A Comparative Analysis of the Efficacy of Three Plant Growth Regulators and Dose Optimization for Improving Agronomic Traits and Seed Yield of Purple-Flowered Alfalfa (Medicago sativa L.)
Abstract
1. Introduction
2. Results
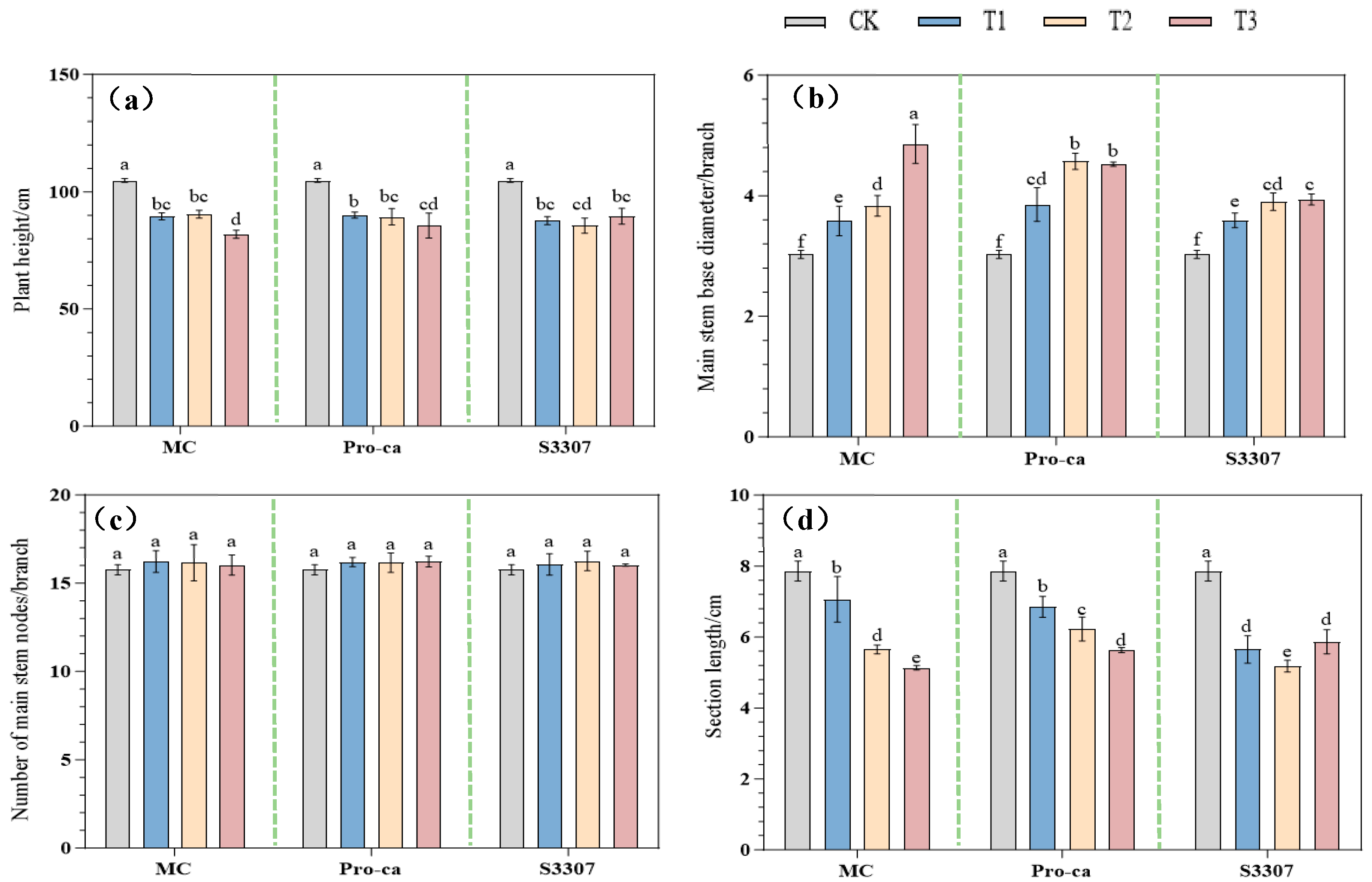
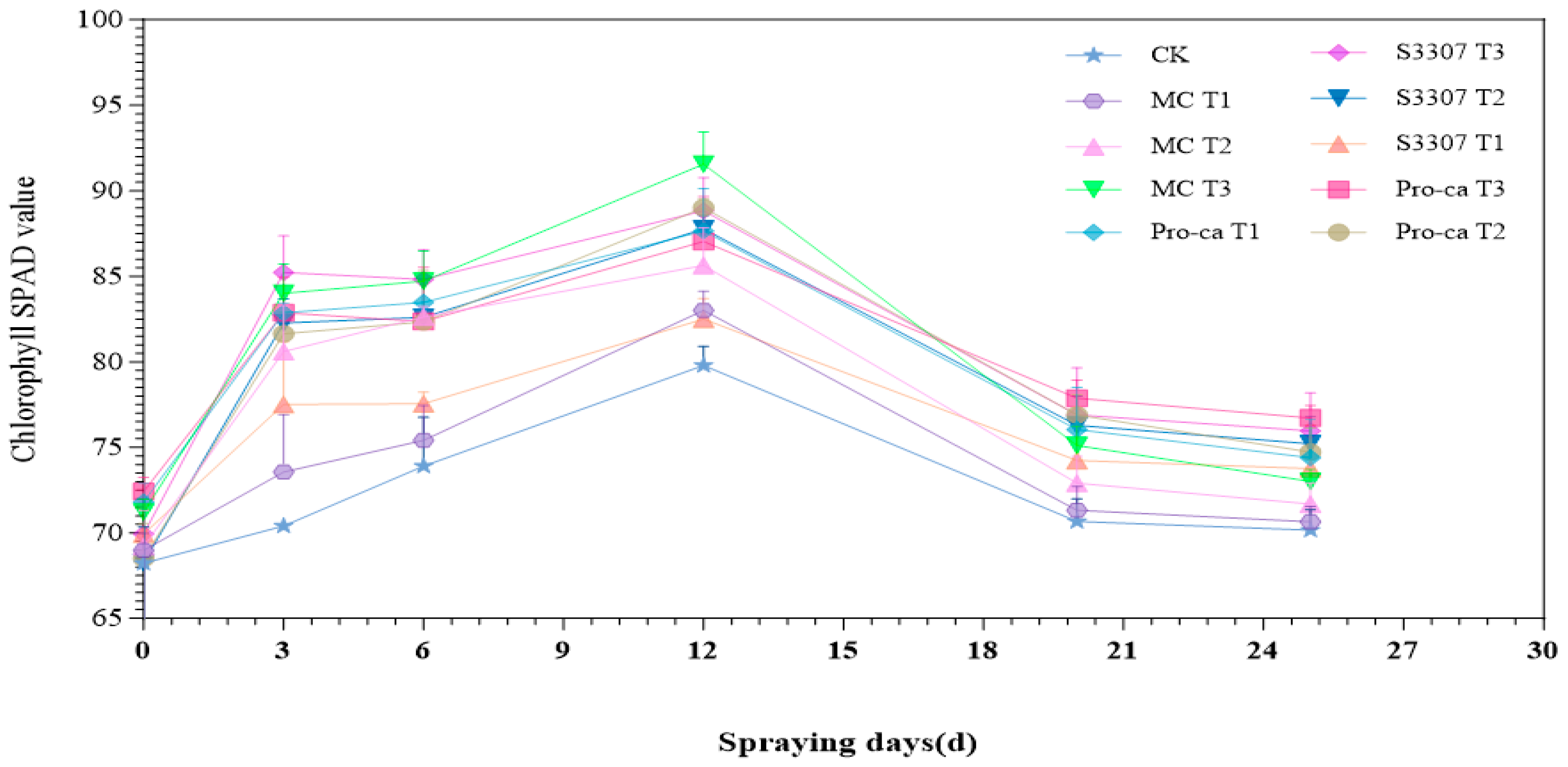
2.1. Effects of Foliar Application of Different Plant Growth Regulators on Agronomic Traits and SPAD Values of Alfalfa
2.1.1. Effects of Plant Growth Regulators on the Key Agronomic Traits
2.1.2. Change Trend of the SPAD Value Under Different Treatments
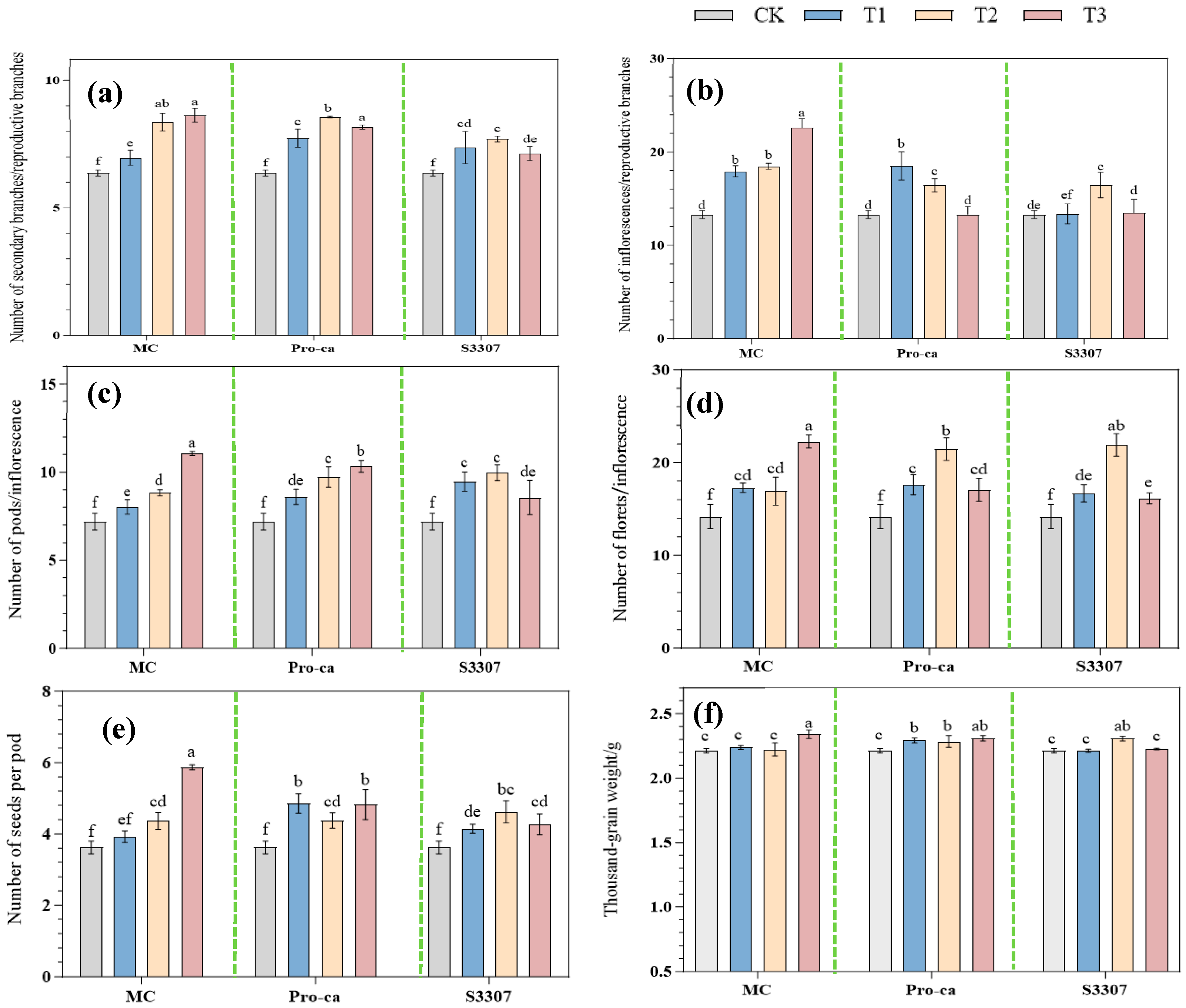
2.2. Effects of Different PGRs on the Seed Yield Components of Alfalfa
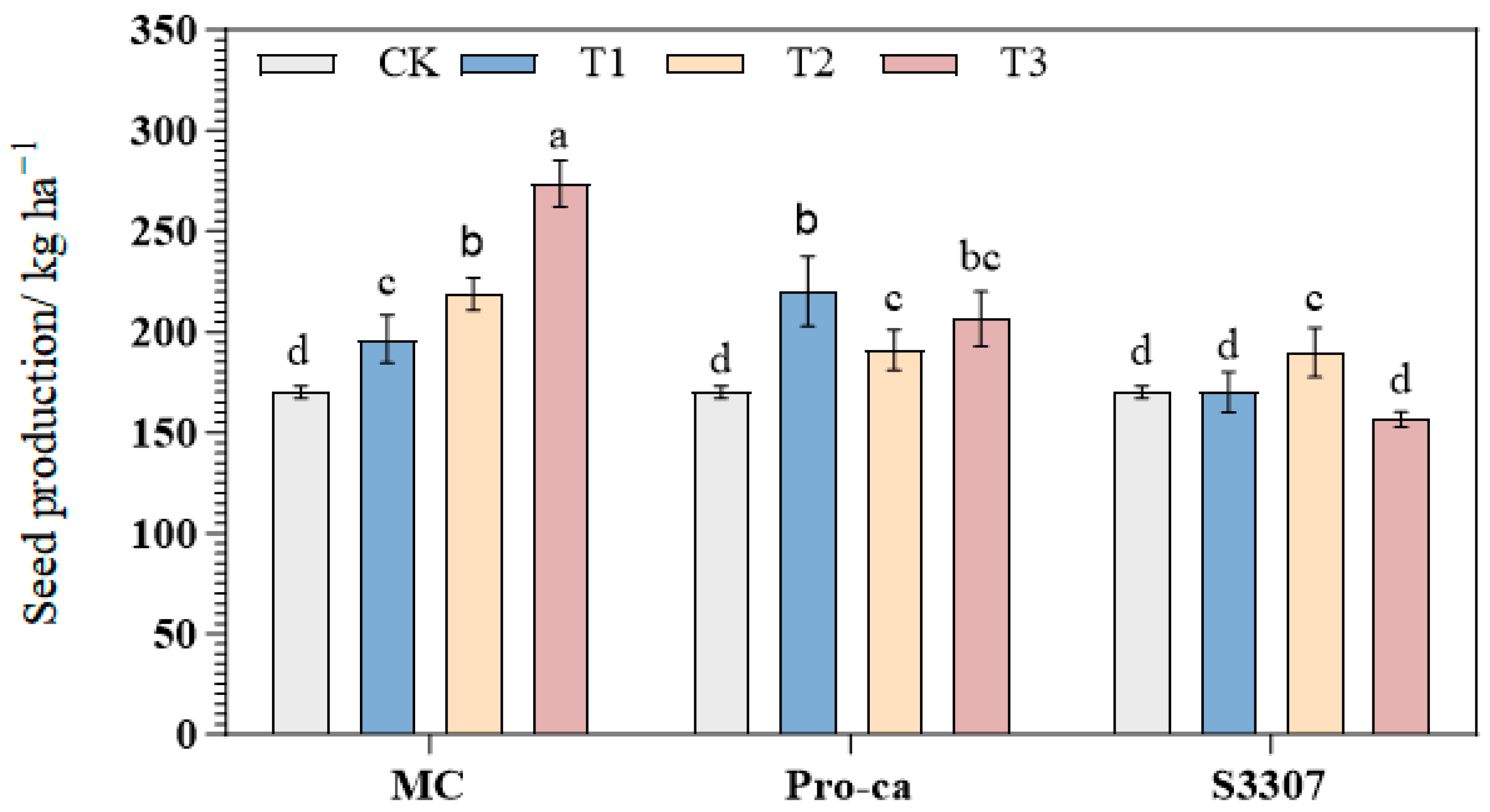
2.3. Effects of Different PGRs on the Seed Yield of Purple Alfalfa
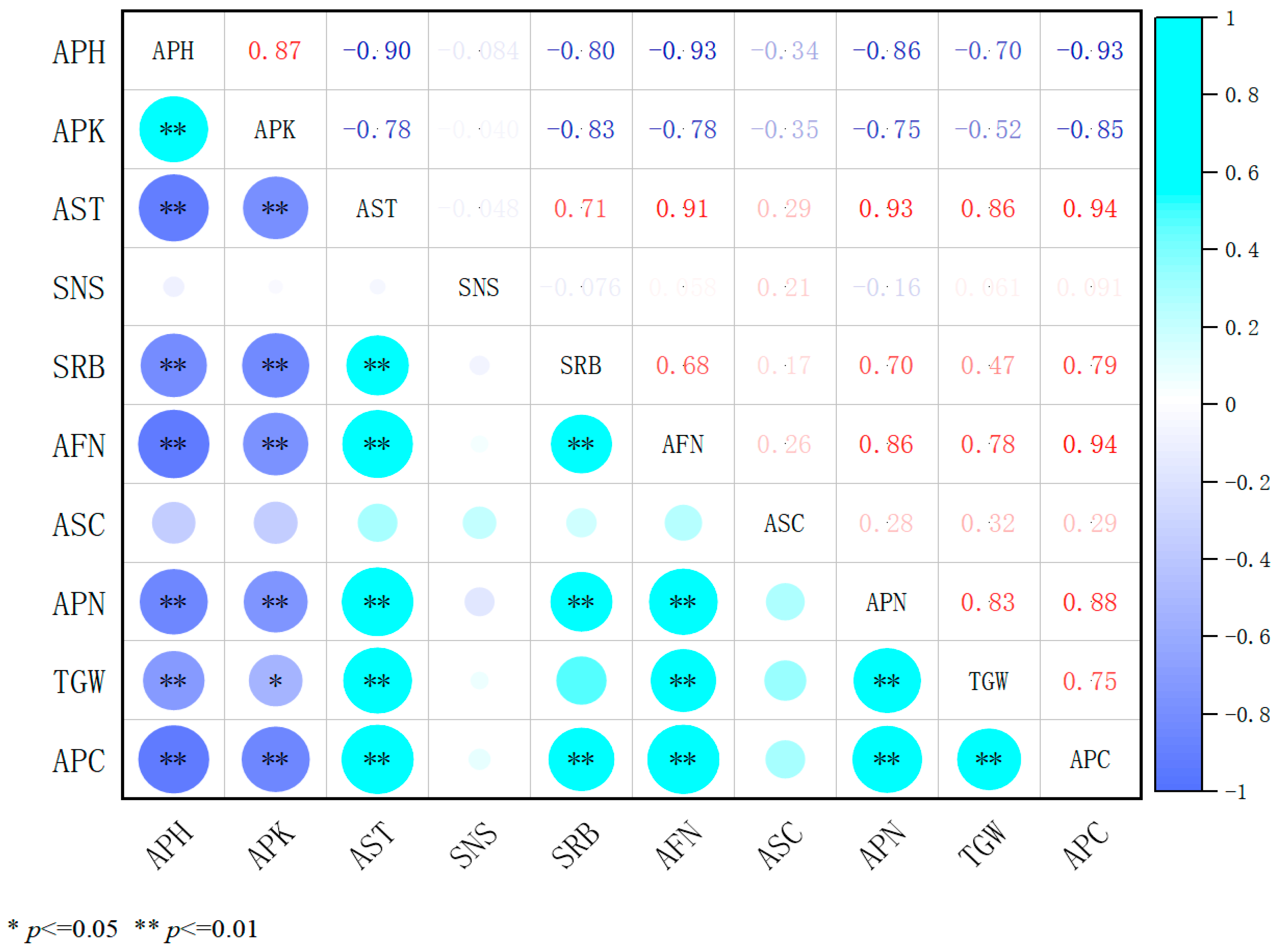
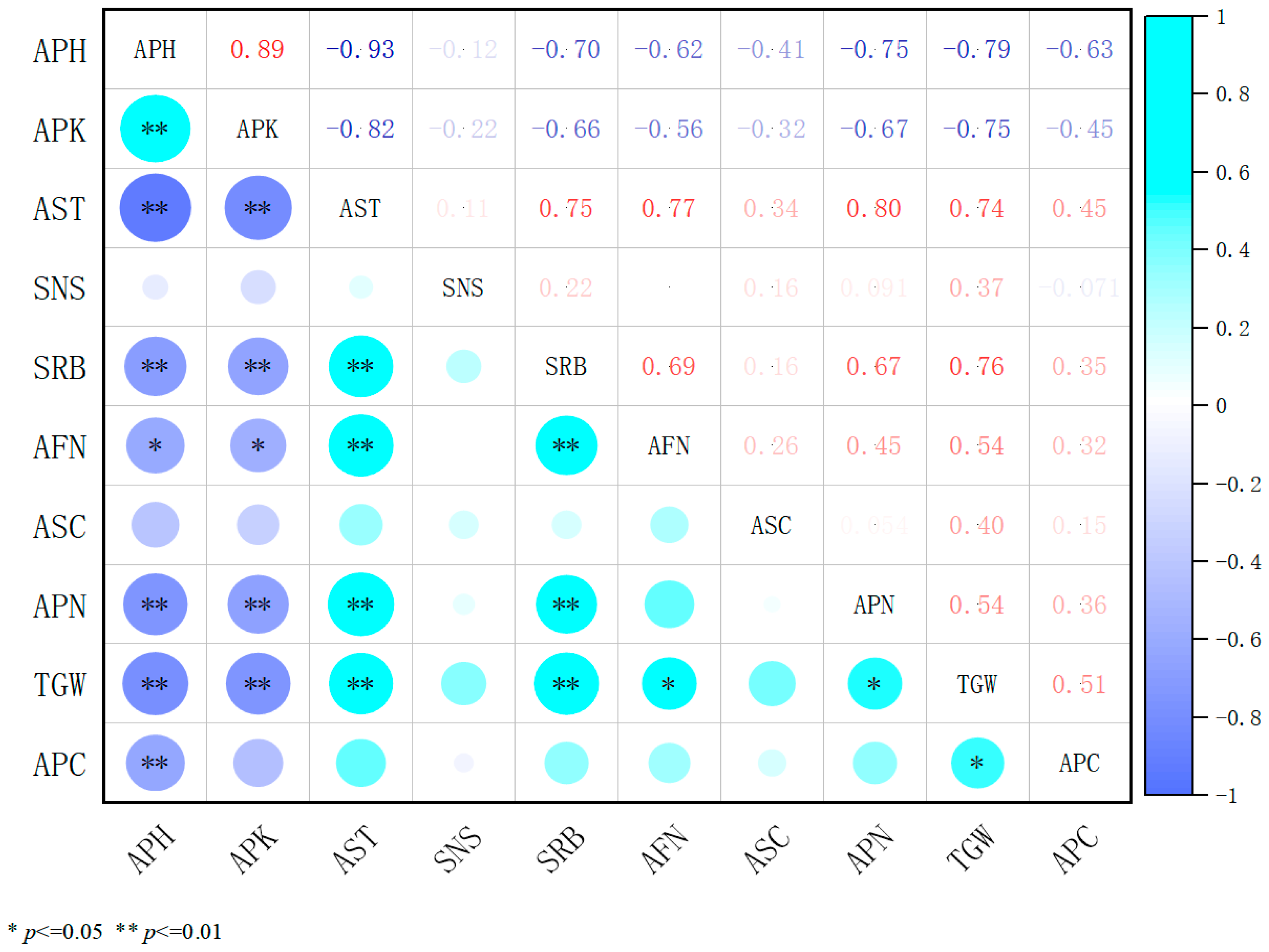
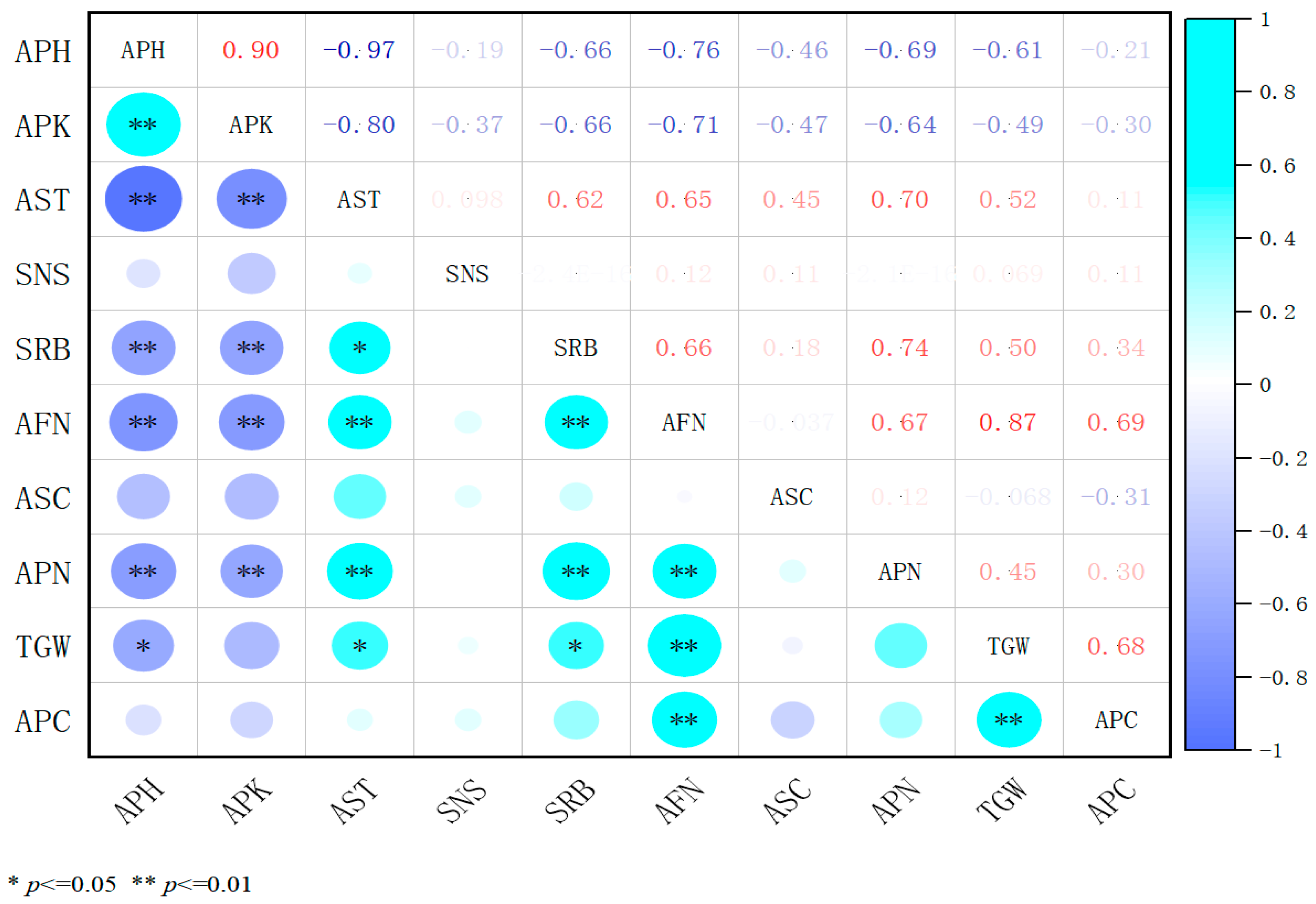
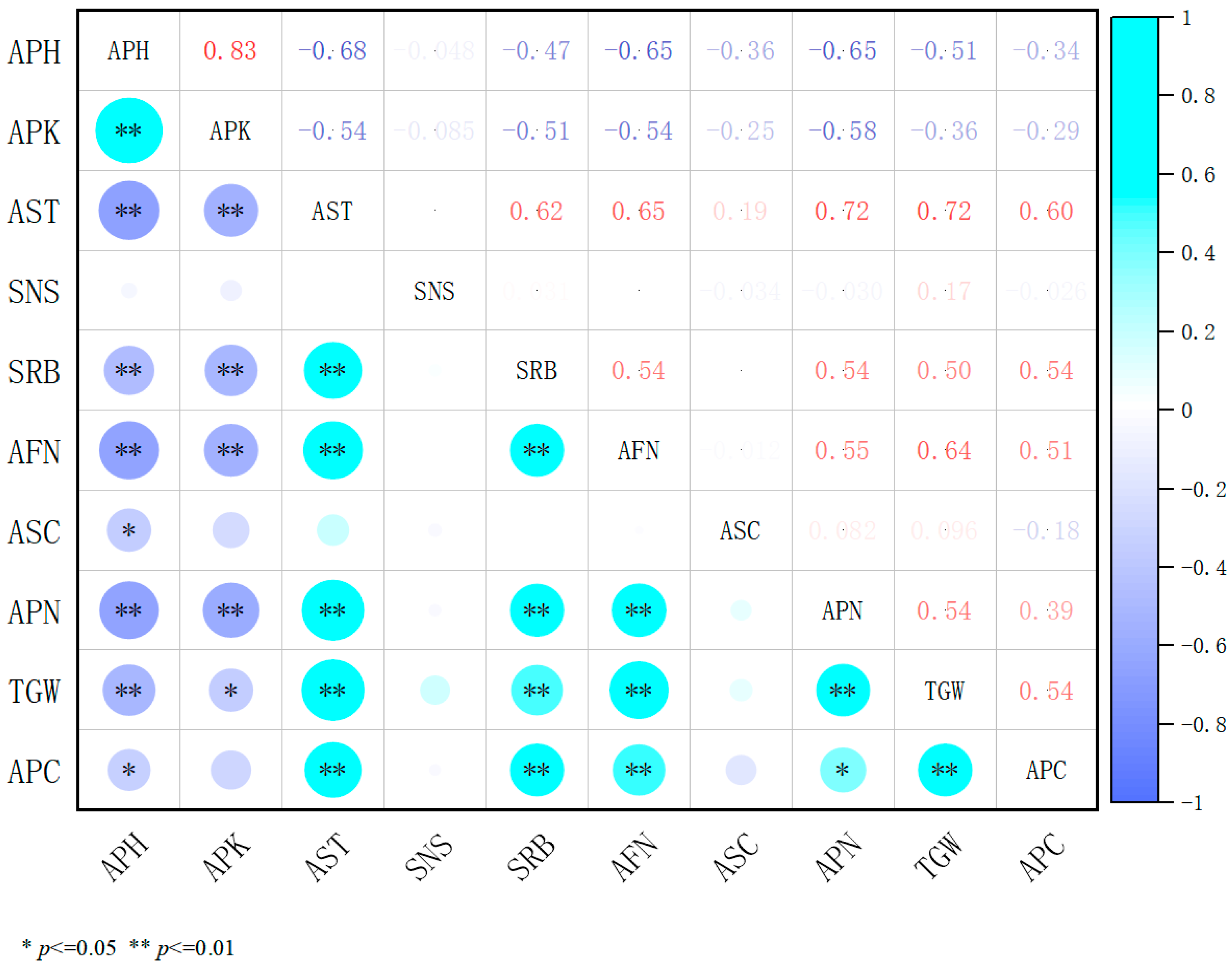
2.4. Correlation Analysis of Three Plant Growth Regulators with Agronomic Traits and Yield Components
2.4.1. Analysis of the Association Between 98% Mepiquat Chloride and Agronomic Traits as Well as Yield Components in Alfalfa
2.4.2. Analysis of the Association Between 5% Prohexadione-Calcium and Agronomic Traits as Well as Yield Components in Alfalfa
2.4.3. Analysis of the Association Between 5% Uniconazole and Agronomic Traits as Well as Yield Components in Alfalfa
2.4.4. An Integrated Correlation Analysis of the Effects of Three Plant Growth Regulators on the Traits and Yield Components of Alfalfa
2.5. Principal Component Analysis for a Comprehensive Evaluation of Seed Yield and Yield Components in Purple Alfalfa
3. Discussion
4. Materials and Methods
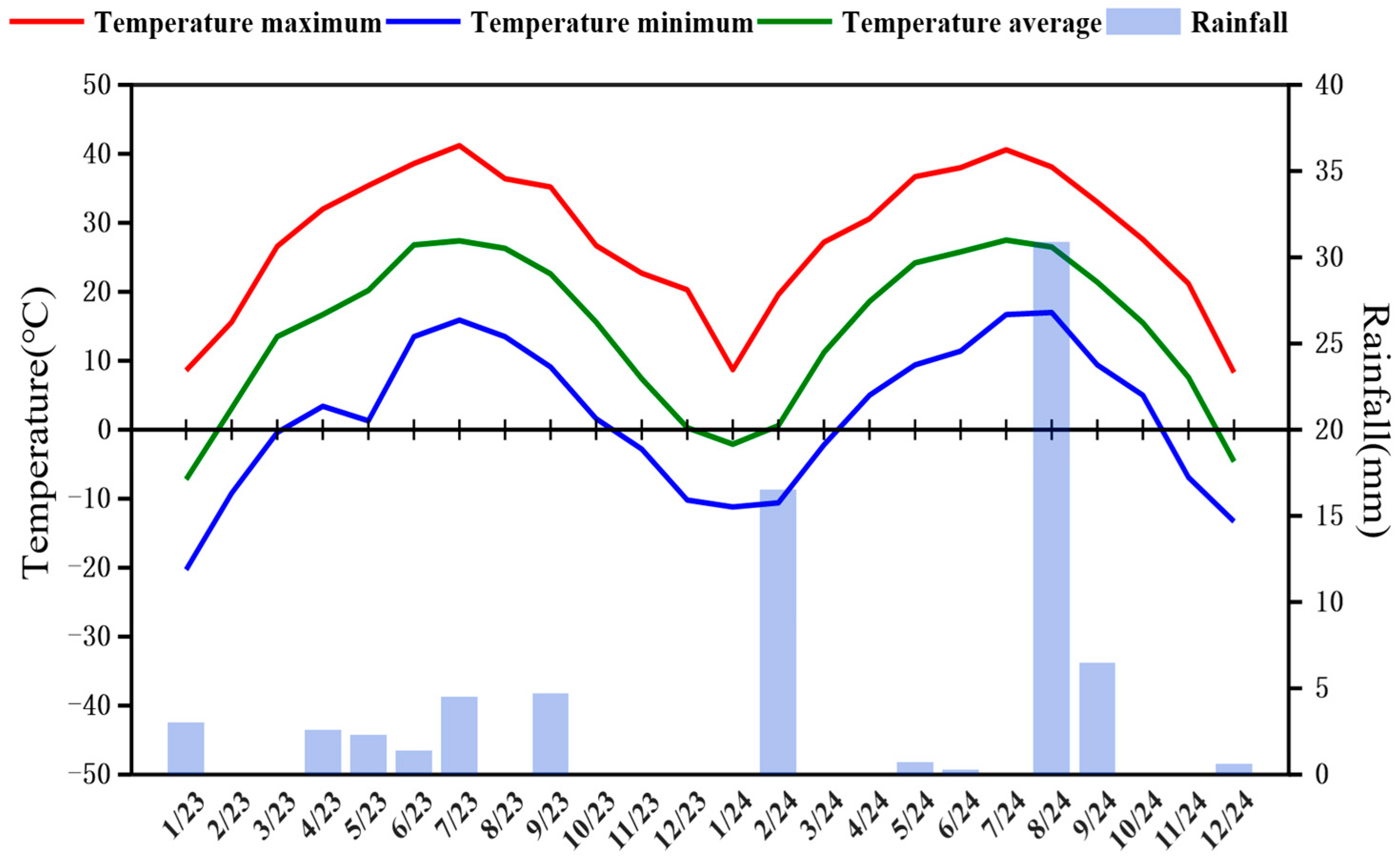
4.1. Overview of the Experimental Site
4.2. Experimental Materials
4.3. Experimental Design
4.4. Measurement Indices and Methods
4.4.1. Alfalfa Growth Traits
4.4.2. Determination of Seed Yield Components
4.5. Data Statistics and Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bora, K.S.; Sharma, A. Phytochemical and pharmacological potential of Medicago sativa: A review. Pharm. Biol. 2011, 49, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Palacios, J.C.O.; McClements, D.J.; Mahfouzi, M.; Moreno, A. Alfalfa as a sustainable source of plant-based food proteins. Trends Food Sci. Technol. 2023, 135, 202–214. [Google Scholar] [CrossRef]
- Hou, P.; Shen, B.; Kong, X.; Sawadgo, W.; Li, W. 2024 Q1 Agricultural Trade Series: US-China Trade Trends and Opportunities for Alfalfa Exports; Plains Press: Auburn, AL, USA, 2024. [Google Scholar]
- Shi, S.; Nan, L.; Smith, K.F. The current status, problems, and prospects of alfalfa (Medicago sativa L.) breeding in China. Agronomy 2017, 7, 1. [Google Scholar] [CrossRef]
- Acharya, J.P.; Lopez, Y.; Gouveia, B.T.; de Bem Oliveira, I.; Resende, M.F.R., Jr.; Muñoz, P.R.; Rios, E.F. Breeding alfalfa (Medicago sativa L.) adapted to subtropical agroecosystems. Agronomy 2020, 10, 742. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, M.M.; Li, P. Policy analysis of China’s grass seed industry from the perspective of policy tools. China Grassl. J. 2023, 45, 109–118. [Google Scholar]
- Monirifar, H.; Mirmozaffari Roudsari, A.; Ghassemi, S.; Tavasolee, A. Harvest time and cultivar effects on growth, physiological traits, yield and quality of alfalfa in saline condition. Int. J. Plant Prod. 2020, 14, 453–462. [Google Scholar] [CrossRef]
- Ventroni, L.M.; Volenec, J.J.; Cangiano, C.A. Fall dormancy and cutting frequency impact on alfalfa yield and yield components. Field Crops Res. 2010, 119, 252–259. [Google Scholar] [CrossRef]
- Peng, W.; Cai, W.; Pan, J.; Su, X.; Dou, L. Molecular Mechanisms of Alfalfa Response to Abiotic Stresses. Plants 2025, 14, 487. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, Y.; Chai, X.; Li, X.; Ullah, A.; Islam, W.; Zhang, Z.; Zeng, F. Effects of different tillage systems and mowing time on nutrient accumulation and forage nutritive value of Cyperus esculentus. Front. Plant Sci. 2023, 14, 1162572. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Hasan, M.K.; Islam, M.R.; Chowdhury, M.K.; Pramanik, M.H.; Iqbal, M.A.; Rajendran, K.; Iqbal, R.; Soufan, W.; Kamran, M.; et al. Water relations and yield characteristics of mungbean as influenced by foliar application of gibberellic acid (GA3). Front. Ecol. Evol. 2023, 11, 1048768. [Google Scholar]
- Desta, B.; Amare, G. Paclobutrazol as a plant growth regulator. Chem. Biol. Technol. Agric. 2021, 8, 1. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Ma, C.; Zhang, Q. Effects of nitrogen and phosphorus addition on agronomic characters, photosynthetic performance and anatomical structure of alfalfa in northern xinjiang, China. Agronomy 2022, 12, 1613. [Google Scholar] [CrossRef]
- Rademacher, W. Growth retardants: Effects on gibberellin biosynthesis and other metabolic pathways. Annu. Rev. Plant Biol. 2000, 51, 501–531. [Google Scholar] [CrossRef] [PubMed]
- Chalise, D.P.; Snider, J.L.; Hand, L.C.; Roberts, P.; Vellidis, G.; Ermanis, A.; Collins, G.D.; Lacerda, L.N.; Cohen, Y.; Pokhrel, A.; et al. Cultivar, irrigation management, and mepiquat chloride strategy: Effects on cotton growth, maturity, yield, and fiber quality. Field Crops Res. 2022, 286, 108633. [Google Scholar] [CrossRef]
- Souza-Schlick, G.D.; Soratto, R.P.; Fernandes, A.M.; Martins, J.D.L. Mepiquat chloride effects on castor growth and yield: Spraying time, rate, and management. Crop Sci. 2018, 58, 880–891. [Google Scholar] [CrossRef]
- Tang, F.; Luo, H. Carbon remobilization in the stems of upland cotton as affected by mepiquat chloride and plant density. Field Crops Res. 2023, 294, 108864. [Google Scholar] [CrossRef]
- Lee, J.M.; Snider, J.L.; Roberts, P.; Hand, L.C.; Culpepper, A.S.; Pokhrel, A.; Chalise, D.P. The effect of pre-drought mepiquat chloride management on cotton sensitivity to drought during peak water demands. Field Crops Res. 2023, 298, 108969. [Google Scholar] [CrossRef]
- Polat, T.; Özer, H.; Öztürk, E.; Sefaoğlu, F. Effects of mepiquat chloride applications on non-oilseed sunflower. Turk. J. Agric. For. 2017, 41, 472–479. [Google Scholar] [CrossRef]
- Mohapatra, J. Bio-Efficacy Evaluation of Mepiquat Chloride on Growth, Yield and Quality of Tomato (Solanum lycopersicum L.) cv. Kashi Aman. Master’s Thesis, Department of Horticulture, Institute of Agricultural Sciences Banaras Hindu University, Varanasi, India, 2022. [Google Scholar]
- Duong, M.V.; Chung, J.-W.; Ha, V.G.; Moon, H.; Yu, J.-K.; So, Y.-S. Prohexadione-Calcium Mitigates the Overgrowth of Corn Seedlings. Agronomy 2024, 14, 371. [Google Scholar] [CrossRef]
- Ito, A.; Sakamoto, D.; Itai, A.; Nishijima, T.; Oyama, N.; Nakamura, Y.; Moriguchi, T.; Nakajima, I. Effects of GA3+4 and GA4+7 Application Either Alone or Combined with Prohexadione-Ca on Fruit Development of Japanese Pear ‘Kosui’. Hortic. J. 2016, 85, 201–208. [Google Scholar] [CrossRef]
- Kim, Y.H.; Khan, A.L.; Hamayun, M.; Kim, J.T.; Lee, J.H.; Hwang, I.C.; Yoon, C.S.; Lee, I.-J. Effects of prohexadione calcium on growth and gibberellins contents of Chrysanthemum morifolium R. cv Monalisa White. Sci. Hortic. 2010, 123, 423–427. [Google Scholar] [CrossRef]
- Deng, P.; Khan, A.; Zhou, H.; Lu, X.; Zhao, H.; Du, Y.; Wang, Y.; Feng, N.; Zheng, D. Application of prohexadione-calcium priming affects Brassica napus L. seedlings by regulating morph-physiological characteristics under salt stress. PeerJ 2024, 12, e17312. [Google Scholar] [CrossRef] [PubMed]
- Winkler, V.W. Reduced risk concept for prohexadione-calcium, a vegetative growth control plant growth regulator in apples. Acta Hortic. 1997, 451, 667–672. [Google Scholar] [CrossRef]
- Zhang, Z. Prohexadione calcium regulates wheat tolerance to drought stress by maintaining water balance and promoting antioxidant metabolism and photosynthesis. Plant Soil Environ. 2024, 70, 673–681. [Google Scholar] [CrossRef]
- Ozbay, N.; Ergun, N. Prohexadione calcium on the growth and quality of eggplant seedlings. Pesqui. Agropecu. Bras. 2015, 50, 932–938. [Google Scholar] [CrossRef]
- Han, Y.; Gao, Y.; Shi, Y.; Du, J.; Zheng, D. Genome-wide transcriptome profiling reveals the mechanism of the effects of uniconazole on root development in Glycine max. J. Plant Biol. 2017, 60, 387–403. [Google Scholar] [CrossRef]
- Liu, S.; Qiao, J.; Zhang, S.; Lu, M.; Yang, Y.; Lai, J.; Guo, Y.; Shi, Y. Application of uniconazole in improving the high-throughput genetic transformation efficiency in maize. Plant Sci. 2024, 349, 112270. [Google Scholar] [CrossRef] [PubMed]
- Pacholak, A.; Burlaga, N.; Frankowski, R.; Zgoła-Grześkowiak, A.; Kaczorek, E. Azole fungicides: (Bio)degradation, transformation products and toxicity elucidation. Sci. Total Environ. 2022, 802, 149917. [Google Scholar] [CrossRef] [PubMed]
- Dembocurski, D. Influência do Uniconazole no Crescimento e Desenvolvimento da Planta na Composição Química de Grãos de Milho. Master’s Thesis, Universidade Estadual do Oeste do Paraná, Cascavel, Brazil, 2023. [Google Scholar]
- Yan, C.; Shan, F.; Wang, C.; Lyu, X.; Wu, Y.; Yan, S.; Ma, C. Positive correlation of lodging resistance and soybean yield under the influence of uniconazole. Agronomy 2024, 14, 754. [Google Scholar] [CrossRef]
- Ahmed, M.D.S. Suitability of Plant Spacings Against Mepiquat Chloride Treated Cotton Plant for Improvement of Yield and Quality. Ph.D. Thesis, Department of Agronomy, Sher-e-Bangla Agricultural University, Dhaka, Bangladesh, 2019. [Google Scholar]
- El-Hifny, Z.M.; Bakheit, B.R.; Hassan, M.S.; Abd El-Rady, W.A. Forage and seed yield variation of alfalfa cultivars in response to planting date. SVU-Int. J. Agric. Sci. 2019, 1, 21–33. [Google Scholar]
- Jing, F.; Shi, S.; A, Y.; Guan, J.; Lu, B.; Wu, B.; Wang, W.; Ma, R.; Nan, P. Analysis of phenotypic and physiological characteristics of plant height difference in alfalfa. Agronomy 2023, 13, 1744. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Zhang, R.; Ma, C.; Dong, S.; Gong, Z. The relationship between internode elongation of soybean stems and spectral distribution of light in the canopy under different plant densities. Plant Prod. Sci. 2021, 24, 326–338. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; Ellis, M.H.; Bonnett, D.G.; Condon, A.G.; Falk, D.; Richards, R.A. The Rht13 dwarfing gene reduces peduncle length and plant height to increase grain number and yield of wheat. Field Crop. Res. 2011, 124, 323–331. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Li, E.; Guo, Y.; Li, W. Plant height is the main factor driving forage yield of Poa species under different row spacings and seeding rates in the Qilian Mountains. Front. Plant Sci. 2025, 16, 1535937. [Google Scholar] [CrossRef] [PubMed]
- Gaur, V.S.; Channappa, G.; Chakraborti, M.; Sharma, T.R.; Mondal, T.K. ‘Green revolution’ dwarf gene sd1 of rice has gigantic impact. Brief. Funct. Genom. 2020, 19, 390–409. [Google Scholar] [CrossRef]
- Asami, T. Toward the next step to the New Green Revolution. Mol. Plant 2023, 16, 802–803. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Du, M.; Zhao, W.; Li, F.; Wang, X.; Eneji, A.E.; Yang, F.; Huang, J.; Meng, L.; Qi, H.; et al. Relationships between plant architecture traits and cotton yield within the plant height range of 80–120 cm desired for mechanical harvesting in the Yellow River Valley of China. Agronomy 2019, 9, 587. [Google Scholar] [CrossRef]
- Kamran, M.; Ahmad, I.; Wang, H.; Wu, X.; Xu, J.; Liu, T.; Ding, R.; Han, Q. Mepiquat chloride application increases lodging resistance of maize by enhancing stem physical strength and lignin biosynthesis. Field Crops Res. 2018, 224, 148–159. [Google Scholar] [CrossRef]
- El-Nwehy, S.S.; Afify, R.R.M. Utilization of gibberellic acid (GA3) and mepiquat chloride (MC) as growth regulators on maize to alleviate salinity stress. J. Breed. Genet. 2023, 55, 1654–1665. [Google Scholar]
- Gwathmey, C.O.; Clement, J.D. Alteration of cotton source–sink relations with plant population density and mepiquat chloride. Field Crops Res. 2010, 116, 101–107. [Google Scholar] [CrossRef]
- Jacyna, T.; Lipa, T. Direct and apparent residual effects of prohexadione-calcium applied to young cropping sweet cherry trees. Acta Agrobot. 2010, 63. [Google Scholar] [CrossRef]
- Huang, X.; Zheng, D.; Feng, N.; Huang, A.; Zhang, R.; Meng, F.; Jie, Y.; Mu, B.; Mu, D.; Zhou, H. Effects of prohexadione calcium spraying during the booting stage on panicle traits, yield, and related physiological characteristics of rice under salt stress. PeerJ 2023, 11, e14673. [Google Scholar] [CrossRef] [PubMed]
- Giannakoula, A.E.; Ilias, I.F.; Maksimović, J.J.D.; Maksimović, V.M.; Živanović, B.D. The effects of plant growth regulators on growth, yield, and phenolic profile of lentil plants. J. Food Compos. Anal. 2012, 28, 46–53. [Google Scholar] [CrossRef]
- Schluttenhofer, C.M.; Massa, G.D.; Mitchell, C.A. Use of uniconazole to control plant height for an industrial/pharmaceutical maize platform. Ind. Crops Prod. 2011, 33, 720–726. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Y.; Wang, L.-F.; Wang, M.; Zhao, M.; Tian, Y.; Li, Y.-F. Transcriptome profiling of the elongating internode of cotton (Gossypium hirsutum L.) seedlings in response to mepiquat chloride. Front. Plant Sci. 2020, 10, 1751. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, J.; Song, Y.; Hai, F.; Wang, L. 5% Prohexadione Calcium EA: Effects on Lodging Resistance, Yield and Its Related Factors of Wheat. J. Agric. 2022, 12, 14. [Google Scholar]
- Yan, Y.; Wan, Y.; Liu, W.; Wang, X.; Yong, T.; Yang, W.; Zhao, L. Influence of seed treatment with uniconazole powder on soybean growth, photosynthesis, dry matter accumulation after flowering and yield in relay strip intercropping system. Plant Prod. Sci. 2015, 18, 295–301. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Toungos, M.D. Plant growth substances in crop production: A Review. Int. J. Innov. Agric. Biol. Res. 2018, 6, 1–8. [Google Scholar]
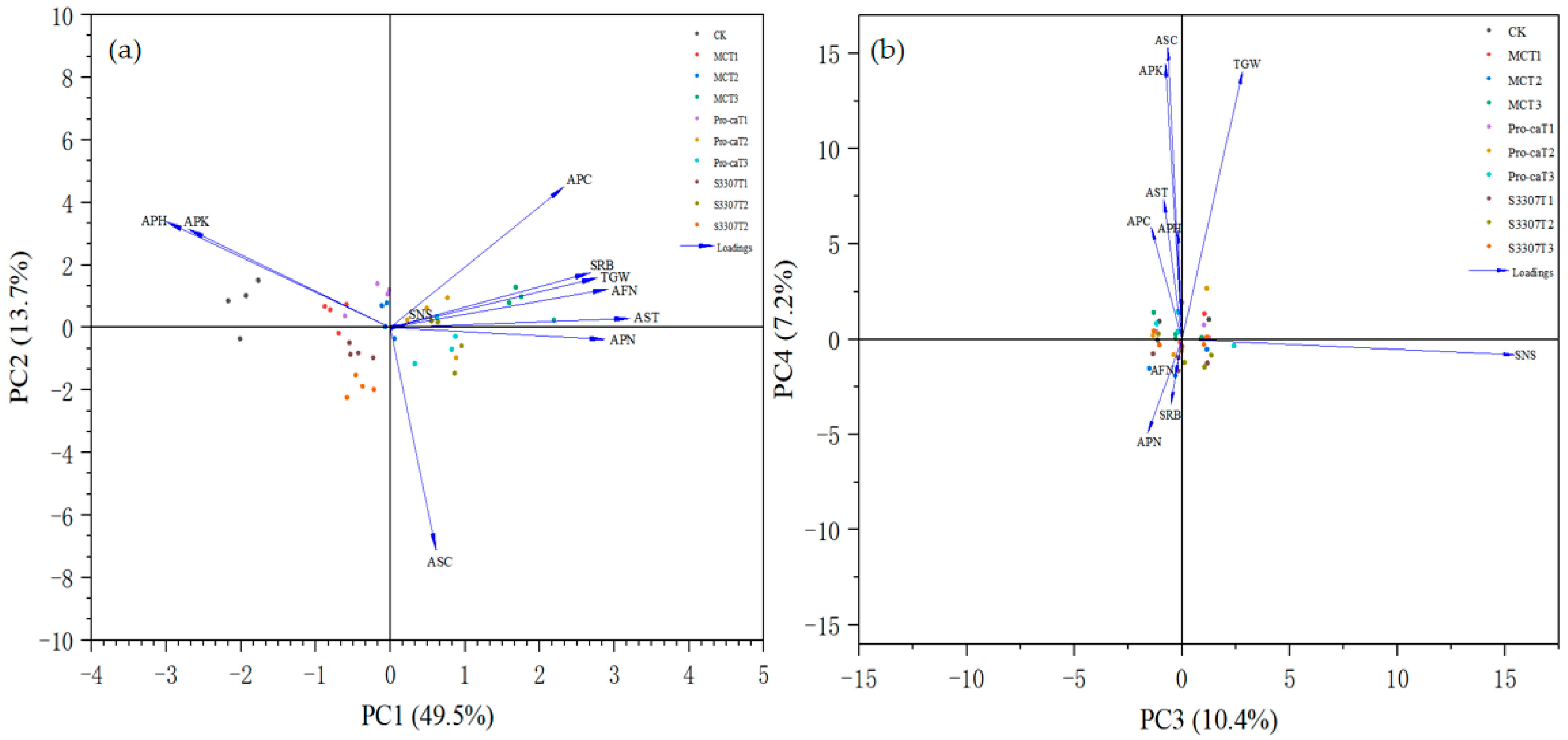
| Name | PCA1 | PCA2 | PCA3 | PCA4 |
|---|---|---|---|---|
| Plant height | −0.37482 | 0.33828 | −0.01204 | 0.20175 |
| Internode length | −0.33816 | 0.31489 | −0.04906 | 0.51011 |
| Stem diameter | 0.40061 | 0.02864 | −0.05374 | 0.25876 |
| Stem nodes | 0.02495 | 0.00859 | 0.97031 | −0.0288 |
| Secondary branching | 0.33465 | 0.1755 | −0.0335 | −0.1206 |
| Simple inflorescence | 0.36452 | 0.12324 | −0.02129 | −0.07091 |
| Number of seeds per pod | 0.07694 | −0.71337 | −0.04231 | 0.54017 |
| Number of pods per inflorescence | 0.35885 | −0.03799 | −0.10129 | −0.17392 |
| Thousand-grain weight | 0.34607 | 0.15899 | 0.17672 | 0.4967 |
| Seed production | 0.29106 | 0.45157 | −0.09057 | 0.20733 |
| Eigenvalue | 4.952 | 1.368 | 1.040 | 0.719 |
| Individual contributions | 49.523 | 13.686 | 10.405 | 7.199 |
| Cumulative contribution | 49.523 | 63.209 | 73.61484 | 80.814 |
| Treatment | F1 | F2 | F3 | F4 | Y |
|---|---|---|---|---|---|
| CK | −4.385 | 0.385 | −0.25 | 0.495 | −2.110 |
| MCT1 | −1.652 | 0.175 | 0.235 | 0.078 | −0.763 |
| MCT2 | −0.975 | 0.133 | −0.227 | −0.758 | −0.108 |
| MCT3 | 4.015 | 0.910 | −0.260 | 0.380 | 2.110 |
| Pro-CaT1 | −0.463 | 0.835 | 0.280 | 0.673 | −0.038 |
| Pro-CaT2 | 1.315 | 0.333 | −0.163 | 0.310 | 0.703 |
| Pro-CaT3 | 1.473 | −0.14 | 0.218 | 0.493 | 0.770 |
| S3307T1 | −0.907 | −0.828 | −0.135 | −0.980 | −0.678 |
| S3307T2 | 1.670 | −0.545 | 0.355 | −0.685 | 0.740 |
| S3307T3 | −0.907 | −1.2575 | 0.050 | −0.0025 | −0.625 |
| Name | Specification | Active Ingredient | Factory |
|---|---|---|---|
| Mepiquat | 5 g.bag | 98% (C7H16NCl) | Sichuan Guoguang Agrochemical Co. (Chengdu, China) |
| Prohexadione-calcium | 10 g.bag | 5% (2(C10H11O5)•Ca | Anyang Quanfeng Biotechnology Co. (Anyang, China) |
| Uniconazole | 10 g.bag | 5% (C15H18ClN3O) | Sichuan Guoguang Agrochemical Co. (Chengdu, China) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.; Sun, Q.; Zhang, S.; An, Y.; Peng, F.; Xiong, J.; Molidaxing, A.; Chen, S.; Wang, Y.; Zhang, B. A Comparative Analysis of the Efficacy of Three Plant Growth Regulators and Dose Optimization for Improving Agronomic Traits and Seed Yield of Purple-Flowered Alfalfa (Medicago sativa L.). Plants 2025, 14, 2258. https://doi.org/10.3390/plants14152258
Peng X, Sun Q, Zhang S, An Y, Peng F, Xiong J, Molidaxing A, Chen S, Wang Y, Zhang B. A Comparative Analysis of the Efficacy of Three Plant Growth Regulators and Dose Optimization for Improving Agronomic Traits and Seed Yield of Purple-Flowered Alfalfa (Medicago sativa L.). Plants. 2025; 14(15):2258. https://doi.org/10.3390/plants14152258
Chicago/Turabian StylePeng, Xianwei, Qunce Sun, Shuzhen Zhang, Youping An, Fengjun Peng, Jie Xiong, Ayixiwake Molidaxing, Shuming Chen, Yuxiang Wang, and Bo Zhang. 2025. "A Comparative Analysis of the Efficacy of Three Plant Growth Regulators and Dose Optimization for Improving Agronomic Traits and Seed Yield of Purple-Flowered Alfalfa (Medicago sativa L.)" Plants 14, no. 15: 2258. https://doi.org/10.3390/plants14152258
APA StylePeng, X., Sun, Q., Zhang, S., An, Y., Peng, F., Xiong, J., Molidaxing, A., Chen, S., Wang, Y., & Zhang, B. (2025). A Comparative Analysis of the Efficacy of Three Plant Growth Regulators and Dose Optimization for Improving Agronomic Traits and Seed Yield of Purple-Flowered Alfalfa (Medicago sativa L.). Plants, 14(15), 2258. https://doi.org/10.3390/plants14152258






