Melatonin Enhances Drought Tolerance by Regulating the Genes Underlying Photosynthesis and Antioxidant Defense in Rubber Tree (Hevea brasiliensis) Seedlings
Abstract
1. Introduction
2. Results
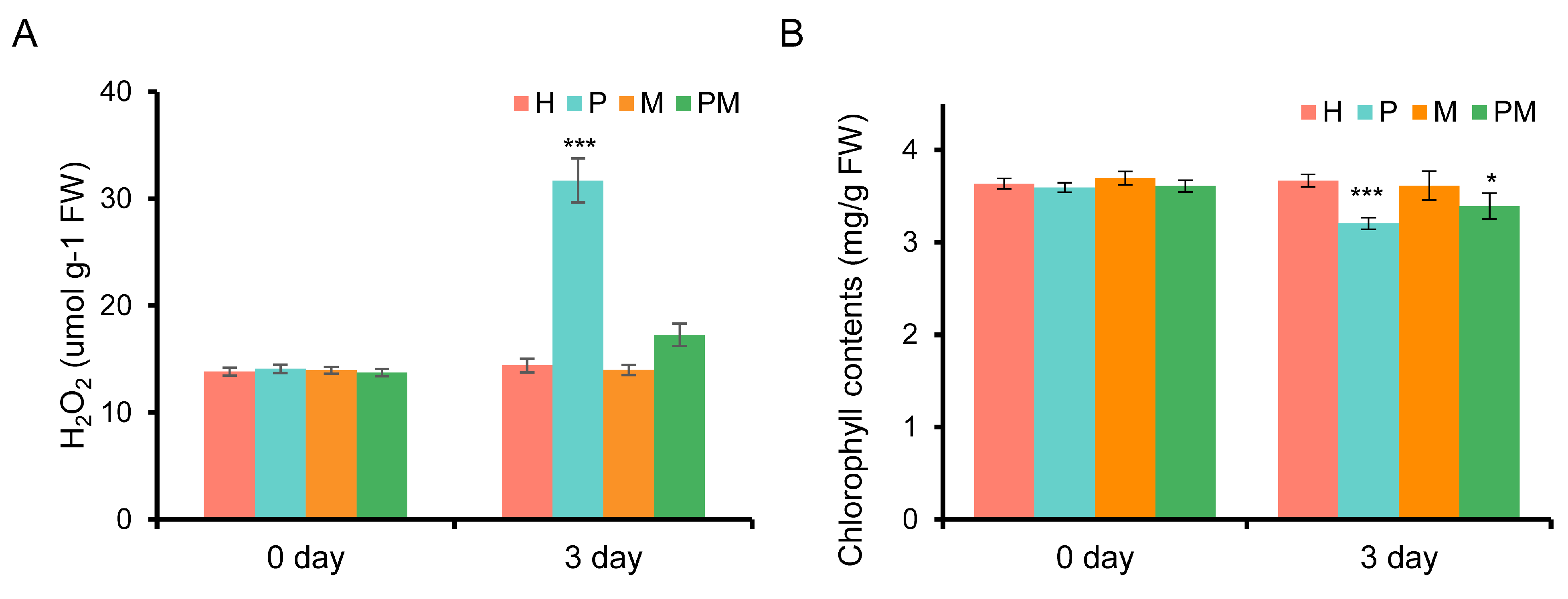
2.1. Exogenous MT Partly Alleviates Chlorophyll Degradation and H2O2 Accumulation Induced by Drought in Rubber Tree Seedlings
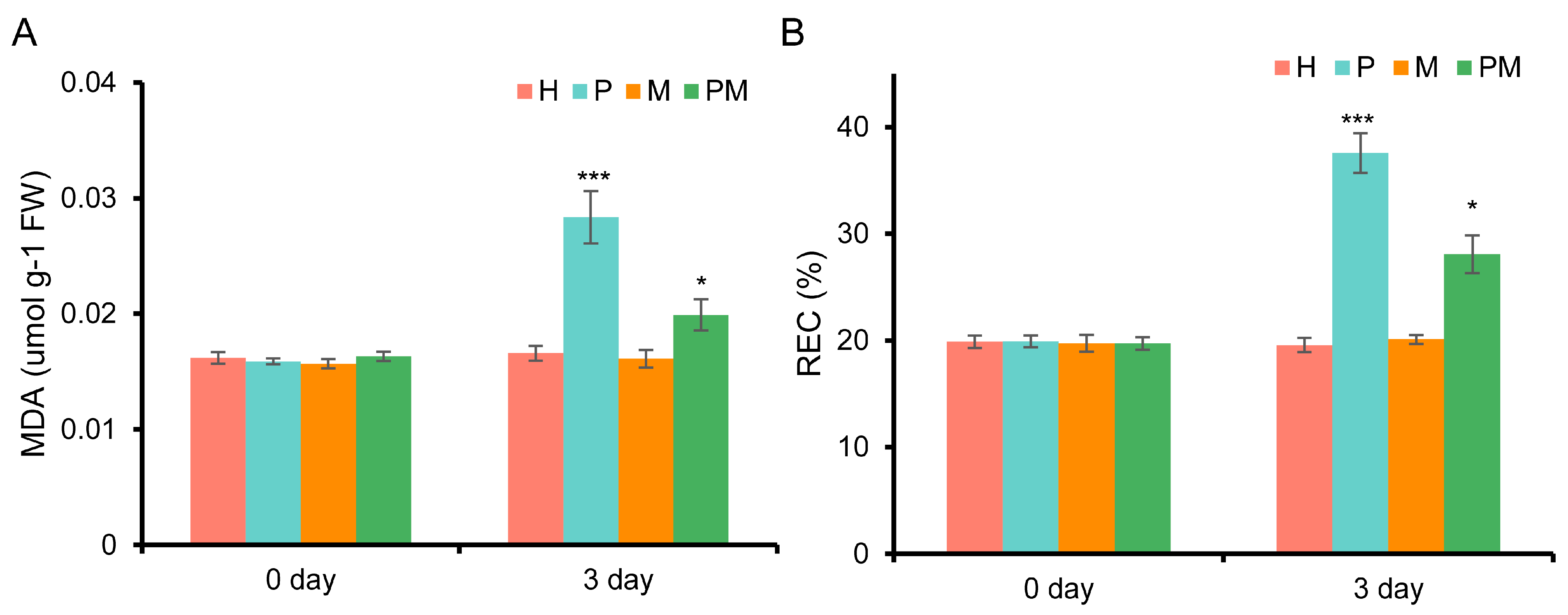
2.2. Exogenous MT Mitigates the Damage to Cell Membranes Induced by Drought in Rubber Tree Seedlings
2.3. Identifying MT-Regulated Genes by Transcriptome Analyses
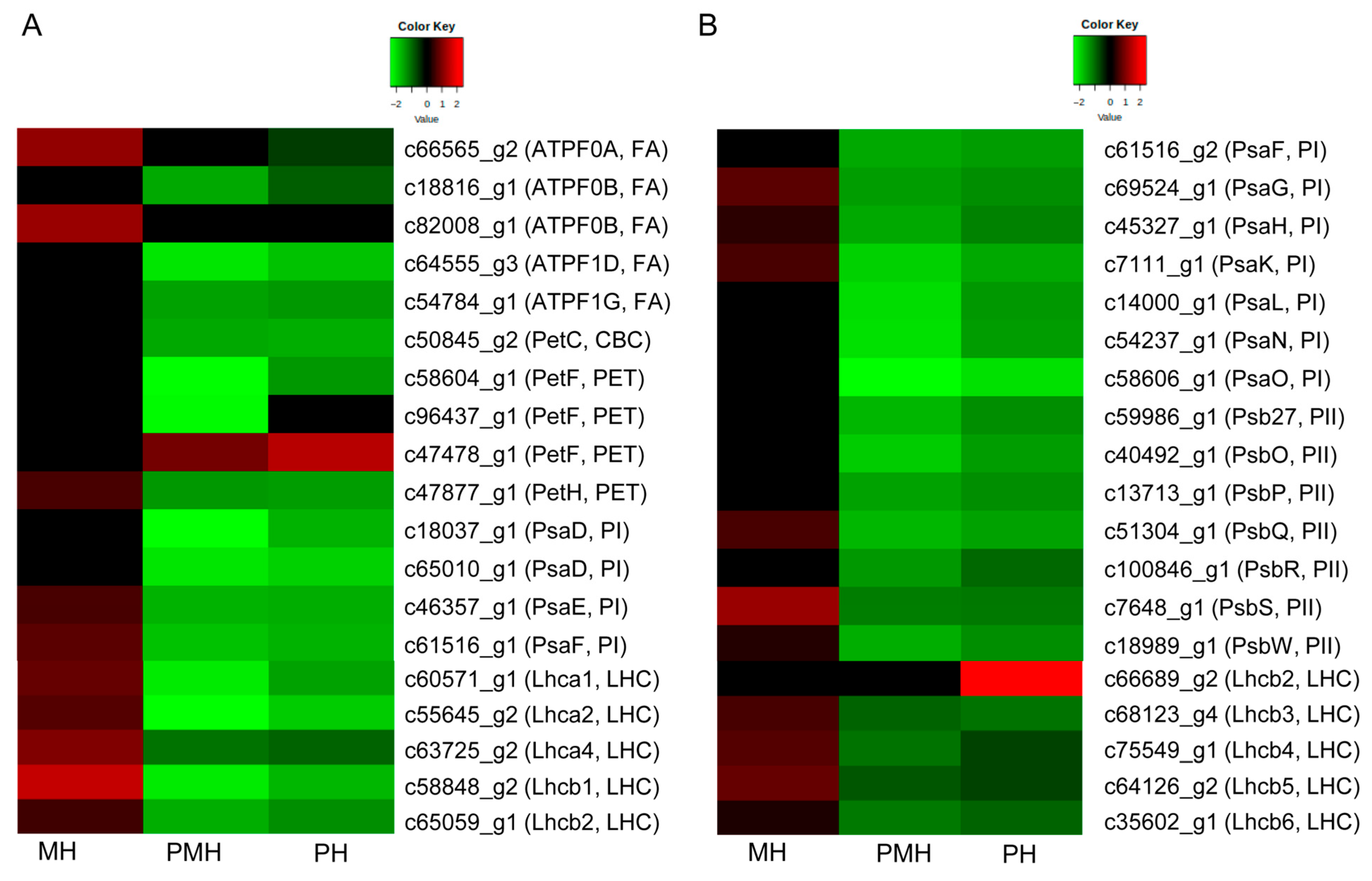
2.4. Genes Related to Photosynthesis
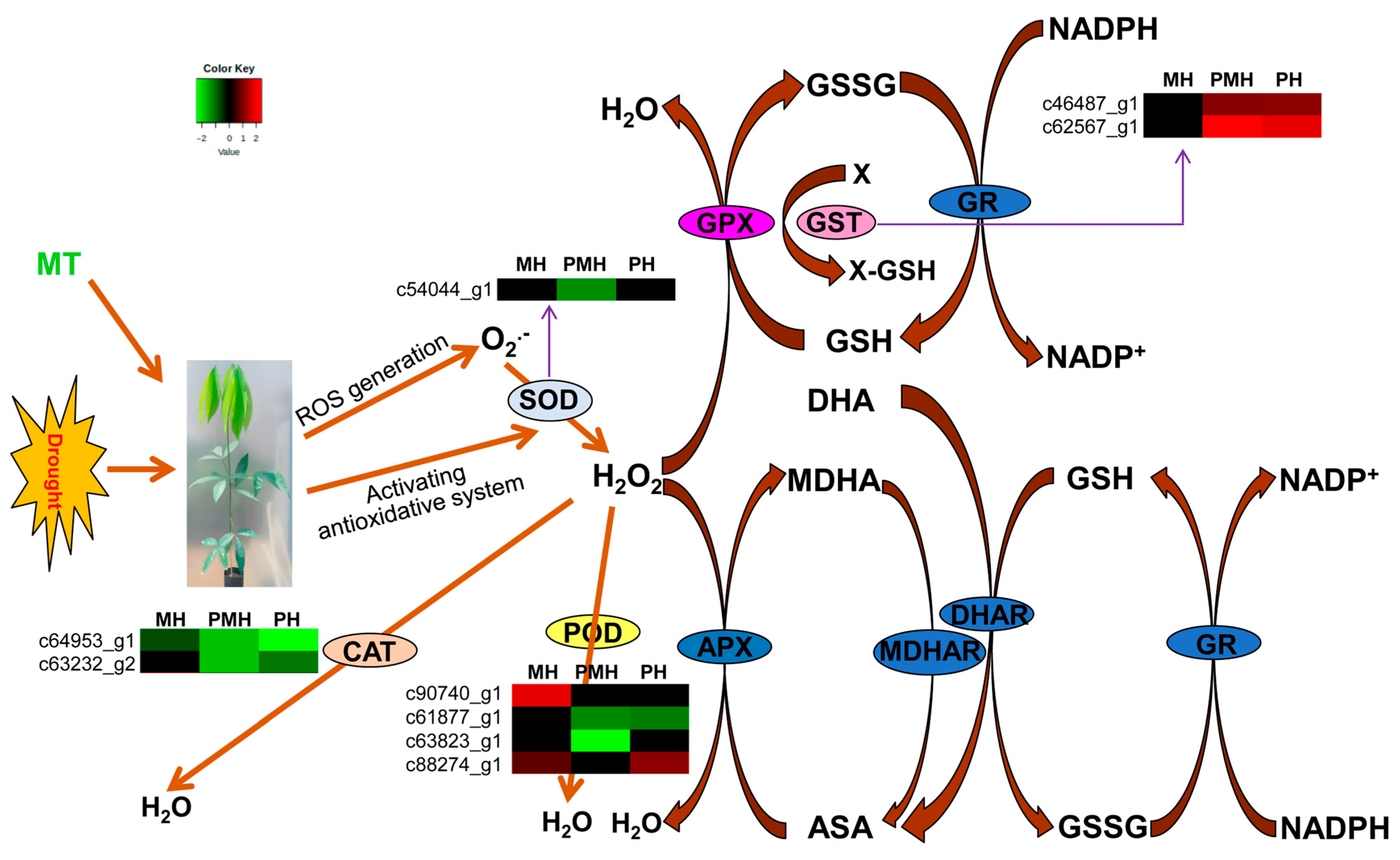
2.5. Genes Involved in Anti-Oxidative Defense System
2.6. Effects of Exogenous MT on Transcription Factors in Rubber Tree Seedlings Under Drought Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Determination of Physiological Parameters Related to Drought
4.3. RNA Extraction, Transcriptome Sequencing, and Data Assembly
4.4. Gene Functional Annotation and Analysis
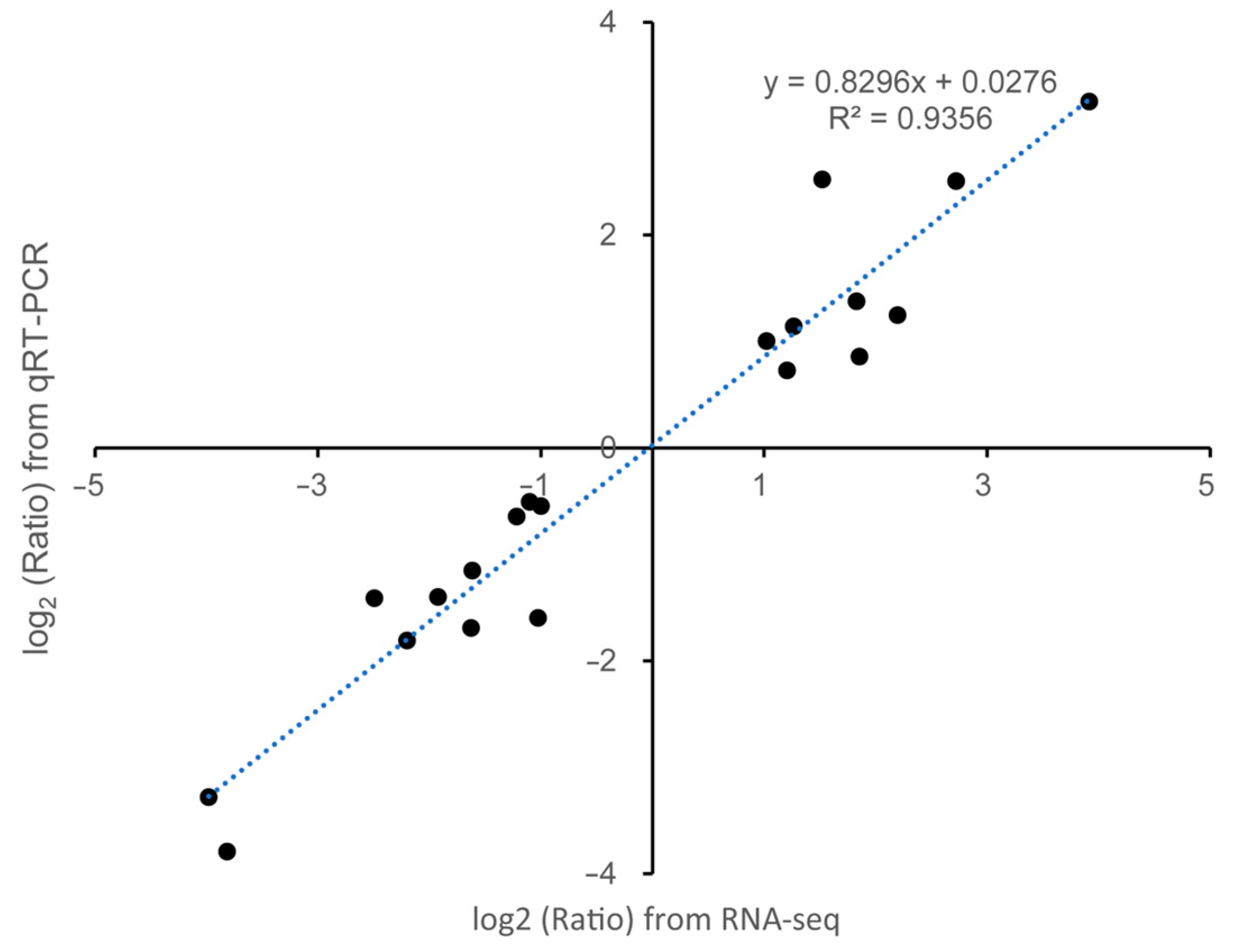
4.5. RT-qPCR Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.M.B.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. IPCC. In Climate Change 2013: The Physical Science Basis, Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Sheffield, J.; Wood, E.F. Projected changes in drought occurrence under future global warming from multi-model, multi-scenario, IPCC AR4 simulations. Clim. Dyn. 2008, 31, 79–105. [Google Scholar] [CrossRef]
- Chen, Y.N.; Li, Y.P.; Li, Z. Analysis of the impact of global climate change on dryland areas. Adv. Earth Sci. 2022, 37, 111–119. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24—Epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2019, 135, 295–303. [Google Scholar] [CrossRef]
- Gu, Z.; Hu, C.; Gan, Y.; Zhou, J.; Tian, G.; Gao, L. Role of microbes in alleviating crop drought stress: A review. Plants 2024, 13, 384. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qiu, L.; Guo, H.; Wang, Y.; Yuan, H.; Yan, D.; Zheng, B. Spermidine induces physiological and biochemical changes in southern highbush blueberry under drought stress. Braz. J. Bot. 2017, 40, 841–851. [Google Scholar] [CrossRef]
- Meng, J.F.; Xu, T.F.; Wang, Z.Z.; Fang, Y.L.; Xi, Z.M.; Zhang, Z.W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef]
- Shao, R.X.; Xin, L.F.; Zheng, H.F.; Li, L.L.; Ran, W.L.; Mao, J.; Yang, Q.H. Changes in chloroplast ultrastructure in leaves of drought-stressed maize inbred lines. Photosynthetica 2016, 54, 74–80. [Google Scholar] [CrossRef]
- Cao, B.L.; Ma, Q.; Zhao, Q.; Wang, L.; Xu, K. Effects of silicon on absorbed light allocation, antioxidant enzymes and ultrastructure of chloroplasts in tomato leaves under simulated drought stress. Sci. Hortic. 2015, 194, 53–62. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, J.; Burgess, P.; Rossi, S.; Huang, B. Interactive effects of melatonin and cytokinin on alleviating drought-induced leaf senescence in creeping bentgrass (Agrostis stolonifera). Environ. Exp. Bot. 2018, 145, 1–11. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Effect of water stress on antioxidant systems and oxidative parameters in fruits of tomato (Solanum lycopersicon L., cv. Micro-tom). Physiol. Mol. Biol. Plants 2013, 19, 363–378. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T.; Ibrahim, A.S. Alleviation of drought-induced oxidative stress in maize (Zea mays L.) plants by dual application of 24-epibrassinolide and spermine. Environ. Exp. Bot. 2015, 113, 47–58. [Google Scholar] [CrossRef]
- Zhang, Y.; Leclercq, J.; Montoro, P. Reactive oxygen species in Hevea brasiliensis latex and relevance to tapping panel dryness. Tree Physiol. 2017, 37, 261–269. [Google Scholar] [CrossRef]
- Carr, M.K.V. The water relations of rubber (Hevea brasiliensis): A review. Exp. Agric. 2012, 48, 176–193. [Google Scholar] [CrossRef]
- Chen, J.W.; Cao, K.F. A possible link between hydraulic properties and leaf habits in Hevea brasiliensis. Funct. Plant Biol. 2015, 42, 718–726. [Google Scholar] [CrossRef]
- Limkaisang, S.; Kom-un, S.; Takamatsu, S.; Furtado, E.L.; Liew, K.W.; Salleh, B.; Sato, Y. Molecular phylogenetic and morphological analyses of Oidium heveae, a powdery mildew of rubber tree. Mycoscience 2005, 46, 220–226. [Google Scholar] [CrossRef]
- Cao, M.; Zhang, J.H. Tree species diversity of tropical forest vegetation in Xishuangbanna, SW China. Biodivers. Conserv. 1997, 6, 995–1006. [Google Scholar] [CrossRef]
- Cao, M.; Zou, X.; Warren, M.; Zhu, H. Tropical forests of Xishuangbanna, China. Biotropica 2006, 38, 306–309. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhang, Y.P.; Li, H.M.; Meng, F.R.; Liu, Y.H.; Wang, C.M. Fog and rainwater chemistry in the tropical seasonal rain forest of Xishuangbanna, southwest China. Water Air Soil Pollut. 2005, 167, 295–309. [Google Scholar] [CrossRef]
- Huang, Z.D.; Pan, Y.Q. Rubber cultivation under climatic stresses in China. In Developments in Crop Science, 1st ed.; Sethuraj, M.R., Mathew, N.M., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; Volume 23, pp. 220–238. [Google Scholar]
- Priyadarshan, P.M. Refinements to Hevea rubber breeding. Tree Genet. Genomes 2017, 13, 20. [Google Scholar] [CrossRef]
- Ahmad, S.; Kamran, M.; Ding, R.; Meng, X.; Wang, H.; Ahmad, I.; Fahad, S.; Han, Q. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. Peer J. 2019, 7, e7793. [Google Scholar] [CrossRef]
- Ahmad, R.; Alsahli, A.A.; Alansi, S.; Altaf, M.A. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic efficiency and antioxidant defense system of pea (Pisum sativum L.). Sci. Hortic. 2023, 322, 112431. [Google Scholar] [CrossRef]
- Ahsan, M.; Younis, A.; Jamal, A.; Alshaharni, M.O.; Algopishi, U.B.; Al-Andal, A.; Sajid, M.; Naeem, M.; Khan, J.A.; Radicetti, E.; et al. Melatonin induces drought stress tolerance by regulating the physiological mechanisms, antioxidant enzymes, and leaf structural modifications in Rosa centifolia L. Heliyon 2025, 11, e41236. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Naz, S.; Altaf, M.M.; Khan, L.U.; Tiwari, R.K.; Lal, M.K.; Shahid, M.A.; Kumar, R.; et al. Melatonin improves drought stress tolerance of tomato by modulation plant growth, root architecture, photosynthesis, and antioxidant defense system. Antioxidants 2022, 11, 309. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Y.E.; Zhao, Y.Q.; Ding, C.B.; Liao, J.Q.; Hu, C.; Zhou, L.J.; Zhang, Z.W.; Yuan, S.; Yuan, M. Exogenous melatonin alleviates oxidative damages and protects photosystem ii in maize seedlings under drought stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Latif Khan, A.; Shahzad, R.; Aaqil Khan, M.; Bilal, S.; Khan, A.; Kang, S.M.; Lee, I.J. Exogenous melatonin induces drought stress tolerance by promoting plant growth and antioxidant defence system of soybean plants. AoB Plants 2021, 13, plab026. [Google Scholar] [CrossRef]
- Kabiri, R.; Hatami, A.; Oloumi, H.; Naghizadeh, M.; Nasibi, F.; Tahmasebi, Z. Foliar application of melatonin induces tolerance to drought stress in Moldavian balm plants (Dracocephalum moldavica) through regulating the antioxidant system. Folia Hortic. 2018, 30, 155–167. [Google Scholar] [CrossRef]
- Kaya, C.; Shabala, S. Melatonin improves drought stress tolerance of pepper (Capsicum annuum) plants via upregulating nitrogen metabolism. Funct. Plant Biol. 2023, 51, FP23060. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Jan, R.; Asif, S.; Farooq, M.; Jang, Y.H.; Kim, E.G.; Kim, N.; Kim, K.M. Exogenous melatonin induces salt and drought stress tolerance in rice by promoting plant growth and defense system. Sci. Rep. 2024, 14, 1214. [Google Scholar] [CrossRef]
- Luo, M.; Wang, D.; Delaplace, P.; Pan, Y.; Zhou, Y.; Tang, W.; Chen, K.; Chen, J.; Xu, Z.; Ma, Y.; et al. Melatonin enhances drought tolerance by affecting jasmonic acid and lignin biosynthesis in wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2023, 202, 107974. [Google Scholar] [CrossRef]
- Roy, M.; Niu, J.; Irshad, A.; Kareem, H.A.; Hassan, M.U.; Xu, N.; Sui, X.; Guo, Z.P.; Amo, A.; Wang, Q.Z. Exogenous melatonin protects alfalfa (Medicago sativa L.) seedlings from drought-induced damage by modulating reactive oxygen species metabolism, mineral balance and photosynthetic efficiency. Plant Stress 2021, 2, 100044. [Google Scholar] [CrossRef]
- Shaffique, S.; Shah, A.A.; Kang, S.M.; Injamum-Ul-Hoque, M.; Shahzad, R.; Azzawi, T.N.I.A.; Yun, B.W.; Lee, I.J. Melatonin: Dual players mitigating drought-induced stress in tomatoes via modulation of phytohormones and antioxidant signaling cascades. BMC Plant Biol. 2024, 24, 1101. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; He, J.; Wang, Z.; Zhang, Z.; Quinet, M.; Meng, Y. Exogenous melatonin enhances drought tolerance and germination in common buckwheat seeds through the coordinated effects of antioxidant and osmotic regulation. BMC Plant Biol. 2025, 25, 613. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Lv, Y.; Bai, J.; Li, T.; Yang, X.; Liu, L.; Zhou, H. Comparative transcriptomics reveals new insights into melatonin-enhanced drought tolerance in naked oat seedlings. Peer J. 2022, 10, e13669. [Google Scholar] [CrossRef]
- Zhao, Q.; Zheng, X.; Wang, C.; Wang, Q.; Wei, Q.; Liu, X.; Liu, Y.; Chen, A.; Jiang, J.; Zhao, X.; et al. Exogenous melatonin improves drought tolerance by regulating the antioxidant defense system and photosynthetic efficiency in fodder soybean seedlings. Plants 2025, 14, 460. [Google Scholar] [CrossRef]
- Campos, C.N.; Ávila, R.G.; de Souza, K.R.D.; Azevedo, L.M.; Alves, J.D. Melatonin reduces oxidative stress and promotes drought tolerance in young Coffea arabica L. plants. Agric. Water Manag. 2019, 211, 37–47. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Liang, D.; Ni, Z.; Xia, H.; Xie, Y.; Lv, X.; Wang, J.; Lin, L.; Deng, Q.; Luo, X. Exogenous melatonin promotes biomass accumulation and photosynthesis of kiwifruit seedlings under drought stress. Sci. Hortic. 2019, 246, 34–43. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Chankova, S.; Parvanova, P.; Yurina, N. Early warning signals of drought induced oxidative stress and genotype tolerance. A review. Bulg. J. Agric. Sci. 2024, 30 (Suppl. S1), 75–82. [Google Scholar]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Gonzalez, D.H. Introduction to transcription factor structure and function. In Plant Transcription Factors; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3–11. [Google Scholar]
- Cahyo, A.N.; Murti, R.H.; Putra, E.T.S.; Oktavia, F.; Ismawanto, S.; Montoro, P. Rubber genotypes with contrasting drought factor index revealed different mechanisms for drought resistance in Hevea brasiliensis. Plants 2022, 11, 3563. [Google Scholar] [CrossRef]
- Yang, H.; Dai, L.J.; Wei, Y.X.; Deng, Z.; Li, D.J. Melatonin enhances salt stress tolerance in rubber tree (Hevea brasiliensis) seedlings. Ind. Crop. Prod. 2020, 145, 111990. [Google Scholar] [CrossRef]
- Wang, L.F. Physiological and molecular responses to drought stress in rubber tree (Hevea brasiliensis Muell. Arg.). Plant Physiol. Biochem. 2014, 83, 243–249. [Google Scholar] [CrossRef]
- Guo, B.; Liu, M.; Yang, H.; Dai, L.; Wang, L. Brassinosteroids regulate the water deficit and latex yield of rubber trees. Int. J. Mol. Sci. 2023, 24, 12857. [Google Scholar] [CrossRef]
- Niu, Y.F.; Zheng, C.; Liu, Z.Y.; Nan, H.; Liu, J. Genome-wide analysis of long noncoding RNAs affecting rubber biosynthesis in Hevea brasiliensis in response to drought stress. Ind. Crops. Prod. 2024, 210, 118084. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul. 2005, 46, 209–221. [Google Scholar] [CrossRef]
- Li, C.; Tan, D.X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Terron, M.P.; Flores, L.J.; Czarnocki, Z. Melatonin and its metabolites: New findings regarding their production and their radical scavenging actions. Acta Biochim. Pol. 2007, 54, 1–9. Available online: https://pubmed.ncbi.nlm.nih.gov/17351668/ (accessed on 9 March 2007). [CrossRef]
- Ying, Y.; Yue, Y.; Huang, X.; Wang, H.; Mei, L.; Yu, W.; Zheng, B.; Wu, J. Salicylic acid induces physiological and biochemical changes in three red bayberry (Myric rubra) genotypes under water stress. Plant Growth Regul. 2013, 71, 181–189. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.J.; Weeda, S.; Yang, C.; Yang, Z.C.; Ren, S.; Guo, Y.D. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Dimassi-Theriou, K.; Therios, I.; Koukourikou-Petridou, M. Melatonin enhances root regeneration, photosynthetic pigments, biomass, total carbohydrates and proline content in the cherry rootstock PHL-C (Prunus avium × Prunus cerasus). Plant Physiol. Biochem. 2012, 61, 162–168. [Google Scholar] [CrossRef]
- Wei, W.; Li, Q.T.; Chu, Y.N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef]
- Muench, S.P.; Trinick, J.; Harrison, M.A. Structural divergence of the rotary ATPases. Q. Rev. Biophys. 2011, 44, 311–356. [Google Scholar] [CrossRef]
- Andersson, J.; Wentworth, M.; Walters, R.G.; Howard, C.A.; Ruban, A.V.; Horton, P.; Jansson, S. Absence of the Lhcb1 and Lhcb2 proteins of the light-harvesting complex of the photosystem II-effects on photosynthesis, grana stacking and fitness. Plant J. 2003, 35, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Ganeteg, U.; Kulheim, C.; Andersson, J.; Jansson, S. Is each light-harvesting complex protein important for plant fitness? Plant Physiol. 2004, 134, 502–509. [Google Scholar] [CrossRef]
- Kovacs, L.; Damkjær, J.; Kereiche, S.; Ilioaia, C.; Ruban, A.V.; Boekema, E.J.; Jansson, S.; Horton, P. Lack of the light-harvesting complex CP24 affects the structure and function of the grana membranes of higher plant chloroplasts. Plant Cell 2006, 18, 3106–3120. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Liu, R.; Yan, L.; Liu, Z.Q.; Jiang, S.C.; Shen, Y.Y.; Wang, X.F.; Zhang, D.P. Light-harvesting chlorophyll a/b-binding proteins are required for stomatal response to abscisic acid in Arabidopsis. J. Exp. Bot. 2012, 63, 1095–1106. [Google Scholar] [CrossRef]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.B.; Xu, P. Transcription factors associated with abiotic and biotic stress tolerance and their potential for crops improvement. Genes 2019, 10, 771. [Google Scholar] [CrossRef]
- Luo, Y.; Hu, T.; Huo, Y.; Wang, L.; Zhang, L.; Yan, R. Transcriptomic and physiological analyses reveal the molecular mechanism through which exogenous melatonin increases drought stress tolerance in Chrysanthemum. Plants 2023, 12, 1489. [Google Scholar] [CrossRef]
- Shi, H.; Chen, K.; Wei, Y.; He, C. Fundamental issues of melatonin-mediated stress signaling in plants. Front. Plant Sci. 2016, 7, 1124. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, C.; Ye, T.T.; Tan, D.X.; Reiter, R.J.; Zhang, H.; Liu, R.Y.; Chan, Z.L. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L.) Pers.] by exogenous melatonin. J. Exp. Bot. 2015, 66, 681–694. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–220. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yang, Z.R.; Li, Z.; Zhang, F.L.; Hao, L.Z. De novo transcriptome assembly and co-expression network analysis of Cynanchum thesioides: Identification of genes involved in resistance to drought stress. Gene 2019, 710, 375–386. [Google Scholar] [CrossRef]
- Shi, H.; Wei, Y.X.; He, C.Z. Melatonin-induced CBF/DREB1s are essential for diurnal change of disease resistance and CCA1 expression in Arabidopsis. Plant Physiol. Biochem. 2016, 100, 150–155. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method. Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Patterson, B.D.; Macrae, E.A.; Ferguson, I.B. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal. Biochem. 1984, 139, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Peng, Y.H.; Zhang, M.H.; Shao, Y.J.; Su, W.A.; Tang, Z.C. Water relations and an expression analysis of plasma membrane intrinsic proteins in sensitive and tolerant rice during chilling and recovery. Cell Res. 2006, 16, 599–608. [Google Scholar] [CrossRef]
- Peever, T.L.; Higgins, V.J. Electrolyte leakage, lipoxygenase, and lipid peroxidation induced in tomato leaf tissue by specific and nonspecific elicitors from Cladosporium fulvum. Plant Physiol. 1989, 90, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.K.; Feng, Z.X.; Wang, X.; Wang, X.W.; Zhang, X.G. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef]
- Mao, X.Z.; Cai, T.; Olyarchuk, J.G.; Wei, L.P. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
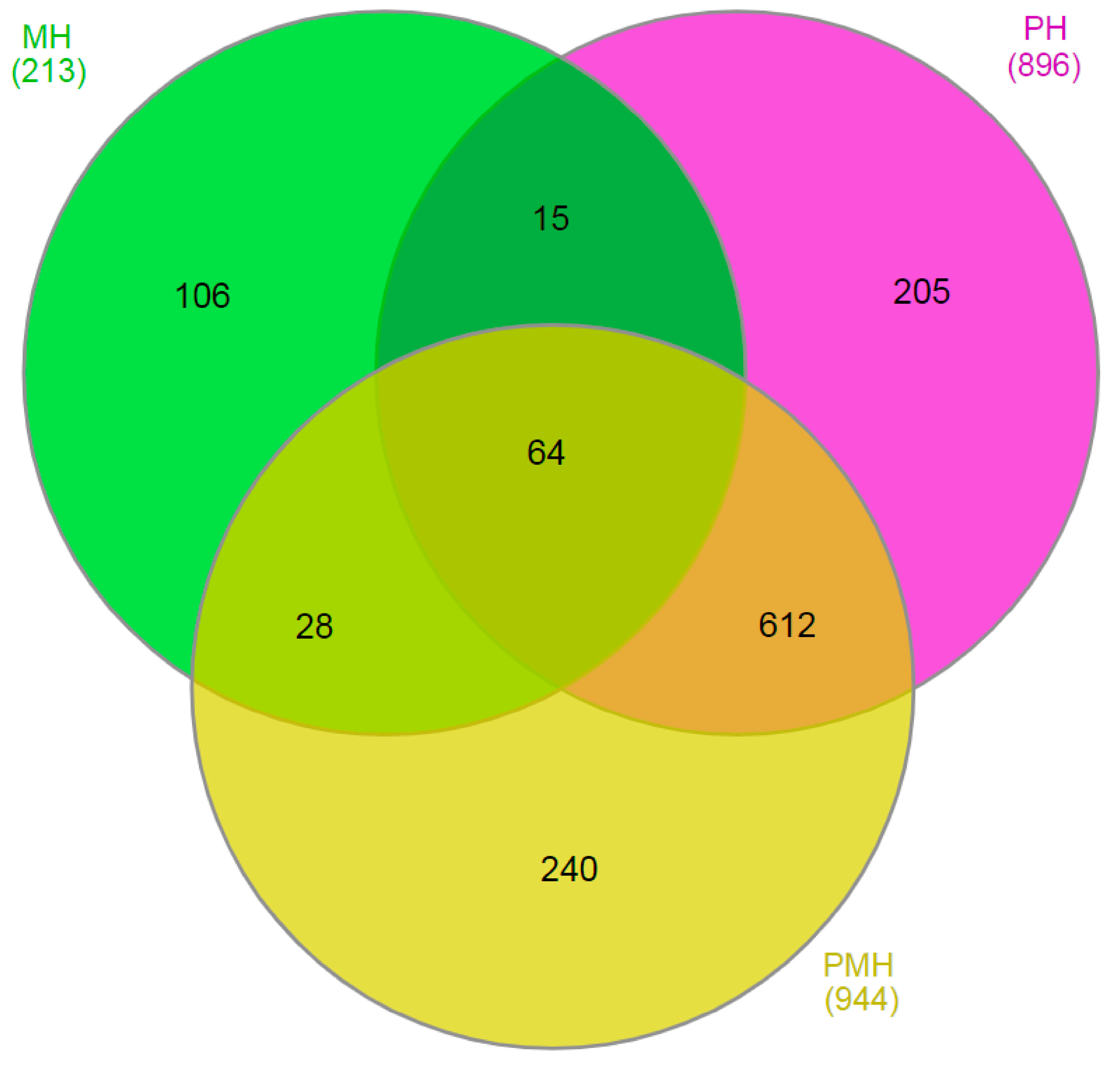
| MH | PH | PMH | |
|---|---|---|---|
| Upregulated | 135 | 421 | 371 |
| Downregulated | 78 | 475 | 573 |
| Total | 213 | 896 | 944 |
| TF Families | Numbers of DEGs in MH | Numbers of DEGs in PMH | Numbers of DEGs in PH |
|---|---|---|---|
| WRKY transcription factor | 1U, 2D | 6U | 7U |
| Myb-transcription factor | 3U, 1D | 3U, 2D | 2U, 1D |
| Transcription factor, putative | 2D | 2U, 2D | 2U, 2D |
| Ethylene-responsive transcription factor | 1D | 5U | 9U |
| Heat shock transcription factor | 2U, 1D | 2U | |
| GATA transcription factor | 2U | 2U | |
| Transcription factor MEIS1 | 4D | 1D | |
| Transcription factor HEX | 2D | 2D | |
| B3 domain-containing transcription factor VRN1 | 1D | ||
| Transcription factor JUNGBRUNNEN 1 | 1U | ||
| Transcription factor Pur-alpha 1 | 1D | ||
| Transcription factor DIVARICATA | 1D | 1U | |
| AP2/ERF and B3 domain-containing TF | 1D | ||
| Transcription factor CPC-like | 1D | ||
| NAC transcription factor | 1D | ||
| MADS box transcription factor | 1D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Xia, Z.; Wang, X.; Yang, H.; Li, Y. Melatonin Enhances Drought Tolerance by Regulating the Genes Underlying Photosynthesis and Antioxidant Defense in Rubber Tree (Hevea brasiliensis) Seedlings. Plants 2025, 14, 2243. https://doi.org/10.3390/plants14142243
Li D, Xia Z, Wang X, Yang H, Li Y. Melatonin Enhances Drought Tolerance by Regulating the Genes Underlying Photosynthesis and Antioxidant Defense in Rubber Tree (Hevea brasiliensis) Seedlings. Plants. 2025; 14(14):2243. https://doi.org/10.3390/plants14142243
Chicago/Turabian StyleLi, Dejun, Zhihui Xia, Xuncheng Wang, Hong Yang, and Yao Li. 2025. "Melatonin Enhances Drought Tolerance by Regulating the Genes Underlying Photosynthesis and Antioxidant Defense in Rubber Tree (Hevea brasiliensis) Seedlings" Plants 14, no. 14: 2243. https://doi.org/10.3390/plants14142243
APA StyleLi, D., Xia, Z., Wang, X., Yang, H., & Li, Y. (2025). Melatonin Enhances Drought Tolerance by Regulating the Genes Underlying Photosynthesis and Antioxidant Defense in Rubber Tree (Hevea brasiliensis) Seedlings. Plants, 14(14), 2243. https://doi.org/10.3390/plants14142243






