaved-Pine Forest. Agric. For. Meteorol. 2004, 126, 175–182. [Google Scholar] [CrossRef]
- Evans, R.D. Physiological Mechanisms Influencing Plant Nitrogen Isotope Composition. Trends Plant Sci. 2001, 6, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Högberg, P. Tansley Review No. 95 15 N Natural Abundance in Soil-Plant Systems. New Phytol. 1997, 137, 179–203. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D. δ15N as an Integrator of the Nitrogen Cycle. Trends Ecol. Evol. 2001, 16, 153–162. [Google Scholar] [CrossRef]
- Evans, R.D.; Bloom, A.J.; Sukrapanna, S.S.; Ehleringer, J.R. Nitrogen Isotope Composition of Tomato (Lycopersicon Esculentum Mill. Cv. T-5) Grown under Ammonium or Nitrate Nutrition. Plant Cell Environ. 1996, 19, 1317–1323. [Google Scholar] [CrossRef]
- Högberg, P.; Högberg, M.N.; Quist, M.E.; Ekblad, A.; Näsholm, T. Nitrogen Isotope Fractionation during Nitrogen Uptake by Ectomycorrhizal and Non-Mycorrhizal Pinus Sylvestris. New Phytol. 1999, 142, 569–576. [Google Scholar] [CrossRef]
- Montoya, J.P.; McCarthy, J.J. Isotopic Fractionation during Nitrate Uptake by Phytoplankton Grown in Continuous Culture. J. Plankton Res. 1995, 17, 439–464. [Google Scholar] [CrossRef]
- Rodriguez-Dominguez, C.M.; Forner, A.; Martorell, S.; Choat, B.; Lopez, R.; Peters, J.M.R.; Pfautsch, S.; Mayr, S.; Carins-Murphy, M.R.; McAdam, S.A.M.; et al. Leaf Water Potential Measurements Using the Pressure Chamber: Synthetic Testing of Assumptions towards Best Practices for Precision and Accuracy. Plant Cell Environ. 2022, 45, 2037–2061. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Martínez, M.; Jiménez-Carrasco, C.; Barradas, M.C.D.; Gallego-Fernández, J.B.; Zunzunegui, M. Ecophysiological Keys to the Success of a Native-Expansive Mediterranean Species in Threatened Coastal Dune Habitats. Plants 2025, 14, 2342. https://doi.org/10.3390/plants14152342
Fernández-Martínez M, Jiménez-Carrasco C, Barradas MCD, Gallego-Fernández JB, Zunzunegui M. Ecophysiological Keys to the Success of a Native-Expansive Mediterranean Species in Threatened Coastal Dune Habitats. Plants. 2025; 14(15):2342. https://doi.org/10.3390/plants14152342
Chicago/Turabian StyleFernández-Martínez, Mario, Carmen Jiménez-Carrasco, Mari Cruz Díaz Barradas, Juan B. Gallego-Fernández, and María Zunzunegui. 2025. "Ecophysiological Keys to the Success of a Native-Expansive Mediterranean Species in Threatened Coastal Dune Habitats" Plants 14, no. 15: 2342. https://doi.org/10.3390/plants14152342
APA StyleFernández-Martínez, M., Jiménez-Carrasco, C., Barradas, M. C. D., Gallego-Fernández, J. B., & Zunzunegui, M. (2025). Ecophysiological Keys to the Success of a Native-Expansive Mediterranean Species in Threatened Coastal Dune Habitats. Plants, 14(15), 2342. https://doi.org/10.3390/plants14152342







