Genus Datura: An Exploration of Genetic Alterations, Bioactive Compounds, and Pharmacological Activity
Abstract
1. Introduction
2. Materials and Methods
3. Genus Datura: Morpho-Taxonomy
| Taxonomic Classification: [32,33] | |
| Kingdom | Plantae |
| Subkingdom | Tracheobionta |
| Superdivision | Spermatophyta |
| Division | Magnoliophyta |
| Subdivision | Angiospermae |
| Class | Magnoliopsida |
| Subclass | Asterids |
| Order | Solanales |
| Family | Solanaceae |
| Genus | Datura |
| Species | Datura sp. |
4. Species-Level Genetic Changes in Datura
| No. | Spesies | Study Sites | Genetic Variability Inducers | Results | Reference |
|---|---|---|---|---|---|
| 1. | D. innoxia | India | Agrobacterium-mediated plant transformation |
| [50] |
| 2. | D. innoxia | France | In vitro micropropagation |
| [40] |
| 3. | D. innoxia | Punjab, Pakistan | Wastewater irrigation |
| [57] |
| 4. | D. innoxia | Khyber Pakhtunkhwa (KP), Pakistan | Elevation gradient, Soil structure |
| [58] |
| 5. | D. innoxia | Mexico | Pollination system among population from various location |
| [38] |
| 6. | D. stramonium | Mexico | Bioinformatics |
| [17] |
| 7. | D. stramonium | California, USA | Agrobacterium-mediated plant transformation |
| [20] |
| 8. | D. stramonium | Mexico and Spain | Flowers biology from different populations |
| [26] |
| 9. | D. stramonium | Himachal Pradesh | Altitude differences |
| [39] |
| 10. | D. stramonium | Indiana, USA | Natural selection |
| [59] |
| 11. | D. stramonium | South Africa | Population study |
| [46] |
| 12. | D. stramonium | Mexico | Different environment |
| [47] |
| 13. | D. stramonium | Teotihuaca’n, State of Mexico | Inbreeding |
| [48] |
| 14. | D. stramonium | El Harrach, Algiers, Algeria | Gamma-irradiation on seeds |
| [49] |
| 15. | D. stramonium | Bulgarian | Differences in explants for in vitro culture |
| [51] |
| 16. | D. stramonium | Mexico | Cross breeding |
| [60] |
| 17. | D. stramonium | Mexico | Adaptability to different environments |
| [61] |
| 18. | D. stramonium | Mexico | Adaptability to different environments |
| [62] |
| 19. | D. stramonium | Italy, Portugal, Spain | Population and Temperature |
| [63] |
| 20. | D. stramonium | Durham, North Carolina | Inbreeding depression |
| [64] |
| 21. | D. stramonium | California, USA | Stigma-anther position |
| [65] |
| 22. | D. wrightii | USA | Environment |
| [66] |
| 23. | D. wrightii | Southern California, USA | Environment (irrigation) |
| [67] |
| 24. | D. wrightii | California, USA | Herbivore and Methyl Jasmonate-Induced |
| [68] |
| 25. | D. metel | Nagoya, Japan | Varieties |
| [37] |
| 26. | Datura spp. | Mexico | Population distribution |
| [16] |
| 27. | Datura spp. | Mexico | Taxonomy |
| [34] |
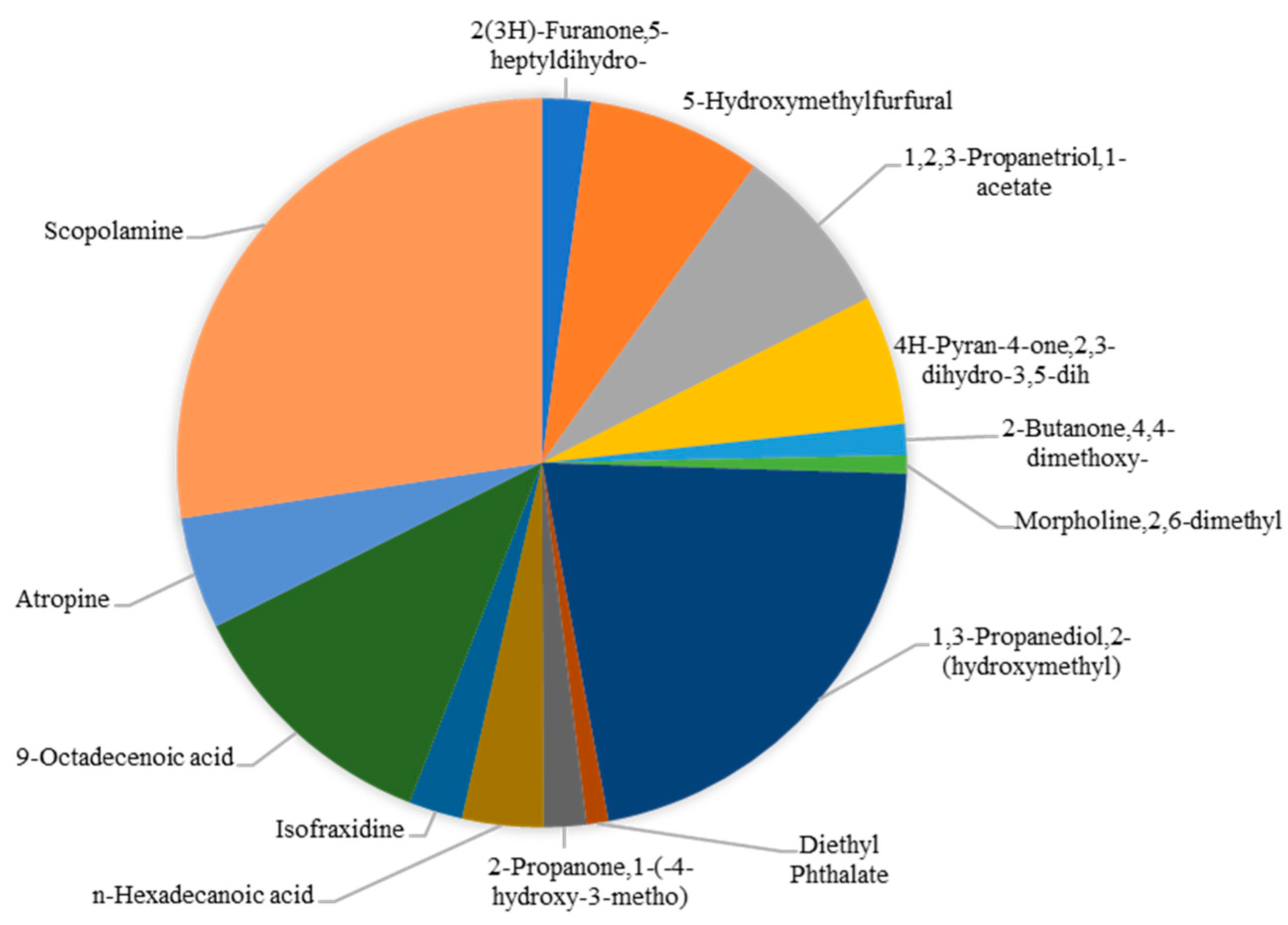
5. The Bioactive Compounds in the Genus Datura and Their Pharmacological Actions
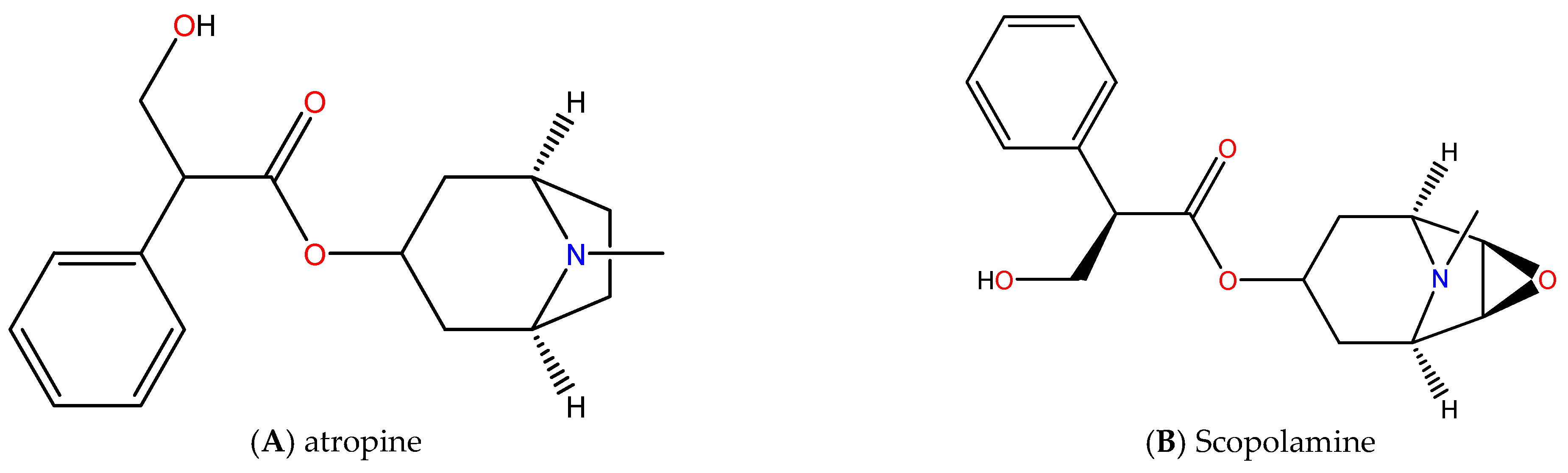

| No. | Datura Species | Datura Source | Pharmacological Activity | Bioactive Compound | Result | Reference |
|---|---|---|---|---|---|---|
| 1. | Datura innoxia Mill. | Fruits, steams, leaves | Brine shrimp lethality assay, anticancer in human leukimia (THP-1) cell line, protein kinase inhibitory assay, antifungal, antibacterial |
|
| [103] |
| 2. | Datura innoxia | Leaves | Antioxidant, enzyme inhibitor |
|
| [104] |
| 3. | Datura stramonium | Leaves | Anticancer in MCF-7, MDA-MB 231 (breast cancer cell lines), PC-3 (prostate cancer cell lines), brine shrimp lethality assay, protein kinase inhibitory assay |
|
| [105] |
| 4. | Datura innoxia | Leaves | Anticancer in MCF-7, MDA-MB 231 (breast cancer cell lines), PC-3 (prostate cancer cell lines), brine shrimp lethality assay, protein kinase inhibitory assay |
|
| [106] |
| 5. | Datura innoxia | Leaves | Anticancer, antioxidant, antiinflammatory | Withametelin |
| [107] |
| 6. | Datura metel | Leaves | Antiinflammatory | Sesquiterpenoids |
| [12] |
| 7. | Datura innoxia | Leaves | Antiinflammatory | Daturaolone |
| [108] |
| 8. | Datura innoxia | Leaves | Acute and subacute oral toxicity in Sprague Dawley rats | Withametelin and daturaolone |
| [109] |
| 9. | Datura fastuosa | Roots | Antioxidant |
|
| [110] |
| 10. | Datura stramonium | Leaves | Anticandidal |
|
| [111] |
| 11. | Datura metel | Leaves | Antibacterial and antioxidant |
|
| [112] |
6. The Role of Biosynthesis Genes in Metabolic Regulation Across Datura Species
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Das, A.; Kar, S.; Bhattacharya, P.; Das, S.; Bora, D.; Datta, B.K. Floral Biology and phenological studies of Datura metel L. in Tripura, Northeast India, with special reference to floral morphotypes. Plant Sci. Today 2024, 11, 132–138. [Google Scholar] [CrossRef]
- Jakabová, S.; Vincze, L.; Farkas, Á.; Kilár, F.; Boros, B.; Felinger, A. Determination of tropane alkaloids atropine and scopolamine by liquid chromatography–mass spectrometry in plant organs of Datura species. J. Chromatogr. A 2012, 1232, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Alam, W.; Khan, H.; Khan, S.A.; Nazir, S.; Akkol, E.K. Datura metel: A review on chemical constituents, traditional uses and pharmacological activities. Curr. Pharm. Des. 2021, 27, 2545–2557. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Cheng, Y.G.; Liu, Y.; Liu, J.Y.; Tan, W.; Guan, S.; Guo, S.; Kuang, H.X. Datura metel L. ameliorates imiquimod-induced psoriasis-like dermatitis and inhibits inflammatory cytokines production through TLR7/8-MyD88-NF-κB-NLRP3 inflammasome pathway. Molecules 2019, 24, 2157. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Li, T.; Sun, Y.; Su, H.; Zeng, Y.; Wang, Q.; Kuang, H. Daturataturin A, a withanolide in Datura metel L., induces HaCaT autophagy through the PI3K-Akt-mTOR signaling pathway. Phytother. Res. 2021, 35, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- White, P.T.; Subramanian, C.; Motiwala, H.F.; Cohen, M.S. Natural withanolides in the treatment of chronic diseases. In Anti-inflammatory Nutraceuticals and Chronic Diseases; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2016; Volume 928, pp. 329–373. [Google Scholar] [CrossRef] [PubMed]
- Alabri, T.H.A.; Al Musalami, A.H.S.; Hossain, M.A.; Weli, A.M.; Al-Riyami, Q. Comparative study of phytochemical screening, antioxidant and antimicrobial capacities of fresh and dry leaves crude plant extracts of Datura metel L. J. King Saud Univ. Sci. 2014, 26, 237–243. [Google Scholar] [CrossRef]
- Roy, S.; Mukherjee, S.; Pawar, S.; Chowdhary, A. Evaluation of in vitro antiviral activity of Datura metel Linn. against Rabies virus. Pharmacogn. Res. 2016, 8, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Prasathkumar, M.; Anisha, S.; Khusro, A.; Mohamed Essa, M.; Babu Chidambaram, S.; Walid Qoronfleh, M.; Sadhasivam, S.; Umar Khayam Sahibzada, M.; Alghamdi, S.; Almehmadi, M.; et al. Anti-Pathogenic, anti-diabetic, anti-inflammatory, antioxidant, and wound healing efficacy of Datura metel L. leaves. Arab. J. Chem. 2022, 15, 1–17. [Google Scholar] [CrossRef]
- Baylie, T.; Kebad, A.; Ayelgn, T.; Tiruneh, M.; Hunie Tesfa, K. Anti-Diabetic effects of the 80% methanolic extract of Datura stramonium Linn (Solanaceae) leaves in streptozotocin-induced diabetic mice. J. Exp. Pharmacol 2023, 15, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Hemeq, Y.; Rauf, A.; Hemeg, H.A.; Akram, Z.; Ahmad, Z.; Naz, H. Anticancer potential of crude extract and triterpenoid isolated from Datura metel Linnaeus. J. Popul. Ther. Clin. Pharmacol. 2023, 30, 1132–1138. [Google Scholar] [CrossRef]
- Tan, J.Y.; Liu, Y.; Cheng, Y.G.; Sun, Y.P.; Pan, J.; Yang, S.H.; Kuang, H.X.; Yang, B.Y. Anti-Inflammatory sesquiterpenoids from the leaves of Datura metel L. Fitoterapia 2020, 142, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mustapa, M.A.; Taupik, M.; Suryadi, A.M.A.; Pakaya, M.S.; Akuba, J.; Djuwarno, E.N.; El Kiyat, W. Cytotoxicity and fragmentation pattern of Datura metel L. leaves using ultra-performance liquid chromatography-mass spectroscopy. J. Appl. Pharm. Sci. 2023, 13, 57–67. [Google Scholar] [CrossRef]
- Tabani, U.; Jani, A.; Purohit, R.; Patel, B.; Sorathiya, R.; Jangbari, N.; Rana, A. Extraction and fraction of Datura metel Linn phytoconstituents and their evaluation as antibacterial compounds. Int. J. Biol. Pharm. Allied. Sci. 2023, 12, 1726–1737. [Google Scholar] [CrossRef]
- Sharan, M.; Jha, N.K.; Jha, S.K.; Jha, A.K. Reversal of promoter hypermethylation of CADM1 and SOCS1 by leaf extract of Datura metel in cervical cancer cells. Chem Biol Lett 2024, 11, 1–7. [Google Scholar] [CrossRef]
- Luna-Cavazos, M.; Bye, R.; Jiao, M. The origin of Datura metel (Solanaceae): Genetic and phylogenetic evidence. Genet. Resour. Crop Evol. 2009, 56, 263–275. [Google Scholar] [CrossRef]
- Velázquez-Márquez, S.; De-La-Cruz, I.M.; Tapia-López, R.; Núñez-Farfán, J. Tropane alkaloids and terpenes synthase genes of Datura stramonium (Solanaceae). PeerJ 2021, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Amuedo, C.G.; Lema, V.S. Pre-Hispanic Datura Ferox, L. in the Southern Andes: Archaeobotanical evidence from an inca archaeological site at Salta, Argentina. Veg. Hist. Archaeobot. 2024, 33, 441–457. [Google Scholar] [CrossRef]
- Ngai, H.L.; Kong, B.L.H.; Lau, D.T.W.; Shaw, P.C. Differentiation of Lingxiaohua and Yangjinhua by chloroplast genome sequencing and dna barcoding markers. Genome 2023, 66, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Rajewski, A.; Carter-House, D.; Stajich, J.; Litt, A. Datura genome reveals duplications of psychoactive alkaloid biosynthetic genes and high mutation rate following tissue culture. BMC Genom. 2021, 22, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kizil, S.; Toncer, O.; Sogut, T. The impact of nitrogen fertilization on the alkaloid content and growth traits of Datura (Datura stramonium L.). J. Agric. Sci. 2024, 69, 45–56. [Google Scholar] [CrossRef]
- Hirano, I.; Iida, H.; Ito, Y.; Park, H.D.; Takahashi, K. Effects of light conditions on growth and defense compound contents of Datura inoxia and D. stramonium. J. Plant Res. 2019, 132, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.K.; Olcerst, A.; McKibben, M.; Hare, J.D.; Barker, M.S.; Bronstein, J.L. A de Novo Long-Read genome assembly of the sacred datura plant (Datura wrightii) reveals a role of tandem gene duplications in the evolution of herbivore-defense response. BMC Genom. 2024, 25, 15. [Google Scholar] [CrossRef] [PubMed]
- Abe, R.; Ohtani, K. An Ethnobotanical study of medicinal plants and traditional therapies on Batan Island, the Philippines. J. Ethnopharmacol. 2013, 145, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Yulizar, Y.; Kusrini, E.; Apriandanu, D.O.B.; Nurdini, N. Datura metel L. Leaves extract mediated CeO2 nanoparticles: Synthesis, characterizations, and degradation activity of DPPH radical. Surf. Interfaces 2020, 19, 1–6. [Google Scholar] [CrossRef]
- Jiménez-Lobato, V.; Martínez-Borda, E.; Núñez-Farfán, J.; Valverde, P.L.; Cruz, L.L.; López-Velázquez, A.; Santos-Gally, R.; Arroyo, J. Changes in Floral Biology and Inbreeding Depression in native and invaded regions of Datura stramonium. Plant Biol. 2018, 20, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Salgotra, R.K.; Chauhan, B.S. Genetic diversity, conservation, and utilization of plant genetic resources. Genes 2023, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Baas, J.; Schotten, M.; Plume, A.; Côté, G.; Karimi, R. Scopus as a curated, high-quality bibliometric data source for academic research in quantitative science studies. Quant Sci. Stud. 2020, 1, 377–386. [Google Scholar] [CrossRef]
- Islam, T.; Ara, I.; Islam, T.; Sah, P.J.; de Almeida, R.S.; Matias, E.F.F.; Ramalho, C.L.G.; Coutinho, H.D.M.; Islam, M.T. Ethnobotanical uses and phytochemical, biological, and toxicological profiles of Datura metel L.: A review. CRTOX 2023, 4, 100106. [Google Scholar] [CrossRef] [PubMed]
- McLean, S.W. Interspecific crosses involving Datura ceratocaula obtained by embryo dissection. Am. J. Bot. 1946, 33, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Loyola-Vargas, V.M. Genetic transformation of Datura species. In Transgenic Medicinal Plants; Biotechnology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 1999; Volume 45. [Google Scholar]
- Hannick, V.C.; Mink, J.N.; Singhurst, J.R.; Holmes, W.C. Annotated checklist of the vascular flora of McLennan County, Texas. Phytoneuron 2013, 29, 1–37. [Google Scholar]
- Classification USDA Natural Resources Conservation Service. Available online: https://plants.usda.gov/java/ClassificationServlet?source=display&classid=DATUR (accessed on 11 May 2025).
- Jiao, M.; Luna-Cavazos, M.; Bye, R. Allozyme variation in Mexican species and classification of Datura (Solanaceae). Plant Syst. Evol. 2002, 232, 155–166. [Google Scholar] [CrossRef]
- Berkov, S.; Zayed, R.; Doncheva, T. Alkaloid patterns in some varieties of Datura stramonium. Fitoterapia 2006, 77, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Verloove, F. Datura wrightii (Solanaceae), a neglected xenophyte, new to Spain. Bouteloua 2008, 4, 37–40. [Google Scholar]
- Hiraoka, N.; Tashimo, K.; Kinoshita, C.; Hiro’oka, M. Genotypes and alkaloid contents of Datura metel varieties. Biol. Pharm. Bull. 1996, 19, 1086–1089. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiménez-Lobato, V.; Núñez-Farfán, J. Mating system of Datura inoxia: Association between selfing rates and herkogamy within populations. PeerJ 2021, 9, e10698. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Sharma, P.; Arora, S. Phytochemical screening of Datura using GC-MS in Shivalik and mid- Himalayan regions of Himachal Pradesh. IOP Conf. Ser. Earth Environ. Sci. 2023, 1110, 1–12. [Google Scholar] [CrossRef]
- Bouami-Guennouni-assimi, R.; Cosson, L.; Jacquin-Dubreuil, A. Selection and improvement of Datura innoxia Mill. morphological variability in in vitro propagated plants. Bull. Soc. Bot. France 1990, 137, 261–269. [Google Scholar] [CrossRef]
- Alexander, J.; Benford, D.; Cockburn, A.; Cravedi, J.-P.; Dogliotti, E.; Di Domenico, A.; Férnandez-Cruz, M.L.; Fürst, P.; FinkGremmels, J.; Galli, C.L.; et al. Scientific opinion of the panel on contaminants in the food chain on a request from the European Commission on tropane alkaloids (from Datura sp.) as undesirable substances in animal feed. EFSA J. 2008, 691, 1–55. [Google Scholar]
- Berkov, S.; Zayed, R. Comparison of tropane alkaloid spectra between Datura innoxia a grown in Egypt and Bulgaria. Z. Naturforsch. 2004, 59, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Cavazos, M.L.; Jiao, M.; Bye, R. Phenetic analysis of Datura section Dutra (Solanaceae) in Mexico. J. Linn. Soc. Bot. 2000, 133, 493–507. [Google Scholar] [CrossRef][Green Version]
- Lockwood, T.E. Generic recognition of Brugmansia. Bot. Mus. Leaf. Harv. Univ. 1973, 23, 273–284. [Google Scholar] [CrossRef]
- Dupin, J.; Smith, S.D. Phylogenetics of Datureae (Solanacease), including description of the new genus Trompettia–re-circumscription of the tribe. Taxon 2018, 67, 359–375. [Google Scholar] [CrossRef]
- Kleunen, M.V.; Fischer, M.; Johnson, S.D. Reproductive assurance through self-fertilization does not vary with population size in the alien invasive plant Datura stramonium. Oikos 2007, 116, 1400–1412. [Google Scholar] [CrossRef]
- Camargo, I.D.; Nattero, J.; Careaga, S.A.; Núñez-Farfán, J. Flower-level developmental plasticity to nutrient availability in Datura stramonium: Implications for the mating system. Ann. Bot. 2017, 120, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Bello-Bedoy, R.; Núñez-Farfán, J. The effect of Inbreeding on defence against multiple enemies in Datura stramonium. J. Evol. Biol. 2011, 24, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Benslimani, N.; Khelifi-Slaoui, M.; Morsli, A.; Djerrad, A.; Al-Ramamneh, E.A.-D.; Makhzoum, A.; Khelifi, L. Effects of gamma irradiation on the alkaloid content in seeds of Datura stramonium and the radiosensitivity of derived seedlings. Plant Sci. Today 2019, 6, 533–540. [Google Scholar] [CrossRef]
- Ducrocq, C.; Sangwan, R.S.; Sangwan-Norreel, B.S. Production of Agrobacterium-mediated transgenic fertile plants by direct somatic embryogenesis from immature zygotic embryos of Datura innoxia. Plant Mol. Biol. 1994, 25, 995–1009. [Google Scholar] [CrossRef] [PubMed]
- Weberm, J.; Georgiev, V.; Pavlov, A.; Bley, T. Flow cytometric investigations of diploid and tetraploid plants and in vitro cultures of Datura stramonium and Hyoscyamus niger. Cytom. Part A 2008, 73, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Kamada, H.; Sudo, H.; Harada, H. Effects of gibberellic acid on hairy root growth in Datura innoxia. J. Plant Physiol. 1989, 134, 633–636. [Google Scholar] [CrossRef]
- Kamada, H.; Ogasawara, T.; Harada, H. Effects of gibberellin A on growth and tropane alkaloid synthesis in Ri transformed plants of Datura innoxia. In Gibberellins; Takahashi, N., Phinney, B.O., MacMillan, J., Eds.; Springer: New York, NY, USA, 1989; Volume 45, pp. 241–248. [Google Scholar]
- Prasad, G.P.; Pratap, G.P.; Marimuthu, S.; Prasad, S.B.; Rao, G.; Mangal, A.K.; Srikanth, N. Molecular identification and Next-Generation Sequence analysis of interspecies genetic variations among three varieties of Datura. Pharmacog. Res. 2020, 12, 158–162. [Google Scholar] [CrossRef]
- Al-Andal, A.; Ewas, M.; Donia, A.E.R.M.; Radwan, A.M.; Suliman, M.N.S.; Nishawy, E.; El- Shabasy, A.; Khames, E. A three-sided story: A biosystematic revision of genus Datura reveals novel tropane alkaloids for the first-time in certain species. Front Plant Sci. 2025, 16, 1555237. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yuanye, D.; Qing, L.; Jinjian, L.; Xiwen, L.; Yitao, W. Complete chloroplast genome sequence of poisonous and medicinal plant Datura stramonium: Organizations and implications for genetic engineering. PLoS ONE 2014, 9, e110656. [Google Scholar] [CrossRef] [PubMed]
- Hassan Farooq, T.; Jabeen, S.; Shakoor, A.; Saleem Arif, M.; Siddique, N.; Shahzad, K.; Umair Riaz, M.; Li, Y. Morpho-anatomical adaptations of dominantly grown wild Datura inoxia to wastewater resource: Productivity and ecological issues. Geosci. Front 2024, 15, 101717. [Google Scholar] [CrossRef]
- Khan, N.; Ullah, R.; Okla, M.K.; Abdel-Maksoud, M.A.; Saleh, I.A.; Abu-Harirah, H.A.; AlRamadneh, T.N.; AbdElgawad, H. Climate and soil factors co-derive the functional traits variations in naturalized downy thorn apple (Datura innoxia Mill.) along the altitudinal gradient in the semi-arid environment. Heliyon 2024, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shonle, I.; Bergelson, J. Evolutionary ecology of the tropane alkaloids of Datura stramonium L. (Solanaceae). Evolution 2000, 54, 778–788. [Google Scholar] [CrossRef] [PubMed]
- De-la-Cruz, I.M.; Hallab, A.; Olivares-Pinto, U.; Tapia-López, R.; Velázquez-Márquez, S.; Piñero, D.; Oyama, K.; Usadel, B.; Núñez-Farfán, J. Genomic signatures of the evolution of defence against its natural enemies in the poisonous and medicinal plant Datura stramonium (Solanaceae). Sci. Rep. 2021, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Castillo, G.; Valverde, P.L.; Cruz, L.L.; Hernández-Cumplido, J.; Andraca-Gómez, G.; Fornoni, J.; Sandoval-Castellanos, E.; Olmedo-Vicente, E.; Flores-Ortiz, C.M.; Núñez-Farfán, J. Adaptive divergence in resistance to herbivores in Datura stramonium. PeerJ 2015, 11, e1411. [Google Scholar] [CrossRef] [PubMed]
- Castillo, G.; Cruz, L.L.; Tapia-López, R.; Olmedo-Vicente, E.; Carmona, D.; Anaya-Lang, A.L.; Fornoni, J.; Andraca-Gómez, G.; Valverde, P.L.; Núñez-Farfán, J. Selection mosaic exerted by specialist and generalist herbivores on chemical and physical defense of Datura stramonium. PLoS ONE 2014, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Loddo, D.; Sousa, E.; Masin, R.; Calha, I.M.; Zanin, G.; Fernández-Quintanilla, C.; Dorado, J. Germination response of local Southern European populations of Datura stramonium at a range of constant temperatures. Weed Res. 2014, 54, 356–365. [Google Scholar] [CrossRef]
- Stone, J.L.; Motten, A.F. Anther-stigma separation is associated with inbreeding depression in Datura stramonium, a predominantly self-fertilizing annual. Evolution 2002, 56, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Motten, A.F.; Stone, J.C. Heritability of stigma position and the effect of stigma-anther separation on outcrossing in a predominantly self-fertilizing weed, Datura stramonium (Solanaceae). Am. J. Bot. 2000, 87, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Elle, E.; Hare, J.D. Environmentally induced variation in floral traits affects the mating system in Datura wrightii. Funct. Ecol. 2002, 16, 79–88. [Google Scholar] [CrossRef]
- Hare, J.D. Variation in herbivore and methyl jasmonate-induced volatiles among genetic lines of Datura wrightii. J. Chem Ecol. 2007, 33, 2028–2043. [Google Scholar] [CrossRef] [PubMed]
- Forkner, R.E.; Hare, J.D. Genetic and environmental variation in acyl glucose ester production and glandular and nonglandular trichome densities in Datura wrightii. J. Chem Ecol. 2000, 26, 2801–2823. [Google Scholar] [CrossRef]
- Kundu, A.; Swarnalakshmi, K.; Rajkhowa, S.; Bhagyasree, S.N.; Nath, T.; Sarmah, B.K.; Ghosh, S. Phytochemicals, UPLC-QTOF-MS/MS analysis, potential acaricidal activity and molecular modeling of Datura metel grown in North-Eastern India. J. Nat. Pestic. Res. 2024, 10, 1–11. [Google Scholar] [CrossRef]
- Tapfuma, K.I.; Mekuto, L.; Makatini, M.M.; Mavumengwana, V. The LC-QTOF-MS/MS analysis data of detectedmetabolites from the crude extract of Datura stramonium leaves. Data Brief 2019, 25, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, A.; Vaganan, M.M.; Sundararaju, P.; Udayakumar, R. Phytochemical analysis and nematicidal activity of ethanolic leaf extracts of Datura metel, Datura innoxia and Brugmansia suaveolens against Meloidogyne incognita. Asian J. Biol. 2017, 2, 1–11. [Google Scholar] [CrossRef]
- Miraldi, E.; Masti, A.; Ferri, S.; Comparini, I.B. Distribution of hyoscyamine and scopolamine in Datura stramonium. Fitoterapia 2001, 72, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Passos, I.D.; Mironidou-Tzouveleki, M. Chapter 71–Hallucinogenic plants in the Mediterranean countries. In Neuropathology of Drug Addictions and Substance Misuse; Academic Press: Cambridge, MA, USA, 2016; pp. 761–772. [Google Scholar] [CrossRef]
- Maheshwari, N.; Khan, A.; Chopade, B.A. Rediscovering the medicinal properties of Datura sp.: A review. J. Med. Plants Res. 2013, 7, 2885–2897. [Google Scholar] [CrossRef]
- Benítez, G.; March-Salas, M.; Villa-Kamel, A.; Cháves-Jiménez, U.; Hernández, J.; Montes-Osuna, N.; Moreno-Chocano, J.; Cariñanos, P. The genus Datura L. (Solanaceae) in Mexico and Spain—Ethnobotanical perspective at the interface of medical and illicit uses. J. Ethnopharmacol. 2018, 218, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Batool, A.; Batool, Z.; Qureshi, R.; Raja, N.I. Phytochemicals, pharmacological properties and biotechnological aspects of a highly medicinal plant: Datura stramonium. J. Plant Sci. 2020, 8, 29–40. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Jones, A.D. Alkaloids of the genus Datura: Review of a rich resource for natural product discovery. Molecules 2021, 26, 2629. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization Model List of Essential Medicines, 21st List; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- WHO. Annex 1 19th WHO Model List of Essential Medicines; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- MacEwan, G.W.; Remick, R.A.; Noone, J.A. Psychosis due to transdermally administered scopolamine. CMAJ 1985, 133, 431–432. [Google Scholar] [PubMed]
- Kassel, L.; Nelson, M.; Shine, J.; Jones, L.R.; Kassel, C. Scopolamine use in the perioperative patient: A systematic review. AORN J. 2018, 108, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Xia, Y.G.; Liu, Y.; Li, L.; Jiang, H.; Yang, L.; Wang, Q.H.; Kuang, H.X. New antiproliferative and immunosuppressive withanolides from the seeds of Datura metel. Phytochem. Lett 2014, 8, 92–96. [Google Scholar] [CrossRef]
- Abubakar, M.; Suleiman, U.; Frank, A.; Ukwuani, A. Hallucinogenic effects of aqueous seeds extract of Datura metel in rats. Internet J. Pharmacol. 2009, 9, 1. [Google Scholar]
- Tijani, A.Y.; Eyineyi, U.G.; Ibrahim, J.A.; Okhale, S.E. Neuro-toxicological impacts of Datura metel Linn. (family: Solanaceae) leaves extract in mice. J. NeuroBehav. Sci. 2015, 2, 1–5. [Google Scholar] [CrossRef]
- Soni, P.; Siddiqui, A.A.; Dwivedi, J.; Soni, V. Pharmacological properties of Datura stramonium L. as a potential medicinal tree: An overview. Asian Pac. J. Trop. Biomed. 2012, 2, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Bawazeer, S.; Rauf, A.; Bawazeer, S. Gastrointestinal motility, muscle relaxation, antipyretic and acute toxicity screening of amyrin type triterpenoid (daturaolone) isolated from Datura metel Linnaeus (Angel’s Trumpet) fruits. Front Pharmacol. 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Javaid, A.; Shafique, S. Herbicidal effects of extracts and residue incorporation of Datura metel against parthenium weed. Nat. Prod. Res. 2010, 24, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Abena, A.A.; Miguel, L.M.; Mouanga, A.; Ouamba, J.M.; Sianard, D.F.; Thiebolt, M.H.; Hondi-Assah, T.C.; Diatewa, M. Neuropsychopharmacological effects of leaves and seeds extracts of Datura fastuosa. Biotechnology 2004, 3, 109–113. [Google Scholar] [CrossRef]
- Ibrahim, M.; Siddique, S.; Rehman, K.; Husnain, M.; Hussain, A.; Akash, M.S.H.; Azam, F. Comprehensive analysis of phytochemical constituents and ethnopharmacological investigation of genus Datura. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 223–283. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.J.; Reynell, C. How do antidepressants influence the BOLD signal in the developing brain? Dev. Cogn. Neurosci. 2017, 25, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Sang, S.; Park, H.-J.; Kwon, S.J.; Suh, N.; Huang, M.-T.; Ho, C.-T.; Yang, C.S. Modulation of arachidonic acid metabolism and nitric oxide synthesis by garcinol and its derivatives. Carcinogenesis 2006, 27, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Dhaliwal, I.; Rana, K.; Delta, A.K.; Kaushik, P. Phytochemistry, pharmacology, and toxicology of Datura species-a review. Antioxidants 2021, 10, 1291. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 174174, Atropine PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Atropine (accessed on 29 May 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 3000322, Boro-Scopol PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Boro-Scopol (accessed on 29 May 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 154417 PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/hyoscyamine (accessed on 29 May 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 71587892, 7-Hydroxyhyoscyamine PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/7-Hydroxyhyoscyamine (accessed on 29 May 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 25084503, Scopoline PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Scopoline (accessed on 29 May 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 79037, 8-Methyl-8-azabicyclo[3.2.1]octane PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/8-Methyl-8-azabicyclo_3.2.1_octane (accessed on 29 May 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 364746 PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/364746 (accessed on 29 May 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 181868, Datumetine PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Datumetine (accessed on 29 May 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 122859, Daturaolone PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Daturaolone (accessed on 29 May 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6918612, Anisodamine PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6918612 (accessed on 29 May 2025).
- Fatima, H.; Khan, K.; Zia, M.; Ur-Rehman, T.; Mirza, B.; Haq, I. Extraction optimization of medicinally important metabolites from Datura innoxia Mill.: An in vitro biological and phytochemical investigation. BMC Complement Altern. Med. 2015, 15, 376. [Google Scholar] [CrossRef] [PubMed]
- Benabderrahim, M.A.; Sarikurkcu, C.; Elfalleh, W.; Ozer, M.S. Datura innoxia and Dipsacus laciniatus: Biological activity and phenolic composition. Biocatal. Agric. Biotech. 2019, 19, 1–6. [Google Scholar] [CrossRef]
- Nasir, B.; Baig, M.W.; Majid, M.; Ali, S.M.; Khan, M.Z.I.; Kaxmi, T.B.; Haq, I. Preclinical anticancer studies on the ethyl acetate leaf extracts of Datura stramonium and Datura inoxia. BMC Complement Med. Ther. 2020, 20, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.W.; Nasir, B.; Waseem, D.; Majid, M.; Khan, M.Z.I.; Haq, I.U. Withametelin: A biologically active withanolide in cancer, inflammation, pain and depression. Saudi Pharm. J. 2020, 28, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.W.; Fatima, H.; Akhtar, N.; Hussain, H.; Okla, M.K.; Al-Hashimi, A.; Al-Qahtani, W.H.; AbdElgawad, H.; Haq, I. Antiinflammatory potential of daturaolone from Datura innoxia Mill: In silico, in vitro and in vivo studies. Pharmaceuticals 2021, 14, 1248. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.W.; Majid, M.; Nasir, B.; Hassan, S.S.U.; Bungau, S.; Haq, I.U. Toxicity evaluation induced by single and 28-days repeated exposure of withametelin and daturaolone in Sprague Dawley rats. Front Pharmacol. 2022, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Melese, G.M.; Aychiluhim, T.B.; Yessuf, A.M.; Eshete, M. 2024. Identification and characterization of phytochemicals in methanolic extract of roots of Datura fastuosa using various techniques. Futur. J. Pharm. Sci. 2024, 10, 108. [Google Scholar] [CrossRef]
- Showkat, S.; Dharumadurai, D.; Kumar, T.S. Phytochemical profiling, spectroscopic identification of active compounds, and mechanism of the anticandidal properties of Datura stramonium L. using SwissADMET prediction and molecular docking analysis. Microb. Pathog. 2025, 198, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Marappa, B.; Gunashree, B.S. Exploration of Potent Antimicrobial and antioxidant bioactive compounds of selected medicinal plants against drug-resistant pathogens. 3 Biotech 2025, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Kohnen-Johannsen, K.L.; Kayser, O. Tropane alkaloids: Chemistry, pharmacology, biosynthesis and production. Molecules 2019, 24, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.A.; Petschenka, G.; Bingham, R.A.; Weber, M.G.; Rasmann, S. Toxic cardenolides: Chemical ecology and coevolution of specialized plant-herbivore interactions. N. Phytol. 2012, 194, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Kariñho-Betancourt, E. Plant-herbivore interactions and secondary metabolites of plants: Ecological and evolutionary perspectives. Bot. Sci. 2018, 96, 35–51. [Google Scholar] [CrossRef]
- Kariñho-Betancourt, E. Coevolution: Plant-herbivore interactions and secondary metabolites of plants. In Co-Evolution of Secondary Metabolites; Mérillon, J.M., Ramawat, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 47–76. [Google Scholar]
- Hashimoto, T.; Nakajima, K.; Ongena, G.; Yamada, Y. Two Tropinone Reductases with distinct Stereospecificities from cultured roots of Hyoscyamus niger. Plant Physiol. 1992, 100, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Boitel-Conti, M.; Laberche, J.-C.; Lanoue, A.; Ducrocq, C.; Sangwan-Norreel, B.S. Influence of feeding precursors on tropane alkaloid production during an abiotic stress in Datura innoxia transformed roots. Plant Cell Tiss. Organ Cult. 2000, 60, 131–137. [Google Scholar] [CrossRef]
- Rahimi, S.; Kim, J.; Mijakovic, I.; Jung, K.H.; Choi, G.; Kim, S.C.; Kim, Y.J. Triterpenoid-biosynthetic UDP-glycosyltransferases from plants. Biotechnol. Adv. 2019, 37, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.E.; Tian, L. Phylogenomic analysis of UDP dependent glycosyltransferases provides insights into the evolutionary landscape of glycosylation in plant metabolism. Plant J. 2019, 100, 1273–1288. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef] [PubMed]
- De-la-Cruz, I.M.; Kariñho-Betancourt, E.; Núñez-Farfán, J.; Oyama, K. Gene family evolution and natural selection signatures in Datura spp. (Solanaceae). Front. Ecol. Evol. 2022, 10, 1–15. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, T.; Zhang, R.; Bu, Q.; Yin, Q.; Zhang, L.; Chen, W. Molecular cloning and functional analysis of hyoscyamine 6β-hydroxylase (H6H) in the poisonous and medicinal plant Datura innoxia Mill. Plant Physiol. Biochem. 2020, 153, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y. KEGG mapper for inferring cellular functions from protein sequences. Protein Sci. 2019, 29, 28–35. [Google Scholar] [CrossRef] [PubMed]
- De-la-Cruz, I.M.; Cruz, L.L.; Martínez-García, L.; Valverde, P.L.; Flores-Ortiz, C.M.; Hernández-Portilla, L.B.; Núñez-Farfán, J. Evolutionary response to herbivory: Population differentiation in microsatellite loci, tropane alkaloids and leaf trichome density in Datura stramonium. Arthropod Plant Interact. 2020, 14, 21–30. [Google Scholar] [CrossRef]
- Kasukabe, Y.; He, L.; Nada, K.; Misawa, S.; Ihara, I.; Tachibana, S. Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol. 2004, 45, 712–722. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assidqi, K.; Sianipar, N.F.; Mangindaan, D.; Enyi, C.U. Genus Datura: An Exploration of Genetic Alterations, Bioactive Compounds, and Pharmacological Activity. Plants 2025, 14, 2244. https://doi.org/10.3390/plants14142244
Assidqi K, Sianipar NF, Mangindaan D, Enyi CU. Genus Datura: An Exploration of Genetic Alterations, Bioactive Compounds, and Pharmacological Activity. Plants. 2025; 14(14):2244. https://doi.org/10.3390/plants14142244
Chicago/Turabian StyleAssidqi, Khoirunnisa, Nesti Fronika Sianipar, Dave Mangindaan, and Chukwunwike Uchenna Enyi. 2025. "Genus Datura: An Exploration of Genetic Alterations, Bioactive Compounds, and Pharmacological Activity" Plants 14, no. 14: 2244. https://doi.org/10.3390/plants14142244
APA StyleAssidqi, K., Sianipar, N. F., Mangindaan, D., & Enyi, C. U. (2025). Genus Datura: An Exploration of Genetic Alterations, Bioactive Compounds, and Pharmacological Activity. Plants, 14(14), 2244. https://doi.org/10.3390/plants14142244







