Abstract
Due to the limited scientific exploration of Argania spinosa (L.) skeel husk, this study presents the first investigation of the metabolite profile of methanol and acetone extracts analyzed by liquid chromatography coupled with electrospray ionization and high-resolution multistage mass spectrometry (LC-ESI/HRMSMS). A total of 43 compounds, including hydroxycinnamic acid and flavonoid derivatives, saponins, and triterpenic acids, were identified, some of which have not been previously reported in this species. The total phenols (TPC) and flavonoids (TFC) content were spectrophotometrically determined. A multi-target approach was applied to investigate the antioxidant potential using 1,1-Diphenyl-2-picrylhydrazyl (DPPH), 2,2-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS), β-carotene bleaching, and Ferric Reducing Ability Power (FRAP) tests. Carbohydrate hydrolyzing enzymes and lipase inhibitory activities were also assessed. The acetone extract exhibited the highest TPC and TFC values, resulting in being the most active in β-carotene bleaching test with IC50 values of 26.68 and 13.82 µg/mL, after 30 and 60 min of incubation, respectively. Moreover, it was the most active against both α-glucosidase and α-amylase enzymes with IC50 values of 12.37 and 18.93 µg/mL, respectively. These results pointed out that this by-product is a rich source of bioactive phytochemicals potentially useful for prevention of type 2 diabetes and obesity.
1. Introduction
Argan tree is a woody species belonging to the Sapotaceae family and represents a tree endemic exclusively to southwestern Morocco, where it forms a unique ecosystem. In this environment, it plays a crucial role in maintaining biodiversity balance as well as supporting the socioeconomic livelihoods of local communities [1].
Several ethnobotanical and ethnopharmacological studies have documented the traditional use of various parts of the plant, highlighting its wide range of uses and applications [2,3]. Local populations use the fruits, seeds, pulp, leaves, bark, wood, and roots of A. spinosa to treat pathological conditions such as diabetes, rheumatism, eczema, burns, hypercholesterolemia, gastritis, ulcers, dysentery, headache, fever, and various dermatological and hair-related disorders [4,5].
The dried seeds are applied externally in powder form to treat eczema, sprains, skin lesions, and burns [3,6], as well as for hair care [7].
This species has increasingly attracted international commercial interest due to the oil extracted from its seeds—known as argan oil—the valorization of which has helped reactivate productive and employment dynamics in rural areas of Morocco. Argan oil is characterized by a lipid profile rich in unsaturated fatty acids, tocopherols, and phytosterols, which make it suitable for diverse applications. Traditionally, it forms part of the Moroccan diet and is appreciated for its nutraceutical properties and distinctive nutty flavor [8]. Its cosmetic applications include skin hydration and the treatment of scars and acne [9].
The nutritional, antioxidant, and dermo cosmetic properties of argan oil, together with its growing presence in international markets, have determined the need for in-depth studies concerning the ripening stage of the fruit, harvesting methods, storage conditions, and extraction processes, all of which significantly affect its quality [10]. In recent years, mechanized extraction processes have been developed, enabling the production of microbiologically safe, stable, and organoleptically acceptable oil [1].
Regarding the by-products of argan processing, argan press cake—the solid residue obtained after seed pressing—is sometimes used as a livestock feed supplement, often combined with the plant’s leaves. The fruit pulp is used in leather tanning and, in some local traditions, also in topical applications [1,5].
Research focused on the use of the Argania spinosa husk—the part commonly referred to as the shell or outer covering of the seed—remains limited or superficial. This component is often overlooked in the argan oil production chain. However, recent studies on other nut species suggest potential applications of this by-product in various fields [11], starting with agronomic uses as a soil amendment. It can significantly contribute to improved water retention capacity and soil fertility. Furthermore, it supports the incorporation of organic matter, enhancing beneficial microbial activity essential for soil and crop health.
The presence of phenolic and antioxidant compounds in the husk is still not well documented in argan, unlike in other nut skins [11]. Isolating and characterizing such compounds could pave the way for new applications in the nutraceutical sector as functional ingredients in dietary supplements or fortified foods [11]. The husk may also serve as an ingredient in dermocosmetic products and promote cellular regeneration.
These applications provide value to an otherwise discarded by-product, contributing to the sustainability of the argan value chain and fostering the development of necessary circular economy policies. In this way, the valorization of the argan husk not only reduces waste in the production chain but may also support the development of innovative and sustainable products, and, in particular, antioxidant products. Indeed, several phenolic compounds, extracted from waste, have been demonstrated to possess significant antioxidant potential [12,13]. Some of these compounds have been identified in Argania spinosa leaves, highlighting their capacity as antidiabetic and antioxidant agents [14,15].
Based on the limitation of scientific data on Argania spinosa husk, the aim of this study was to investigate the metabolite profile of methanol (CH3OH) and acetone (CH3)2CO extracts using ultra-high-performance liquid chromatography coupled to hybrid quadrupole-Orbitrap mass spectrometer, using negative electrospray ionization mode, in tandem mass spectrometry mode [UHPLC-(−)ESI/Q Exactive MS/MS] as well as the in vitro biological potential of this by-product as antioxidant and antidiabetic agent.
2. Results and Discussion
2.1. Chemical Composition of Argania spinosa Husk Extracts
Previous studies have highlighted that the A. spinosa kernel is rich in phenolics, flavonoids, and saponins [16,17,18,19,20]. The CH3OH and (CH3)2CO extracts of A. spinosa husk have been analyzed using UHPLC-(−)ESI/Q Exactive MS/MS. By carefully examining accurate mass measurements, fragmentation patterns, and cross-referencing with literature data, 43 metabolites have been identified. These were categorized into different chemical classes, including hydroxycinnamic acid derivatives (2, 3, 6, 7), flavanol (1, 4, 5, 8, 9) and flavonol (10–14, 19, 21, 30) derivatives, saponins (15–18, 20, 22–29), and triterpenic acids (31–43) (Figure 1 and Table 1).
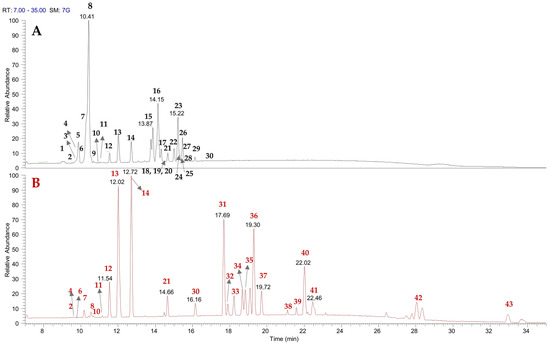
Figure 1.
LC-ESI/HRMSMS profiles of A. spinosa husk methanol (A) and acetone extracts (B).

Table 1.
Metabolites identified in A. spinosa husk extracts by UHPLC-(−)ESI/Q Exactive MS/MS analysis.
Careful analysis of the LC-HRMS spectra suggested the presence of the flavanols catechin (4) and epicatechin (8), as confirmed by comparison with standards, along with their procyanidin derivatives—procyanidin B1 (1), procyanidin B2 (5), and procyanidin C1 (9)—as supported by key product ions in the LC-HRMS/MS spectra and by literature data. Indeed, these compounds have been previously reported in A. spinosa fruits [16]. Furthermore, the analysis of flavonoid derivatives enabled the identification of flavonol derivatives and revealed the presence of one or more sugar moieties linked to the aglycone. In detail, compounds 10–14, and 19 displayed characteristic fragmentation patterns, with base peaks indicating the neutral loss of sugar units such as hexose (162 Da), deoxyhexose (146 Da), or pentose (132 Da). In addition, the above-mentioned compounds exhibited a base peak at m/z 301, corresponding to the molecular formula C15H9O7, consistent with [quercetin-H]−. Compound 30 was unequivocally identified as the aglycone quercetin using the standard. Even if the occurrence of compounds 1, 4, 5, 8–14, 19, 21, and 30 has been documented in A. spinosa fruit, this is the first report of their presence in the husk [16,17].
Peaks 2, 3, 6, and 7 displayed MS/MS fragmentation patterns typical of hydroxycinnamic acid derivatives. They exhibited a neutral loss of 180 Da, corresponding to a hexose moiety, indicating the presence of glycosylated derivatives, along with product ions characteristic of hydroxycinnamic acid linked to hexose. Specifically, the product ions at m/z 145.0283, 161.0340, 175.0390, and 205.0497 corresponded to the mono-dehydrated forms of coumaroyl, caffeoyl, feruloyl, and sinapoyl units in compounds 2, 3, 6, and 7, respectively [21].
LC-HRMS/MS analysis of CH3OH extract enabled the identification of compounds 15–18, 20, and 22–29 as saponins. Fragmentation data from these saponins provided insights into the type of aglycone and the number of sugar units. The majority of saponins revealed in the methanol extract of A. spinosa were derived from protobassic acid (compounds 22, 23, 24, 26, 28, and 29) or 16α-hydroxyprotobassic acid (compounds 15–18, and 20). Minor saponins derived from other sapogenins, such as bayogenin (compounds 25 and 27), were also detected. All mentioned saponins—except compound 20—have been previously identified in A. spinosa kernel [22,23]. However, only compounds 15, 22, and 26 have been previously reported in the husk of argan fruit [24]. Consequently, compounds 16–18, 20, 23–25, and 27–29 are reported here for the first time in A. spinosa husk.
LC-HRMS/MS allowed the assignment of compounds 31–43, detected only in (CH3)2CO extract, as triterpenic acids. In particular, compounds 32, 34, and 40 were identified as the aglycones of arganines, 16-α-hydroxyprotobassic acid, protobassic acid, and bayogenin, respectively; while compounds 37 and 41 were assigned as protobassic acid and bayogenin isomers, respectively. The base peaks and LC-HRMS/MS spectra of compounds 31, 33, 35, 36, 38, and 39 allowed their tentative assignment as triterpenic acids chemically related to the aforementioned aglycones, with slight modifications. Compounds 31–41 have never been reported in A. spinosa. Notably, the presence of zanhic acid (31) and medicagenic acid (33), typical aglycones of Medicago sativa saponins [22], is here reported for the first time in A. spinosa. Finally, compounds 42 and 43 were identified as maslinic and oleanolic acid, respectively, which have been previously reported in A. spinosa fruits [18], but never in argan husk.
Among the analyzed extracts, flavonoids and most hydroxycinnamic acid derivatives were present in both extracts; saponins were mainly found in the CH3OH extract, and triterpenic acids were detected exclusively in the (CH3)2CO extract, reflecting the polarity of the solvents used for extraction.
2.2. Antioxidant Activity of Argania spinosa Husk Extracts
Determination of Total Phenols Content (TPC) and Total Flavonoids Content (TFC) revealed that (CH3)2CO extract exhibited the highest bioactive content with values of 20.42 mg CAE/g dry extract and 12.7 mg QE/g dry extract, respectively, whereas values of 7.62 mg CAE/g dry extract and 1.78 mg QE/g dry extract were detected for CH3OH extract.
The analysis of antioxidant potential was carried out using a multi-target approach. Samples exhibited antioxidant activity in a concentration-dependent manner. Methanol extract resulted in the most active in both radical scavenging activity test with IC50 values of 198.34 and 302.23 µg/mL for DPPH and ABTS test, respectively, (Table 2, Figures S1a and S2a).

Table 2.
Antioxidant potential of Argania spinosa (L.) husk CH3OH and (CH3)2CO extracts.
The same sample exhibited a promising FRAP activity with a value comparable to that of the positive control BHT (54.88 vs. 63.44 μM Fe (II)/g). The β-carotene bleaching assay investigates the ability of the antioxidants to inhibit lipid peroxidation in the phase of initiation as well as in the phase of propagation [25]. As shown in Figure S1a and b, both extracts exhibited a promising protective effect in a concentration-dependent manner.
Previously, El Monfalouti et al. [24] investigated the content of bioactive compounds and antioxidant potential of shell and kernels from argan. Authors found a TPC of 8.2 mg GAE/g in kernels. These samples exhibited ABTS radical scavenging potential. More recently, Mirpoor et al. [25] confirmed the antioxidant effect of argan seed oil cakes. Methanolic extracts from seed and kernel obtained from A. spinosa from Morocco were investigated for their total phytochemical content and bioactivity. TPC values of 49.36 and 207.52 mg GAE/g extract for seeds and kernels, respectively, were found, whereas values of 18.41 and 103.43 mg QE/g extract were detected in the same samples for TFC. Notably, no DPPH or ABTS scavenging effect was found in argan seed, whereas kernels exhibited a promising radical scavenging potential with IC50 values of 11.69 and 62.51 µg/mL for DPPH and ABTS, respectively, [26]. On the contrary, pulp methanol extract and its n-butanol fraction exhibited a promising radical scavenging effect with EC50 values of 9.73 and 5.35 μg/mL for DPPH assay, and 0.338 and 0.271 μg/mL for ABTS test, respectively. The same study evidenced FRAP value is in the same range of potency of our sample [27].
Antioxidant activity may arise from a number of properties exhibited by major compounds such as catechin, sinapoyl-O-glucoside, quercetin-glycosides, bayogenin, and procyanidin isomer. Flavonoids such as catechin and quercetin derivatives have shown important protective activities, demonstrating the enhanced antioxidant effect in ethanol-treated rats and in H2O2-treated liver cells. Quercetin and catechin cooperatively inhibited IKKα/p53 pathway and activated the Nrf2 signaling pathway. IKKα was a critical negative regulator in their joint action [28]; sinapoyl-O-glucoside, a derivative of the sinapic acid, identified in various Brassica species, acted as an antioxidant by scavenging free radicals and reducing oxidative stress, including protection against oxidative stress-related diseases [29]; bayogenin isomers have instead demonstrated very important activities: in addition to the antioxidant activity [30], they have shown antimicrobial activity against bacterial and fungal strains Bacillus subtilis, Escherichia coli, Mucor miehei, and Candida albicans [30], and in vitro hepatoprotective effects against CCl4 induced toxicity [31].
2.3. Hypoglycaemic and Hypolipidemic Effect of Argania spinosa Husk Extracts
The extracts obtained from argan skin by-products were also assessed against enzymes involved in carbohydrate and fat digestion. All extracts exhibited enzyme inhibitory effects in a concentration-dependent manner. Acetone extract resulted in being the most active against both α-glucosidase and α-amylase enzymes with IC50 values of 12.37 and 18.93 µg/mL, respectively. Both values are lower than the positive control acarbose (Table 3, Figure S2a,b).

Table 3.
Hypoglycaemic and hypolipidemic effect of Argania spinosa (L.) husk CH3OH and (CH3)2CO extracts.
Methanol extract exhibited the highest lipase inhibitory activity with an IC50 value of 19.21 µg/mL. Previously, Daoudi et al. [32] investigated the effect of unroasted (UNR)/roasted(R) A. spinose seeds oil against carbohydrate-hydrolyzing enzymes (α-glucosidase and α-amylase). The results evidenced that both samples inhibited α-glucosidase with IC50 values of 0.081 and 0.117 mg/mL, for UNR and R samples, respectively. A similar effect was observed, also, α-amylase with IC50 values of 5.87 and 23.98 mg/mL for UNR and R samples, respectively. In vivo studies evidenced that the oral intake of both oils (2 mL/Kg) significantly attenuated the hyperglycemia induced by the sucrose and the starch in the normal and STZ-diabetic rats.
In agreement with these results, Kamal et al. [33] demonstrated that saponin-rich extract obtained from argan cake by-products was able to inhibit carbohydrate-hydrolysis enzymes with IC50 values of 209.10 and 0.89 mg/mL against α-amylase and α-glucosidase, respectively. Moreover, the extract significantly reduced blood glucose concentration in diabetic mice with activity comparable to metformin. The effect of Argan fruit methanol extract against α-glucosidase (EC50 values of 0.15 vs. 0.23 mg/mL) was also demonstrated [26].
3. Materials and Methods
3.1. Plants Material
Argania spinosa (L.) Skeels fruits were collected near Ounagha, Morocco, 31°31′39″ N, 9°33′41″ O 242 m s/l. in May 2023 (Figure 2). Authentication was performed by Prof. Vincenzo Ilardi, and a voucher specimen has been deposited in the University of Palermo (reference number PAL109781).

Figure 2.
Argania spinosa (L.) Skeel fruit. Photo by Prof. Vincenzo Ilardi.
3.2. Extraction of Plant Material
Fresh fruits of Argania spinosa (1.2 kg) were manually peeled, and the husk (420 g) was manually removed (Figure 3), freeze-dried to give 225 g of dry material, and extracted successively with n-hexane (Merck, Milan, Italy), for one week (800 mL × 3-times), acetone (Merck, Milan, Italy) (800 mL × 3-times), and methanol (Merck, Milan, Italy) (800 mL × 3-times). The resulting solutions were evaporated to give 3.8 and 2.2 g for the acetone and methanol extracts, respectively.

Figure 3.
Argania spinosa (L.) husks. Photo by Dr. Natale Badalamenti.
3.3. LC-ESI/HRMS/MS Analysis
Qualitative UHPLC-(−)ESI/Q Exactive MS/MS analysis was conducted using an ultra-high-performance liquid chromatography system (UltiMate 3000 UHPLC, Dionex, Sunnyvale, CA, USA) coupled to an electrospray ionization source and a high-resolution mass spectrometer (Q Exactive Hybrid Quadrupole-Orbitrap, Thermo Fisher Scientific, Waltham, MA, USA). Chromatographic separations were performed on a Luna 5 µm C18(2) 100 Å column (150 mm × 2.1 mm; Phenomenex, Aschaffenburg, Germany) at a flow rate of 0.2 mL/min. A binary solvent system was employed, consisting of eluent A (water with 0.1% formic acid, Merck, Milan, Italy) and eluent B (acetonitrile with 0.1% formic acid, Merck, Milan, Italy). The column temperature was maintained at 30 °C throughout the separation (45 min) and re-equilibration (10 min) phases. The gradient elution program included a linear increase from 5% to 95% B over 30 min, held at 95% B for 5 min, followed by a return to 5% B over 5 min. The autosampler was set to inject 5 µL of the extracts (1.0 mg/mL), with each sample analyzed in triplicate. In negative ion mode, the electrospray ionization (ESI) source parameters were set as follows: sheath gas at 50 arbitrary units, auxiliary gas at 10 arbitrary units, and capillary temperature at 300 °C. The mass spectrometer operated over a mass range of m/z 120–1600, with a resolution of 70,000 and an automatic gain control (AGC) target of 3 × 106 [21]. Data-dependent acquisition (DDA), with a resolution of 17,500, was employed. MS/MS spectra were acquired for the five most intense ions from the high-resolution MS scan, using a normalized collision energy of 30%, a minimum signal threshold of 3 × 104, and an isolation window of 2.0 m/z [21].
3.4. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
The Total Phenolic Content (TPC) was assessed using the Folin–Ciocalteu method as previously described [12]. Briefly, extract was mixed with Folin–Ciocalteu reagent, sodium carbonate, and distilled water. After 2 h of incubation at room temperature, the absorbance was read at 765 nm by using the UV-Vis Jenway 6003 spectrophotometer (Nottingham, UK). Results were reported as mg of chlorogenic acid equivalent (CAE) equivalents/g dry extract.
For Total Flavonoid Content (TFC), the extract was mixed with sodium nitrite and distilled water. After incubation for 5 min at room temperature, aluminum chloride was added. After 5 min, sodium hydroxide 1 M and water were added. The absorbance was read at 510 nm by using the UV-Vis Jenway 6003 spectrophotometer (Nottingham, UK). Results were reported as mg quercetin equivalents (QE)/g dry extract [12].
3.5. Antioxidant Activity
3.5.1. Radical Scavenging Potential
The radical scavenging activity was assessed by 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) (ABTS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) tests.
For the ABTS test, the procedure previously reported was performed [12]. In this test the ABTS·+ radical cation solution with an absorbance value of 0.70 ± 0.03 nm at 734 nm was added to the extract (25 µL) at different concentrations (5–400 µg/mL) and left to react for 6 min at 25 °C. After that, the absorbance was read at 734 nm by using the UV-Vis Jenway 6003 spectrophotometer (Nottingham, UK).
In the DPPH test, extracts at different concentrations (62.5–1000 µg/mL) were mixed with DPPH solution (1.0 × 10−4 M), and after 30 min, the absorbance was read at 517 nm by using the UV-Vis Jenway 6003 spectrophotometer (Nottingham, UK) [12].
Ascorbic acid was used as positive control in both tests. Results are expressed as the concentration of extract at which the neutralization of free radicals is 50% (IC50) values (µg/mL).
3.5.2. β-Carotene Bleaching Test
For the β-carotene bleaching test, a mixture of β-carotene, linoleic acid, and Tween 20 was prepared. Extracts at different concentrations (2.5–100 µg/mL) were mixed with emulsion and left to react for 30 and 60 min. The absorbance was read at 470 nm by using the UV-Vis Jenway 6003 spectrophotometer (Nottingham, UK) against a blank [12]. Propyl gallate was the positive control. Results are reported as IC50 (µg/mL).
3.5.3. FRAP Test
In the Ferric Reducing Ability Power (FRAP) test, tripyridyltriazine (TPTZ) reagent, FeCl3, HCl, and acetate buffer were mixed to obtain the FRAP solution. Then the extract (2.5 mg/mL) was added to the diluted FRAP solution. After 30 min of incubation at 25 °C, the absorbance was measured at 595 nm by using the UV-Vis Jenway 6003 spectrophotometer (Nottingham, UK) [12]. Butylated hydroxytoluene (BHT) was used as a positive control. The FRAP value was expressed as µM Fe (II)/g.
3.6. Carbohydrate Hydrolyzing Enzymes Inhibitory Activities
The α-amylase and α-glucosidase inhibitory activity was assessed as previously reported [12]. In the α-amylase inhibitory test, the enzyme (EC 3.2.1.1), starch, and colorimetric reagent (CRS) were mixed. After that, samples at different concentrations were added and left to react with the enzyme at room temperature for 5 min. The absorbance was read at 540 nm by using the UV-Vis Jenway 6003 spectrophotometer (Nottingham, UK).
In the α-glucosidase inhibitory test, maltose solution, α-glucosidase (EC 3.2.1.20), O-dianisidine (DIAN), and peroxidase/glucose oxidase (PGO) system-color reagent solution were prepared. Samples, at different concentrations, were mixed with maltose solution and enzyme solution. The resulting solution was left to react at 37 °C for half an hour min. To arrest the reaction, perchloric acid solution was added, and after centrifugation for 10 min at 3000× g, the supernatant was collected and mixed with DIAN and PGO and left to incubate at 37 °C for half an hour. The absorbance was read at λ = 492 nm by using the UV-Vis Jenway 6003 spectrophotometer (Nottingham, UK). Acarbose was used as a positive control in both tests.
3.7. Lipase Inhibitory Activity Test
The porcine pancreatic lipase inhibitory activity was determined following the procedure previously described [12]. Briefly, a solution of 4-nitrophenyl octanoate (NPC) in dimethyl sulfoxide (DMSO), lipase enzyme (EC 3.1.1.3), and Tris-HCl buffer (pH 8.5) was prepared. Samples, at different concentrations, were mixed with enzyme, NPC solution, and Tris-HCl buffer and left to react at 37 °C for half hour. After that the absorbance was read at λ = 405 nm by using the UV-Vis Jenway 6003 spectrophotometer (Nottingham, UK). Orlistat was used as a positive control.
3.8. Statistical Analysis
Samples were analyzed in triplicate. Results were expressed as the mean ± standard deviation (S.D.) (n = 3). Prism GraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA, USA) was used to calculate the concentration giving 50% neutralization/inhibition (IC50). Tukey’s test was used to determine any significant difference on chemical parameters among investigated samples.
4. Conclusions
This work offers an in-depth look at the phytochemistry, antioxidant, hypoglycemic, and lipase inhibitory properties of Argania spinosa (L.) husks. Argan oil is known for its medicinal properties, and in recent years, the potential offered by both the leaves and the fruit shells of this tree has been valorized. Little or no scientific research has been conducted on the husk. Forty-three compounds divided into different chemical classes, such as hydroxycinnamic acid derivatives, flavanol derivatives, saponins, and triterpenic acids, were identified.
Among them, several flavonoids and saponins (1, 4, 5, 8–14, 19, 21, 30), previously identified only in the Argan fruits, have been identified here in the husk for the first time. The occurrence of triterpenes like mediagenic acid and zahnic acid has been highlighted here for the first time in A. spinosa.
The husk acetone extract exhibited the highest inhibitory activity against carbohydrate-hydrolyzing enzymes, whereas methanol extract exhibited the most interesting activity against pancreatic lipase.
These results pointed out that this Argan by-product is still a rich source of bioactive phytochemicals with potential use in pharmaceutical, cosmetic, and food industries for the development of agents for the prevention of type 2 diabetes and obesity.
However, further in vivo studies are still necessary to confirm the by-product in vitro activity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14152288/s1, Figure S1. Radical scavenging potential assessed by ABTS (a) and DPPH (b) test by Argania spinosa husk MeOH and acetone extracts; Figure S2. Percentage of inhibition of A. spinosa husk’s extracts in β-carotene bleaching test at (a) 30 min incubation (b) 60 min incubation.
Author Contributions
Conceptualization, N.B., F.S. and M.B.; methodology, N.B. and V.I.; software, N.B., R.P. and M.B.; validation, N.B., R.T., A.C. and M.B.; formal analysis, R.P. and A.C.; resources, N.B. and M.B.; data curation, S.P., R.T. and F.S.; writing—original draft preparation, M.B., S.P. and A.C.; writing—review and editing, M.R.L. and V.I.; supervision, S.P., N.B., M.B. and F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received external funding from the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4—Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union—NextGenerationEU; Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP UNIPA B73C22000790001 for the University of Palermo, CUP UNISA D43C22001260001 for the University of Salerno. Project title “National Biodiversity Future Center—NBFC”. This work was supported by a grant from Progetto Finanziato da Next Generation EU PNRR—Missione 4 “Istruzione e Ricerca”—Componente C2 -investimento 1.1 (PNRR M4.C2.1.1), Fondo per il Programma Nazionale di Ricerca e Progetti di Rilevante Interesse Nazionale (PRIN)—codice P2022CKMPW_002—CUP B53D23025620001.

Data Availability Statement
All data and materials are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hallouch, O.; Ibourki, M.; Bijla, L.; Oubannin, S.; Asbbane, A.; Mazar, A.; Prasad Devkota, K.; Guillaume, D.; Goh, K.W.; Bouyahya, A.; et al. A review on the utilization of the by-products generated during the production of Argan oil. J. Agric. Food Res. 2025, 20, 101770. [Google Scholar] [CrossRef]
- Charrouf, Z.; Guillaume, D. Ethnoeconomical, ethnomedical, and phytochemical study of Argania spinosa (L.) Husk. J. Ethnopharmacol. 1999, 67, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Ouhaddou, H.; Boubaker, H.; Msanda, F.; El Mousadik, A. An ethnobotanical study of medicinal plants of the Agadir Ida Ou Tanane province (southwest Morocco). J. Appl. Biosci. 2014, 84, 7707–7722. [Google Scholar] [CrossRef]
- Barkaoui, M.; Katiri, A.; Boubaker, H.; Msanda, F. Ethnobotanical survey of medicinal plants used in the traditional treatment of diabetes in Chtouka Ait Baha and Tiznit (Western Anti-Atlas), Morocco. J. Ethnopharmacol. 2017, 198, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Moukal, A. L’arganier, Argania spinosa L. (Husk), usage thérapeutique, cosmétique et alimentaire. Phytothérapie 2004, 2, 135–141. [Google Scholar] [CrossRef]
- Abouri, M.; El Mousadik, A.; Msanda, F.; Boubaker, H.; Saadi, B.; Cherifi, K. An ethnobotanical survey of medicinal plants used in the Tata Province, Morocco. Int. J. Med. Plants Res. 2012, 1, 99–123. [Google Scholar]
- El Kabouss, A.; Charrouf, Z.; Faid, M.; Garneau, F.X.; Collin, G. Chemical composition and antimicrobial activity of the leaf essential oil of Argania spinosa L. Husk. J. Essent. Oil Res. 2002, 14, 147–149. [Google Scholar] [CrossRef]
- Boukhobza, M.; Pichon-Prun, N. L’arganier ressource économique et médicinale pour le Maroc. Phytothérapie 1988, 27, 21–26. [Google Scholar]
- Soheir, K.; Sirine, H. Enquête Ethnobotanique sur l’Utilisation Traditionnelle des Cosmétiques Naturels en Algérie. Ph.D. Thesis, Université D’Oran, Oran, Algeria, 2019. [Google Scholar]
- Oubannin, S.; Bijla, L.; Gagour, J.; Hajir, J.; Ait Aabd, N.; Sakar, E.; Salama, M.A.; Gharby, S. A comparative evaluation of proximate composition, elemental profiling and oil physicochemical properties of black cumin (Nigella sativa L.) seeds and argan (Argania spinosa L. Husk) kernels. Chem. Data Collect. 2022, 41, 100920. [Google Scholar] [CrossRef]
- Vuono, L.F.; Sicari, V.; Mincione, A.; Tundis, R.; Pino, R.; Badalamenti, N.; Bruno, M.; Sottile, F.; Piacente, S.; Settanni, L.; et al. Reuse of almond skin to formulate a new gluten- and lactose-free bakery product. Foods 2024, 13, 3796. [Google Scholar] [CrossRef]
- Bottone, A.; Cerulli, A.; Durso, G.; Masullo, M.; Montoro, P.; Napolitano, A.; Piacente, S. Plant Specialized Metabolites in Hazelnut (Corylus avellana) Kernel and Byproducts: An Update on Chemistry, Biological Activity, and Analytical Aspects. Planta Med. 2019, 85, 840–855. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, P.; Wu, C.L.; Chang, H.T.; Chang, S.T. Antioxidant activity of the ethanolic ex-tract from the bark of Chamaecyparis obtusa var. formosana. J. Sci. Food Agric. 2008, 88, 1400–1405. [Google Scholar] [CrossRef]
- Azizi, S.; Dalli, M.; Roubi, M.; Moon, S.; Berrichi, A.; Maleb, A.; Kim, S.H.; Gseyra, N.; Kim, B. Insights on Phytochemistry and Pharmacological Properties of Argania spinosa L. Skeels: A Comprehensive Review. ACS Omega 2024, 9, 36043–36065. [Google Scholar] [CrossRef] [PubMed]
- Joguet, N.; Maugard, T. Characterization and quantification of phenolic compounds of Argania spinosa leaves by HPLC-PDA-ESI-MS analyses and their antioxidant activity. Chem. Nat. Compd. 2013, 48, 1069–1071. [Google Scholar] [CrossRef]
- Khallouki, F.; Haubner, R.; Ricarte, I.; Erben, G.; Klika, K.; Ulrich, C.M.; Owen, R.W. Identification of polyphenolic compounds in the flesh of Argan (Morocco) fruits. Food Chem. 2015, 179, 191–198. [Google Scholar] [CrossRef]
- Khallouki, F.; Voggel, J.; Breuer, A.; Klika, K.D.; Ulrich, C.M.; Owen, R.W. Comparison of the major polyphenols in mature Argan fruits from two regions of Morocco. Food Chem. 2017, 221, 1034–1040. [Google Scholar] [CrossRef]
- Chafchaouni-Moussaoui, I.; Charrouf, Z.; Guillaume, D. Triterpenoids from Argania spinosa: 20 years of research. Nat. Prod. Commun. 2013, 8, 43–46. [Google Scholar] [CrossRef]
- Henry, M.; Kowalczyk, M.; Maldini, M.; Piacente, S.; Stochmal, A.; Oleszek, W. Saponin inventory from Argania spinosa kernel cakes by liquid chromatography and mass spectrometry. Phytochem. Anal. 2013, 24, 616–622. [Google Scholar] [CrossRef]
- Alaoui, A.; Charrouf, Z.; Soufiaoui, M.; Carbone, V.; Malorni, A.; Pizza, C.; Piacente, S. Triterpenoid saponins from the shells of Argania spinosa seeds. J. Agric. Food Chem. 2002, 50, 4600–4603. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Cerulli, A.; Dirsch, V.M.; Heiss, E.H.; Masullo, M.; Piacente, S. LC-MS- and 1H NMR-Based Metabolomics to Highlight the Impact of Extraction Solvents on Chemical Profile and Antioxidant Activity of Daikon Sprouts (Raphanus sativus L.). Antioxidants 2023, 12, 1542. [Google Scholar] [CrossRef]
- Bialy, Z.; Jurzysta, M.; Oleszek, W.; Piacente, S.; Pizza, C. Saponins in alfalfa (Medicago sativa L.) root and their structural elucidation. J. Agric. Food Chem. 1999, 47, 3185–3192. [Google Scholar] [CrossRef]
- Prieto, M.A.; Rodríguez-Amado, I.; Vázquez, J.A.; Murado, M.A. β-Carotene assay revisited. application to characterize and quantify antioxidant and prooxidant activities in a microplate. J. Agric. Food Chem. 2012, 60, 8983–8993. [Google Scholar] [CrossRef]
- El Monfalouti, H.; Charrouf, Z.; Belviso, S.; Ghirardello, D.; Scursatone, B.; Guillaume, D.; Denhez, C.; Zeppa, G. Analysis and antioxidant capacity of the phenolic compounds from argan fruit (Argania spinosa (L.) Skeels). Eur. J. Lipid Sci. Technol. 2012, 114, 446–452. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Giosafatto, C.V.L.; Mariniello, L.; D’Agostino, A.; D’Agostino, M.; Cammarota, M.; Schiraldi, C.; Porta, R. Argan (Argania spinosa L.) Seed Oil Cake as a Potential Source of Protein-Based Film Matrix for Pharmaco-Cosmetic Applications. Int. J. Mol. Sci. 2022, 23, 8478. [Google Scholar] [CrossRef] [PubMed]
- El Idrissi, Y.; El Moudden, H.; Harhar, H.; Zarrouk, A.; Tabyaoui, M. Comparison and correlation of phytochemical content with antioxidant potential of different parts of Argan tree, Argania spinosa L. Casp. J. Environ. Sci. 2020, 19, 261–266. [Google Scholar]
- Alaoui, A.; Sahri, N.; Mahdi, I.; Fahsi, N.; El Herradi, E.H.; Sobeh, M. Argan: Phytochemical pro-filing and evaluation of the antioxidant, hypoglycemic, and antibacterial properties of its fruit pulp extracts. Heliyon 2023, 10, e23612. [Google Scholar] [CrossRef]
- Guan, H.; Zhang, W.; Liu, H.; Jiang, Y.; Li, F.; Wang, D.; Liu, Y.; He, F.; Wu, M.; Ivan Neil Waterhouse, G.; et al. Simultaneous binding of quercetin and catechin to FOXO3 enhances IKKα transcription inhibition and suppression of oxidative stress-induced acute alcoholic liver injury in rats. J. Adv. Res. 2025, 67, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.I.; Wiesenborn, D.P.; Kim, Y.S. Antioxidant activity of phenolic compounds from canola (Brassica napus) seed. Food Sci. Biotechnol. 2014, 23, 1753–1760. [Google Scholar] [CrossRef]
- Wansi, J.D.; Chiozem, D.D.; Tcho, A.T.; Toze, F.A.; Devkota, K.P.; Ndjakou, B.L.; Wandji, J.; Sewald, N. Antimicrobial and antioxidant effects of phenolic constituents from Klainedoxa gabonensis. Pharm. Biol. 2010, 48, 1124–1129. [Google Scholar] [CrossRef]
- Kokanova-Nedialkova, Z.; Nedialkov, P.; Kondeva-Burdina, M.; Simeonova, R. Hepatoprotective activity of a purified methanol extract and saponins from the roots of Chenopodium bonus-henricus L. Z. Naturforsch. C J. Biosci. 2019, 74, 329–337. [Google Scholar] [CrossRef]
- Daoudi, N.E.; Bouhrim, M.; Ouassou, H.; Legssyer, A.; Mekhfi, H.; Ziyyat, A.; Aziz, M.; Bnouham, M. Inhibitory effect of roasted/unroasted Argania spinosa seeds oil on α- glucosidase, α-amylase and intestinal glucose absorption activities. S. Afr. J. Bot. 2020, 135, 413–420. [Google Scholar] [CrossRef]
- Kamal, R.; Kharbach, M.; Heyden, Y.V.; Yu, H.; Bouklouze, A.; Cherrah, Y.; Alaoui, K. In Vitro & In Vivo Anti-Hyperglycemic Potential of Saponins Cake and Argan Oil from Argania spinosa. Foods 2021, 10, 1078. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).