Allometric Growth of Annual Pinus yunnanensis After Decapitation Under Different Shading Levels
Abstract
1. Introduction
2. Methodology
2.1. Research Site
2.2. Individualized Design
2.3. Collection and Treatment of Seeds
2.4. Decapitation and Shading of Seedlings
2.5. Determination of Seedling Biomass
2.6. Plant Growth Assessment
Determination of Phenotypic Plasticity Index
2.7. Statistics and Analysis of Data
3. Results
3.1. Effects of Shading on Biomass Allocation
3.2. Relationship Between Phenotypic Plasticity Analysis and Biomass Allocation Under Different Shading Levels
3.3. Allometric Relationships Between Different Components and Plant Biomass Under Different Shading Levels
4. Discussion
4.1. Factors Affecting Biomass Allocation
4.2. The Relationship Between Phenotypic Plasticity Analysis and Plant Allometric Growth
4.3. How Shading Intensity Affects Plant Allometric Growth
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Akaeme, F.C.; Georgin, J.; de Oliveira, J.S.; Franco, D.S.P. Biomass Hydrochar: A Critical Review of Process Chemistry, Synthesis Methodology, and Applications. Sustainability 2025, 17, 1660. [Google Scholar] [CrossRef]
- Ding, X.; Chen, E.; Zhao, L.; Fan, Y.; Wang, J.; Ma, Y. Three-Stage Up-Scaling and Uncertainty Estimation in Forest Aboveground Biomass Based on Multi-Source Remote Sensing Data Considering Spatial Correlation. Remote Sens. 2025, 17, 671. [Google Scholar] [CrossRef]
- Mura, C.; Strømme, C.B.; Anfodillo, T. Stable Allometric Trajectories in Picea abies (L.) Karst Trees along an Elevational Gradient. Forests 2020, 11, 1231. [Google Scholar] [CrossRef]
- Tang, G.; Wang, Y.; Lu, Z.; Cheng, S.; Hu, Z.; Chen, S.; Chen, L.; Tang, J.; Xu, Y.; Cai, N. Effects of Combined Nitrogen–Phosphorus on Biomass Accumulation, Allocation, and Allometric Growth Relationships in Pinus yunnanensis Seedlings after Top Pruning. Plants 2024, 13, 2450. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, Y.; Wang, J.; Liu, Y.; Wang, H.; Song, Y.; Lu, Z.; Yang, Y. Biomass Allocation and Allometry in Juglans mandshurica Seedlings from Different Geographical Provenances in China. Forests 2024, 15, 1434. [Google Scholar] [CrossRef]
- Hao, Y.; Sun, Z.; Tan, Z.-H. Biomass Allocation of China’s Fores as Indicated by a Literature-Based Allometry Database. Fores 2024, 15, 942. [Google Scholar] [CrossRef]
- Liu, G.-Z.; Zhao, K.; Zhang, S.-Q.; Liang, Y.-M.; Yue, Y.-J.; Liu, G.-H.; Qin, F.-C. Biomass Allocation and Allometric Relationship of Salix gordejevii Branches in Sandy Habita Heterogeneity in Northern China. Sustainability 2024, 16, 5483. [Google Scholar] [CrossRef]
- Poorter, H.; Jagodzinski, A.M.; Ruiz-Peinado, R.; Kuyah, S.; Luo, Y.; Oleksyn, J.; Usoltsev, V.A.; Buckley, T.N.; Reich, P.B.; Sack, L. How does biomass distribution change with size and differ among species? An analysis for 1200 plant species from five continents. New Phytol. 2015, 208, 736–749. [Google Scholar] [CrossRef]
- Zhou, C.; Kong, D.; Li, J.; Su, X.; Cai, N.; Xu, Y. The morphological and physiological responses of Pinus yunnanensis to different levels of shading after decapitation. Ind. Crops Prod. 2025, 224, 120374. [Google Scholar] [CrossRef]
- Zhou, C.; Gu, X.; Li, J.; Su, X.; Chen, S.; Tang, J.; Chen, L.; Cai, N.; Xu, Y. Physiological Characteristics and Transcriptomic Responses of Pinus yunnanensis Lateral Branching to Different Shading Environmen. Plan 2024, 13, 1588. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, D.; Yu, Z.; Huang, J.; Li, J.; Sun, Y.; Wang, X.; Wang, Q.; Wang, X. Effects of different light intensity on the growth, physiological and biochemical properties, and stomatal ultrastructure of Rhododendron micranthum saplings. J. For. Res. 2025, 36, 25. [Google Scholar] [CrossRef]
- Xue, G.; Wu, J.; Zhou, B.; Zhu, X.; Zeng, J.; Ma, Y.; Wang, Y.; Jia, H. Effects of Shading on the Growth and Photosynthetic Fluorescence Characteristics of Castanopsis hystrix Seedlings of Top Community-Building Species in Southern Subtropical China. Forests 2023, 14, 1659. [Google Scholar] [CrossRef]
- Wang, L.; Liang, B.; Liu, J.; Jin, H.; Zhu, Z.; Hao, S.; Wang, S.; Sheng, X.; Zhou, X.; Zhu, H.; et al. The Combination of Shading and Potassium Application Regulated the Bulb Active Ingredien Allocation in Fritillaria thunbergii Miq. by Affecting Rhizosphere Microecology. Microorganisms 2025, 13, 125. [Google Scholar] [CrossRef]
- Page, V.; Blösch, R.M.; Feller, U. Regulation of shoot growth, root development and manganese allocation in wheat (Triticum aestivum) genotypes by light intensity. Plant Growth Regul. 2012, 67, 209–215. [Google Scholar] [CrossRef]
- Rosado, D.; Ackermann, A.; Spassibojko, O.; Rossi, M.; Pedmale, U.V. WRKY transcription factors and ethylene signaling modify root growth during the shade-avoidance response. Plant Physiol. 2022, 188, 1294–1311. [Google Scholar] [CrossRef]
- Carins Murphy, M.R.; Jordan, G.J.; Brodribb, T.J. Differential leaf expansion can enable hydraulic acclimation to sun and shade. Plant Cell Environ. 2012, 35, 1407–1418. [Google Scholar] [CrossRef]
- Luo, D.; Huang, G.; Zhang, Q.; Zhou, G.; Peng, S.; Li, Y. Plasticity of mesophyll cell density and cell wall thickness and composition play a pivotal role in regulating plant growth and photosynthesis under shading in rapeseed. Ann Bot. 2023, 132, 963–978. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Li, Y.; Li, G.; Liu, D.; Zhao, M.; Cai, N. Growth Promotion of Yunnan Pine Early Seedlings in Response to Foliar Application of IAA and IBA. Int. J. Mol. Sci. 2012, 13, 6507–6520. [Google Scholar] [CrossRef]
- Tulik, M.; Karczewski, J.; Szeliga, N.; Jura-Morawiec, J.; Jarzyna, I. Morphological Characteristics and Allometric Relationships of Shoot in Two Undergrowth Plan: Polygonatum odoratum and Polygonatum multiflorum. Fores 2018, 9, 783. [Google Scholar] [CrossRef]
- Fang, Z.; Zhong, C.; Cheng, C.; Lv, X.; Chen, S. Effects of shading on growth and biomass allocation of Aegiceras corniculatum seedlings. Mol. Plant Breed. 2024, 22, 7837–7844. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, C.; Qian, S.S.; Luo, Y.; Zhou, R.; Tang, J.; Bao, W. Large-scale patterns of understory biomass and its allocation across China’s forests. Sci. Total Environ. 2022, 804, 150169. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, W.; Xie, L.Z.; Ni, Z.C.; Wei, Q.Z.; Wang, W.; Tian, H. Exindividualimental and numerical evaluation of the crystalline silicon PV window under the climatic conditions in southwest China. Energy 2019, 183, 584–598. [Google Scholar] [CrossRef]
- Xue, X.; Meng, C.; Cen, Y.; Xiao, J.; Li, X.; Chen, W. Modulation of hydrothermal conditions on the inhibiting and promoting effects of cumulative drought on vegetation productivity in southwest China. Ecol. Indic. 2024, 169, 112924. [Google Scholar] [CrossRef]
- Roeber, V.M.; Bajaj, I.; Rohde, M.; Schmülling, T.; Cortleven, A. Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant Cell Environ. 2021, 44, 645–664. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Han, X.; Yang, J.; Wu, X.; Yao, Q.; Zhao, C.; Zhong, Y.; Ning, P.; Tian, S. Differentiation analysis of VOCs in Kunming during rainy and dry seasons based on monitoring high temporal resolution. Atmos. Pollut. Res. 2024, 15, 101996. [Google Scholar] [CrossRef]
- Pallas, B.; Silva, D.D.; Valsesia, P.; Yang, W.W.; Guillaume, O.; Lauri, P.É.; Vercambre, G.; Génard, M.; Costes, E. Simulation of carbon allocation and organ growth variability in apple tree by connecting architectural and source–sink models. Ann. Bot. 2016, 118, 317–330. [Google Scholar] [CrossRef]
- Ju, X.T.; Kou, C.L.; Christie, P.; Dou, Z.; Zhang, F. Changes in the soil environment from excessive application of fertilizers and manures to two contrasting intensive cropping systems on the North China Plain. Environ. Pollut. 2007, 145, 497–506. [Google Scholar] [CrossRef]
- Gotoh, E.; Suetsugu, N.; Yamori, W.; Ishishita, K.; Kiyabu, R.; Fukuda, M.; Higa, T.; Shirouchi, B.; Wada, M. Chloroplast Allocation Response Enhances Leaf Photosynthesis and Plant Biomass Production. Plant Physiol. 2018, 178, 1358–1369. [Google Scholar] [CrossRef]
- Warren, C.R. How does P affect photosynthesis and metabolite profiles of Eucalyptus globulus? Tree Physiol. 2011, 31, 727–739. [Google Scholar] [CrossRef]
- Kang, R.; Li, M.; Guo, S.; Xia, D.; Liu, L.; Dong, W.; Xu, W.; Lv, Y. Effect of Brassinolide on Stoichiometric Stability Characteristics of Tall Fescue under Drought Stress in Ecological Restoration. Sustainability 2024, 16, 5942. [Google Scholar] [CrossRef]
- Ledón-Rettig, C.C.; Ragsdale, E.J. Physiological Mechanisms and the Evolution of Plasticity. In Phenotypic Plasticity & Evolution: Causes, Consequences, Controversies; Pfennig, D.W., Ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 113–137. [Google Scholar] [CrossRef]
- Fordyce, J.A. The evolutionary consequences of ecological interactions mediated through phenotypic plasticity. J. Exp. Biol. 2006, 209 Pt 12, 2377–2383. [Google Scholar] [CrossRef] [PubMed]
- Nussey, D.H.; Wilson, A.J.; Brommer, J.E. The evolutionary ecology of individual phenotypic plasticity in wild populations. J. Evol. Biol. 2007, 20, 831–844. [Google Scholar] [CrossRef]
- Price, T.D. Phenotypic plasticity, sexual selection and the evolution of colour patterns. J. Exp. Biol. 2006, 209 Pt 12, 2368–2376. [Google Scholar] [CrossRef]
- Claveau, Y.; Messier, C.; Comeau, P.G. Interacting influence of light and size on aboveground biomass distribution in sub-boreal conifer saplings with contrasting shade tolerance. Tree Physiol. 2005, 25, 373–384. [Google Scholar] [CrossRef]
- Saldaña-Acosta, A.; Meave, J.A.; Sánchez-Velásquez, L.R. Seedling biomass allocation and vital rates of cloud forest tree species: Responses to light in shade house conditions. For. Ecol. Manag. 2009, 258, 1650–1659. [Google Scholar] [CrossRef]
- Brown, C.E.; Mickelbart, M.V.; Jacobs, D.F. Leaf physiology and biomass allocation of backcross hybrid American chestnut (Castanea dentata) seedlings in response to light and water availability. Tree Physiol. 2014, 34, 1362–1375. [Google Scholar] [CrossRef]
- King, D.A. Branch growth and biomass allocation in Abies amabilis saplings in contrasting light environments. Tree Physiol. 1997, 17, 251–258. [Google Scholar] [CrossRef]
- Cifuentes, L.; Moreno, F. Trait coordination at leaf level explains the resistance to excess light stress in shade-tolerant tropical tree species. Tree Physiol. 2022, 42, 1325–1336. [Google Scholar] [CrossRef]
- Danyagri, G.; Dang, Q.-L. Effects of elevated carbon dioxide concentration and soil temindividualature on the growth and biomass responses of mountain maple (Acer spicatum) seedlings to light availability. J. Plant Ecol. 2014, 7, 535–543. [Google Scholar] [CrossRef]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef]
- Li, X.; Rong, H.; Zhang, J.; Wang, D.; Li, Y. Modulating the local coordination environment of single-atom catalysts for enhanced catalytic performance. Nano Res. 2020, 13, 1842–1855. [Google Scholar] [CrossRef]
- Eghbal, E.; Aliniaeifard, S.; Mehrjerdi, M.Z.; Abdi, S.; Hassani, S.B.; Rassaie, T.; Gruda, N.S. Growth, phytochemical, and phytohormonal responses of basil to different light durations and intensities under constant daily light integral. BMC Plant Biol. 2024, 24, 935. [Google Scholar] [CrossRef] [PubMed]
- Kebrom, T.H.; Burson, B.L.; Finlayson, S.A. Phytochrome B represses Teosinte Branched1 expression and induces sorghum axillary bud outgrowth in response to light signals. Plant Physiol. 2006, 140, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Kebrom, T.H. Shade signals activate distinct molecular mechanisms that induce dormancy and inhibit flowering in vegetative axillary buds of sorghum. Plant Direct. 2024, 8, e626. [Google Scholar] [CrossRef]
- Leuschner, C.; Hertel, D.; Schmid, I.; Koch, O.; Muhs, A.; Hölscher, D. Stand fine root biomass and fine root morphology in old-growth beech forests as a function of precipitation and soil fertility. Plant Soil 2004, 258, 43–56. [Google Scholar] [CrossRef]
- Lee, H.J.; Ha, J.H.; Kim, S.G.; Choi, H.K.; Kim, Z.H.; Han, Y.J.; Kim, J.I.; Oh, Y.; Fragoso, V.; Shin, K.; et al. Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots. Sci. Signal. 2016, 9, ra106. [Google Scholar] [CrossRef]
- Yang, J.; Qiao, H.; Wu, C.; Huang, H.; Nzambimana, C.; Jiang, C.; Wang, J.; Tang, D.; Zhong, W.; Du, K.; et al. Physiological and Transcriptome Responses of Sweet Potato [Ipomoea batatas (L.) Lam] to Weak-Light Stress. Plants 2024, 13, 2214. [Google Scholar] [CrossRef]
- Gao, J.; Shi, J.; Dong, S.; Liu, P.; Zhao, B.; Zhang, J. Effect of Different Light Intensities on Root Characteristics and Grain Yield of Summer Maize (Zea mays L.). Sci. Agric. Sin. 2017, 50, 2104–2113. [Google Scholar] [CrossRef]
- Feldpausch, T.R.; Banin, L.; Phillips, O.L.; Baker, T.R.; Lewis, S.L.; Quesada, C.A.; Affum-Baffoe, K.; Arets, E.J.M.M.; Berry, N.J.; Bird, M.; et al. Height-diameter allometry of tropical forest trees. Biogeosciences 2011, 8, 1081–1106. [Google Scholar] [CrossRef]
- Asigbaase, M.; Dawoe, E.; Abugre, S.; Kyereh, B.; Nsor, C.A. Allometric relationships between stem diameter, height and crown area of associated trees of cocoa agroforests of Ghana. Sci. Rep. 2023, 13, 14897. [Google Scholar] [CrossRef]
- Babin, R.; Hoopen, G.M.T.; Cilas, C.; Enjalric, F.; Gendre, P.; Lumaret, J.-P. Impact of shade on the spatial distribution of Sahlbergella singularis in traditional cocoa agroforests. Agric. For. Entomol. 2010, 12, 69–79. [Google Scholar] [CrossRef]
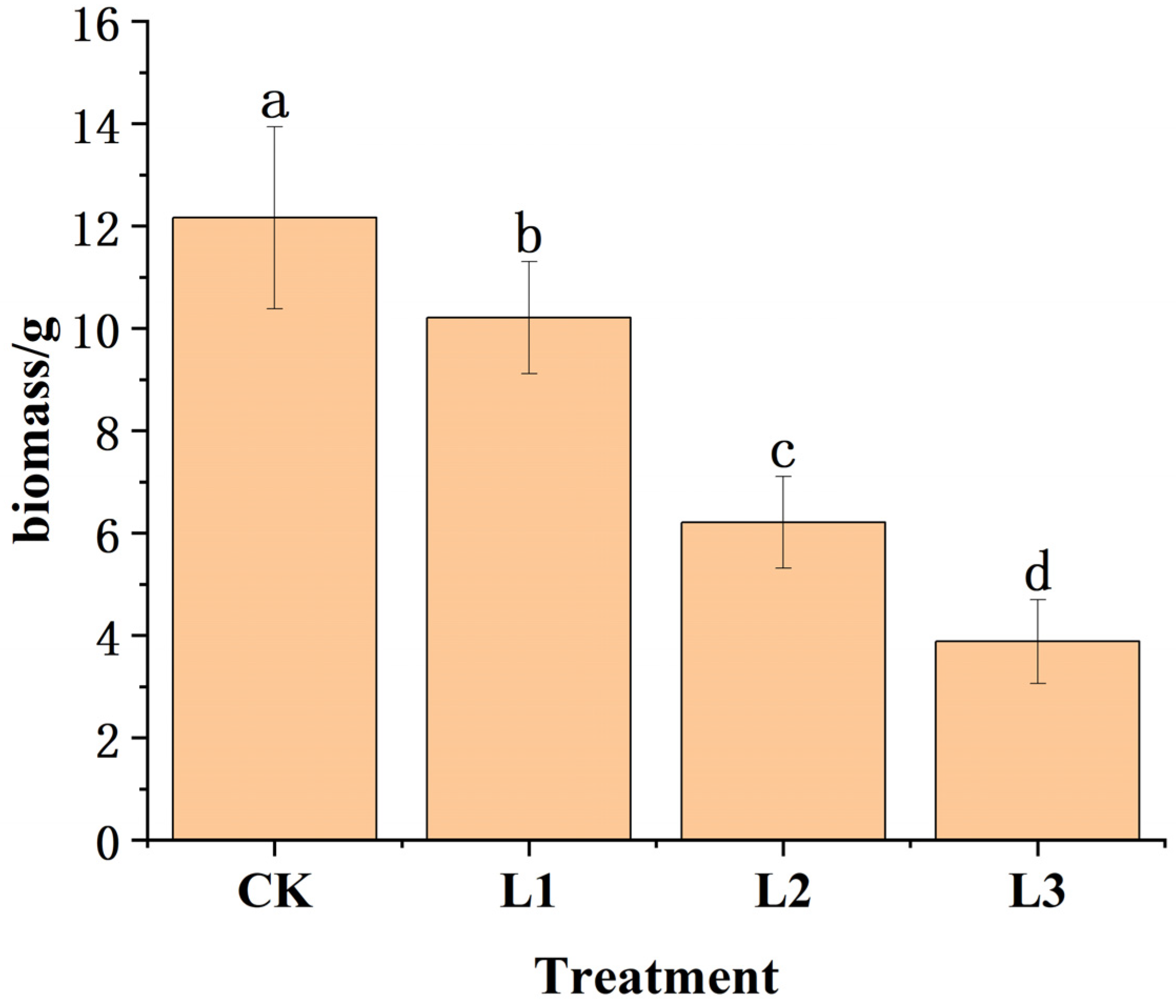
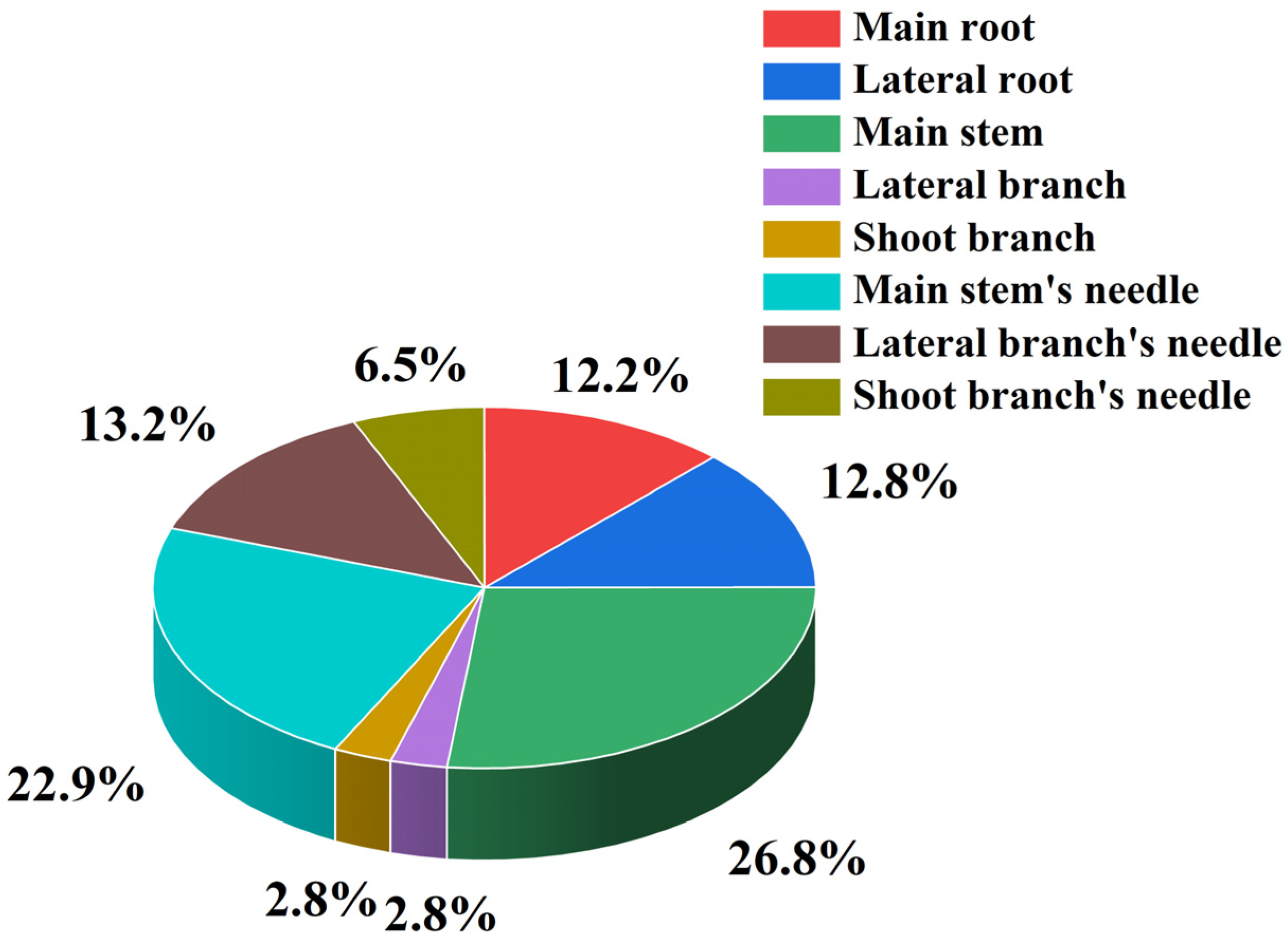

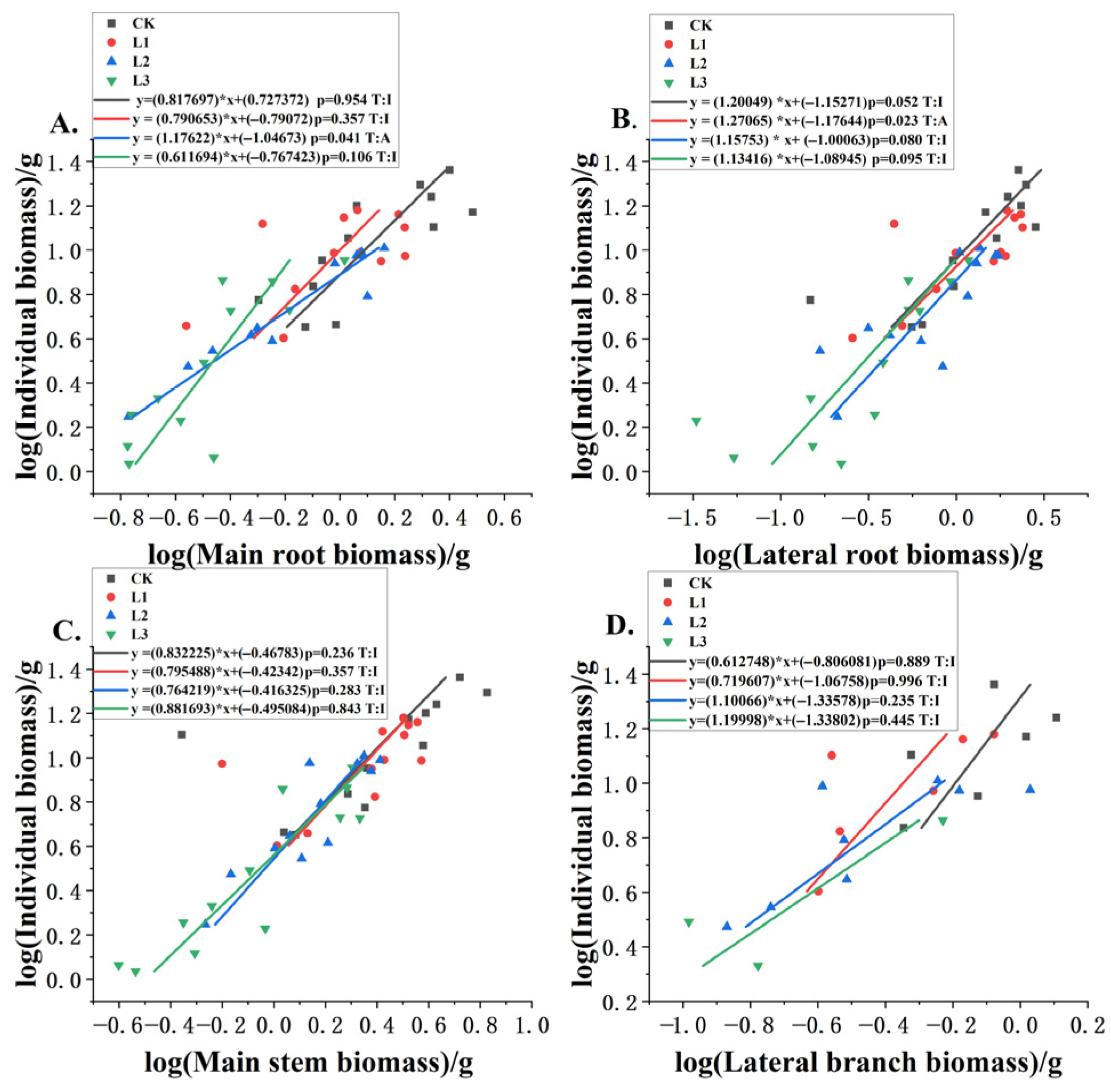
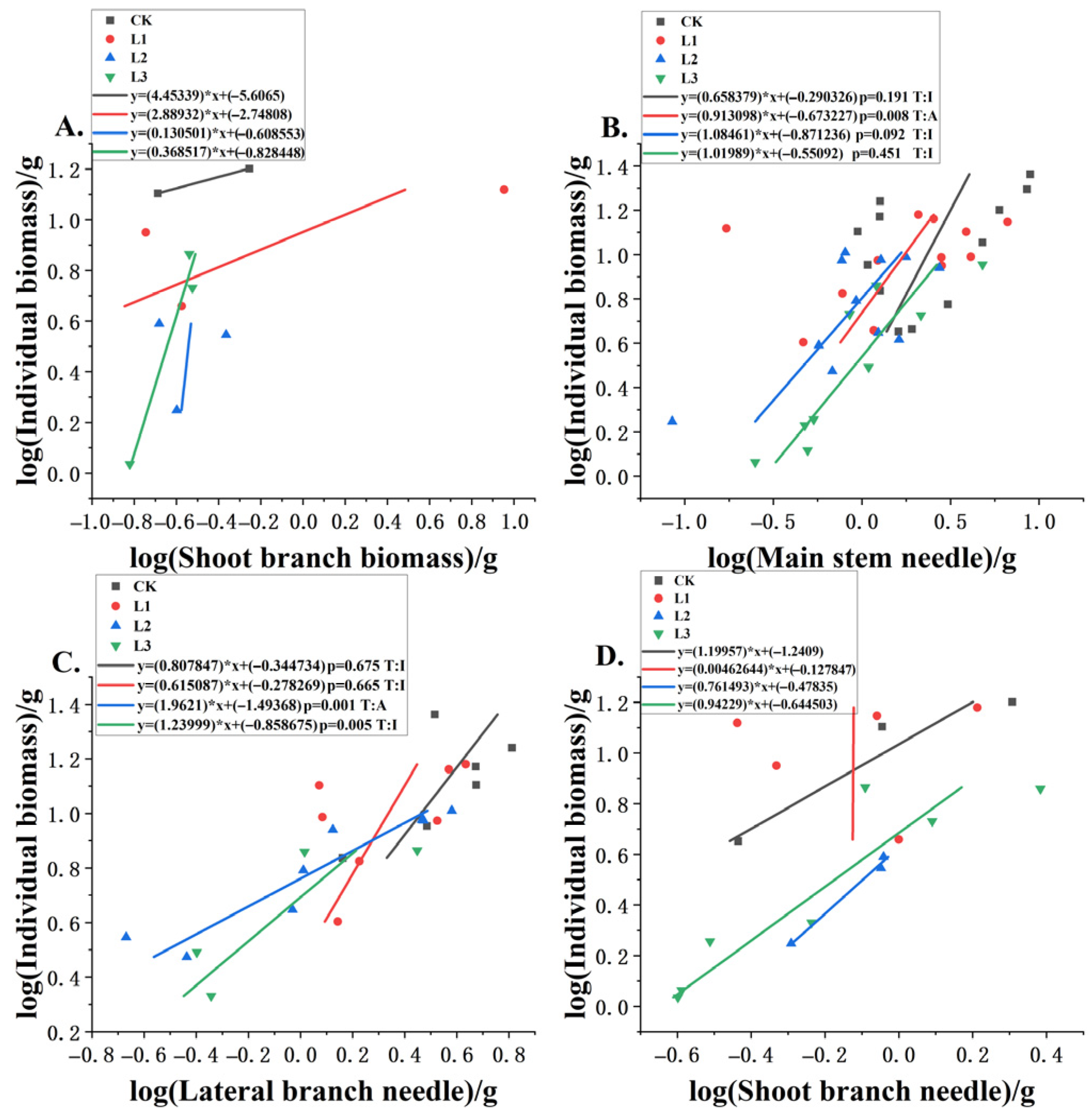

| PI | Main Root | Lateral Root | Main Stem | Lateral Branch | Shoot Branch | Main Stem Needle | Lateral Branch Needle | Shoot Branch Needle | Individual Biomass |
|---|---|---|---|---|---|---|---|---|---|
| CK | 0.58 | 0.65 | 0.77 | 1.00 | 1.00 | 0.85 | 1.00 | 1.00 | 0.63 |
| L1 | 0.69 | 0.73 | 0.30 | 0.78 | 1.00 | 0.86 | 0.83 | 0.71 | 0.33 |
| L2 | 0.63 | 0.62 | 0.58 | 0.83 | 1.00 | 0.81 | 0.84 | 1.00 | 0.60 |
| L3 | 0.74 | 0.76 | 0.79 | 1.00 | 1.00 | 0.85 | 1.00 | 1.00 | 0.78 |
| Lateral Root | n | R2 | P | Slope | Intercepts | H0_b | F | p | Type |
|---|---|---|---|---|---|---|---|---|---|
| CK | 12 | 0.634 | 0.002 | 0.6632 | 0.9736 | 1.000 | 4.871 | 0.052 | I |
| L1 | 12 | 0.546 | 0.006 | 0.5817 | 0.9386 | 1.000 | 7.129 | 0.023 | A |
| L2 | 12 | 0.669 | 0.001 | 0.7064 | 0.8406 | 1.000 | 3.794 | 0.080 | I |
| L3 | 12 | 0.661 | 0.001 | 0.7168 | 0.8692 | 1.000 | 3.391 | 0.095 | I |
| Main Stem | n | R2 | P | Slope | Intercepts | H0_b | F | p | Type |
|---|---|---|---|---|---|---|---|---|---|
| CK | 12 | 0.372 | 0.035 | 0.7329 | 0.7431 | 1.000 | 1.588 | 0.236 | I |
| L1 | 12 | 0.395 | 0.029 | 0.7903 | 0.6966 | 1.000 | 0.933 | 0.357 | I |
| L2 | 12 | 0.803 | 0.000 | 1.1722 | 0.5645 | 1.000 | 1.289 | 0.283 | I |
| L3 | 12 | 0.821 | 0.000 | 1.0276 | 0.5531 | 1.000 | 0.042 | 0.843 | I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Zhou, C.; Yang, B.; Li, J.; Xu, Y.; Cai, N. Allometric Growth of Annual Pinus yunnanensis After Decapitation Under Different Shading Levels. Plants 2025, 14, 2251. https://doi.org/10.3390/plants14152251
Wang P, Zhou C, Yang B, Li J, Xu Y, Cai N. Allometric Growth of Annual Pinus yunnanensis After Decapitation Under Different Shading Levels. Plants. 2025; 14(15):2251. https://doi.org/10.3390/plants14152251
Chicago/Turabian StyleWang, Pengrui, Chiyu Zhou, Boning Yang, Jiangfei Li, Yulan Xu, and Nianhui Cai. 2025. "Allometric Growth of Annual Pinus yunnanensis After Decapitation Under Different Shading Levels" Plants 14, no. 15: 2251. https://doi.org/10.3390/plants14152251
APA StyleWang, P., Zhou, C., Yang, B., Li, J., Xu, Y., & Cai, N. (2025). Allometric Growth of Annual Pinus yunnanensis After Decapitation Under Different Shading Levels. Plants, 14(15), 2251. https://doi.org/10.3390/plants14152251







