Use of Foliar Biostimulants in Durum Wheat: Understanding Its Potential in Improving Agronomic and Quality Responses Under Mediterranean Field Conditions
Abstract
1. Introduction
2. Results
2.1. Weather Conditions
2.2. Crop Traits
3. Discussion
4. Materials and Methods
4.1. Location and Experimental Design
- (T1), extracts of seaweed Codium fragile (Suringar) Hariot and plant Opuntia ficus-barbarica A. Berger at the dose of 1 kg ha−1. After they were harvested, the algae were thoroughly washed with fresh water and then ground. Mechanical pressure was used to extract the desired compounds from the seaweed biomass. The weight ratio of the algae and plant pressed biomass mixtures was 5:1;
- (T2), micronized vaterite, calcium carbonate (Ca 29%), with particle size lower than 5 µm, at the dose of 2 kg ha−1;
- (T3), culture broth of Pseudomonas protegens (109 CFU g−1, determined by serial dilution method on Petri dish), rich in auxins and cytokinins, at the dose of 1 kg ha−1. Pseudomonas protegens was fermented at room temperature in a continuously stirred vessel with a substrate containing water, molasses, ammonium sulfate and tryptophan;
- (T4), humic and fulvic acid enriched in micronutrients such as iron and zinc at the dose of 1 kg ha−1. The product derived from the extraction of leonardite with KOH. It contained 62% organic matter (DM basis), with 1.1% of organic nitrogen and the following nutrients: P2O5 (238 ppm), SO3 (681 ppm), CaO (939 ppm), Fe (253 ppm), Cu (96 ppm), Mg (78 ppm), Zn (71 ppm), B (71 ppm), Mo (28 ppm) and Mn (25 ppm);
- (T5), organic nitrogen fertilizer (N 5%) derived from sugar-beet processing and containing 35% of glycine betaine, at a dose of 5 kg ha−1. The product also contains 15% C and 1.5% K2O.
4.2. Sampling and Measurements
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A Meta-Analysis of Projected Global Food Demand and Population at Risk of Hunger for the Period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Carmo-Filho, A.d.S.; Ribas, G.G. Intensification and Sustainability of Production Systems—A Bibliometric Analysis. Agronomy 2024, 14, 1968. [Google Scholar] [CrossRef]
- Gao, Y.; Cabrera Serrenho, A. Greenhouse Gas Emissions from Nitrogen Fertilizers Could Be Reduced by up to One-Fifth of Current Levels by 2050 with Combined Interventions. Nat. Food 2023, 4, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Venkata Supraja, K.; Paramasivan, B. Integrated Microalgal Biorefinery for the Production and Application of Biostimulants in Circular Bioeconomy. Bioresour. Technol. 2021, 339, 125588. [Google Scholar] [CrossRef]
- Ulukan, H. Wheat Production Trends and Research Priorities: A Global Perspective. In Advances in Wheat Breeding; Springer Nature: Singapore, 2024; pp. 1–22. [Google Scholar]
- Martínez-Moreno, F.; Ammar, K.; Solís, I. Global Changes in Cultivated Area and Breeding Activities of Durum Wheat from 1800 to Date: A Historical Review. Agronomy 2022, 12, 1135. [Google Scholar] [CrossRef]
- Sellami, M.H.; Di Mola, I.; Ottaiano, L.; Cozzolino, E.; De Vita, P.; Mori, M. Assessing Temporal Variability in Durum Wheat Performance and Stability through Multi-Trait Mean Performance Selection in Mediterranean Climate. Front. Agron. 2024, 6, 1466040. [Google Scholar] [CrossRef]
- Rossini, A.; Ruggeri, R.; Belocchi, A.; Rossini, F. Response of Durum Wheat Cultivars to Climate Change in a Mediterranean Environment: Trends of Weather and Crop Variables at the Turn of 21st Century. J. Agron. Crop Sci. 2024, 210, e12786. [Google Scholar] [CrossRef]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Schütz, L.; Gattinger, A.; Meier, M.; Müller, A.; Boller, T.; Mäder, P.; Mathimaran, N. Improving Crop Yield and Nutrient Use Efficiency via Biofertilization—A Global Meta-Analysis. Front. Plant Sci. 2018, 8, 2204. [Google Scholar] [CrossRef]
- Ngoune Liliane, T.; Shelton Charles, M. Factors Affecting Yield of Crops. In Agronomy—Climate Change and Food Security; IntechOpen: London, UK, 2020. [Google Scholar]
- Bhupenchandra, I.; Chongtham, S.K.; Devi, E.L.; R., R.; Choudhary, A.K.; Salam, M.D.; Sahoo, M.R.; Bhutia, T.L.; Devi, S.H.; Thounaojam, A.S.; et al. Role of Biostimulants in Mitigating the Effects of Climate Change on Crop Performance. Front. Plant Sci. 2022, 13, 967665. [Google Scholar] [CrossRef]
- Najafi Vafa, Z.; Sohrabi, Y.; Mirzaghaderi, G.; Heidari, G. Soil Microorganisms and Seaweed Application With Supplementary Irrigation Improved Physiological Traits and Yield of Two Dryland Wheat Cultivars. Front. Plant Sci. 2022, 13, 855090. [Google Scholar] [CrossRef]
- Szczepanek, M.; Wszelaczyńska, E.; Pobereżny, J. Effect of Seaweed Biostimulant Application in Spring Wheat. AgroLife Sci. J. 2018, 7, 131–136. [Google Scholar]
- Trivedi, K.; Vijay Anand, K.G.; Vaghela, P.; Ghosh, A. Differential Growth, Yield and Biochemical Responses of Maize to the Exogenous Application of Kappaphycusalvarezii Seaweed Extract, at Grain-Filling Stage under Normal and Drought Conditions. Algal Res. 2018, 35, 236–244. [Google Scholar] [CrossRef]
- Alharbi, K.; Amin, M.A.; Ismail, M.A.; Ibrahim, M.T.S.; Hassan, S.E.D.; Fouda, A.; Eid, A.M.; Said, H.A. Alleviate the Drought Stress on Triticum aestivum L. Using the Algal Extracts of Sargassum latifolium and Corallina elongate Versus the Commercial Algal Products. Life 2022, 12, 1757. [Google Scholar] [CrossRef]
- Khalid, M.; Rehman, H.M.; Ahmed, N.; Nawaz, S.; Saleem, F.; Ahmad, S.; Uzair, M.; Rana, I.A.; Atif, R.M.; Zaman, Q.U.; et al. Using Exogenous Melatonin, Glutathione, Proline, and Glycine Betaine Treatments to Combat Abiotic Stresses in Crops. Int. J. Mol. Sci. 2022, 23, 12913. [Google Scholar] [CrossRef]
- Tisarum, R.; Theerawitaya, C.; Samphumphung, T.; Takabe, T.; Cha-Um, S. Exogenous Foliar Application of Glycine Betaine to Alleviate Water Deficit Tolerance in Two Indica Rice Genotypes under Greenhouse Conditions. Agronomy 2019, 9, 138. [Google Scholar] [CrossRef]
- Rashid, I.; Murtaza, G.; Dar, A.A.; Wang, Z. The Influence of Humic and Fulvic Acids on Cd Bioavailability to Wheat Cultivars Grown on Sewage Irrigated Cd-Contaminated Soils. Ecotoxicol. Env. Saf. 2020, 205, 111347. [Google Scholar] [CrossRef]
- Noreen, S.; Ali, B.; Hasnain, S. Growth Promotion of Vigna mungo (L.) by Pseudomonas Spp. Exhibiting Auxin Production and ACC-Deaminase Activity. Ann. Microbiol. 2012, 62, 411–417. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in Plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Sadak, M.S.; Talaat, I.M. Attenuation of Negative Effects of Saline Stress in Wheat Plant by Chitosan and Calcium Carbonate. Bull. Natl. Res. Cent. 2021, 45, 136. [Google Scholar] [CrossRef]
- Rossini, A.; Ruggeri, R.; Rossini, F. Combining Nitrogen Fertilization and Biostimulant Application in Durum Wheat: Effects on Morphophysiological Traits, Grain Production, and Quality. Ital. J. Agron. 2025, 20, 100027. [Google Scholar] [CrossRef]
- Garcia, S.N.; Osburn, B.I.; Jay-Russell, M.T. One Health for Food Safety, Food Security, and Sustainable Food Production. Front. Sustain. Food Syst. 2020, 4, 1. [Google Scholar] [CrossRef]
- Golian, M.; Mezeyová, I.; Andrejiová, A.; Hegedűsová, A.; Adamec, S.; Štefániková, J.; Árvay, J. Effects of Selected Biostimulants on Qualitative and Quantitative Parameters of Nine Cultivars of the Genus Capsicum Spp. Open Agric. 2024, 9, 20220266. [Google Scholar] [CrossRef]
- Mathlouthi, F.; Ruggeri, R.; Rossini, A.; Rossini, F. A New Fertilization Approach for Bread Wheat in the Mediterranean Environment: Effects on Yield and Grain Protein Content. Agronomy 2022, 12, 2152. [Google Scholar] [CrossRef]
- Sobolewska, M.; Wenda-Piesik, A.; Jaroszewska, A.; Stankowski, S. Effect of Habitat and Foliar Fertilization with K, Zn and Mn on Winter Wheat Grain and Baking Qualities. Agronomy 2020, 10, 276. [Google Scholar] [CrossRef]
- Rossini, A.; Ruggeri, R.; Rossini, F. Discriminating among Alternative Dressing Solutions for Cereal Seed Treatment: Effect on Germination and Seedling Vigor of Durum Wheat. Int. J. Plant Biol. 2024, 15, 230–241. [Google Scholar] [CrossRef]
- Rossini, A.; Ruggeri, R.; Mzid, N.; Rossini, F.; Di Miceli, G. Codium fragile (Suringar) Hariot as Biostimulant Agent to Alleviate Salt Stress in Durum Wheat: Preliminary Results from Germination Trials. Plants 2024, 13, 283. [Google Scholar] [CrossRef]
- Spada, M.; Marín-Sanz, M.; Bigini, V.; Quagliata, G.; Coppa, E.; Barro, F.; Savatin, D.; Ruggeri, R.; Sestili, F.; Rossini, F.; et al. Use of Biostimulants for Water Stress Mitigation in Two Durum Wheat (Triticum durum Desf.) Genotypes with Different Drought Tolerance. Plant Stress 2024, 14, 100566. [Google Scholar] [CrossRef]
- Nasiroleslami, E.; Mozafari, H.; Sadeghi-Shoae, M.; Habibi, D.; Sani, B. Changes in Yield, Protein, Minerals, and Fatty Acid Profile of Wheat (Triticum aestivum L.) under Fertilizer Management Involving Application of Nitrogen, Humic Acid, and Seaweed Extract. J. Soil. Sci. Plant Nutr. 2021, 21, 2642–2651. [Google Scholar] [CrossRef]
- Pačuta, V.; Rašovský, M.; Michalska-Klimczak, B.; Wyszyňski, Z. Grain Yield and Quality Traits of Durum Wheat (Triticum durum Desf.) Treated with Seaweed- and Humic Acid-Based Biostimulants. Agronomy 2021, 11, 1270. [Google Scholar] [CrossRef]
- Shafiq, S.; Akram, N.A.; Ashraf, M.; García-Caparrós, P.; Ali, O.M.; Latef, A.A.H.A. Influence of Glycine Betaine (Natural and Synthetic) on Growth, Metabolism and Yield Production of Drought-Stressed Maize (Zeamays L.) Plants. Plants 2021, 10, 2540. [Google Scholar] [CrossRef] [PubMed]
- Alsudays, I.M.; Alshammary, F.H.; Alabdallah, N.M.; Alatawi, A.; Alotaibi, M.M.; Alwutayd, K.M.; Alharbi, M.M.; Alghanem, S.M.S.; Alzuaibr, F.M.; Gharib, H.S.; et al. Applications of Humic and Fulvic Acid under Saline Soil Conditions to Improve Growth and Yield in Barley. BMC Plant Biol. 2024, 24, 191. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Shahbaz, M.; Asadullah; Imran, A.; Marghoob, M.U.; Imtiaz, M.; Mubeen, F. Potential of Salt Tolerant PGPR in Growth and Yield Augmentation of Wheat (Triticum aestivum L.) Under Saline Conditions. Front. Microbiol. 2020, 11, 2019. [Google Scholar] [CrossRef]
- Tarakhovskaya, E.R.; Maslov, Y.I.; Shishova, M.F. Phytohormones in Algae. Russ. J. Plant Physiol. 2007, 54, 163–170. [Google Scholar] [CrossRef]
- Cai, T.; Xu, H.; Peng, D.; Yin, Y.; Yang, W.; Ni, Y.; Chen, X.; Xu, C.; Yang, D.; Cui, Z.; et al. Exogenous Hormonal Application Improves Grain Yield of Wheat by Optimizing Tiller Productivity. Field Crops Res. 2014, 155, 172–183. [Google Scholar] [CrossRef]
- Zarea, M.J. The Regulatory Roles of Phytohormones in the Wheat Grain-Filling Process. J. Plant Growth Regul. 2025, 44, 2609–2626. [Google Scholar] [CrossRef]
- Marzec, M.; Alqudah, A. Key Hormonal Components Regulate Agronomically Important Traits in Barley. Int. J. Mol. Sci. 2018, 19, 795. [Google Scholar] [CrossRef]
- Bakhoum, G.S.; Tawfik, M.M.; Kabesh, M.O.; Sadak, M.S. Potential Role of Algae Extract as a Natural Stimulating for Wheat Production under Reduced Nitrogen Fertilizer Rates and Water Deficit. Biocatal. Agric. Biotechnol. 2023, 51, 102794. [Google Scholar] [CrossRef]
- Fox, A.R.; Soto, G.; Valverde, C.; Russo, D.; Lagares, A.; Zorreguieta, Á.; Alleva, K.; Pascuan, C.; Frare, R.; Mercado-Blanco, J.; et al. Major Cereal Crops Benefit from Biological Nitrogen Fixation When Inoculated with the Nitrogen-fixing Bacterium Pseudomonas protegens Pf-5 X940. Environ. Microbiol. 2016, 18, 3522–3534. [Google Scholar] [CrossRef]
- Bakaeva, M.; Chetverikov, S.; Timergalin, M.; Feoktistova, A.; Rameev, T.; Chetverikova, D.; Kenjieva, A.; Starikov, S.; Sharipov, D.; Hkudaygulov, G. PGP-Bacterium Pseudomonas protegens Improves Bread Wheat Growth and Mitigates Herbicide and Drought Stress. Plants 2022, 11, 3289. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Zhu, M.; Li, Q.; Wang, X.; Wan, J.; Zhang, Y. Glycine Betaine-Mediated Root Priming Improves Water Stress Tolerance in Wheat (Triticum aestivum L.). Agriculture 2021, 11, 1127. [Google Scholar] [CrossRef]
- Mansour, H. Influence of Different Habitats on the Chemical Constituents of Codium tomentosum. Egypt. J. Bot. 2018, 58, 275–285. [Google Scholar] [CrossRef]
- Oukarroum, A.; El Madidi, S.; Strasser, R.J. Exogenous Glycine Betaine and Proline Play a Protective Role in Heat-Stressed Barley Leaves (Hordeum vulgare L.): A Chlorophyll a Fluorescence Study. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2012, 146, 1037–1043. [Google Scholar] [CrossRef]
- Dolatabadian, A.; Sanavy, S.A.M.M.; Gholamhoseini, M.; Joghan, A.K.; Majdi, M.; Kashkooli, A.B. The Role of Calcium in Improving Photosynthesis and Related Physiological and Biochemical Attributes of Spring Wheat Subjected to Simulated Acid Rain. Physiol. Mol. Biol. Plants 2013, 19, 189–198. [Google Scholar] [CrossRef]
- Delfine, S.; Tognetti, R.; Desiderio, E.; Alvino, A. Effect of Foliar Application of N and Humic Acids on Growth and Yield of Durum Wheat. Agron. Sustain. Dev. 2005, 25, 183–191. [Google Scholar] [CrossRef]
- Layek, J.; Das, A.; Ghosh, A.; Sarkar, D.; Idapuganti, R.G.; Boragohain, J.; Yadav, G.S.; Lal, R. Foliar Application of Seaweed Sap Enhances Growth, Yield and Quality of Maize in Eastern Himalayas. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 89, 221–229. [Google Scholar] [CrossRef]
- Graziano, S.; Marmiroli, N.; Visioli, G.; Gullì, M. Proteins and Metabolites as Indicators of Flours Quality and Nutritional Properties of Two Durum Wheat Varieties Grown in Different Italian Locations. Foods 2020, 9, 315. [Google Scholar] [CrossRef]
- Regent Instruments Inc. WinRHIZO 2016 Basic, Reg, Pro & Arabidopsis for Root Measurement User Manual; Regent Instruments Inc.: Québec, QC, Canada, 2016. [Google Scholar]
- Boudiar, R.; Cabeza, A.; Fernández-Calleja, M.; Pérez-Torres, A.; Casas, A.M.; González, J.M.; Mekhlouf, A.; Igartua, E. Root Trait Diversity in Field Grown Durum Wheat and Comparison with Seedlings. Agronomy 2021, 11, 2545. [Google Scholar] [CrossRef]
- Hobson, D.J.; Harty, M.A.; Langton, D.; McDonnell, K.; Tracy, S.R. The Establishment of Winter Wheat Root System Architecture in Field Soils: The Effect of Soil Type on Root Development in a Temperate Climate. Soil. Use Manag. 2023, 39, 198–208. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing. 2021. Available online: https://www.R-project.org/ (accessed on 17 September 2023).

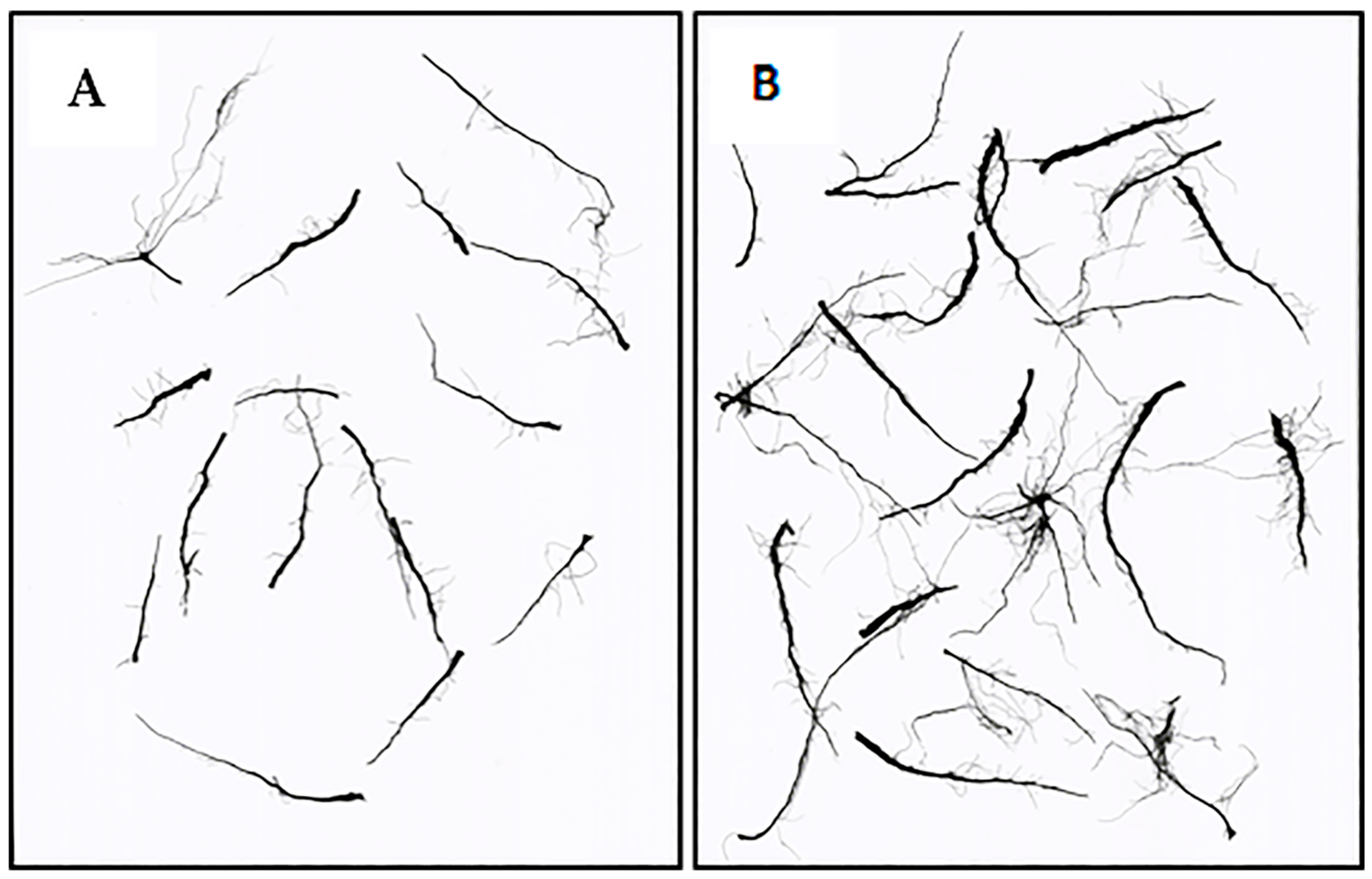

| Biostimulant | |
|---|---|
| Root length | *** |
| Grain yield | ** |
| Number of spikes m−2 | *** |
| Number of kernels spike−1 | ns |
| Thousand kernel weight | ** |
| Grain protein content | *** |
| Test weight | ** |
| T0 | T1 | T2 | T3 | T4 | T5 | |
|---|---|---|---|---|---|---|
| Root length (cm) | 175 ± 52.9 C | 385 ± 52.9 A | 318 ± 52.9 AB | 309 ± 52.9 AB | 249 ± 52.9 BC | 301 ± 52.9 AB |
| Grain yield (t ha−1) | 4.11 ± 0.4 C | 5.26 ± 0.4 A | 5.08 ± 0.4 AB | 4.47 ± 0.4 AC | 4.3 ± 0.4 BC | 5.08 ± 0.4 AC |
| NSM (n. m−2) | 176 ± 14.7 B | 221 ± 14.7 A | 223 ± 14.7 A | 181 ± 14.7 B | 181 ± 14.7 B | 222 ± 14.7 A |
| TKW (g) | 49.7 ± 1.25 B | 55.7 ± 1.25 A | 53.2 ± 1.25 A | 54.4 ± 1.25 A | 54.8 ± 1.25 A | 55.3 ± 1.25 A |
| Protein content (%) | 12.4 ± 0.38 C | 13.3 ± 0.38 A | 12.9 ± 0.38 AB | 12.4 ± 0.38 C | 12.7 ± 0.38 BC | 12.4 ± 0.38 C |
| Test weight (kg hL−1) | 77.6 ± 0.91 B | 78.5 ± 0.91 A | 78.2 ± 0.91 AB | 78.2 ± 0.91 AB | 78.0 ± 0.91 AB | 78.1 ± 0.91 AB |
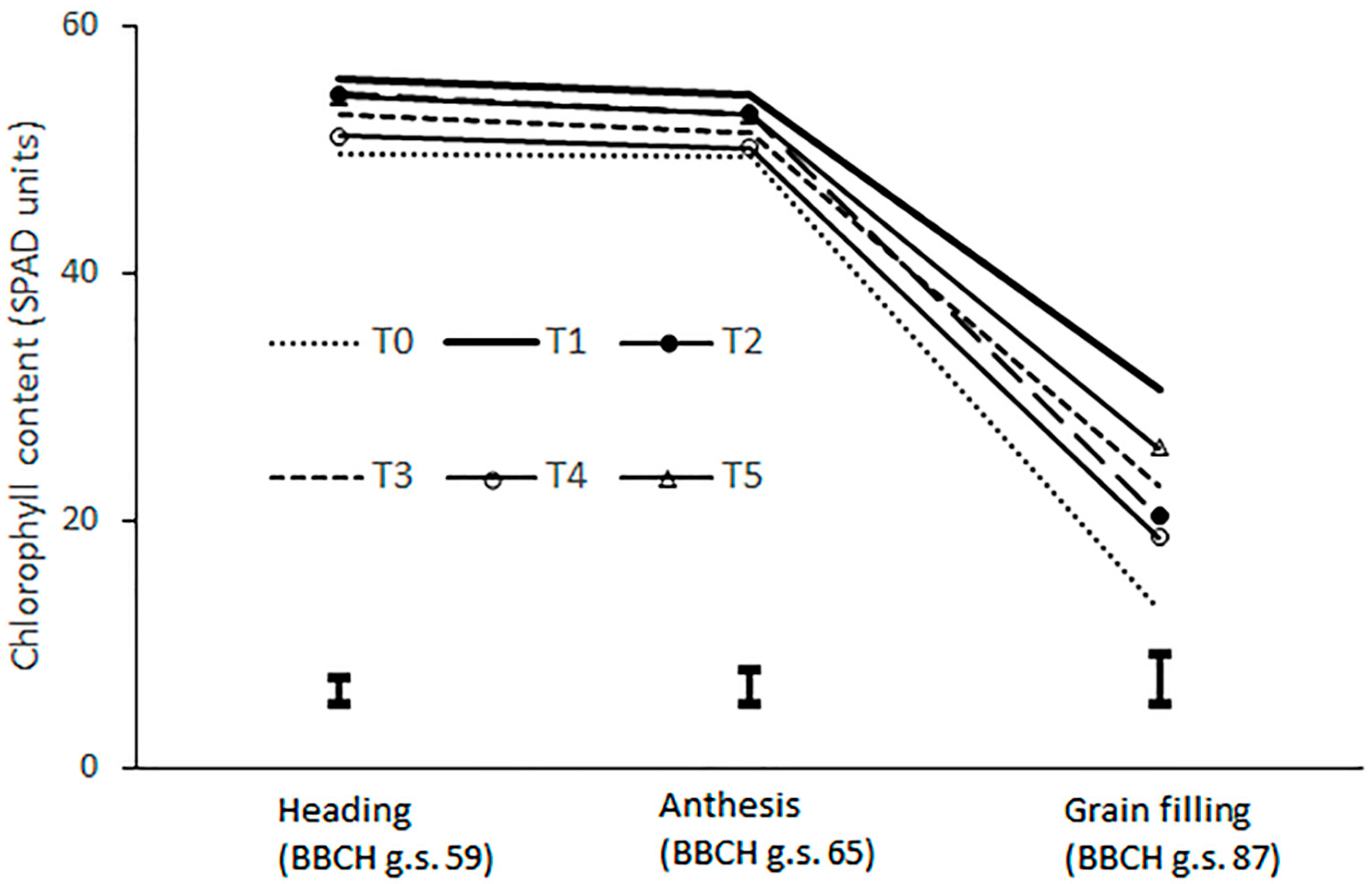
| Heading | Anthesis | Grain Filling | |
|---|---|---|---|
| (BBCH stage 59) | (BBCH stage 65) | (BBCH stage 87) | |
| Biostimulants | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossini, A.; Ruggeri, R.; Rossini, F. Use of Foliar Biostimulants in Durum Wheat: Understanding Its Potential in Improving Agronomic and Quality Responses Under Mediterranean Field Conditions. Plants 2025, 14, 2276. https://doi.org/10.3390/plants14152276
Rossini A, Ruggeri R, Rossini F. Use of Foliar Biostimulants in Durum Wheat: Understanding Its Potential in Improving Agronomic and Quality Responses Under Mediterranean Field Conditions. Plants. 2025; 14(15):2276. https://doi.org/10.3390/plants14152276
Chicago/Turabian StyleRossini, Angelo, Roberto Ruggeri, and Francesco Rossini. 2025. "Use of Foliar Biostimulants in Durum Wheat: Understanding Its Potential in Improving Agronomic and Quality Responses Under Mediterranean Field Conditions" Plants 14, no. 15: 2276. https://doi.org/10.3390/plants14152276
APA StyleRossini, A., Ruggeri, R., & Rossini, F. (2025). Use of Foliar Biostimulants in Durum Wheat: Understanding Its Potential in Improving Agronomic and Quality Responses Under Mediterranean Field Conditions. Plants, 14(15), 2276. https://doi.org/10.3390/plants14152276







