StMAPKK1 Enhances Thermotolerance in Potato (Solanum tuberosum L.) by Enhancing Antioxidant Defense and Photosynthetic Efficiency Under Heat Stress
Abstract
1. Introduction
2. Results
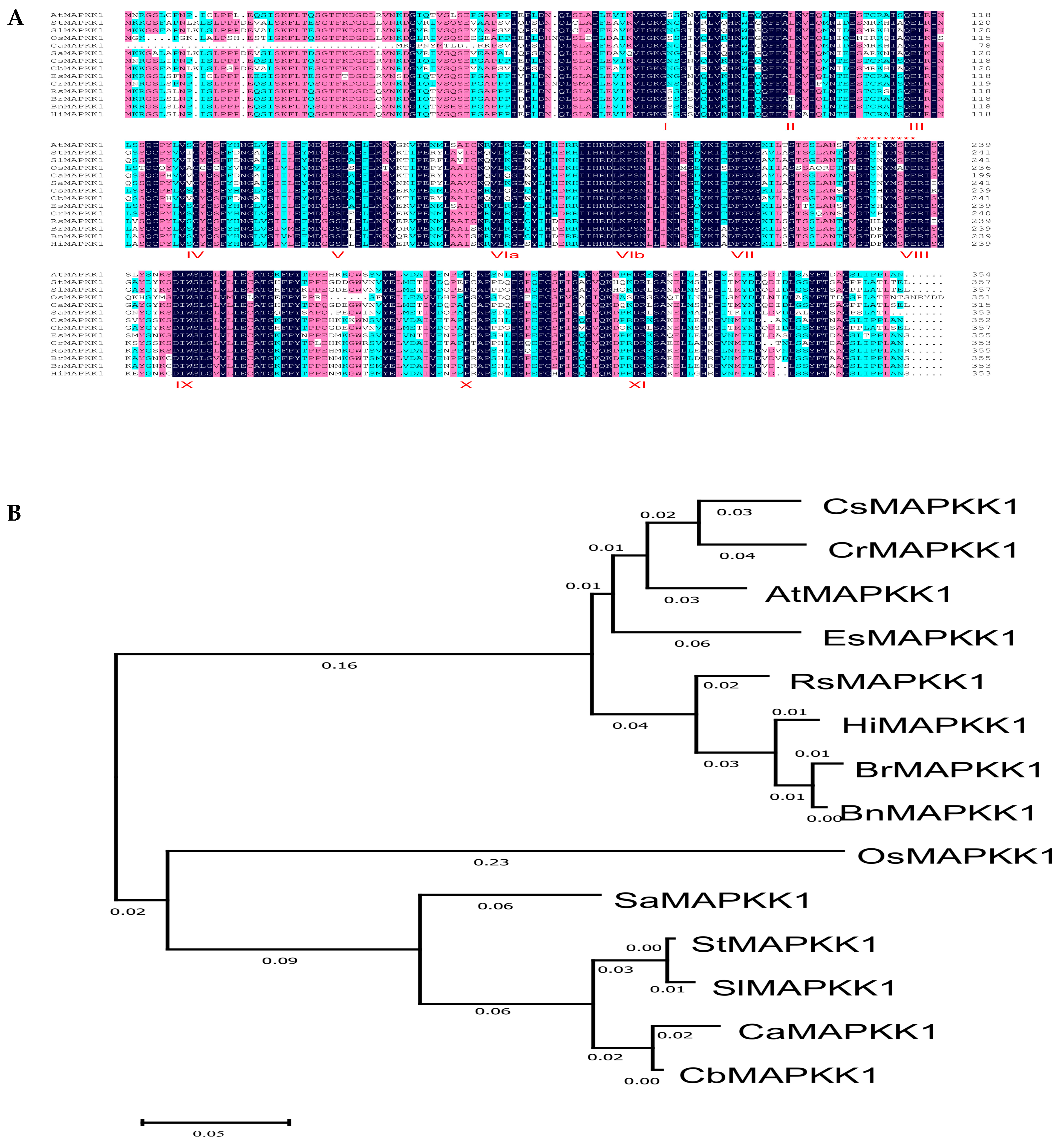
2.1. Sequence Alignment and Phylogenetic Analysis of StMAPKK1
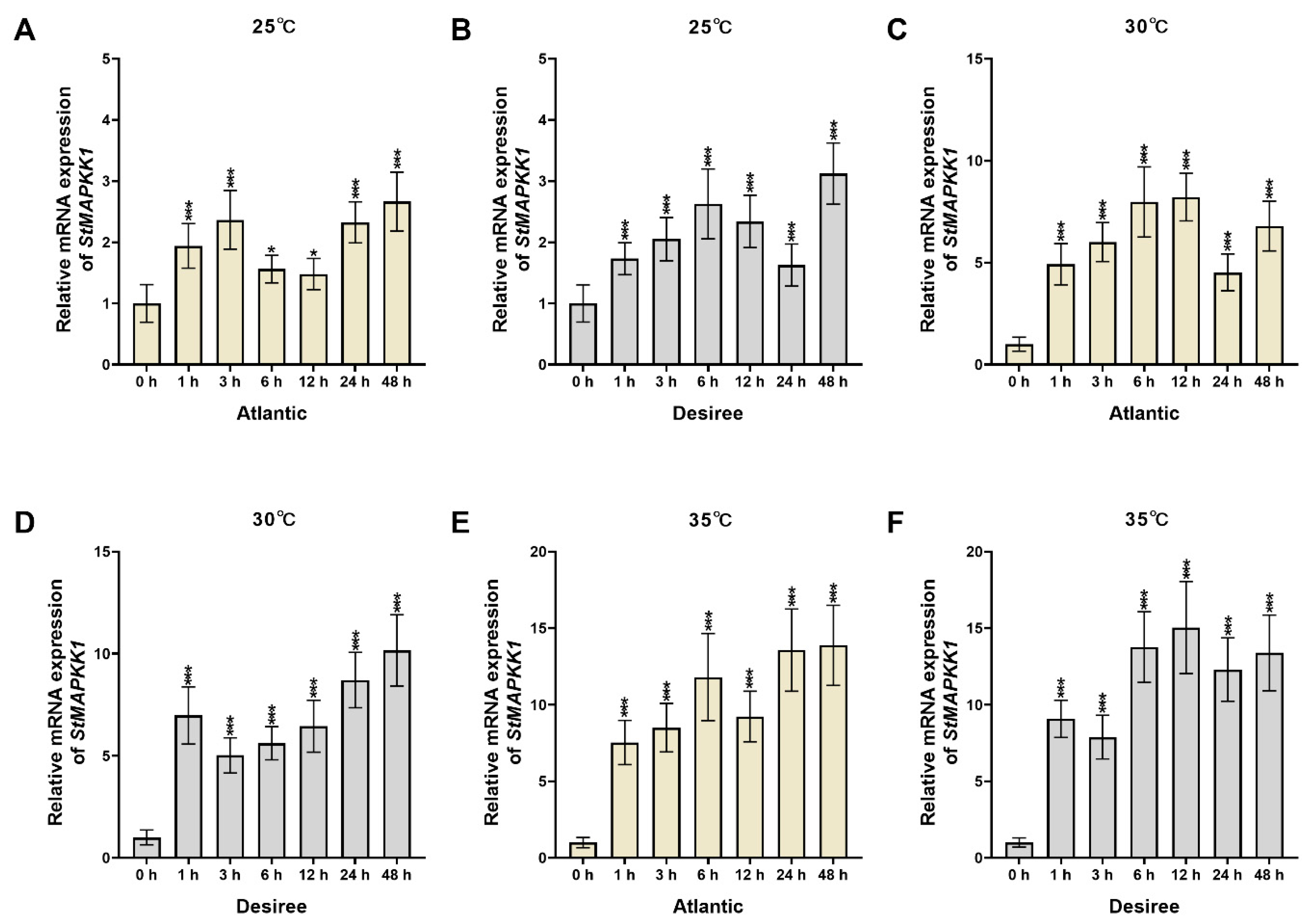
2.2. Expression Profiling of StMAPKK1 Under Heat Stress in Potato Cultivars
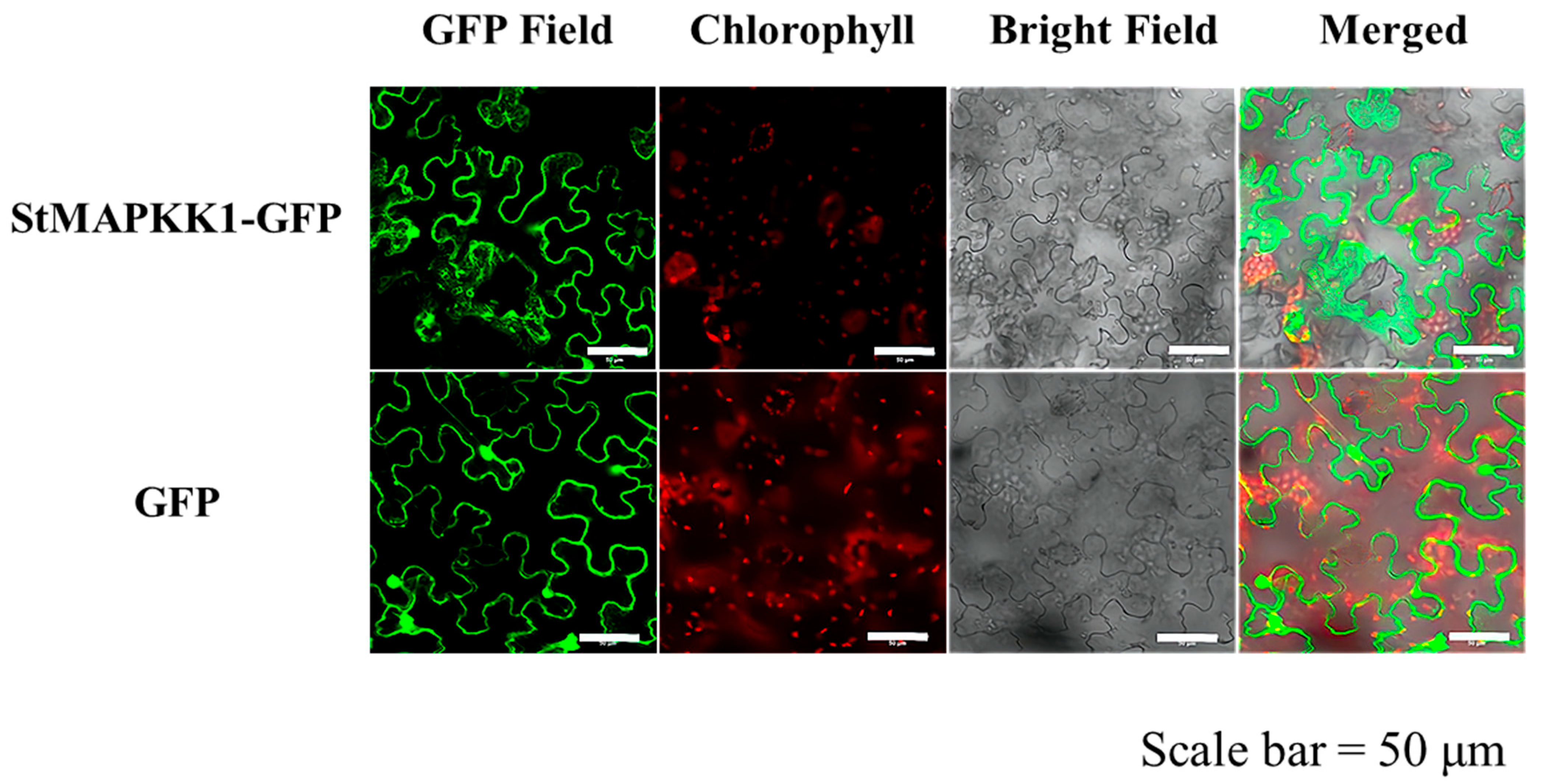
2.3. Subcellular Localization of StMAPKK1 and Generation of Transgenic Potato Lines
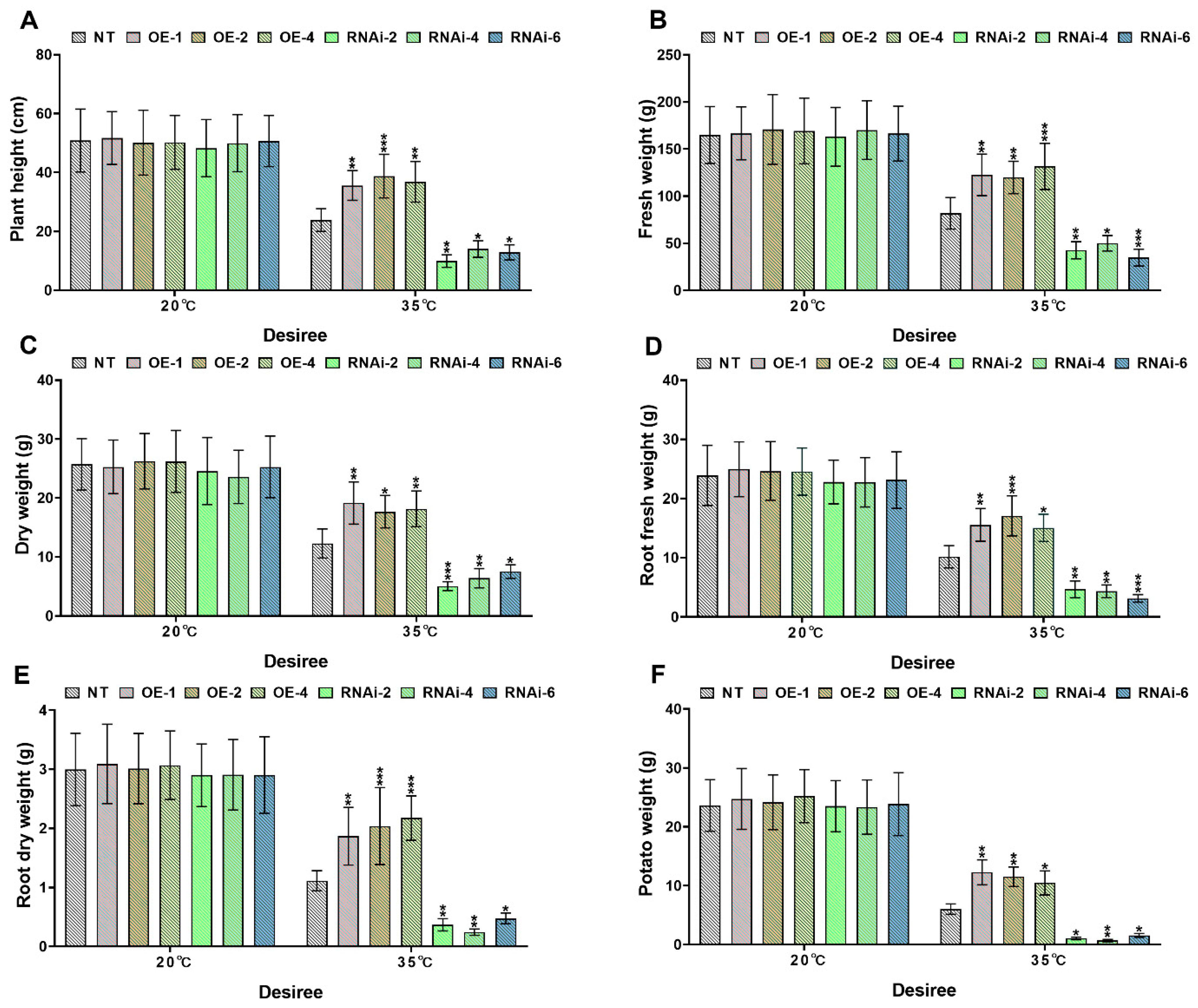
2.4. StMAPKK1-Mediated Signaling Enhances Thermotolerance and Biomass Accumulation
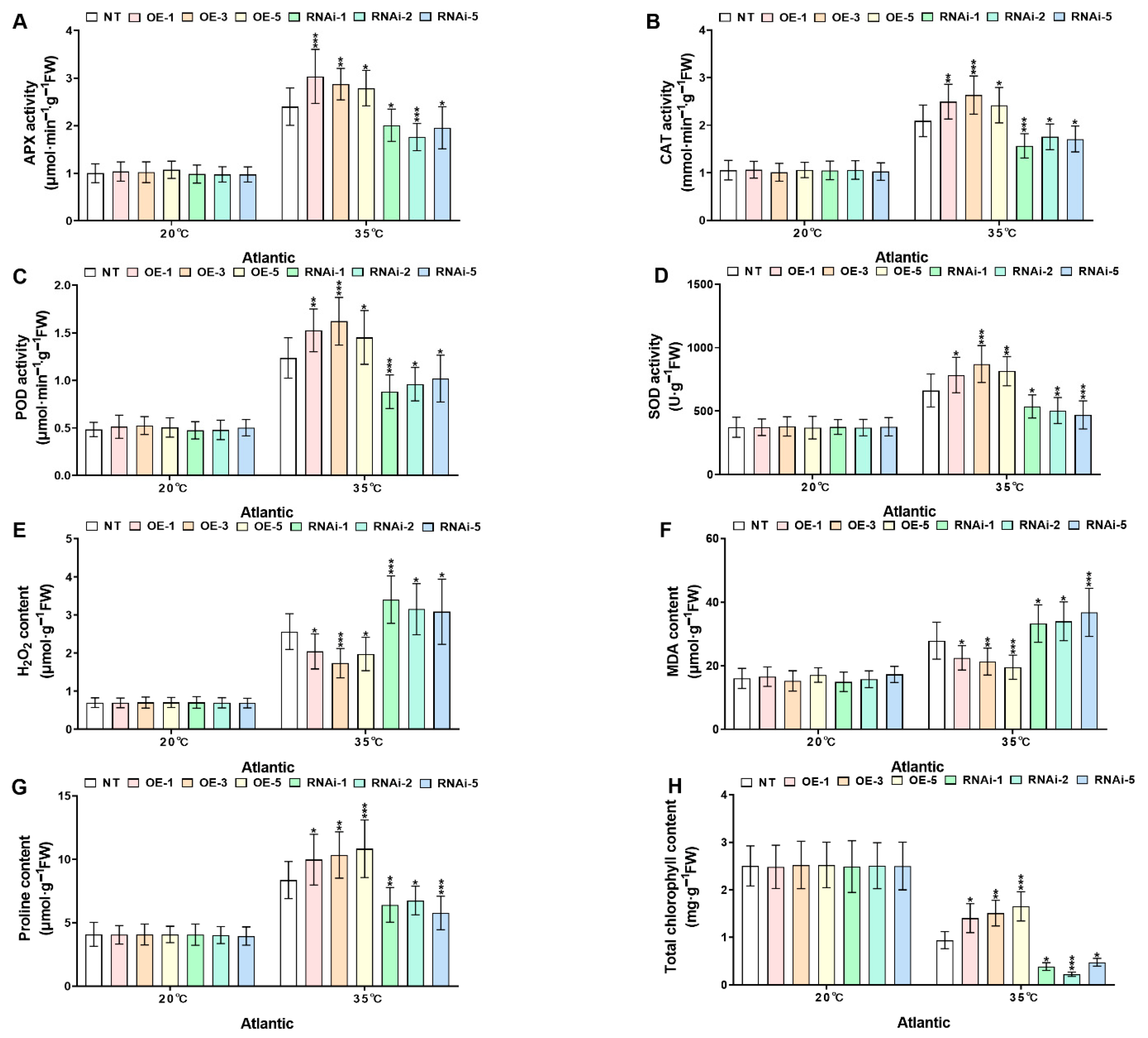
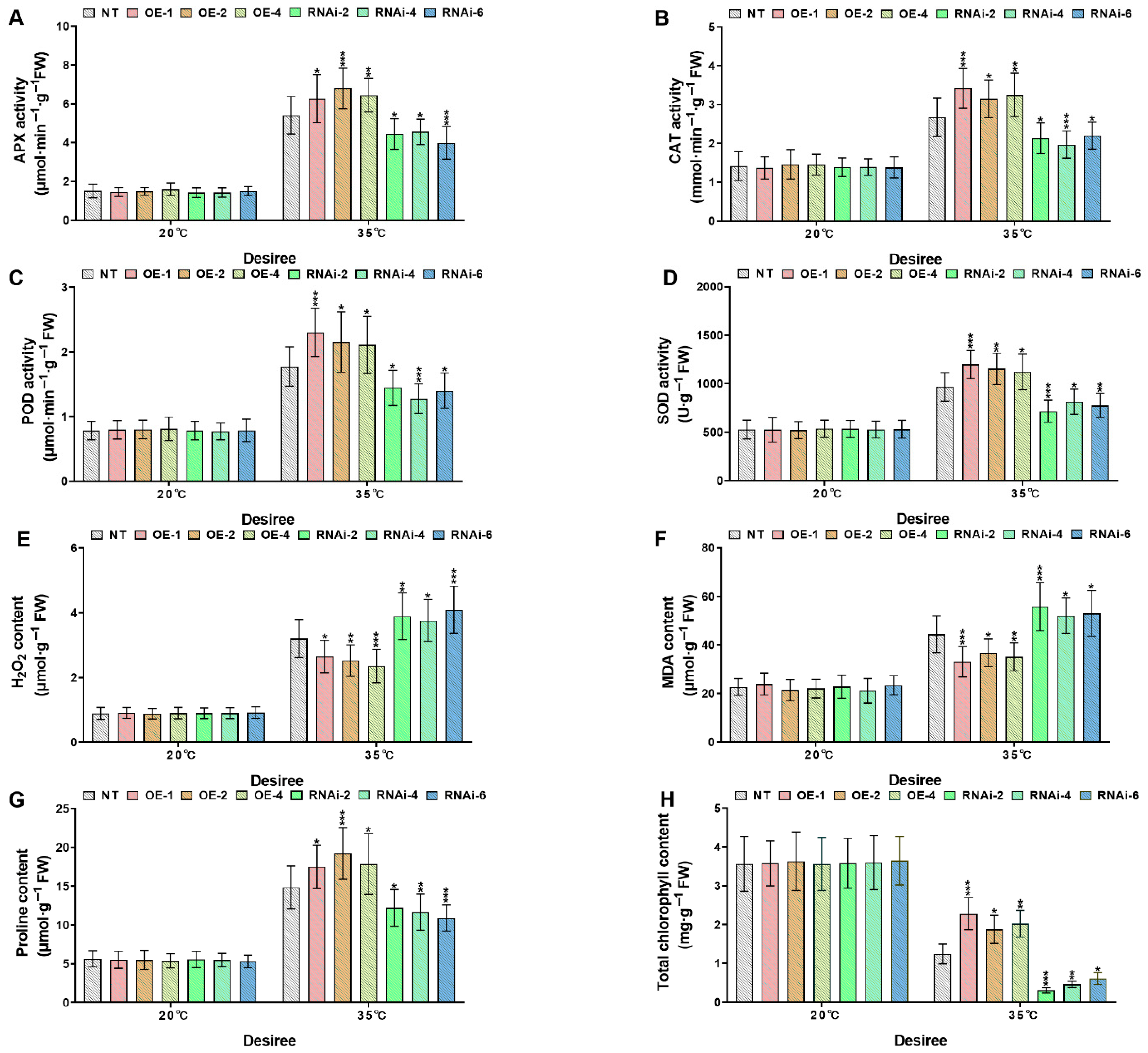
2.5. StMAPKK1 Enhances Potato Thermotolerance by Modulating Antioxidant Defense, Osmoprotectant Biosynthesis, Oxidative Stress Markers, and Chlorophyll Stability
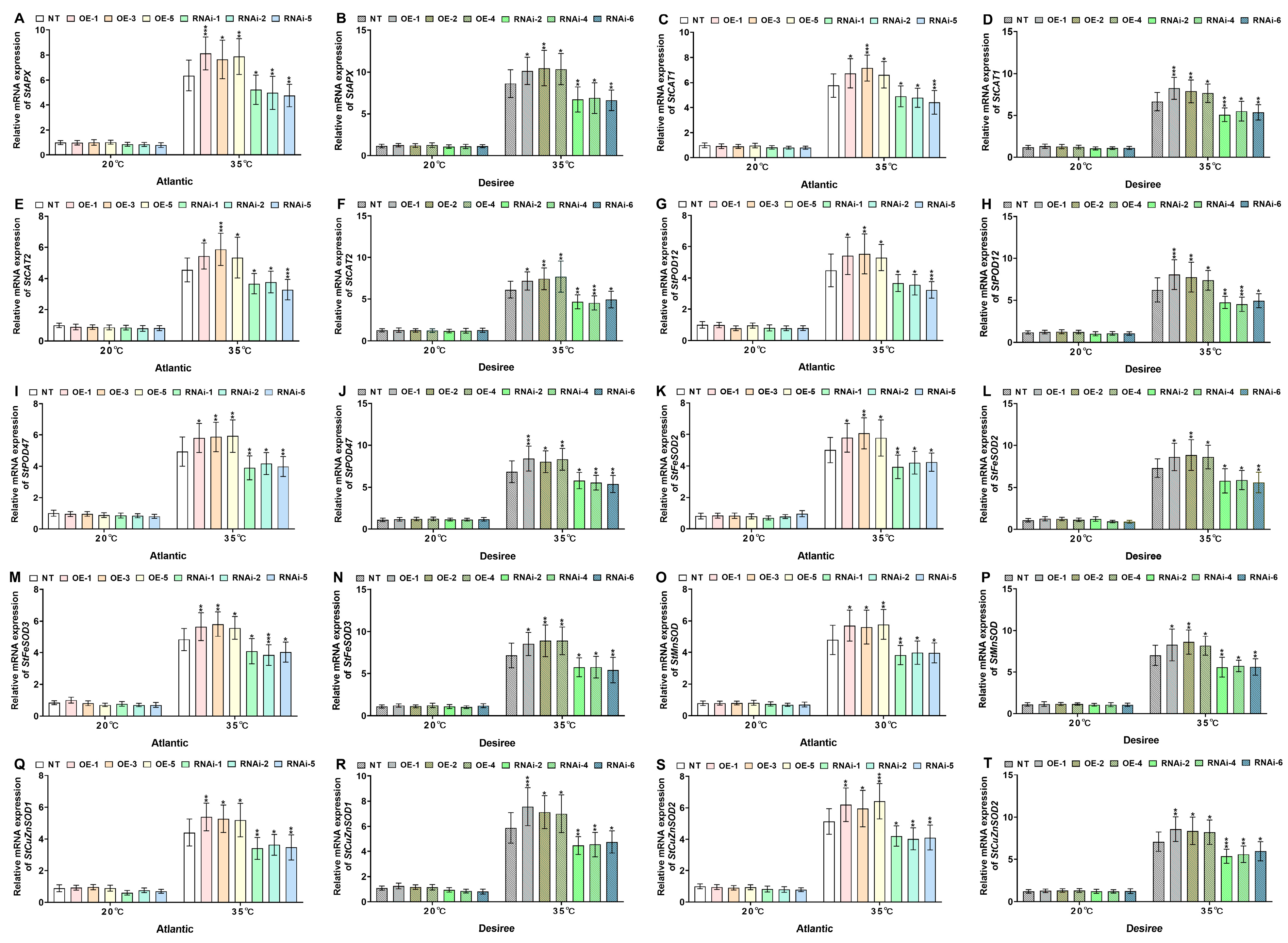
2.6. StMAPKK1 Regulates the Stress-Responsive Gene Network to Confer Heat Tolerance in Potato
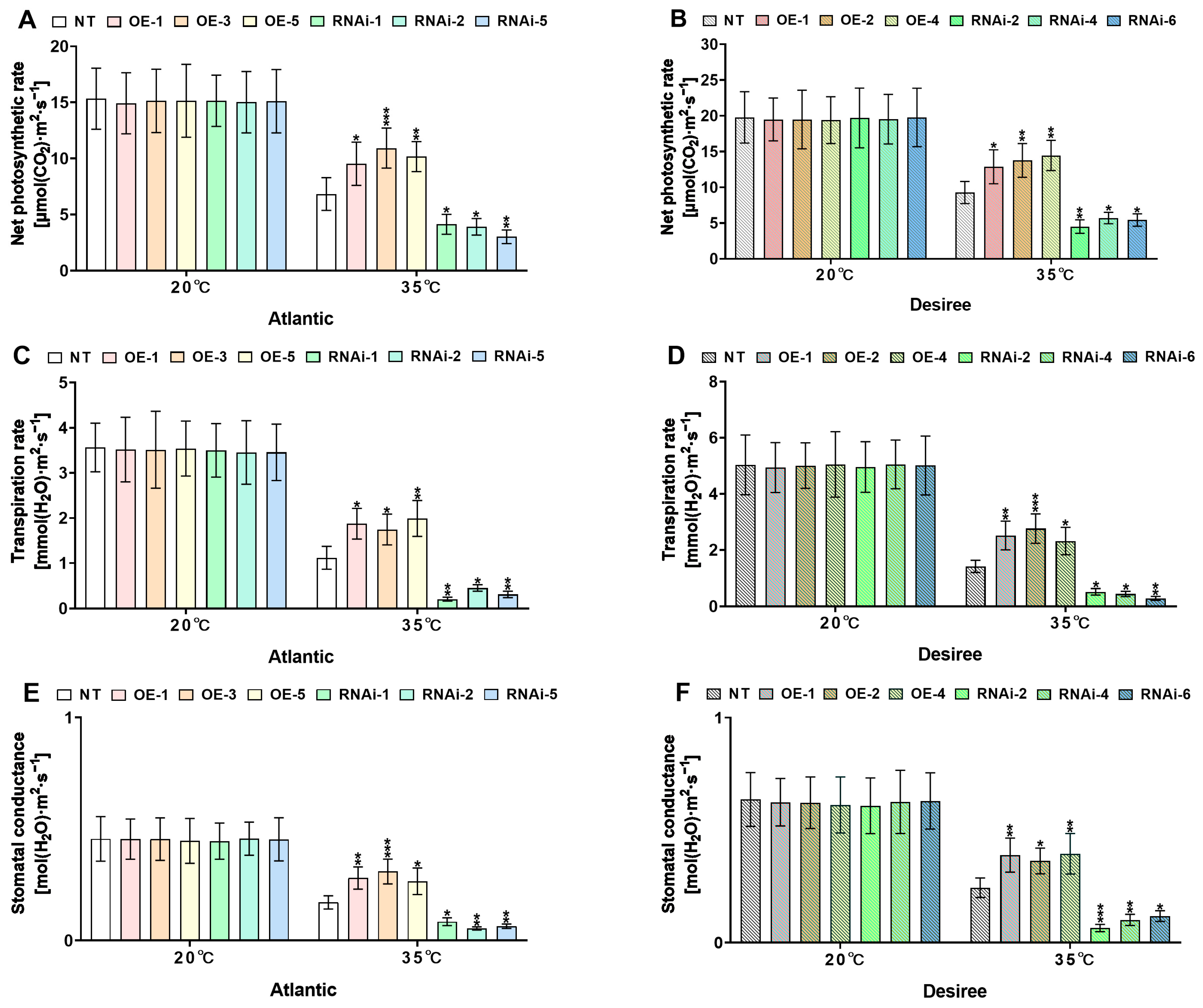
2.7. StMAPKK1 Maintains Photosynthesis Under Heat Stress in Potato
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Stress Treatments
4.2. Phylogenetic Analysis and Sequence Comparison
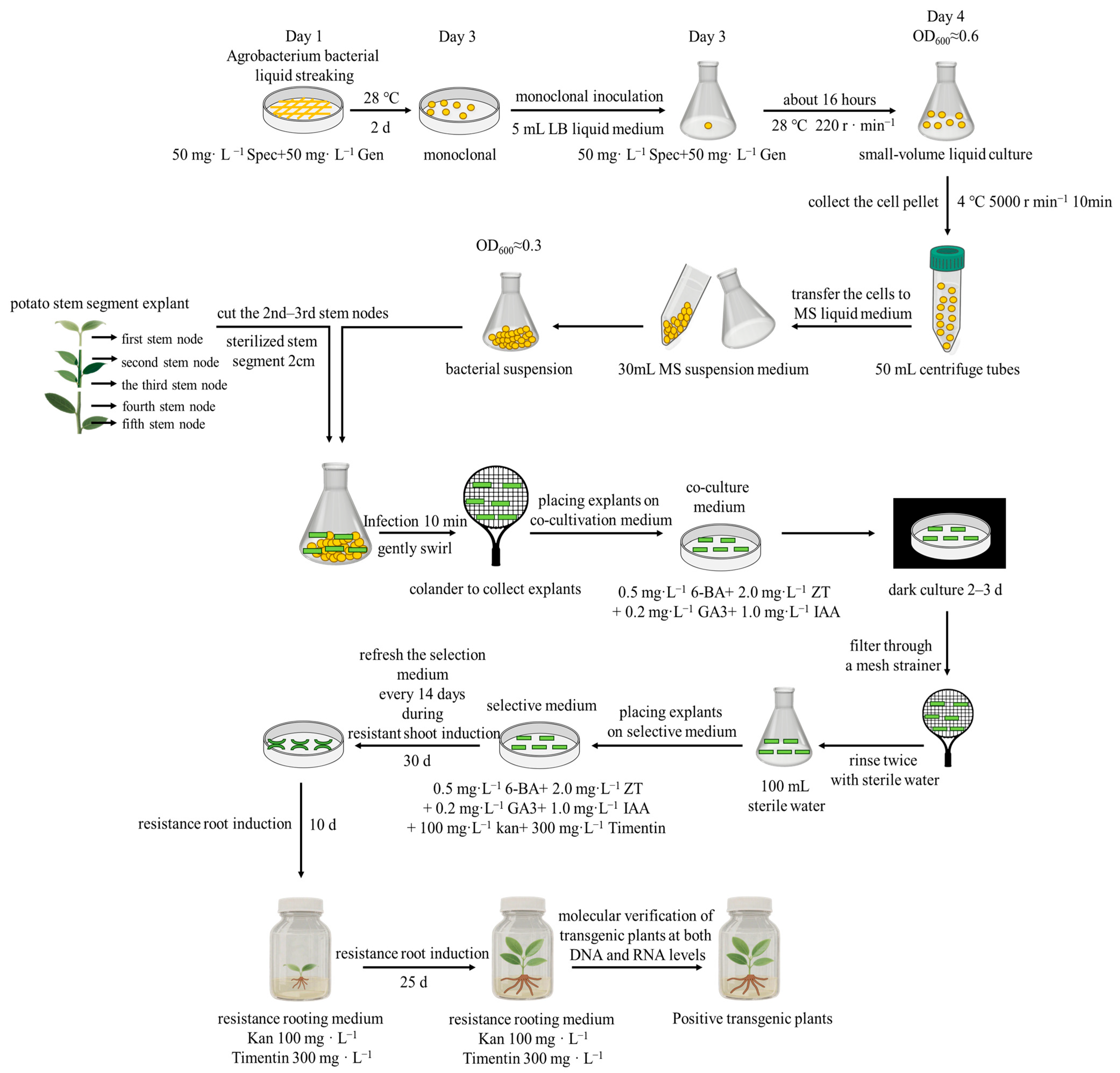
4.3. Generation of StMAPKK1 Transgenic Potato Plants
4.4. qRT-PCR Analysis of Gene Expression
4.5. Subcellular Localization of StMAPKK1
4.6. Analysis of Growth Parameters
4.7. Measurement of Photosynthetic Parameters and Chlorophyll Content
4.8. Biochemical Analysis of Stress Markers and Antioxidant Enzyme Activities
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAPKK | Mitogen-activated protein kinase kinase |
| OE | Overexpressing |
| RNAi | RNA interference |
| NT | Non-transgenic |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| APX | Ascorbate peroxidase |
| CAT | Catalase |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| MDA | Malondialdehyde |
| H2O2 | Hydrogen peroxide |
| HSFA3 | Heat shock transcription factor A3 |
| HSP | Heat shock protein |
| ROS | Reactive oxygen species |
| Vec. | Vector control |
References
- Li, L.; Zhu, T.; Wen, L.; Zhang, T.; Ren, M. Bio-fortification of potato nutrition. J. Adv. Res. 2024, S2090-1232, 487–489. [Google Scholar] [CrossRef]
- Devaux, A.; Goffart, J.P.; Kromann, P.; Andrade-Piedra, J.; Polar, V.; Hareau, G. The potato of the future: Opportunities and challenges in sustainable agri-food systems. Potato Res. 2021, 64, 681–720. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kukreja, S.; Goutam, U. Impact of heat stress on potato (Solanum tuberosum L.): Present scenario and future opportunities. J. Hortic. Sci. Biotechnol. 2020, 95, 407–424. [Google Scholar] [CrossRef]
- Dahal, K.; Li, X.Q.; Tai, H.; Creelman, A.; Bizimungu, B. Improving potato stress tolerance and tuber yield under a climate change scenario–a current overview. Front. Plant Sci. 2019, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, K.; AbuQamar, S.; Jarrar, M.; Al-Rajab, A.J.; Trémouillaux-Guiller, J. MAPK cascades and major abiotic stresses. Plant Cell Rep. 2014, 33, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhu, W.; Zhang, X.; Chen, X.; Wang, W.; Lin, H.; Wang, J.; Ye, W. Molecular characterization, expression, and interaction of MAPK, MAPKK, and MAPKKK genes in upland cotton. Genomics 2021, 113, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Jonak, C.; Okrész, L.; Bögre, L.; Hirt, H. Complexity, cross-talk and integration of plant MAP kinase signaling. Curr. Opin. Plant Biol. 2002, 5, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, S. Mitogen-activated protein kinase cascades in plant signaling. J. Integr. Plant Biol. 2022, 64, 301–341. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Raina, S.K.; Sultan, S.M. Arabidopsis MAPK signaling pathways and their cross-talks in abiotic stress response. J. Plant Biochem. Biotechnol. 2020, 29, 700–714. [Google Scholar] [CrossRef]
- MAPK Group. Mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci. 2002, 7, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.P.; Richa, T.; Kumar, K.; Raghuram, B.; Sinha, A.K. In silico analysis reveals 75 members of the mitogen-activated protein kinase kinase kinase gene family in rice. DNA Res. 2010, 17, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, D.; Wang, L.; Li, D. Genome-Wide Analysis of Mitogen-Activated Protein Kinase Gene Family in Maize. Plant Mol. Biol. Rep. 2013, 31, 1446–1460. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Pan, C.; Guan, X.; Wang, Y.; Liu, S.; He, Y.; Chen, J.; Chen, L.; Lu, G. Genome-wide identification of MAPKK and MAPKKK gene families in tomato and transcriptional profiling analysis during development and stress response. PLoS ONE 2014, 9, e103032. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, C.; Wang, Y.; Ye, L.; Wu, J.; Chen, L.; Zou, T.; Lu, G. Genome-wide identification of MAPK, MAPKK, and MAPKKK gene families and transcriptional profiling analysis during development and stress response in cucumber. BMC Genom. 2015, 16, 386. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Yue, H.; Zhao, X.; Wang, M.; Song, W.; Nie, X. Genome-Wide Identification and Analysis of MAPK and MAPKK Gene Families in Bread Wheat (Triticum aestivum L.). Genes 2017, 8, 284. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, W.; Tie, W.; Ding, Z.; Ding, X.; Liu, Y.; Yan, Y.; Wu, C.; Peng, M.; Xu, B.; et al. The MAPKKK and MAPKK gene families in banana: Identification, phylogeny and expression during development, ripening and abiotic stress. Sci. Rep. 2017, 7, 1159. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Osuna, A.; Calatrava, V.; Galvan, A.; Fernandez, E.; Llamas, A. Identification of the MAPK Cascade and its Relationship with Nitrogen Metabolism in the Green Alga Chlamydomonas reinhardtii. Int. J. Mol. Sci. 2020, 21, 3417. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ren, S.; Han, Y.; Zhang, Q.; Qin, L.; Xing, Y. Identification and analysis of mitogen-activated protein kinase (MAPK) cascades in Fragaria vesca. Int. J. Mol. Sci. 2017, 18, 1766. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Kato, H.; Imai, R. Biochemical identification of the OsMKK6-OsMPK3 signaling pathway for chilling stress tolerance in rice. Biochem. J. 2012, 443, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Rao, K.P.; Sharma, P.; Sinha, A.K. Differential regulation of rice mitogen-activated protein kinase kinase (MKK) by abiotic stress. Plant Physiol. Biochem. 2008, 46, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Pan, J.; Zhang, M.; Xing, X.; Zhou, Y.; Liu, Y. ZmMKK4, a novel group C mitogen-activated protein kinase kinase in maize (Zea mays), confers salt and cold tolerance in transgenic Arabidopsis. Plant Cell Environ. 2011, 34, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.; Lu, W.; Meng, F.; Wu, C.A.; Guo, X. Cotton GhMKK5 affects disease resistance, induces HR-like cell death, and reduces the tolerance to salt and drought stress in transgenic Nicotiana benthamiana. J. Exp. Bot. 2012, 63, 3935–3951. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, W.; Zhang, N.; Jin, H.; Duan, H.; Chen, Z.; Chen, S.; Wang, Q.; Tang, J.; Zhou, J.; et al. StMAPKK5 responds to heat stress by regulating potato growth, photosynthesis, and antioxidant defenses. Front. Plant Sci. 2024, 15, 1392425. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, K.; Zhu, L.; Zhang, N.; Si, H. StMAPKK5 Positively Regulates Response to Drought and Salt Stress in Potato. Int. J. Mol. Sci. 2024, 25, 3662. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Luo, X.; Zhang, H.; Chen, M.; Yin, W.; Cao, Z.; Deng, R.; Li, Y.; Li, F. Genome-wide identification and analysis of the MAPK and MAPKK gene families in potato (Solanum tuberosum L.). Agronomy 2022, 13, 93. [Google Scholar] [CrossRef]
- Ding, R.; Li, J.; Wang, J.; Li, Y.; Ye, W.; Yan, G.; Yin, Z. Molecular traits of MAPK kinases and the regulatory mechanism of GhMAPKK5 alleviating drought/salt stress in cotton. Plant Physiol. 2024, 196, 2030–2047. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xi, M.; Liu, T.; Wu, X.; Ju, L.; Wang, D. The central role of transcription factors in bridging biotic and abiotic stress responses for plants’ resilience. New Crops 2024, 1, 100005. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, T.; Guo, Y.; Wang, M.; Brachhold, K.; Chu, C.; Hanson, A.; Kumar, S.; Lin, R.; Long, E.; et al. 100 essential questions for the future of agriculture. Mod. Agric. 2023, 1, 4–12. [Google Scholar] [CrossRef]
- Kaier, A.; Beck, S.; Ingold, M.; Corral, J.M.; Reinert, S.; Sonnewald, U.; Sonnewald, S. Identification of heat stress-related genomic regions by genome-wide association study in Solanum tuberosum. Genomics 2024, 116, 110954. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Song, H.; Feng, J.; Zhou, C.; Yang, M.J.; Shi, P.; Yu, Z.L.; Li, Y.R.; Guo, Y.J.; Li, H.Z.; et al. Genome-wide analysis of the hard clam mitogen-activated protein kinase kinase gene family and their transcriptional profiles under abiotic stress. Mar. Environ. Res. 2022, 176, 105606. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Tena, G.; Plotnikova, J.; Willmann, M.R.; Chiu, W.L.; Gomez-Gomez, L.; Boller, T.; Ausubel, F.M.; Sheen, J. MAP kinase signaling cascade in Arabidopsis innate immunity. Nature 2002, 415, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, M.; Gu, C.; Jiang, H.; Sun, J.; Li, J. Genome-wide identification of MAPK family genes and their response to abiotic stresses in tea plant (Camellia sinensis). Open Life Sci. 2022, 17, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jia, L.; Chen, Q.; Qiao, Q.; Huang, X.; Zhang, S. Genome-wide identification of the mitogen-activated protein kinase kinase kinase (MAPKKK) in pear (Pyrus bretschneideri) and their functional analysis in response to black spot. Planta 2022, 257, 5. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Gotoh, Y.; Nishida, E. Interaction of MAP kinase with MAP kinase kinase: Its possible role in the control of nucleocytoplasmic transport of MAP kinase. EMBO J. 1997, 16, 1901–1908. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhou, B.; Duan, L.; Zhu, H.; Zhang, Z. MtMAPKK4 is an essential gene for growth and reproduction of Medicago truncatula. Physiol. Plant. 2017, 159, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, L.; Asseng, S.; Zhang, D.; Ma, W.; Tang, L.; Cao, W.; Zhu, Y. Modelling the effects of post-heading heat stress on biomass partitioning, and grain number and weight of wheat. J. Exp. Bot. 2020, 71, 6015–6031. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, W.; He, X.; Wang, F.; Zhou, Y.; Guo, X.; Guo, X. The cotton mitogen-activated protein kinase kinase 3 functions in drought tolerance by regulating stomatal responses and root growth. Plant Cell Physiol. 2016, 57, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Wang, G.; Wang, L.; Pan, J.; Liu, Y.; Li, D. ZmMKK1, a novel group A mitogen-activated protein kinase kinase gene in maize, conferred chilling stress tolerance and was involved in pathogen defense in transgenic tobacco. Plant Sci. 2014, 214, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Su, H.; Han, L.; Wang, C.; Sun, Y.; Liu, F. Differential expression profiles of poplar MAP kinase kinases in response to abiotic stresses and plant hormones, and overexpression of PtMKK4 improves the drought tolerance of poplar. Gene 2014, 545, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Xing, Q.F.; Zhou, C.Y.; Wang, K.X.; Xu, T.; Yang, P.; Qi, Z.Y.; Shao, S.J.; Ahammed, G.J.; Zhou, J. Melatonin mediates elevated carbon dioxide-induced photosynthesis and thermotolerance in tomato. J. Pineal Res. 2023, 74, e12858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Pan, J.; Kong, X.; Zhou, Y.; Liu, Y.; Sun, L.; Li, D. ZmMKK3, a novel maize group B mitogen-activated protein kinase kinase gene, mediates osmotic stress and ABA signal responses. J. Plant Physiol. 2012, 169, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xue, C.; Chen, M.; Yang, Q. StHsfB5 Promotes Heat Resistance by Directly Regulating the Expression of Hsp Genes in Potato. Int. J. Mol. Sci. 2023, 24, 16528. [Google Scholar] [CrossRef] [PubMed]
- Netshimbupfe, M.H.; Berner, J.; Gouws, C. The interactive effects of drought and heat stress on photosynthetic efficiency and biochemical defense mechanisms of Amaranthus species. Plant Environ. Interact. 2022, 3, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Chu, X.; Li, Y.; Wang, C.; Guo, X. Cotton GhMKK1 induces the tolerance of salt and drought stress, and mediates defense responses to pathogen infection in transgenic Nicotiana benthamiana. PLoS ONE 2013, 8, e68503. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, F.F.; Zhu, X.; Zhang, N.; Si, H. Screening and bioinformatics analysis of proteins interacting with StMAPKK1 in potato by yeast two-hybrid system. Mol. Plant Breed. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Dereeper, A.; Audic, S.; Claverie, J.M.; Blanc, G. Blast-explorer helps you build datasets for phylogenetic analysis. BMC Evol. Biol. 2010, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Cao, C.; Yang, H.; Wang, J.; Wei, W.; Zhu, D.; Gao, P.; Zhao, Y. Molecular cloning and potential role of DiSOC1s in flowering regulation in Davidia involucrata Baill. Plant Physiol. Biochem. 2020, 157, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Klocko, A.L.; Brunner, A.M.; Ma, C.; Magnuson, A.C.; Howe, G.T.; An, X.; Strauss, S.H. RNA Interference suppression of AGAMOUS and SEEDSTICK alters floral organ identity and impairs floral organ determinacy, ovule differentiation, and seed hair development in Populus. New Phytol. 2019, 222, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Zhenye, Q.; Huimin, D.; Lulu, X.; Gaofeng, L.; Guohong, W.; Feng, Z. Texture Analysis of Steaming and Baking Potato Tuber. J. Nucl. Agric. Sci. 2023, 37, 981–989. [Google Scholar] [CrossRef]
- Zhu, X.; Duan, H.; Zhang, G.; Jin, H.; Xu, C.; Chen, S.; Zhou, C.; Chen, Z.; Tang, J.; Zhang, Y. StMAPK1 functions as a thermos-tolerant gene in regulating heat stress tolerance in potato (Solanum tuberosum). Front. Plant Sci. 2023, 14, 1218962. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photo-oxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, D.; Charfeddine, M.; Jbir, R.; Saidi, M.N.; Pirrello, J.; Charfeddine, S.; Bouzayen, M.; Gargouri-Bouzid, R. Identification and functional characterization of ten AP2/ERF genes in potatoes. Plant Cell Tissue Organ. Cult. 2015, 123, 155–172. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro, in Methods in Enzymology; Packer, L., Ed.; Academic Press: San Diego, CA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. In Methods of Biochemical Analysis; Glick, D., Ed.; Interscience Publishers: New York, NY, USA, 1954; Volume 1, pp. 357–424. [Google Scholar] [CrossRef]
- Zhu, X.; Hong, X.; Liu, X.; Li, S.; Yang, J.; Wang, F.; Yue, Y.; Zhang, N.; Si, H. Calcium-dependent protein kinase 32 gene maintains photosynthesis and tolerance of potato in response to salt stress. Sci. Hortic. 2021, 285, 110179. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Majeed, Y.; Wang, K.; Duan, X.; Guan, N.; Luo, J.; Zheng, H.; Zou, H.; Jin, H.; Chen, Z.; et al. StMAPKK1 Enhances Thermotolerance in Potato (Solanum tuberosum L.) by Enhancing Antioxidant Defense and Photosynthetic Efficiency Under Heat Stress. Plants 2025, 14, 2289. https://doi.org/10.3390/plants14152289
Zhu X, Majeed Y, Wang K, Duan X, Guan N, Luo J, Zheng H, Zou H, Jin H, Chen Z, et al. StMAPKK1 Enhances Thermotolerance in Potato (Solanum tuberosum L.) by Enhancing Antioxidant Defense and Photosynthetic Efficiency Under Heat Stress. Plants. 2025; 14(15):2289. https://doi.org/10.3390/plants14152289
Chicago/Turabian StyleZhu, Xi, Yasir Majeed, Kaitong Wang, Xiaoqin Duan, Nengkang Guan, Junfu Luo, Haifei Zheng, Huafen Zou, Hui Jin, Zhuo Chen, and et al. 2025. "StMAPKK1 Enhances Thermotolerance in Potato (Solanum tuberosum L.) by Enhancing Antioxidant Defense and Photosynthetic Efficiency Under Heat Stress" Plants 14, no. 15: 2289. https://doi.org/10.3390/plants14152289
APA StyleZhu, X., Majeed, Y., Wang, K., Duan, X., Guan, N., Luo, J., Zheng, H., Zou, H., Jin, H., Chen, Z., & Zhang, Y. (2025). StMAPKK1 Enhances Thermotolerance in Potato (Solanum tuberosum L.) by Enhancing Antioxidant Defense and Photosynthetic Efficiency Under Heat Stress. Plants, 14(15), 2289. https://doi.org/10.3390/plants14152289





