The Molecular Mechanism and Effects of Root Pruning Treatment on Blueberry Tree Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Individual Morphometry
2.3. Determination of Photosynthetic and Chlorophyll Fluorescence Parameters
2.4. RNA Extraction, cDNA Library Construction, and Transcriptome Sequencing
2.5. Functional Analysis of Differentially Expressed Genes
2.6. Identification of Transcription Factors Related to Vascular Development
2.7. Quantitative RT-PCR Analysis
2.8. Data Processing and Statistical Analysis
3. Results
3.1. The Effect of Different Degrees of Root Pruning on Blueberry Plant Growth and Development
3.2. Growth and Development of Blueberry Plants Under CT4 Root Pruning
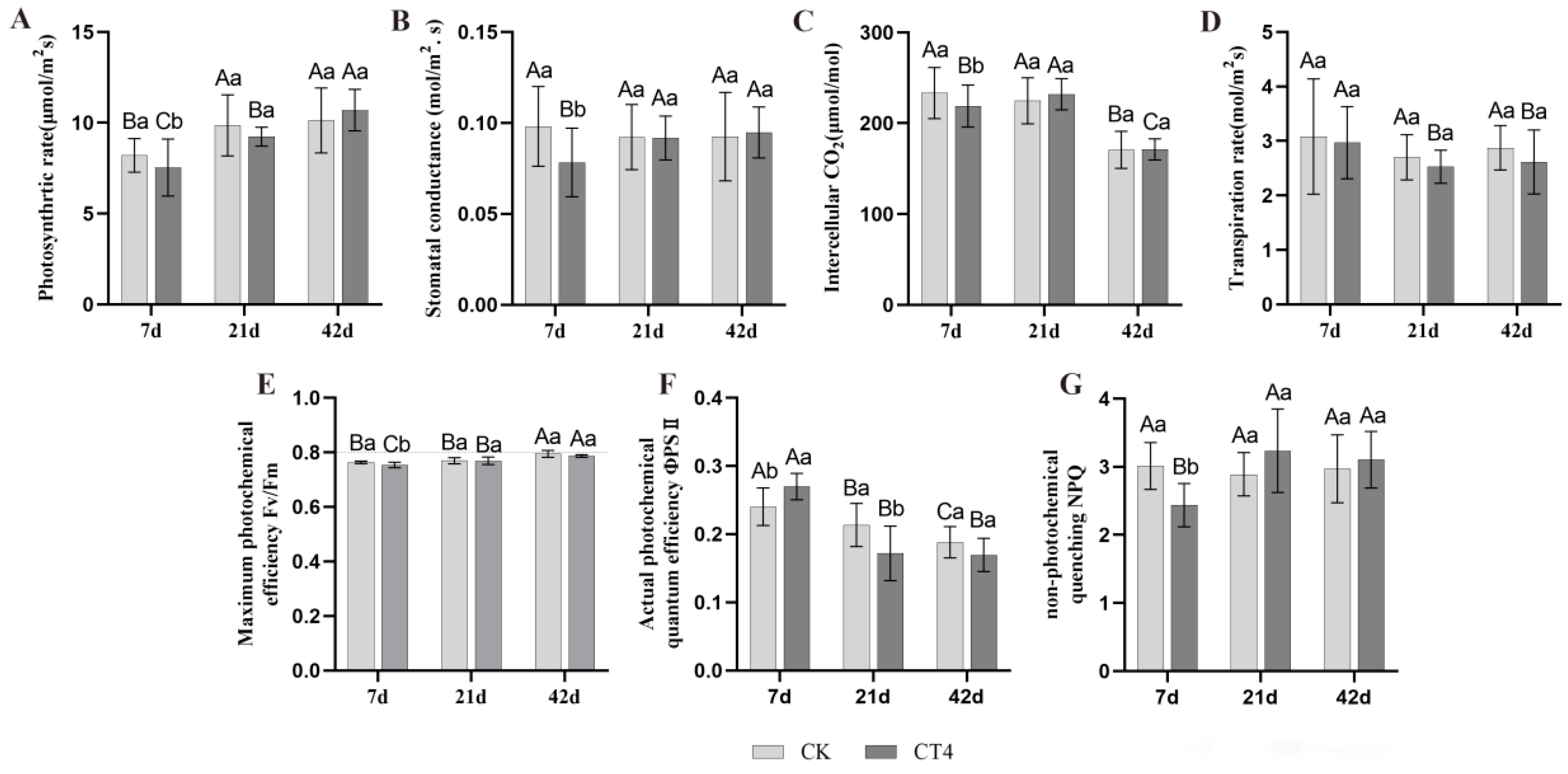
3.3. Dynamic Changes in the Photosynthesis of Blueberry Plants Under CT4 Root Pruning Degree
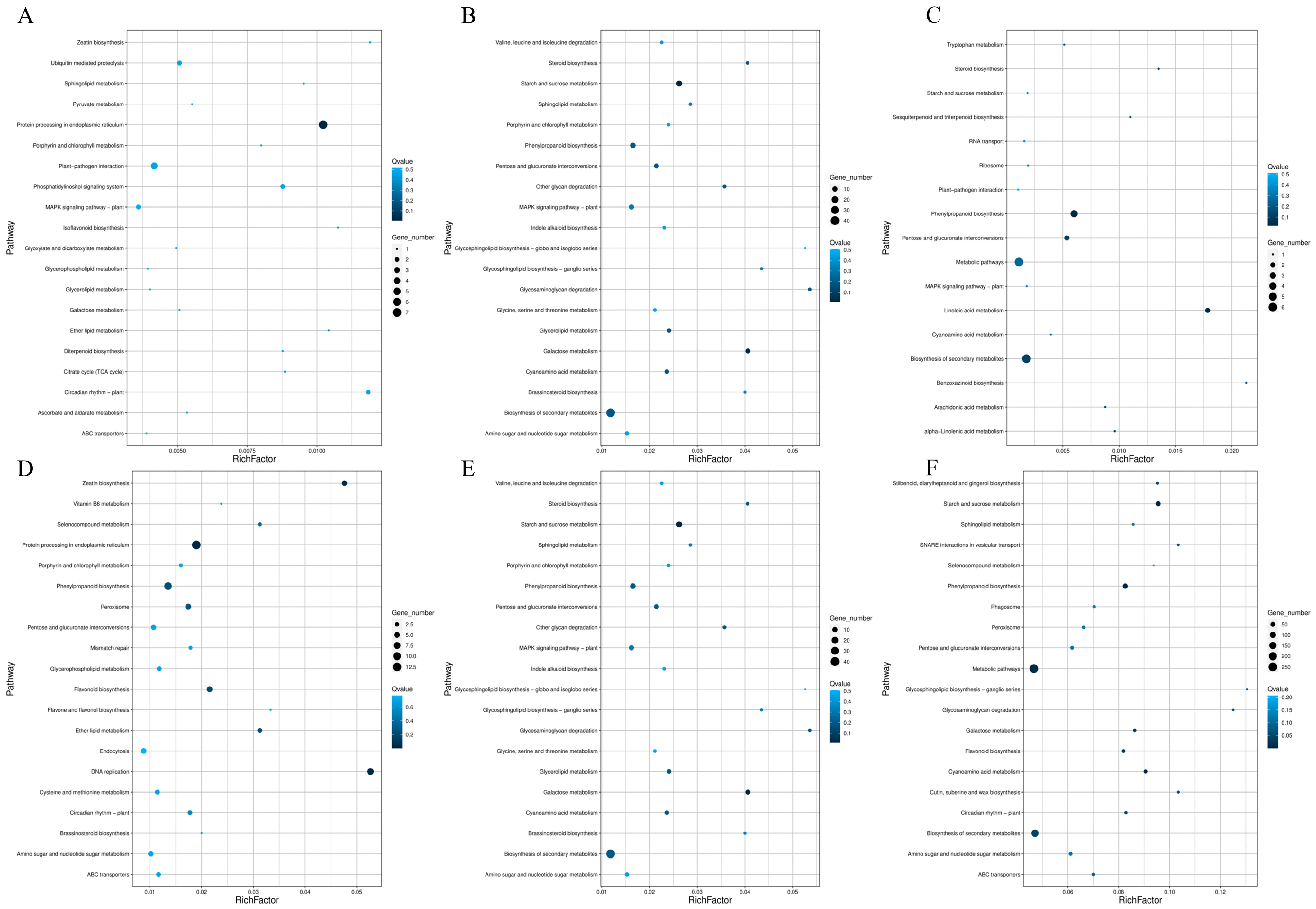
3.4. Transcriptome Sequencing Analysis
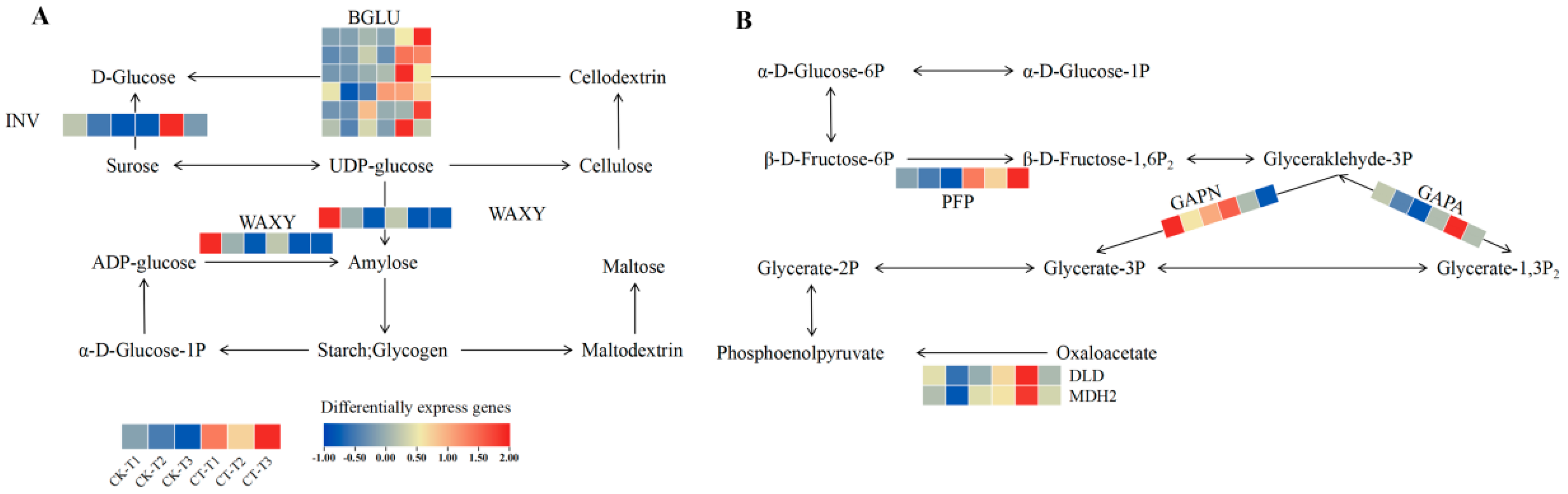
3.5. Effect of Root Pruning on the Expression of STARCH, Sucrose, and Carbon Metabolism Pathway Genes in Blueberry Roots
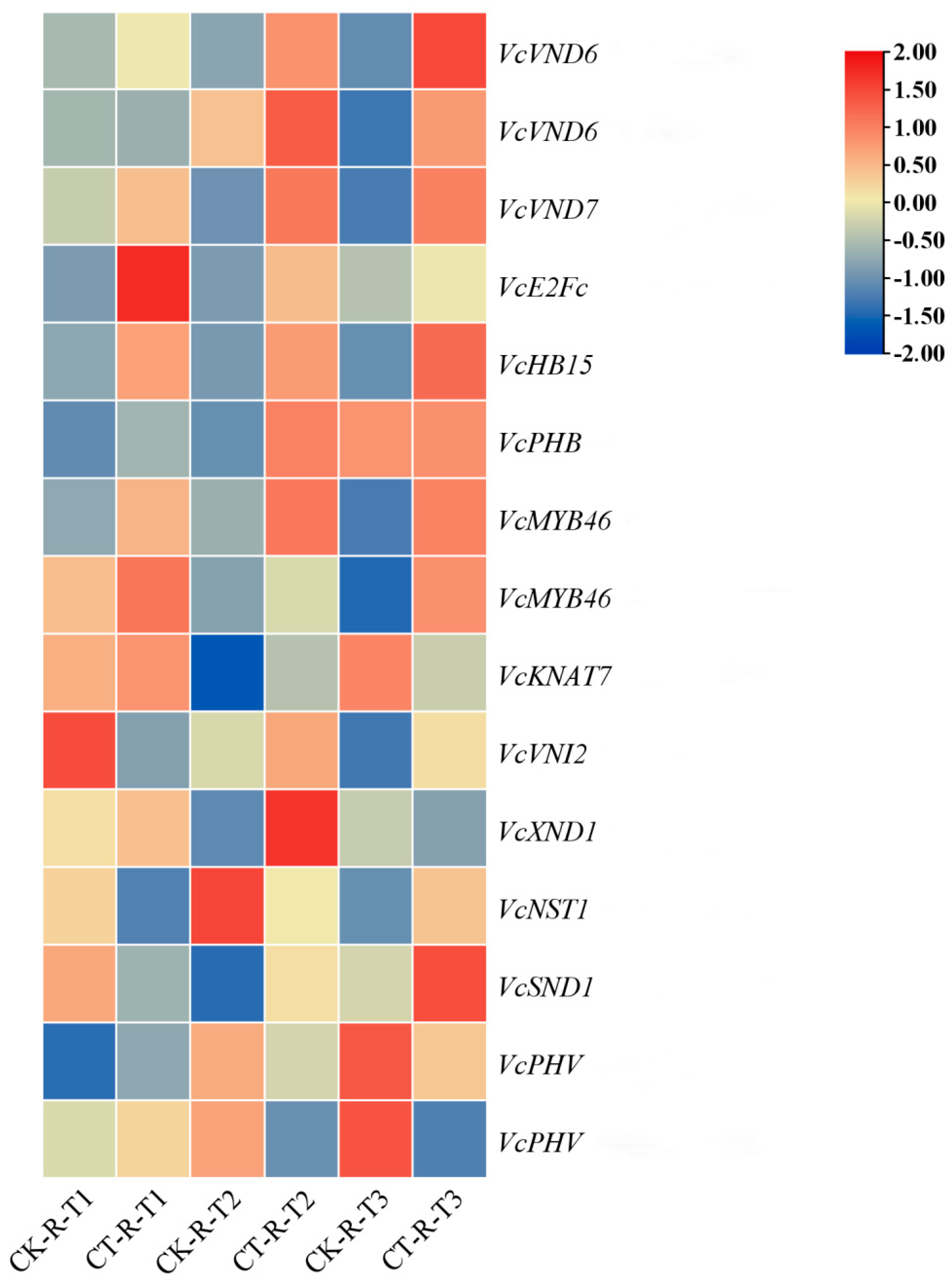
3.6. Effect of Root Pruning on the Expression of Transcription Factors for Vascular Tissue Development in Blueberry Roots
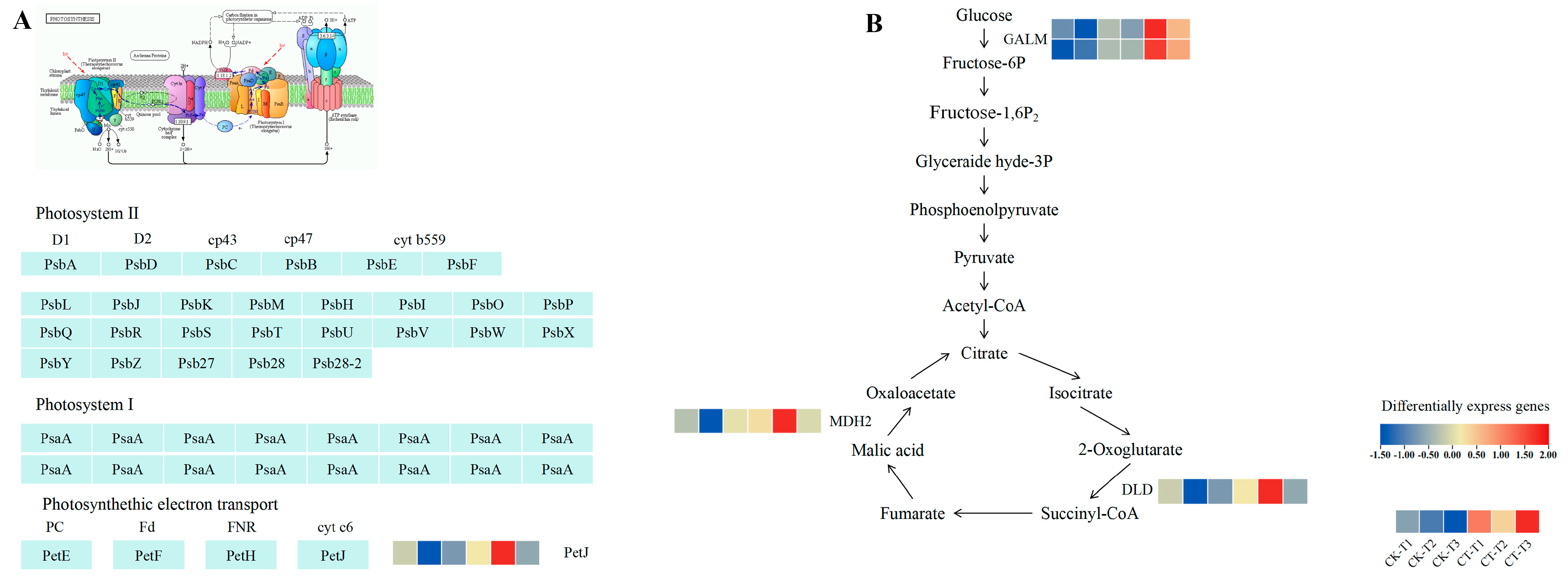
3.7. Effect of Root Pruning on Gene Expression in Photosynthesis, Glycolysis, and Tricarboxylic Acid Cycle Pathways in Blueberry Leaves
3.8. RT-qPCR Analysis
4. Discussion
4.1. Proper Root Pruning Promotes Blueberry Plant Growth and Development
4.2. Changes in the Expression of Genes Related to Vascular Tissue Development Led to Changes in Root Development
4.3. Proper Root Pruning Promotes Plant Photosynthesis
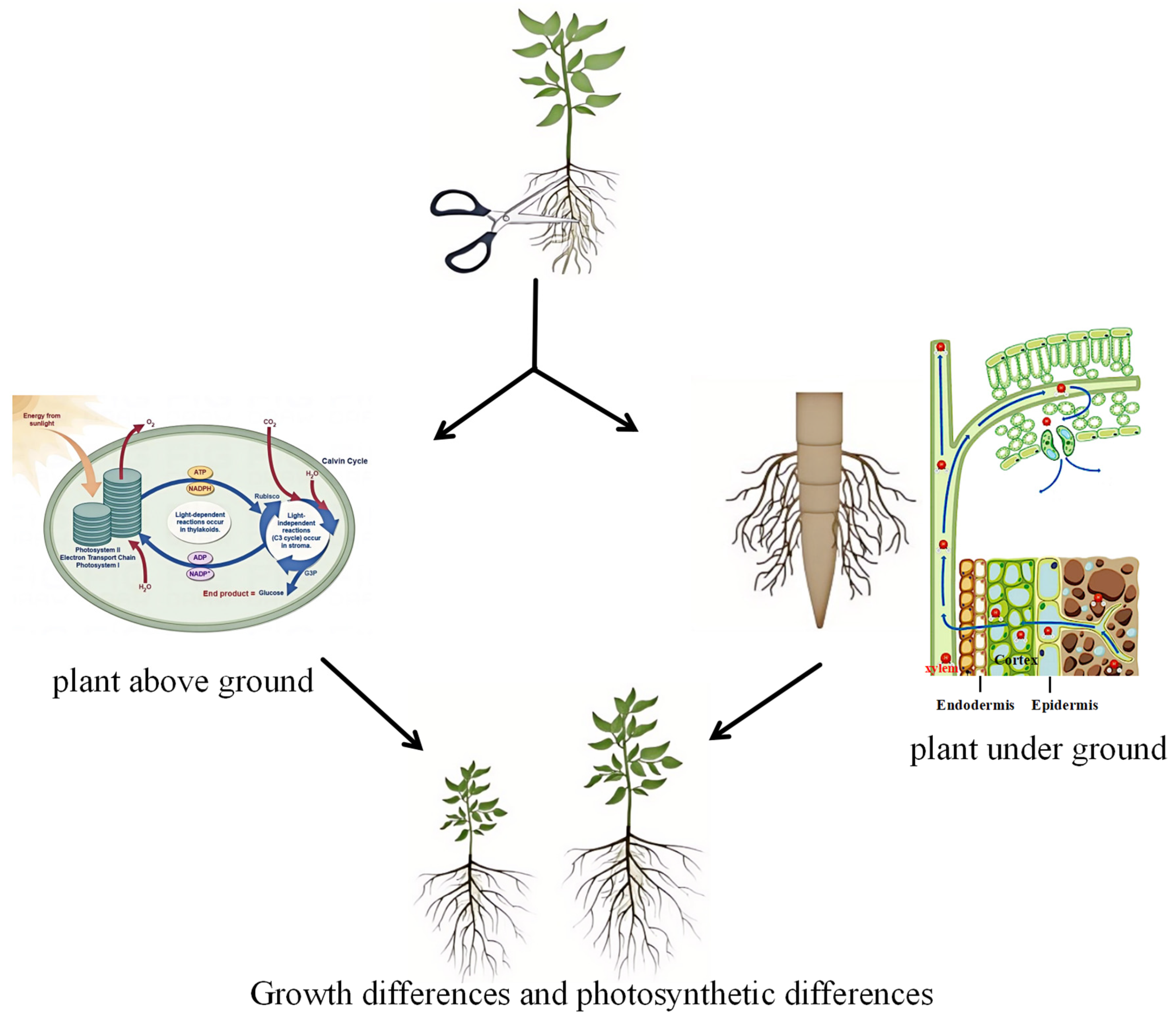
4.4. Coordinated Development of Aboveground and Belowground Plant Structures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cai, T.; Xu, H.; Peng, D.; Yin, Y.; Yang, W.; Ni, Y.; Xu, C.; Chen, X.; Xu, C.; Yang, D.; et al. Exogenous hormonal application improves grain yield of wheat by optimizing tiller productivity. Field Crops Res. 2014, 155, 172–183. [Google Scholar] [CrossRef]
- Yu, H.; Hu, Y.; Qi, L.; Zhang, K.; Jiang, J.; Li, H.; Zhang, X.; Zhang, Z. Hyperspectral detection of moisture content in rice straw nutrient bowl trays based on PSO-SVR. Sustainability 2023, 15, 8703. [Google Scholar] [CrossRef]
- Löf, M.; Thomsen, A.; Madsen, P. Sowing and transplanting of broadleaves (Fagus sylvatica L., Quercus robur L., Prunus avium L. and Crataegus monogyna Jacq.) for afforestation of farmland. For. Ecol. Manag. 2004, 188, 113–123. [Google Scholar] [CrossRef]
- Allen, K.S.; Harper, R.W.; Bayer, A.; Brazee, N.J. A review of nursery production systems and their influence on urban tree survival. Urban For. Urban Green. 2017, 21, 183–191. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, J.; Wan, F.; Xian, L.; Liu, Y. Effects of Root Pruning and Size on Growth Traits of Hybrid Poplar Seedlings. Forests 2024, 15, 1770. [Google Scholar] [CrossRef]
- Zhang, R.; Peng, F.R.; Yan, P.; Cao, F.; Liu, Z.; Le, D.; Tan, P. Effects of root pruning on germinated pecan seedlings. HortScience 2015, 50, 1549–1552. [Google Scholar] [CrossRef]
- Miller, B.M.; Graves, W.R. Root pruning and auxin alter root morphology of hickories. HortScience 2019, 54, 1517–1520. [Google Scholar] [CrossRef]
- Yan, M.; Zhang, C.; Li, H.; Zhang, L.; Ren, Y.; Chen, Y.; Cai, H.; Zhang, S. Root pruning improves maize water-use efficiency by root water absorption. Front. Plant Sci. 2023, 13, 1023088. [Google Scholar] [CrossRef]
- Paul, A.; Kaur, N.; Gill, P.P.S.; Gaur, K.; Asin, L.; Alegre, S.; Montserrat, R.; Asrey, R.; Patel, V.B.; Barman, K.; et al. Effect of girdling and root pruning on vegetative growth, fruit metabolites and yield of pear grown under sub-tropical conditions. Agric. Res. J. 2022, 59, 903–910. [Google Scholar] [CrossRef]
- Inurreta-Aguirre, H.D.; Lauri, P.É.; Dupraz, C.; Gosme, M. Impact of shade and tree root pruning on soil water content and crop yield of winter cereals in a Mediterranean alley crop** system. Agrofor. Syst. 2022, 96, 747–757. [Google Scholar] [CrossRef]
- Marchioretto, L.D.R.; De Rossi, A.; Conte, E.D. Chemical root pruning improves quality and nutrient uptake of Cape Gooseberry (Physalis peruviana) seedlings. Sci. Hortic. 2020, 261, 108948. [Google Scholar] [CrossRef]
- Hu, C.; Ding, M.; Qu, C.; Sadras, V.; Yang, X.; Zhang, S. Yield and water use efficiency of wheat in the Loess Plateau: Responses to root pruning and defoliation. Field Crops Res. 2015, 179, 6–11. [Google Scholar] [CrossRef]
- Mucha, J.; Jagodziński, A.M.; Bułaj, B.; Łakomy, P.; Talaśka, A.M.; Oleksyn, J.; Zadworny, M. Functional response of Quercus robur L. to taproot pruning: A 5-year case study. Ann. For. Sci. 2018, 75, 22. [Google Scholar] [CrossRef]
- Dong, T.; Duan, B.; Zhang, S.; Korpelainen, H.; Niinemets, Ü.; Li, C. Growth, biomass allocation and photosynthetic responses are related to intensity of root severance and soil moisture conditions in the plantation tree Cunninghamia lanceolata. Tree Physiol. 2016, 36, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Fanello, D.D.; Kelly, S.J.; Bartoli, C.G.; Cano, M.G.; Alonso, S.M.; Guiamet, J.J. Plasticity of root growth and respiratory activity: Root responses to above-ground senescence, fruit removal or partial root pruning in soybean. Plant Sci. 2020, 290, 110296. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and oxidative burst: Roots in plant development. Plant Divers. 2020, 42, 33–43. [Google Scholar] [CrossRef]
- Zhou, W.; Lozano-Torres, J.L.; Blilou, I.; Zhang, X.; Zhai, Q.; Smant, J.; Li, C.; Scheres, B. A jasmonate signaling network activates root stem cells and promotes regeneration. Cell 2019, 177, 942–956.e14. [Google Scholar] [CrossRef]
- Xu, D.; Miao, J.; Yumoto, E.; Yokota, T.; Asahina, M.; Watahiki, M. YUCCA9-mediated auxin biosynthesis and polar auxin transport synergistically regulate regeneration of root systems following root cutting. Plant Cell Physiol. 2017, 58, 1710–1723. [Google Scholar] [CrossRef]
- Zhao, Q. Lignification: Flexibility, biosynthesis and regulation. Trends Plant Sci. 2016, 21, 713–721. [Google Scholar] [CrossRef]
- Emonet, A.; Hay, A. Development and diversity of lignin patterns. Plant Physiol. 2022, 190, 31–43. [Google Scholar] [CrossRef]
- Orellana, R.J.M.; Winkelmann, T.; Rath, T. Laser wounding pattern in relation to vascular tissue development for the stimulation of adventitious root formation in rose cuttings. Sci. Hortic. 2024, 338, 113647. [Google Scholar] [CrossRef]
- Meychik, N.; Nikolaeva, Y.; Kushunina, M. The significance of ion-exchange properties of plant root cell walls for nutrient and water uptake by plants. Plant Physiol. Biochem. 2021, 166, 140–147. [Google Scholar] [CrossRef]
- Schäfer, E.D.; Owen, M.R.; Band, L.R.; Farcot, E.; Bennett, M.J.; Lynch, J.P. Modeling root loss reveals impacts on nutrient uptake and crop development. Plant Physiol. 2022, 190, 2260–2278. [Google Scholar] [CrossRef] [PubMed]
- Melkikh, A.V.; Sutormina, M.I. From leaves to roots: Biophysical models of transport of substances in plants. Prog. Biophys. Mol. Biol. 2022, 169, 53–83. [Google Scholar] [CrossRef] [PubMed]
- Jura-Morawiec, J.; Wiland-Szymańska, J. A contribution to understanding the structure of amphivasal secondary bundles in monocotyledons. Mod. Phytomorphol. 2014, 6, 75. [Google Scholar]
- Petit, G.; Crivellaro, A. Comparative axial widening of phloem and xylem conduits in small woody plants. Trees 2014, 28, 915–921. [Google Scholar] [CrossRef]
- Steyn, J.; Hoffman, E.; Lötze, E. Root growth dynamics of two southern highbush blueberry (V. corymbosum L. interspecific hybrids) cultivars in the Western Cape, South Africa. Sci. Hortic. 2024, 336, 113344. [Google Scholar] [CrossRef]
- Bryla, D.R.; Valenzuela-Estrada, L.R.; Vargas, O.L. Root production, distribution, and turnover in conventional and organic northern highbush blueberry systems. Acta Hortic. 2017, 1180, 169–176. [Google Scholar] [CrossRef]
- Elsysy, M.; Einhorn, T.C. Air-Pruning Containers Modify Root and Scion Growth and Alter Resource Allocation of Bench-Grafted Apple Plants. Horticulturae 2022, 8, 797. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. 1938, 347, 1–32. [Google Scholar]
- Chan, L.F.; Wei, M.L.; Lu, C.T. Genotypic variation in coefficients for estimating leaf area of rice. J. Agric. Res. China 2001, 50, 24–37. [Google Scholar]
- Thomas, J.R.; Namken, L.N.; Oerther, G.F. Estimating leaf water content by reflectance measurements 1. Agron. J. 1971, 63, 845–847. [Google Scholar] [CrossRef]
- Tang, Y.; Tian, G.; Min, C.; Song, J.; Chen, D.; Luo, J. Impacts of root pruning intensity and direction on the phytoremediation of moderately Cd-polluted soil by Celosia argentea. Int. J. Phytoremediat. 2021, 24, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Testerink, C.; Zhang, Y. How roots and shoots communicate through stressful times. Trends Plant Sci. 2021, 26, 940–952. [Google Scholar] [CrossRef]
- Prince, M.; Anming, D.; Yingzhen, K. Expansins: Roles in plant growth and potential applications in crop improvement. Plant Cell Rep. 2016, 35, 949–965. [Google Scholar] [CrossRef]
- Sergio, S.; Audrey, C.; Matthieu, R.; Lilian, R.; Aline, B.; Laurent, N.; Thierry, D. Root tip contact with low-phosphate media reprograms plant root architecture. Nat. Genet. 2007, 39, 792–796. [Google Scholar] [CrossRef]
- Houston, K.; Tucker, M.R.; Chowdhury, J. The plant cell wall: A complex and dynamic structure as revealed by the responses of genes under stress conditions. Front. Plant Sci. 2016, 7, 984. [Google Scholar] [CrossRef]
- Winter, H.; Huber, S.C. Regulation of sucrose metabolism in higher plants: Localization and regulation of activity of key enzymes. Crit. Rev. Plant Sci. 2000, 19, 31–67. [Google Scholar] [CrossRef]
- Haigler, C.H.; Ivanova-Datcheva, M.; Hogan, P.S.; Salnikov, V.V.; Hwang, S.; Martin, K.; Delmer, D.P. Carbon partitioning to cellulose synthesis. Plant Cell Walls 2001, 29–51. [Google Scholar]
- Salnikov, V.V.; Grimson, M.J.; Delmer, D.P.; Haigler, C.H. Sucrose synthase localizes to cellulose synthesis sites in tracheary elements. Phytochemistry 2001, 57, 823–833. [Google Scholar] [CrossRef]
- Salnikov, V.V.; Grimson, M.J.; Seagull, R.W.; Haigler, C.H. Localization of sucrose synthase and callose in freeze-substituted secondary-wall-stage cotton fibers. Protoplasma 2003, 221, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Udagawa, M.; Nishikubo, N.; Horiguchi, G.; Yamaguchi, M.; Ito, J.; Mimura, T.; Fukuda, H.; Demura, T. Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev. 2005, 19, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Mitsuda, N.; Iwase, A.; Yamamoto, H.; Yoshida, M.; Seki, M.; Shinozaki, K.; Ohme-Takagi, M. NAC transcription factors, NST1 and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis. Plant Cell 2007, 19, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, S.; Ohashi, Y. Interaction of the Arabidopsis E2F and DP proteins confers their concomitant nuclear translocation and transactivation. Plant Physiol. 2002, 128, 833–843. [Google Scholar] [CrossRef]
- Heckmann, S.; Lermontova, I.; Berckmans, B.; De Veylder, L.; Bäumlein, H.; Schubert, I. The E2F transcription factor family regulates CENH3 expression in Arabidopsis thaliana. Plant J. 2011, 68, 646–656. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Ohtani, M.; Mitsuda, N.; Kubo, M.; Ohme-Takagi, M.; Fukuda, H.; Demura, T. VND-INTERACTING2, a NAC domain transcription factor, negatively regulates xylem vessel formation in Arabidopsis. Plant Cell 2010, 22, 1249–1263. [Google Scholar] [CrossRef]
- Zhao, C.; Avci, U.; Grant, E.H.; Haigler, C.H.; Beers, E.P. XND1, a member of the NAC domain family in Arabidopsis thaliana, negatively regulates lignocellulose synthesis and programmed cell death in xylem. Plant J. 2008, 53, 425–436. [Google Scholar] [CrossRef]
- Taylor-Teeples, M.; Lin, L.; de Lucas, M.; Turco, G.; Toal, T.W.; Gaudinier, A.; Young, N.F.; Trabucco, G.M.; Veling, M.T.; Lamothe, R.; et al. An Arabidopsis gene regulatory network for secondary cell wall synthesis. Nature 2015, 517, 571–575. [Google Scholar] [CrossRef]
- Zhong, R.; Richardson, E.A.; Ye, Z.H. The MYB46 transcription factor is a direct target of SND1 and regulates secondary wall biosynthesis in Arabidopsis. Plant Cell 2007, 19, 2776–2792. [Google Scholar] [CrossRef]
- McCarthy, R.L.; Zhong, R.; Ye, Z.H. MYB83 is a direct target of SND1 and acts redundantly with MYB46 in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell Physiol. 2009, 50, 1950–1964. [Google Scholar] [CrossRef]
- Ko, J.H.; Kim, W.C.; Han, K.H. Ectopic expression of MYB46 identifies transcriptional regulatory genes involved in secondary wall biosynthesis in Arabidopsis. Plant J. 2009, 60, 649–665. [Google Scholar] [CrossRef]
- Zhong, R.; Lee, C.; Zhou, J.; McCarthy, R.; Ye, Z. A battery of transcription factors involved in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell 2008, 20, 2763–2782. [Google Scholar] [CrossRef]
- Benson, A.R.; Morgenroth, J.; Koeser, A.K. The effects of root pruning on growth and physiology of two Acer species in New Zealand. Urban For. Urban Green. 2019, 38, 64–73. [Google Scholar] [CrossRef]
- Jing, D.; Du, Z.; Wang, M.; Wang, Q.; Ma, H.; Liu, F.; Ma, B.; Dong, Y. Regulatory effects of root pruning on leaf nutrients, photosynthesis, and growth of trees in a closed-canopy poplar plantation. PLoS ONE 2018, 13, e0197515. [Google Scholar] [CrossRef] [PubMed]
- Vitali, V.; Ramirez, J.A.; Perrette, G.; Delagrange, S.; Paquette, A.; Messier, C. Complex above-and below-ground growth responses of two urban tree species following root, stem, and foliage damage—An experimental approach. Front. Plant Sci. 2019, 10, 1100. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.A.; Handa, I.T.; Posada, J.M.; Delagrange, S.; Delagrange, D.; Messier, C. Carbohydrate dynamics in roots, stems, and branches after maintenance pruning in two common urban tree species of North America. Urban For. Urban Green. 2018, 30, 24–31. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, L.; Shi, C.; Wang, X.; Li, B.; Zuo, S.; Li, Q.; Han, J.; Wang, H.; Lou, X. The Molecular Mechanism and Effects of Root Pruning Treatment on Blueberry Tree Growth. Plants 2025, 14, 2269. https://doi.org/10.3390/plants14152269
Chu L, Shi C, Wang X, Li B, Zuo S, Li Q, Han J, Wang H, Lou X. The Molecular Mechanism and Effects of Root Pruning Treatment on Blueberry Tree Growth. Plants. 2025; 14(15):2269. https://doi.org/10.3390/plants14152269
Chicago/Turabian StyleChu, Liwei, Chengjing Shi, Xin Wang, Benyin Li, Siyu Zuo, Qixuan Li, Jiarui Han, Hexin Wang, and Xin Lou. 2025. "The Molecular Mechanism and Effects of Root Pruning Treatment on Blueberry Tree Growth" Plants 14, no. 15: 2269. https://doi.org/10.3390/plants14152269
APA StyleChu, L., Shi, C., Wang, X., Li, B., Zuo, S., Li, Q., Han, J., Wang, H., & Lou, X. (2025). The Molecular Mechanism and Effects of Root Pruning Treatment on Blueberry Tree Growth. Plants, 14(15), 2269. https://doi.org/10.3390/plants14152269





