Response of Patch Characteristics of Carex alatauensis S. R. Zhang to Establishment Age in Artificial Grasslands on the Qinghai–Tibet Plateau, China
Abstract
1. Introduction
2. Results
2.1. Tillering Structure Parameters of C. alatauensis Under Different Restoration Years
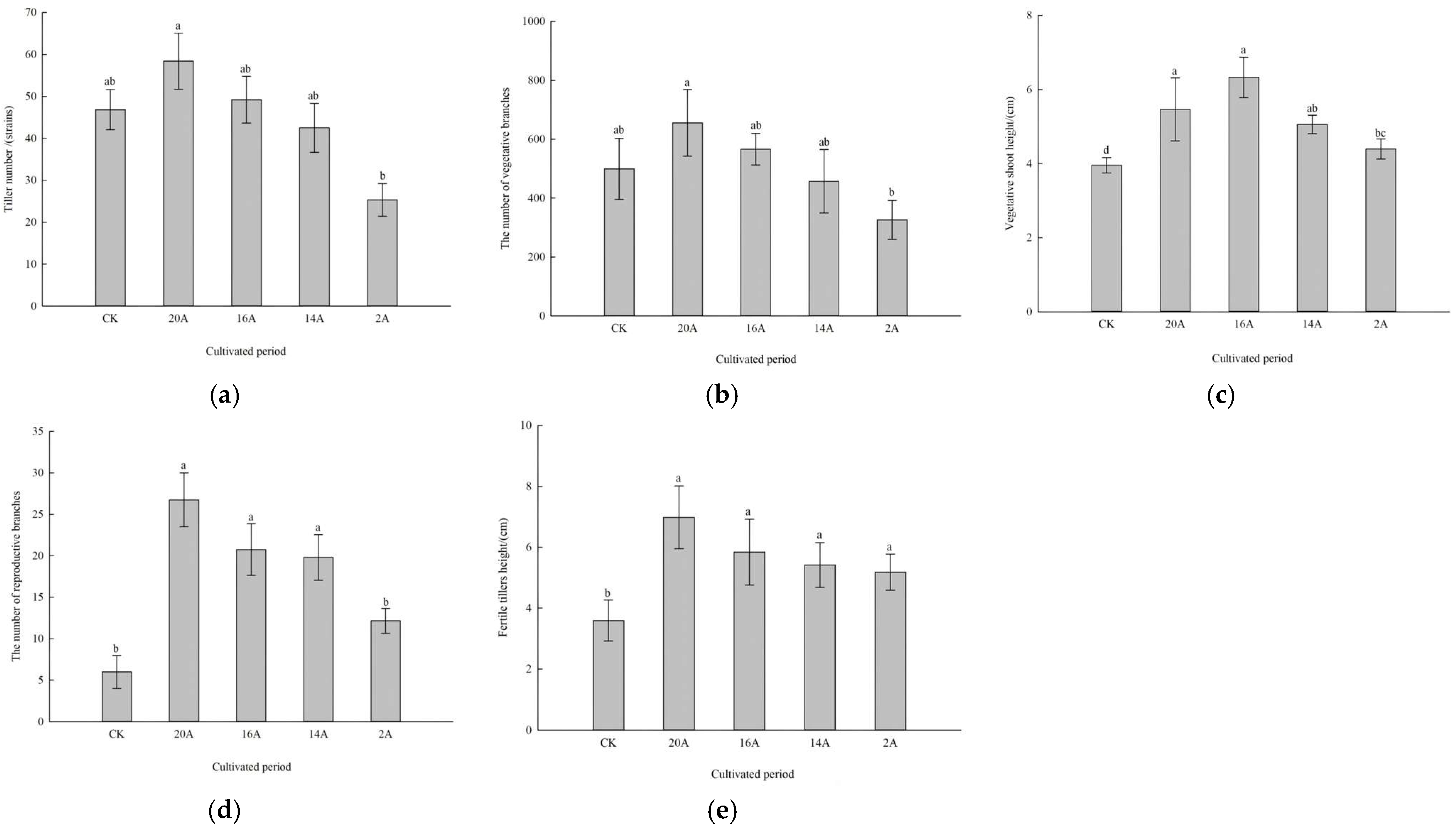
2.1.1. Characteristics of Tillers, Reproductive Shoots and Vegetative Shoots of C. alatauensis Under Different Restoration Years
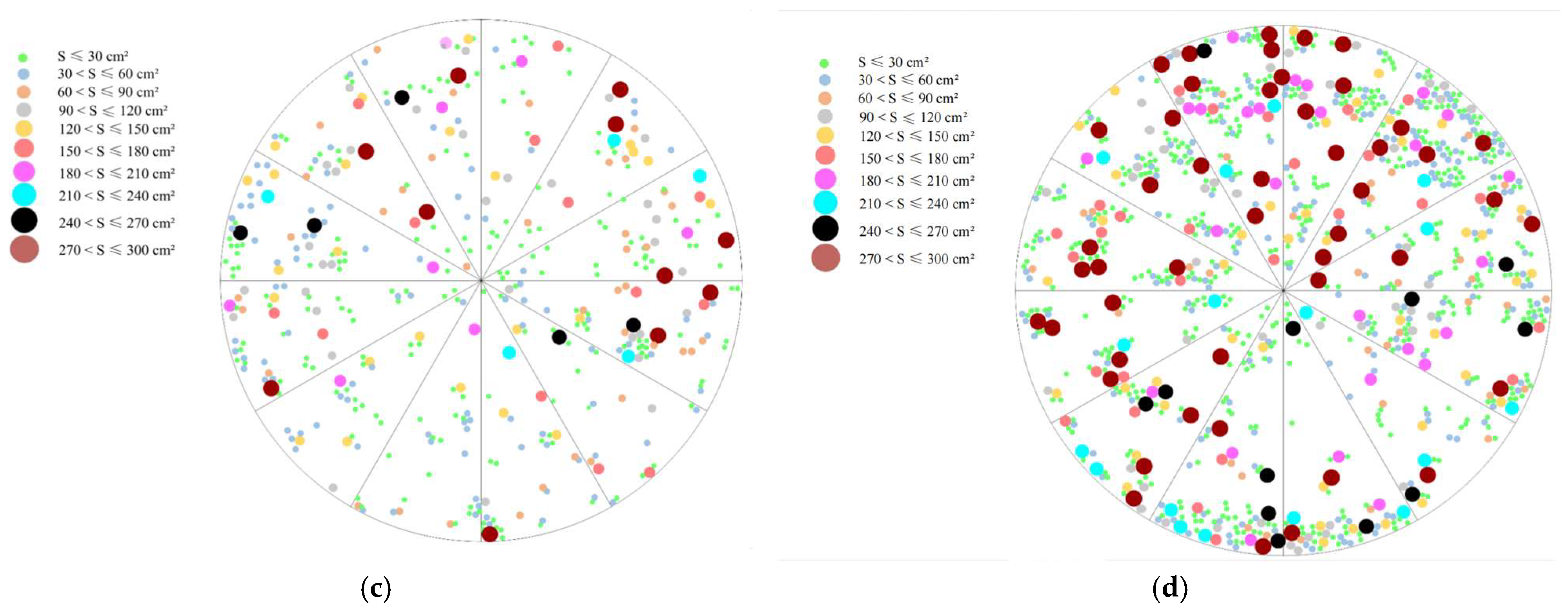
2.1.2. Spatial Distribution and Expansion Characteristics of C. alatauensis Patches in Artificial Grasslands with Different Establishment Ages
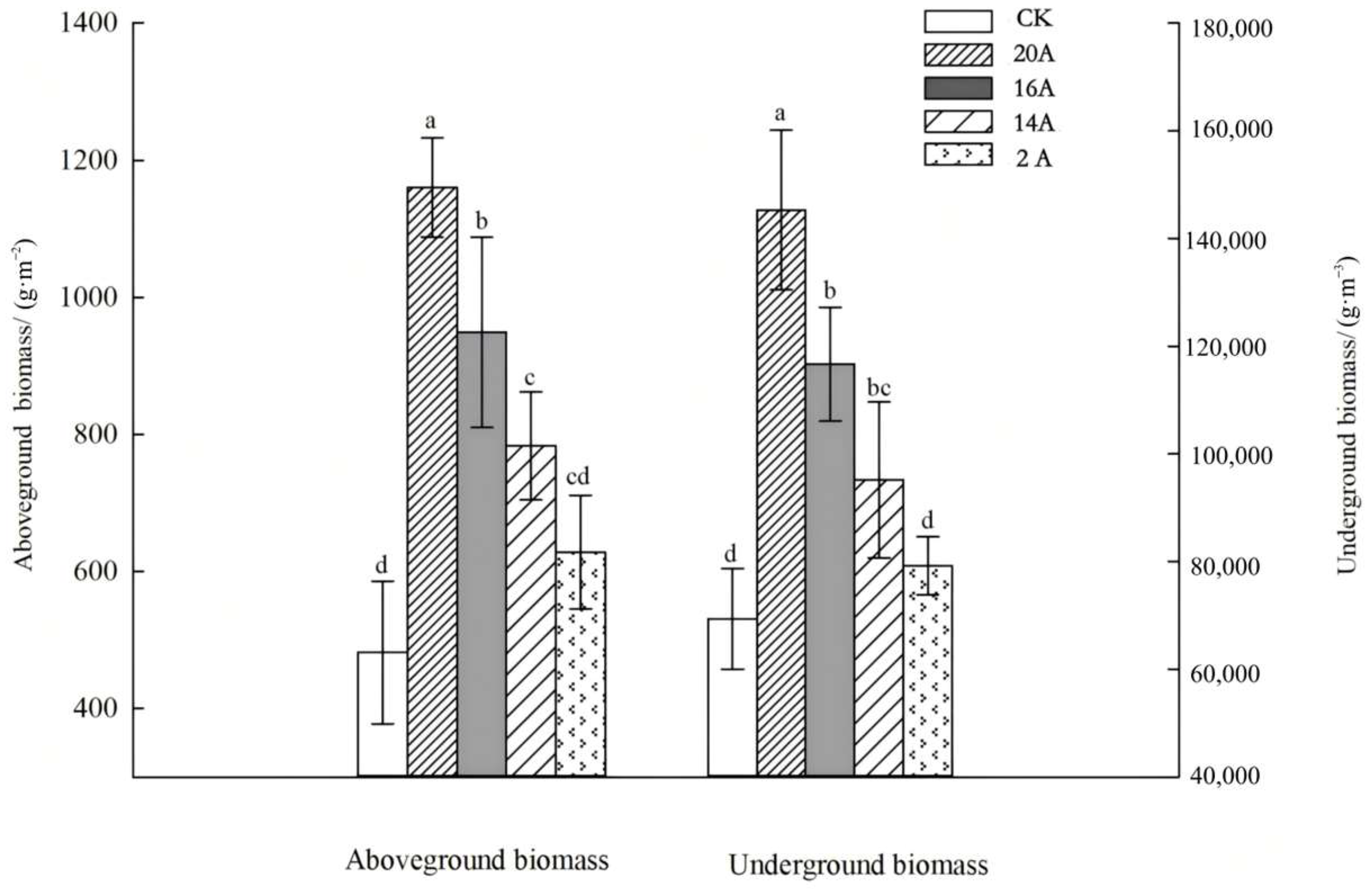
2.1.3. Biomass Characteristics of C. alatauensis Across Different Restoration Ages
2.2. Community Structure and Diversity Characteristics Under Different Restoration Years
2.2.1. Plant Community Height, Coverage and Aboveground Biomass Characteristics
2.2.2. Characteristics of Grassland Plant Diversity
2.3. Physicochemical Characteristics of Soil Under Different Restoration Years
2.3.1. Soil pH Characteristics
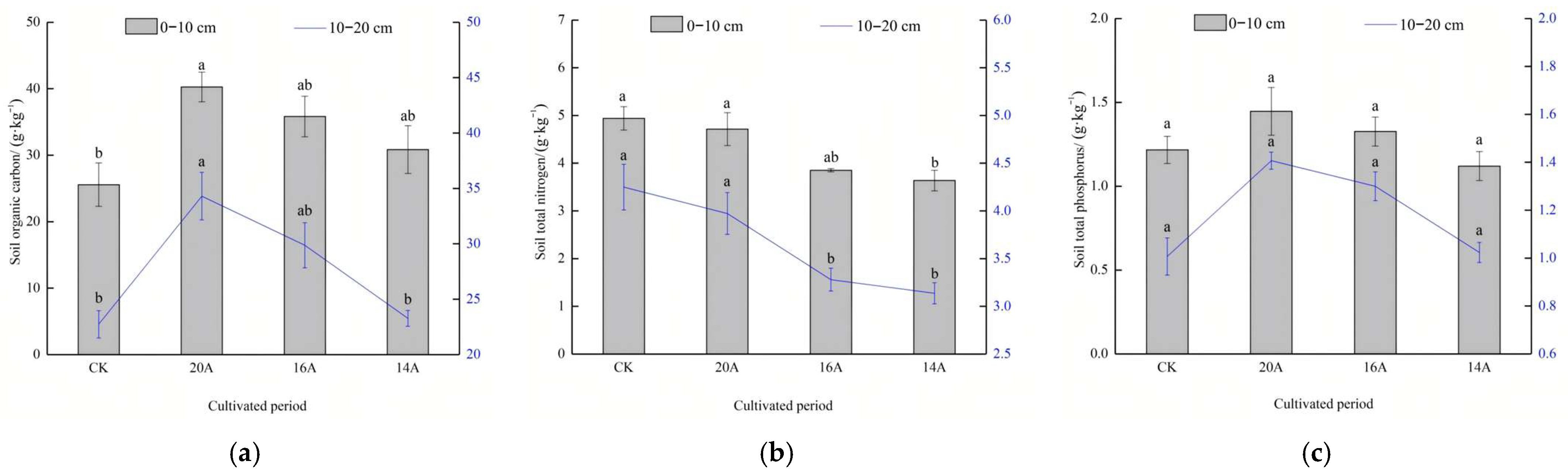
2.3.2. Characteristics of Soil Organic Carbon, Total Nitrogen and Total Phosphorus
2.3.3. Characteristics of Soil Ammonium Nitrogen, Nitrate Nitrogen and Available Phosphorus
2.4. Characteristics of C. alatauensis Tiller Structure Correlated with Plant Community Characteristics and Soil Physicochemical Properties
3. Discussion
3.1. Reproductive and Expansion Characteristics of C. alatauensis
3.2. Characteristics of Plant Community Structure and Diversity
3.3. Physicochemical Characteristics of Artificial Grassland Soil and Its Synergistic Restoration Mechanism with Vegetation Cover
4. Materials and Methods
4.1. General Situation of Test Site
4.2. Experimental Design
4.3. Index Determination and Methods
4.3.1. Functional Characteristics of C. alatauensis
4.3.2. Community Characteristics of C. alatauensis
- (1)
- Simpson’s diversity index (D): D = 1 − ΣPi2
- (2)
- Shannon–Wiener diversity index (H): H = −ΣPilnpi
- (3)
- Pielou’s evenness index (E): E = −ΣPilnpi/lnS
- (4)
- Species richness index (R): R = total number of observed species in the plot
4.3.3. Soil Characteristics
4.4. Data Analysis Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, M.; Xu, Y.D.; Li, S.; Dong, S.K.; Yang, W.K.; Zhang, G.R.; Li, S.Y.; Dou, J.; Zhao, X.L. Active restoration facilitates sedge colonization in degraded alpine meadows on the Qinghai-Tibetan Plateau. J. Environ. Manag. 2025, 388, 126028. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Li, C.D.; Zhao, X.Q.; Liu, W.T.; Yang, X.X.; Lv, W.D.; Sun, S.N.; Cao, Q.; Dong, Q.M. Fungal diversity shapes ecosystem multifunctionality in alpine grasslands under different herbivore assemblages: A case study from the Qinghai-Tibetan Plateau. Catena 2025, 256, 109109. [Google Scholar] [CrossRef]
- Bai, M.M.; Wei, K.T.; Ma, K.K.; Xu, C.L.; Yu, X.J. Rest grazing from the critical period of soil thawing promotes the propagation of Kobresia humilis in alpine meadow. Ecol. Eng. 2022, 179, 106634. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, G.X.; Yang, Y.; Yang, Y.; Peng, A.H.; Zhang, L. Effect of simulative warming on growth and antioxidative characteristics of Kobresia pygmaea and K. tibetica in the permafrost region of Qinghai-Tibetan Plateau, China. J. Appl. Ecol. 2017, 28, 1161–1167. [Google Scholar] [CrossRef]
- Li, J.X.; Wu, L.L.; Zhou, Y.Z.; Xie, Y.L.; Lu, F.W.; Chang, F.F.; Yang, X.; Han, X.Z.; Cheng, M.X. Kobresia humilis via root-released flavonoids recruit Bacillus for promoted growth. Microbiol. Res. 2024, 287, 127866. [Google Scholar] [CrossRef]
- Chen, Y.P.; He, Q.; Yang, Y.S.; Wang, J.B.; Zhang, X.J. Plant community and soil water characteristics under different grazing intensities ofalpine meadow on southern slope of Oilian Mountains. Grassl. Sci. 2023, 40, 2000–2013. [Google Scholar]
- Li, M.; Wang, J.B.; Zhang, X.J.; Zhang, Y.; Wang, Z.R.; Yang, Y.S. Effects of the traits of reproductive and vegetative branches of Kobresia humilis under different herbivore assemblage grazing in alpine grassland. J. Ecol. 2024, 44, 10162–10177. [Google Scholar] [CrossRef]
- Liu, W.W.; Wang, F.C.; Yang, X.X.; Liu, Y.Z.; Feng, B.; Yu, Y.; Zhang, C.P.; Cao, Q.; Dong, Q.M. Effects of the traits of reproductive and vegetative branches of Kobresia humilis under different herbivore assemblage grazing in alpine grassland. Acta Agrestia Sin. 2022, 30, 2231–2238. [Google Scholar]
- Pang, X.P.; Jia, T.T.; Li, Q.Q.; Luo, M.W.; Xiao, Y.; Zhao, X.; Guo, Z.G. Effect of available burrow densities of plateau pika (Ochotona curzoniae) on characteristics and distribution pattern of Kobresia pygmaea community. Acta Ecol. Sin. 2015, 35, 873–884. [Google Scholar] [CrossRef][Green Version]
- Augusteyn, J.; Rich, M.; Mitchell, C.; Mulder, E.; Nolan, B.; Lim, L.; Melzer, R. Does reducing grazing pressure or predation conserve kowaris a case study at Diamantina National Park. Aust. J. Zool. 2022, 70, 56–73. [Google Scholar] [CrossRef]
- Yan, S.; Xia, F.; Wei, W.; Wang, J.L.; Wu, H.Y.; Ran, L.L.; Xue, Y.Y.; Shi, H.; Zheng, S.K.; Wang, J.Q.; et al. Differences along an erosion gradient in alpine meadow plant community diversity and factors influencing diversity. Acta Pharm. Sin. 2025, 34, 1–13. [Google Scholar] [CrossRef]
- Shang, Z.H.; Long, R.J. Formation causes and recovery of the “Black Soil Type” degraded alpine grassland in Qinghai-Tibetan Plateau. Front. Agric. China 2007, 1, 197–202. [Google Scholar] [CrossRef]
- Gao, X.X.; Dong, S.K.; Xu, Y.D.; Wu, S.G.; Wu, X.H.; Zhang, X.; Zhi, Y.L.; Li, S.; Liu, S.L.; Li, Y.; et al. Resilience of revegetated grassland for restoring severely degraded alpine meadows is driven by plant and soil quality along recovery time: A case study from the Three-river Headwater Area of Qinghai-Tibetan Plateau. Agric. Ecosyst. Environ. 2019, 279, 169–177. [Google Scholar] [CrossRef]
- Xie, L.L.; Ma, Y.S.; Wang, Y.L.; Ma, Y.; Wang, X.L. Changes in soil bacterial and fungal community composition and functional groups during the artificial restoration of degraded grassland of “black-soil mountain”. Ecol. Evol. 2024, 14, e70361. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.H.; Wu, Q.G.; Hu, J.Y.; Yu, L.F.; Bie, P.F.; Wang, H.; Deng, D.Z. Changes in soil physical and chemical properties during the process of alpine meadow degradation along the eastern Qinghai-Tibet plateau. Eurasian Soil Sci. 2018, 51, 1440–1446. [Google Scholar] [CrossRef]
- Zhou, X.B.; Wang, X.L.; Ma, Y.S.; Wang, Y.L.; Ma, Y.; Xie, L.L. Fertilization can accelerate the pace of soil microbial community response to rest-grazing duration in the three-river source region of China. Ecol. Evol. 2023, 13, e10734. [Google Scholar] [CrossRef] [PubMed]
- She, Y.D.; Li, X.L.; Zhang, J.; Zhou, H.K. Effects of soil characteristics on grassland productivity in long-term artificial grassland establishment. Glob. Ecol. Conserv. 2024, 54, e03136. [Google Scholar] [CrossRef]
- Yang, P.N.; Li, X.L.; Li, C.Y.; Zhang, J. Effects of long-term exclosure on main plant functional groups and their biochemical properties in a patchily degraded alpine meadow in the source zone of the yellow river, west China. Agronomy 2023, 13, 2781. [Google Scholar] [CrossRef]
- Li, X.W.; Li, X.L.; Shi, Y.; Zhao, S.J.; Liu, J.L.; Lin, Y.Y.; Li, C.L.; Zhang, C.H. Effects of microtopography on soil microbial communities in alpine meadows on the Qinghai-Tibetan Plateau. Catena 2024, 239, 107945. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 1999. [Google Scholar]
- Li, X.L.; Yang, Y.W.; Zhang, J.; Shi, L.Q. Growth characteristics of Kobresia humilis clones with different degrees of degradation. Heilongjiang J. Anim. Husb. Vet. Med. 2002, 10, 19. [Google Scholar] [CrossRef]
- Xing, Y.F.; Shi, J.J.; De, K.J.; Wang, W.; Wang, X.L.; Tang, J.W.; Li, S.Y. Pattern and intraspecific association of Kobresia humilisin perennial artificial grassland spatial distribution. J. Grassl. Sci. 2022, 30, 253–258. [Google Scholar]
- Gao, X.Z.; Li, X.L.; Ma, G.H.; Ma, G.X. Reproductive law of Kobresia humilis and Kobresia alpina clones in different degraded alpine meadows. Grass Ind. Anim. Husb. 2008, 1, 7–11. [Google Scholar]
- Xu, Z.W.; Li, X.L.; Sun, H.Q. Community composition and species diversity analysis of “black soil type” degraded grassland. Heilongjiang J. Anim. Sci. Vet. Med. 2004, 6, 58–59. [Google Scholar] [CrossRef]
- Abigail, A.D.; Paul, K.; Brian, R.; Wilson, O.C. Paddock trees promote pasture biomass accumulation and improve soil properties in grazing systems. Plant Soil 2025, 1–20. [Google Scholar] [CrossRef]
- She, Y.D.; Zhou, H.K.; Zhang, Z.H.; Qin, R.M.; Chang, T.; Su, H.Y.; Wei, J.J.; Li, H.L.; Ma, L. Plant community characteristics, soil nutrients and their interactions in artificial grassland with different establishment years in the Three Rivers Headwater Region. Grassl. Sci. 2023, 69, 165–177. [Google Scholar] [CrossRef]
- Li, H.N.; Li, X.L.; Yang, Y.W.; Ma, G.H.; Ma, G.X. Changes of plant biomass and seed productivity in Kobresia humuilis and K. pygmaedunder different degenerative gradations in alpine meadow. Hubei Agric. Sci. 2003, 5, 84–87. [Google Scholar]
- Zhao, W.; Yin, Y.L.; Li, S.X.; Song, J.Q.; Dong, Y.L.; Su, S.F. Establishing artificial grassland on extremely degraded alpine meadow changes the soil fungal community and function in the qilian mountain area. Land Degrad. Dev. 2025, 36, 919–931. [Google Scholar] [CrossRef]
- Xing, Y.F.; Shi, J.J.; Ma, Y.; Ou, W.Y.; Liu, Q.Q.; Lyu, L.Y.; Zhang, H.R.; Cai, Z.C. Keystone species and driving factors of artificial grassland on the Qinghai–Tibetan plateau, China. Diversity 2024, 16, 758. [Google Scholar] [CrossRef]
- Seahra, S.E.; Yurkonis, K.A.; Newman, J.A. Species patch size at seeding affects diversity and productivity responses in establishing grasslands. J. Ecol. 2016, 104, 479–486. [Google Scholar] [CrossRef]
- Zhou, H.K.; Zhao, X.Q.; Tang, Y.H.; Gu, S.; Zhou, L. Alpine grassland degradation and its control in the source region of the Yangtze and Yellow Rivers, China. Grail Sci. 2005, 51, 191–203. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Shi, J.J.; Ma, Y.S.; Wang, Y.L.; Wang, X.L.; Lyu, L.Y.; He, M.H.; Cai, Z.C. Relationship between plant diversity and community stability in alpine mining areas. J. Mt. Sci. 2025, 22, 1–12. [Google Scholar] [CrossRef]
- Zheng, L.L.; Song, M.H.; Wu, C.P.; Meng, J.; Guo, Y.; Zu, J.X.; Yu, F.H. Soil nutrient heterogeneity affects community stability through changing asynchrony in an alpine meadow on the Qinghai-Tibetan Plateau. Glob. Ecol. Conserv. 2024, 53, e03045. [Google Scholar] [CrossRef]
- Liu, X.Q.; Zhang, X.; Zhang, L.F.; Li, Y.N.; Zhao, L.; Xu, S.X.; Li, H.Q.; Ma, R.R.; Niu, B.; Gao, Y.B.; et al. Effects of exclosure duration on the community structure and species diversity of an alpine meadow in the Qinghai-Tibet Plateau. Acta Ecol. Sin. 2016, 36, 5150–5162. [Google Scholar] [CrossRef]
- Wang, C.T.; Cao, G.M.; Wang, Q.L.; Shi, J.J.; Du, Y.G.; Long, R.J. Characteristics of artificial grassland plant communities with different establishm entduration and their relationships with soil properties in the source region of Three Rivers in China. J. Appl. Ecol. 2007, 11, 2426–2431. [Google Scholar] [CrossRef]
- Luo, S.H.; Li, L.Q.; Ma, Y.S.; Li, S.X.; Wang, X.L.; Wang, Y.L. Vegetation community characteristics of Poa pratensis cultivated grassland in Qinghai grassland with different growth years. Herbology 2018, 5, 24–29. [Google Scholar] [CrossRef]
- Zhang, K.C.; Pan, J.P.; An, Z.S.; Zhang, Y.; Yu, Y.P. Effects of alpine meadow degradation on the soil physical and chemical properties in Maqu, China. Res. Cold Arid. Reg. 2024, 16, 269–277. [Google Scholar] [CrossRef]
- Li, J.S.; Shao, X.Q.; Huang, D.; Shang, J.Y.; Liu, K.S.; Zhang, Q.; Yang, X.M.; Li, H.; He, Y.X. The addition of organic carbon and nitrogen accelerates the restoration of soil system of degraded alpine grassland in Qinghai-Tibet Plateau. Ecol. Eng. 2020, 158, 106084. [Google Scholar] [CrossRef]
- Sun, H.F.; Li, X.L.; Jin, L.Q.; Zhang, J.; Li, Q.D. Variation of vegetation community and soil nutrients of artificial grassland in source area of Yellow River. Bull. Soil Water Conserv. 2019, 39, 25–30. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yao, B.H.; Zhang, C.J.; Wang, F.; Sun, X.M.; Su, J.H. Seasonal changes of soil physicochemical properties and enzyme activity of “Bare land” degraded meadow in gannan. J. Grassl 2021, 29, 220–227. [Google Scholar]
- Liu, Y. Effects of Different Land Use Patterns on Landscape Pattern and Soil Spatial Heterogeneity in Desert Steppe; Inner Mongolia Agricultural University: Huhehaote, China, 2023. [Google Scholar]
- Xing, Y.F.; Wang, X.L.; Liu, Y.Q.; Hua, R.; Wang, C.; Wu, J.L.; Shi, J.J. Characteristics of plant community and soil organic carbon and nitrogen in artificial grassland with different establishment years. J. Grassl. 2020, 28, 521–528. [Google Scholar]
- Li, S.Y.; Shi, J.J.; Wang, H.B.; He, Y.L.; Tang, Y.Q.; Xing, Y.F.; Wu, J.L.; He, M.H.; Zhang, H.R. Vegetation characteristics of black soil land artificial grassland of different years in source area of Three Rivers. Acta Agric. Boreali-Occident. Sin. 2024, 33, 922–932. [Google Scholar] [CrossRef]
- Yu, Z.H.; Yu, Y.; Cao, Q.; Liu, Y.Z.; Feng, B.; Zhang, X.F.; Dong, Q.M. Evaluation of vegetation-soil synergistic coupling in winter rangeland of the Qilian Mountains under different winter grazing management systems. Acta Prataculturae Sin. 2024, 33, 1–12. [Google Scholar] [CrossRef]







| Sample Plot Number | Patch Type | Proportion% | Growth Rate% | |
|---|---|---|---|---|
| 2019 | 2020 | |||
| CK | Small | 14.23 | 14.83 | 4.22 |
| Middle | 18.58 | 19.15 | 3.05 | |
| Large | 67.19 | 63.85 | −4.97 | |
| 20A | Small | 31.15 | 32.10 | 3.06 |
| Middle | 20.08 | 19.53 | −2.73 | |
| Large | 48.77 | 49.87 | 2.25 | |
| 16A | Small | 32.31 | 32.09 | 2.09 |
| Middle | 20.46 | 19.65 | −3.93 | |
| Large | 47.23 | 47.96 | 1.54 | |
| 14A | Small | 33.23 | 33.91 | 2.04 |
| Middle | 22.84 | 21.91 | −4.04 | |
| Large | 43.94 | 45.43 | 3.40 | |
| 2A | Small | — | — | — |
| Middle | — | — | — | |
| Large | — | — | — | |
| Serial Number of Plots | Species Richness | Simpson Index | Shannon–Wiener Index | Pielou Index |
|---|---|---|---|---|
| CK | 21.33 ± 1.11 a | 0.93 ± 0.01 a | 2.78 ± 0.02 a | 0.91 ± 0.02 a |
| 20A | 20.67 ± 2.89 a | 0.90 ± 0.01 a | 2.60 ± 0.17 a | 0.87 ± 0.01 a |
| 16A | 19.33 ± 1.11 a | 0.84 ± 0.04 a | 2.40 ± 0.10 a | 0.81 ± 0.04 a |
| 14A | 19.00 ± 3.33 a | 0.83 ± 0.07 a | 2.39 ± 0.25 a | 0.79 ± 0.06 a |
| 2A | 18.67 ± 0.44 b | 0.80 ± 0.02 b | 2.36 ± 0.19 b | 0.73 ± 0.07 b |
| Establishment Year | Establishment Duration | Altitude /m | Longitude and Latitude | Dominant Species |
|---|---|---|---|---|
| degraded grassland (CK) | 0A | 3727 | 100°12′41.2″ E 34°27′53.2″ N | L. Virgaurea, Artemisia hedinii Ostenf., A. pendulum |
| 2000 | 20A | 3735 | 100°13′5.6″ E 34°27′59.6″ N | E. Nutans, C. alatauensis,
C. parvula |
| 2004 | 16A | 3735 | 100°13′5.3″ E 34°27′59.0″ N | E. nutans, P. crymophila, C. parvula |
| 2006 | 14A | 3747 | 100°12′34.4″ E 34°27′38.8″ N | E. Nutans, Festuca ovina L., A. pendulum |
| 2018 | 2A | 3720 | 100°15′1.73″ E 34°26′9.86″ N | E. Nutans, P. kansuensis, Lancea tibetica Hook. f. & Thomson |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, L.; Wang, C.; Gao, P.; Li, F.; Liu, Q.; Shi, J. Response of Patch Characteristics of Carex alatauensis S. R. Zhang to Establishment Age in Artificial Grasslands on the Qinghai–Tibet Plateau, China. Plants 2025, 14, 2257. https://doi.org/10.3390/plants14152257
Lyu L, Wang C, Gao P, Li F, Liu Q, Shi J. Response of Patch Characteristics of Carex alatauensis S. R. Zhang to Establishment Age in Artificial Grasslands on the Qinghai–Tibet Plateau, China. Plants. 2025; 14(15):2257. https://doi.org/10.3390/plants14152257
Chicago/Turabian StyleLyu, Liangyu, Chao Wang, Pei Gao, Fayi Li, Qingqing Liu, and Jianjun Shi. 2025. "Response of Patch Characteristics of Carex alatauensis S. R. Zhang to Establishment Age in Artificial Grasslands on the Qinghai–Tibet Plateau, China" Plants 14, no. 15: 2257. https://doi.org/10.3390/plants14152257
APA StyleLyu, L., Wang, C., Gao, P., Li, F., Liu, Q., & Shi, J. (2025). Response of Patch Characteristics of Carex alatauensis S. R. Zhang to Establishment Age in Artificial Grasslands on the Qinghai–Tibet Plateau, China. Plants, 14(15), 2257. https://doi.org/10.3390/plants14152257






