Spatiotemporal Variations in Nectar Robbing and Its Effects on Reproduction in Salvia castanea Diels (Lamiaceae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Species
2.2. Effect of Nectar Robbing on Floral Visits
2.3. Spatiotemporal Variations in Robbing Rate and Their Effects on Reproduction
2.4. Effects of Nectar Robbing on Nectar Availability and Flower Longevity
2.5. Amount of Pollen Needed for Sufficient Fertilization, and Deposited Pollen Counts and Their Seed Set After Single Visit
2.6. Statistical Analysis
3. Results
3.1. Effect of Nectar Robbing on Floral Visits
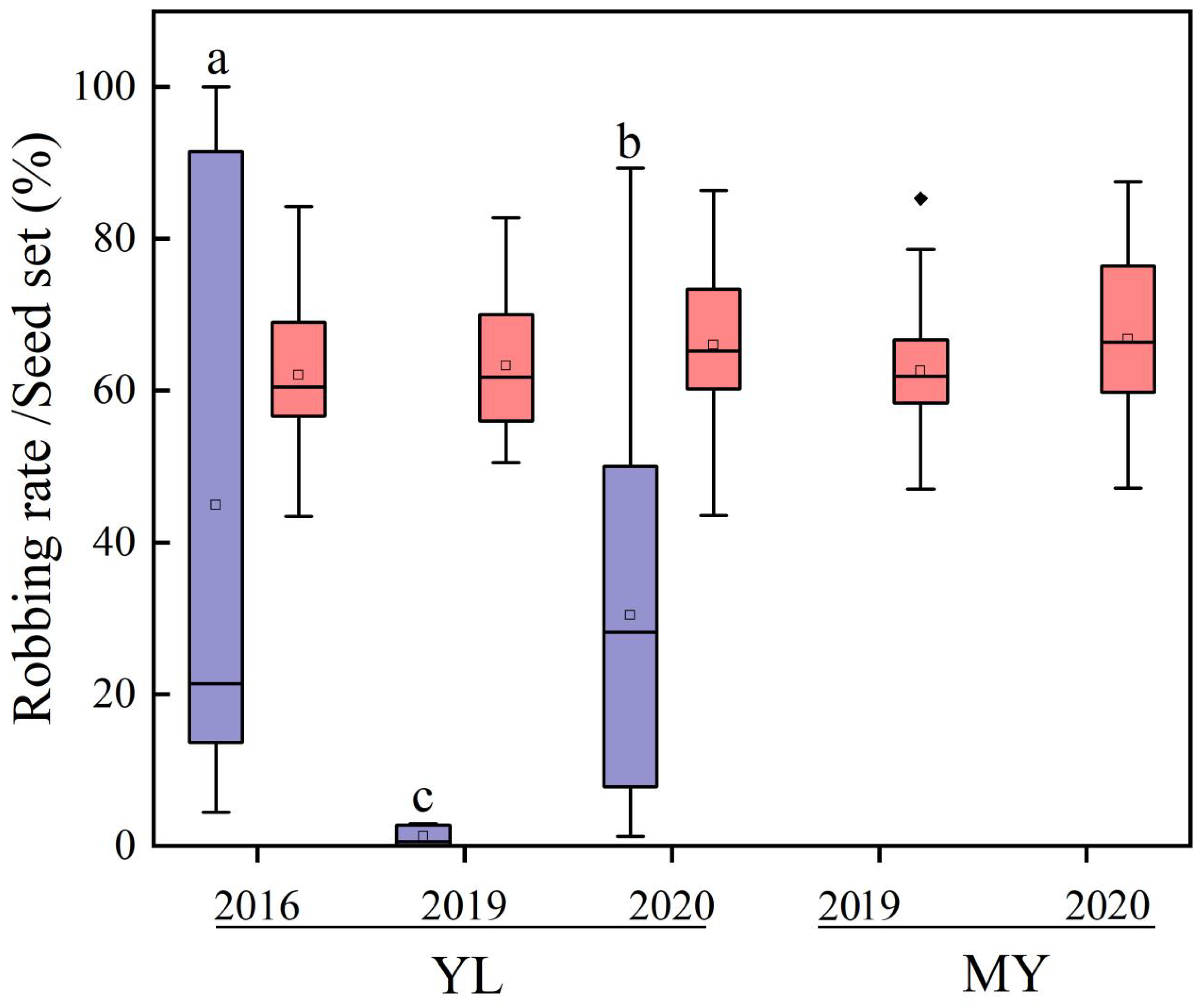
3.2. Spatiotemporal Variations in Robbing Rate and Their Effects on Reproduction
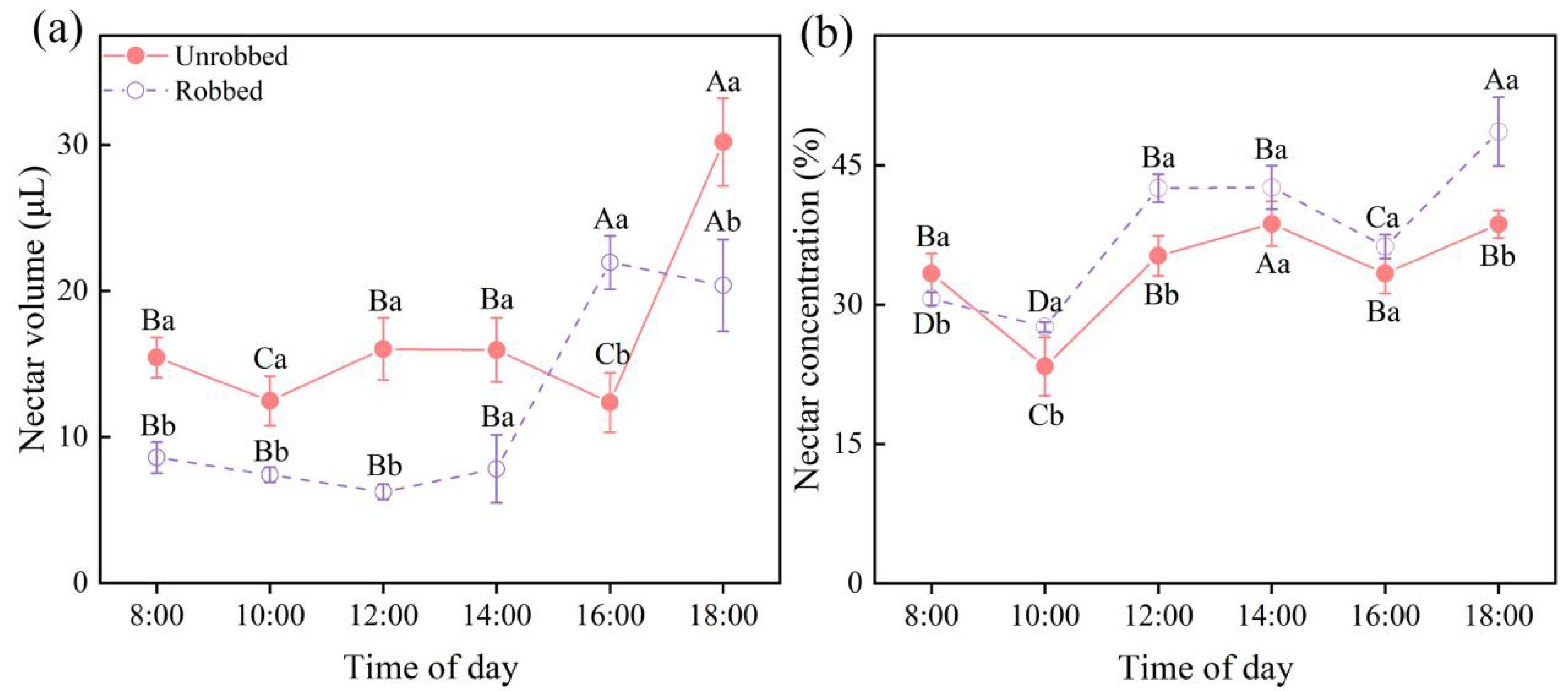
3.3. Effects of Nectar Robbing on Nectar Availability and Flower Longevity
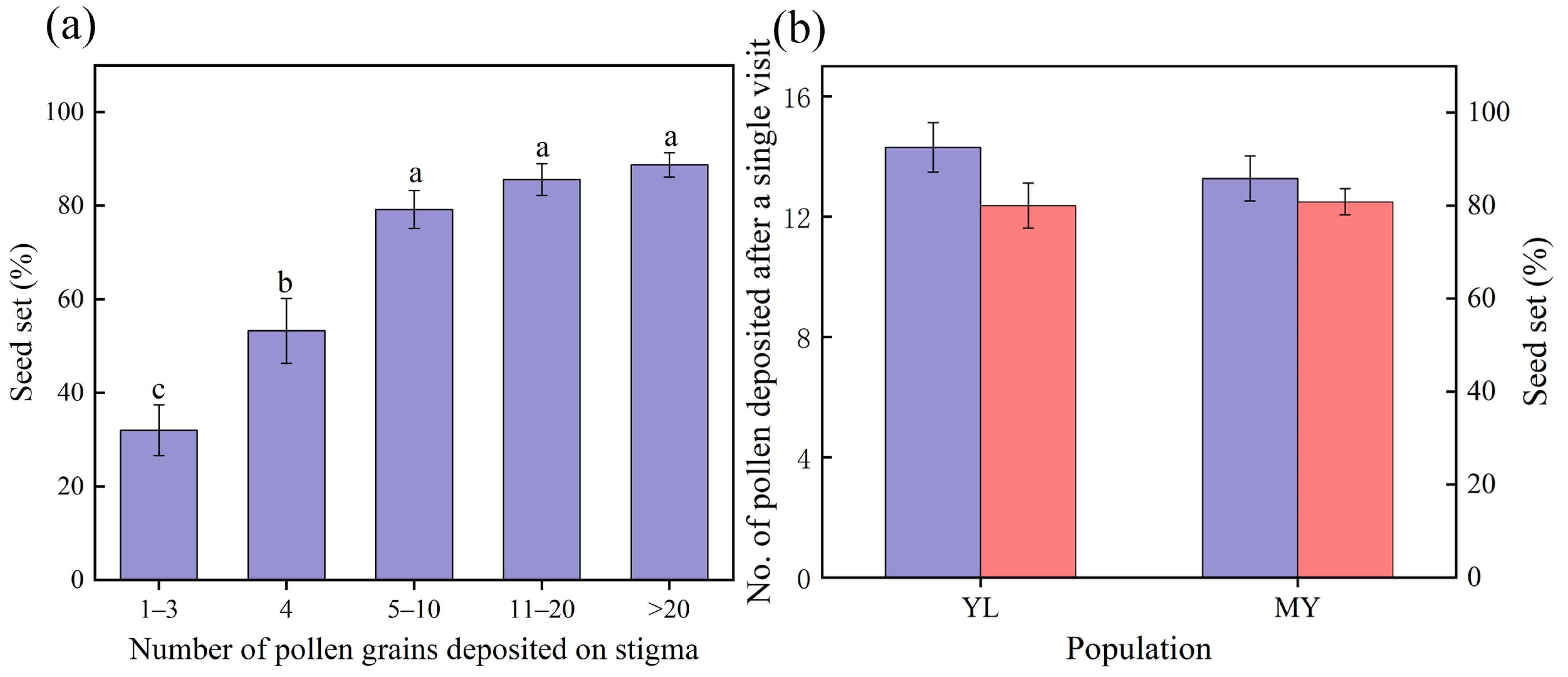
3.4. Amount of Pollen Needed for Sufficient Fertilization, and Deposited Pollen Counts and Their Seed Set After a Single Visit
4. Discussion
4.1. Effects of Nectar Robbing on Pollinators and Their Spatiotemporal Variation
4.2. Effect of Spatiotemporal Variations in Nectar Robbing on Reproduction
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inouye, D.W. The Terminology of Floral Larceny. Ecology 1980, 61, 1251–1253. [Google Scholar] [CrossRef]
- Irwin, R.E.; Bronstein, J.L.; Manson, J.S.; Richardson, L. Nectar Robbing: Ecological and Evolutionary Perspectives. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 271–292. [Google Scholar] [CrossRef]
- Irwin, R.E.; Maloof, J.E. Variation in nectar robbing over time, space, and species. Oecologia 2002, 133, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Nossa, S.V.; Sánchez, J.M.; Navarro, L. Nectar robbing: A common phenomenon determined by accessibility constraints, nectar volume and density of energy rewards. Oikos 2016, 125, 1044–1055. [Google Scholar] [CrossRef]
- Navarro, L. Pollination ecology of Anthyllis vulneraria subsp. vulgaris (Fabaceae): Nectar robbers as pollinators. Am. J. Bot. 2000, 87, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Maloof, J.E.; Inouye, D.W. Are nectar robbers cheaters or mutualists? Ecology 2000, 81, 2651–2661. [Google Scholar] [CrossRef]
- Wang, X.; Yao, R.; Lv, X.; Yi, Y.; Tang, X. Nectar robbing by bees affects the reproductive fitness of the distylous plant Tirpitzia sinensis (Linaceae). Ecol. Evol. 2023, 13, e10714. [Google Scholar] [CrossRef]
- Ye, Z.M.; Jin, X.F.; Wang, Q.F.; Yang, C.F.; Inouye, D.W. Nectar replenishment maintains the neutral effects of nectar robbing on female reproductive success of Salvia przewalskii (Lamiaceae), a plant pollinated and robbed by bumble bees. Ann. Bot. 2017, 119, 1053–1059. [Google Scholar] [CrossRef]
- Ye, Z.; Jin, X.; Inouye, D.W.; Wang, Q.; Yang, C. Variation in composition of two bumble bee species across communities affects nectar robbing but maintains pollinator visitation rate to an alpine plant, Salvia przewalskii. Ecol. Èntomol. 2018, 43, 363–370. [Google Scholar] [CrossRef]
- Zhong, J.R.; Jin, X.F.; Orr, M.C.; Li, X.Q.; He, Y.D.; Wang, S.W.; Wang, Q.F.; Yang, C.F.; Ye, Z.M. The ethics of theft: Reevaluating the impacts of floral larceny on plant reproductive success. Plant Divers. 2025, 47, 148–158. [Google Scholar] [CrossRef]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Fenster, C.B.; Armbruster, W.S.; Wilson, P.; Dudash, M.R.; Thomson, J.D. Pollination syndromes and floral specialization. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 375–403. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, Q.; Huang, Y.; Ma, Y.; Claßen-Bockhoff, R.; Tian, R.; Wei, Y. Effective hawkmoth pollination in the primarily bee-pollinated Salvia daiguii—An example of adaptive generalization. Plant Species Biol. 2023, 38, 18–26. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Yu, Q.; Zhao, J.-M.; Guo, Y.-H. Differential effects of nectar robbing by the same bumble-bee species on three sympatric Corydalis species with varied mating systems. Ann. Bot. 2009, 104, 33–39. [Google Scholar] [CrossRef] [PubMed]
- McDade, L.A.; Kinsman, S. The impact of floral parasitism in two neotropical hummingbird-pollinated plant species. Evolution 1980, 34, 944–958. [Google Scholar]
- Barker, J.L.; Dornhaus, A.; Bronstein, J.L.; Muth, F. Learning about larceny: Experience can bias bumble bees to rob nectar. Behav. Ecol. Sociobiol. 2018, 72, 67–78. [Google Scholar] [CrossRef]
- Maloof, J.E. The effects of a bumble bee nectar robber on plant reproductive success and pollinator behavior. Am. J. Bot. 2001, 88, 1960–1965. [Google Scholar] [CrossRef]
- Inouye, D.W. The ecology of nectar robbing. In The Biology of Nectaries; Beattey, B., Elias, T., Eds.; Columbia University Press: New York, NY, USA, 1983; pp. 153–173. [Google Scholar]
- Zimmerman, M.; Cook, S. Pollinator foraging, experimental nectar-robbing and plant fitness in Impatiens capensis. Am. Midl. Nat. 1985, 113, 84–91. [Google Scholar] [CrossRef]
- Carrió, E.; Güemes, J. Nectar robbing does not affect female reproductive success of an endangered Antirrhinum species, Plantaginaceae. Plant Ecol. Divers. 2020, 103, 80–84. [Google Scholar] [CrossRef]
- Fitch, G.; Vandermeer, J. Changes in partner traits drive variation in plant–nectar robber interactions across habitats. Basic Appl. Ecol. 2021, 53, 1–11. [Google Scholar] [CrossRef]
- Heiling, J.M.; Ledbetter, T.A.; Richman, S.K.; Ellison, H.K.; Bronstein, J.L.; Irwin, R.E. Why are some plant-nectar robber interactions commensalisms? Oikos 2018, 127, 1679–1689. [Google Scholar] [CrossRef]
- Cuevas, E.; Rosas-Guerrero, V. Spatio-temporal variation of nectar robbing in Salvia gesneriflora and its effects on nectar production and legitimate visitors. Plant Biol. 2016, 18, 9–14. [Google Scholar] [CrossRef]
- Sakhalkar, S.P.; Janeček, Š.; Klomberg, Y.; Mertens, J.E.J.; Hodeček, J.; Tropek, R. Cheaters among pollinators: Floral traits drive spatiotemporal variation in nectar robbing and thieving in Afrotropical rainforests. Ecosphere 2023, 14, 1–15. [Google Scholar] [CrossRef]
- Xiao, H.-W.; Huang, Y.-B.; Chang, Y.-H.; Chen, Y.; Abbott, R.J.; Wei, Y.-K.; Ma, Y.-P. Occurrence and prevention of delayed autonomous selfing in Salvia umbratica (Lamiaceae). Front. Plant Sci. 2021, 12, 635310. [Google Scholar] [CrossRef] [PubMed]
- Drew, B.T. Evolution, pollination biology and species richness of Salvia (Lamiaceae). Int. J. Plant Sci. 2020, 181, 767–769. [Google Scholar] [CrossRef]
- Xiao, H.W.; Huang, Y.B.; Wang, Q.; Wei, Y.K. Diversity of visiting insects and changes of pollinator behavior in alpine species Salvia castanea Diels (Lamiaceae). Acta Ecol. Sin. 2022, 45, 1841–1853. [Google Scholar] [CrossRef]
- Xiao, H.-W.; Huang, Y.-B.; Liu, Q.-S.; Claßen-Bockhoff, R.; Tian, R.-N.; Wei, Y.-K. Mixed mating pattern in bumblebee-pollinated Salvia species from China. Biol. J. Linn. Soc. 2024, 143, 1–13. [Google Scholar] [CrossRef]
- Han, X.Y.; Xiao, H.W.; Tian, R.N. Flower colour diversity, generation factors, and breeding applications in Salvia: A global perspective. Planta 2025, submitted to journal. [Google Scholar]
- Claßen-Bockhoff, R.; Speck, T.; Tweraser, E.; Wester, P.; Thimm, S.; Reith, M. The staminal lever mechanism in Salvia L. (Lamiaceae): A key innovation for adaptive radiation? Org. Divers. Evol. 2004, 4, 189–205. [Google Scholar] [CrossRef]
- Wester, P.; Claßen-Bockhoff, R. Hummingbird pollination in Salvia haenkei (Lamiaceae) lacking the typical lever mechanism. Plant Syst. Evol. 2006, 257, 133–146. [Google Scholar] [CrossRef]
- Wester, P.; Claßen-Bockhoff, R. Bird pollination in south African Salvia species. Flora 2006, 201, 396–406. [Google Scholar] [CrossRef]
- Celep, F.; Atalay, Z.; Dikmen, F.; Doğan, M.; Classen-Bockhoff, R. Flies as pollinators of melittophilous Salvia species (Lamiaceae). Am. J. Bot. 2014, 101, 2148–2159. [Google Scholar] [CrossRef]
- Fragoso-Martínez, I.; Martínez-Gordillo, M.; Salazar, G.A.; Sazatornil, F.; Jenks, A.A.; Peña, M.d.R.G.; Barrera-Aveleida, G.; Benitez-Vieyra, S.; Magallón, S.; Cornejo-Tenorio, G.; et al. Phylogeny of the Neotropical sages (Salvia subg. Calosphace; Lamiaceae) and insights into pollinator and area shifts. Plant Syst. Evol. 2018, 304, 43–55. [Google Scholar] [CrossRef]
- Kriebel, R.; Drew, B.T.; Drummond, C.P.; González-Gallegos, J.G.; Celep, F.; Mahdjoub, M.M.; Rose, J.P.; Xiang, C.; Hu, G.; Walker, J.B.; et al. Tracking temporal shifts in area, biomes, and pollinators in the radiation of Salvia (sages) across continents: Leveraging anchored hybrid enrichment and targeted sequence data. Am. J. Bot. 2019, 106, 573–597. [Google Scholar] [CrossRef]
- Barrionuevo, C.N.; Santiago, B.V.; Federico, S. Floral biology of Salvia stachydifolia, a species visited by bees and birds: Connecting sexual phases, nectar dynamics and breeding system to visitors’ behaviour. J. Plant Ecol. 2021, 14, 580–590. [Google Scholar] [CrossRef]
- Surina, B.; Balant, M.; Glasnović, P.; Gogala, A.; Fišer, Ž.; Satovic, Z.; Liber, Z.; Radosavljević, I.; Classen-Bockhoff, R. Lack of pollinators selects for increased selfing, restricted gene flow and resource allocation in the rare Mediterranean sage Salvia brachyodon. Sci. Rep. 2024, 14, 5017. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Zhao, S.; Xiao, H.; Liu, D.; Huang, Y.; Wei, Y.; Ma, Y. Unusual patterns of hybridization involving two alpine Salvia species: Absence of both F1 and backcrossed hybrids. Front. Plant Sci. 2022, 13, 1010577. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, J.; Tan, S.; Cadotte, M.W.; Xu, K.; Gao, L.; Li, D. Trait variation and functional diversity maintenance of understory herbaceous species coexisting along an elevational gradient in Yulong Mountain, Southwest China. Plant Divers. 2016, 38, 303–311. [Google Scholar] [CrossRef]
- Inouye, D.W. The effect of proboscis and corolla tube lengths on patterns and rates of flower visitation by bumblebees. Oecologia 1980, 45, 197–201. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, S.; Fang, Q.-E.; Bai, X.-M. Evolutionary response of staminal lever mechanism of different species in Salvia to spatial variation in pollinators. Chin. J. Plant Ecol. 2012, 36, 681–689. [Google Scholar] [CrossRef]
- Ling, T.C.; Phokasem, P.; Sinpoo, C.; Yang, Y.-P.; Disayathanoowat, T. Pollinator differentiation drive reproductive isolation between two sympatric Salvia species (Lamiaceae). Plants 2022, 11, 2423. [Google Scholar] [CrossRef]
- Xiao, H.-W.; Liu, Q.-S.; Huang, Y.-B.; Tian, R.-N. Effects of ecological factors on the pollination biology and seed production of Salvia daiguii: A critically endangered ornamental species from China. Sci. Hortic. 2024, 333, 1–10. [Google Scholar] [CrossRef]
- Claßen-Bockhoff, R.; Wester, P.; Tweraser, E. The staminal lever mechanism in Salvia L. (Lamiaceae)—A review. Plant Biol. 2003, 5, 33–41. [Google Scholar] [CrossRef]
- Schemske, D.W.; Horvitz, C.C. Temporal variation in selection on a floral character. Evolution 1989, 43, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Aigner, P.A. Optimality modeling and fitness trade-offs: When should plants become pollinator specialists? Oikos 2001, 95, 177–184. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Irwin, R.E.; Flanagan, R.J.; Karron, J.D. Ecology and evolution of plant-pollinator interactions. Ann. Bot. 2009, 103, 1355–1363. [Google Scholar] [CrossRef]
- Castro, S.; Silveira, P.; Navarro, L. Floral traits variation, legitimate pollination, and nectar robbing in Polygala vayredae (Polygalaceae). Ecol. Res. 2009, 24, 47–55. [Google Scholar] [CrossRef]
- Irwin, R.E.; Brody, A.K. Nectar robbing in Ipomopsis aggregata: Effects on pollinator behavior and plant fitness. Oecologia 1998, 116, 519–527. [Google Scholar] [CrossRef]
- Navarro, L.; Medel, R. Relationship between floral tube length and nectar robbing in Duranta erecta L. (Verbenaceae). Biol. J. Linn. Soc. 2009, 96, 392–398. [Google Scholar] [CrossRef]
- Gonzalez-Gomez, P.L.; Valdivia, C.E. Direct and indirect effects of nectar robbing on the pollinating behavior of Patagona gigas (Trochilidae). Biotropica 2005, 37, 693–696. [Google Scholar] [CrossRef]
- Irwin, R.E. Hummingbird avoidance of nectarrobbed plants: Spatial location or visual cues. Oikos 2000, 91, 499–506. [Google Scholar] [CrossRef]
- Ashman, T.L.; Schoen, D.J. How long should flowers live? Nature 1994, 371, 788–791. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Irwin, R.E.; Flanagan, R.J.; Karron, J.D. Towards the flower economics spectrum. New Phytol. 2021, 229, 665–672. [Google Scholar] [CrossRef]
- Aximoff, I.A.; Freitas, L. Is pollen removal or seed set favoured by flower longevity in a hummingbird-pollinated Salvia species? Ann. Bot. 2010, 106, 413–419. [Google Scholar] [CrossRef]


| Population | YL | MY | ||||
|---|---|---|---|---|---|---|
| Year | 2014 | 2015 | 2016 | 2020 | 2019 | 2020 |
| Average No. of flowers observed per video per day | 74 (n = 3 d) | 52 (n = 3 d) | 70 (n = 4 d) | 68 (n = 6 d) | 170 (n = 8 d) | 71 (n = 6 d) |
| Total No. of flower visits by all insects during the observation period | 548 | 855 | 4853 | 14268 | 808 | 3110 |
| Total No. of flower visits by pollinators | 519 (94.71%) | 50 (5.85%) | 217 (4.46%) | 14245 (99.84%) | 808 (100%) | 3110 (100%) |
| Total No. of flower visits by robbers | 29 (5.29%) | 805 (94.15%) | 4652 (95.54%) | 23 (0.16%) | 0 | 0 |
| No. of visits per flower per day | 2.43 ± 0.47 c | 0.30 ± 0.09 d | 1.35 ± 0.95 c | 34.91 ± 1.50 a | 0.54 ± 0.13 cd | 7.26 ± 0.47 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.-W.; Huang, Y.-B. Spatiotemporal Variations in Nectar Robbing and Its Effects on Reproduction in Salvia castanea Diels (Lamiaceae). Plants 2025, 14, 2266. https://doi.org/10.3390/plants14152266
Xiao H-W, Huang Y-B. Spatiotemporal Variations in Nectar Robbing and Its Effects on Reproduction in Salvia castanea Diels (Lamiaceae). Plants. 2025; 14(15):2266. https://doi.org/10.3390/plants14152266
Chicago/Turabian StyleXiao, Han-Wen, and Yan-Bo Huang. 2025. "Spatiotemporal Variations in Nectar Robbing and Its Effects on Reproduction in Salvia castanea Diels (Lamiaceae)" Plants 14, no. 15: 2266. https://doi.org/10.3390/plants14152266
APA StyleXiao, H.-W., & Huang, Y.-B. (2025). Spatiotemporal Variations in Nectar Robbing and Its Effects on Reproduction in Salvia castanea Diels (Lamiaceae). Plants, 14(15), 2266. https://doi.org/10.3390/plants14152266






