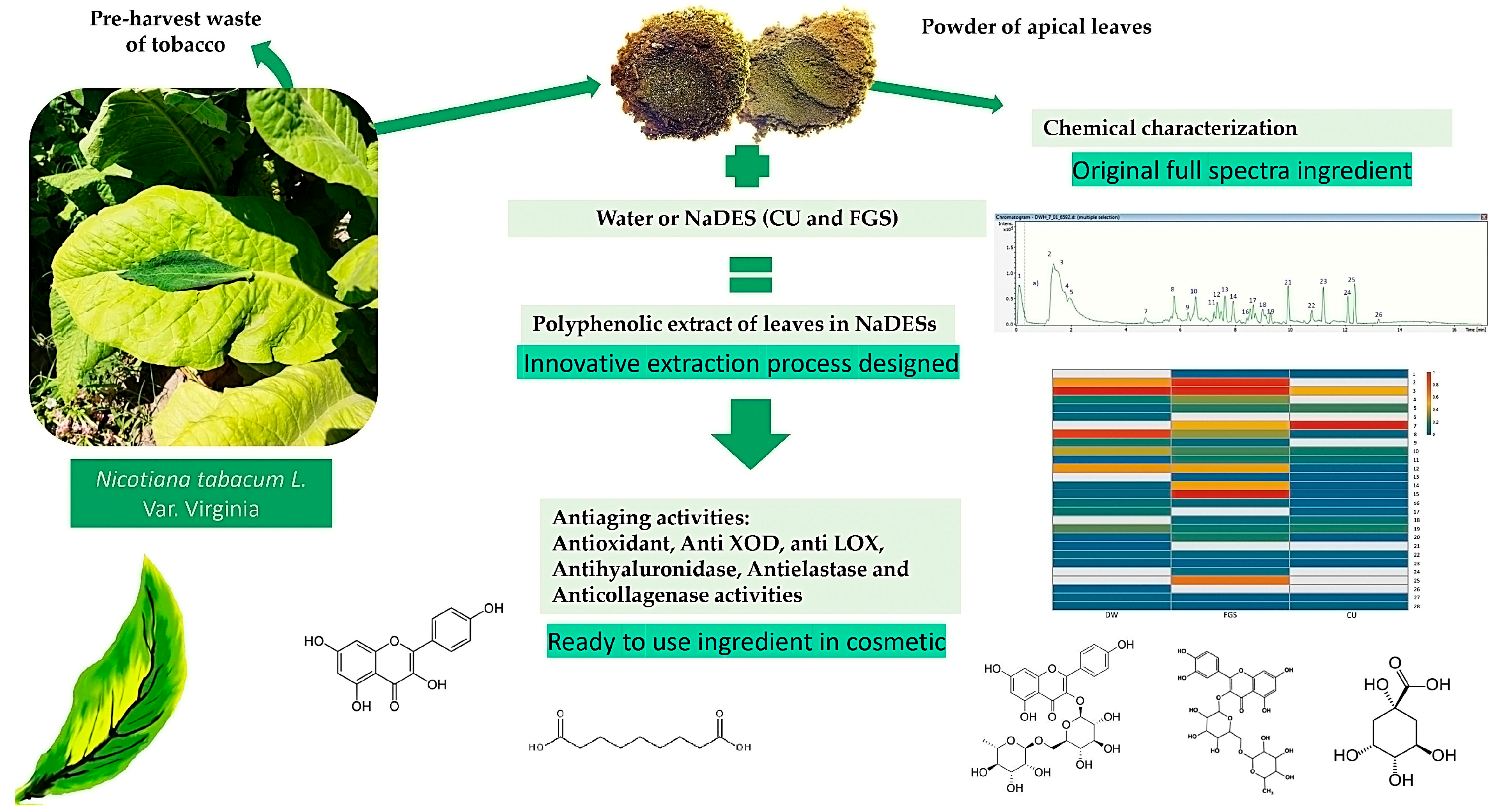
Green Extract from Pre-Harvest Tobacco Waste as a Non-Conventional Source of Anti-Aging Ingredients for Cosmetic Applications
Abstract
1. Introduction
2. Results and Discussion
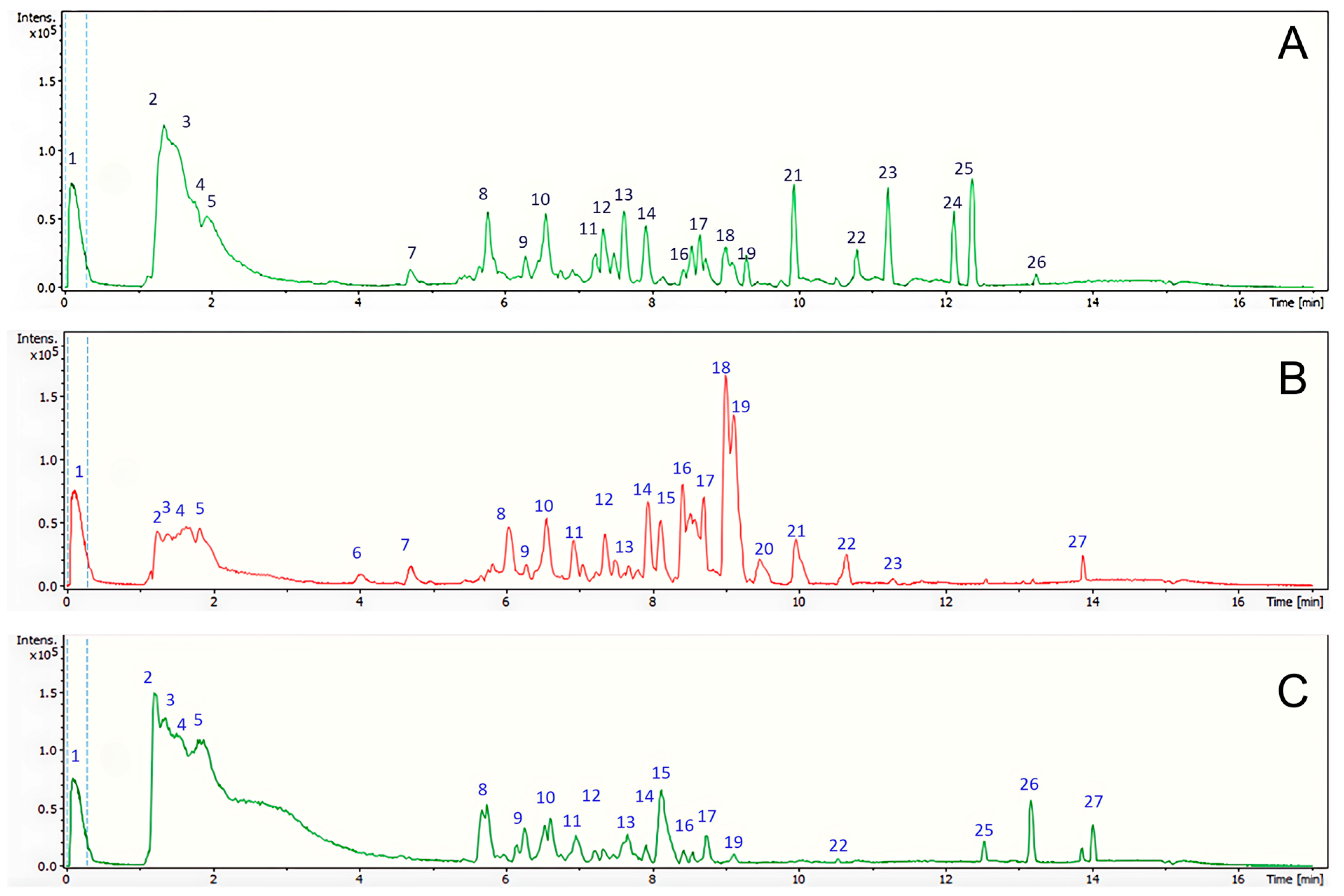
2.1. Phytochemical Composition of Nicotiana tabacum Green Extracts
2.2. Effect of Tobacco Leaf Waste Extracts on Enzymatic Activities Related to Anti-Inflammatory Mechanisms
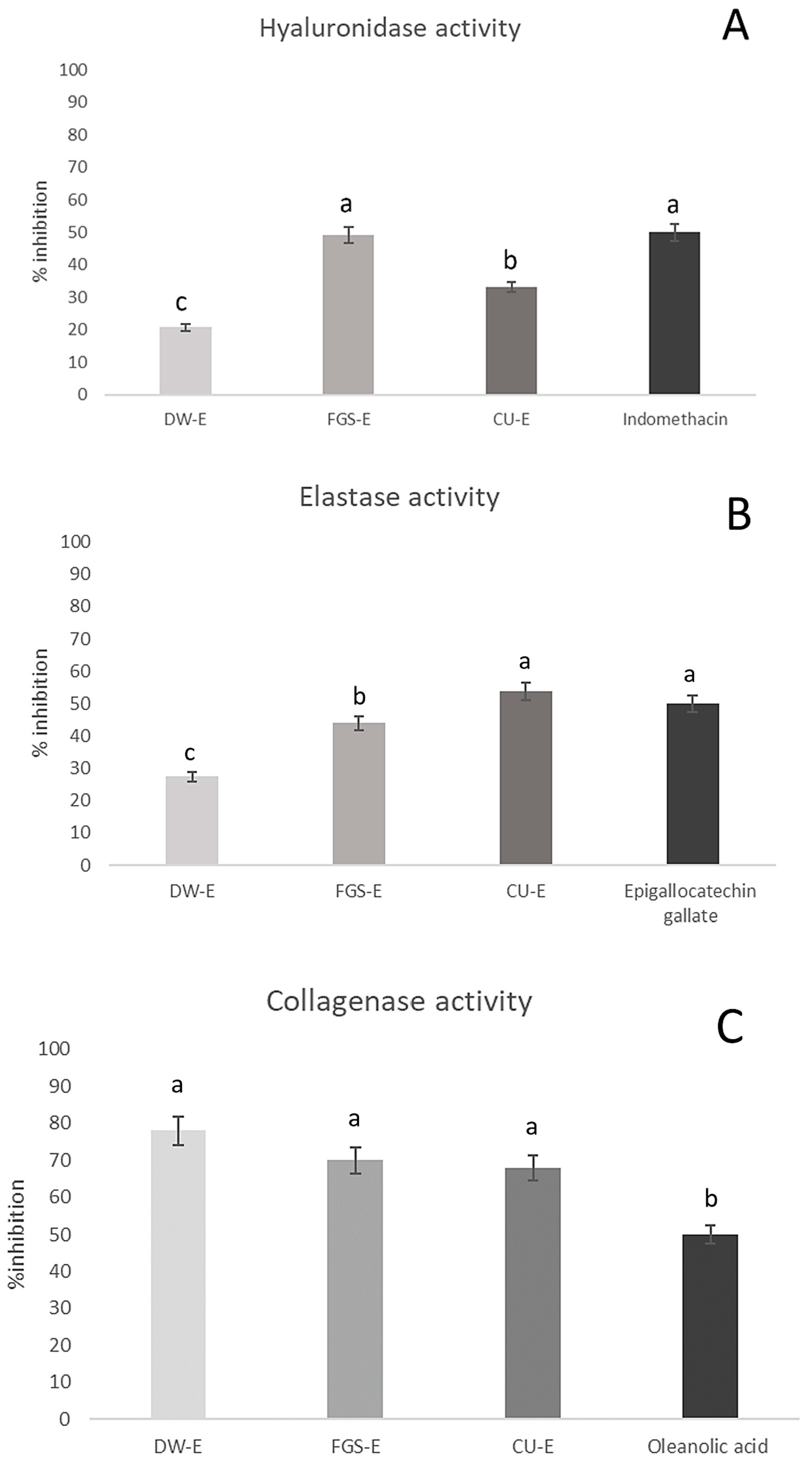
2.3. Activity on Skin Aging-Related Enzymes
2.4. Toxicity Assays
3. Materials and Methods
3.1. Reagents
3.2. Plant Material
3.3. NaDESs Preparation
3.4. Powder Extraction
3.5. Determination of Chemical Composition
3.5.1. Total Polyphenol and Flavonoid Quantification
3.5.2. UHPLC-MS
3.6. Anti-Inflammatory Activity
3.6.1. Xanthine Oxidase (XO) Inhibition
3.6.2. Lipoxygenase (LOX) Inhibition
3.7. Activity of Tobacco Leaf Extracts on Skin Aging-Related Enzymes
3.7.1. Elastase Inhibition
3.7.2. Hyaluronidase Inhibition
3.7.3. Collagenase Inhibition
3.8. Mutagenicity
3.8.1. Salmonella Typhimurium Assay
3.8.2. Caenorhabditis elegans Toxicity Test
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, M.; Freeman, M.K.; Fleming, T.D.; Robinson, M.; Dwyer-Lindgren, L.; Thomson, B.; Wollum, A.; Sanman, E.; Wulf, S.; Lopez, A.D.; et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA 2014, 311, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Datos Argentina. Agroindustria. Tabaco. 2025. Available online: https://datos.gob.ar/ro/dataset/agroindustria-tabaco---produccion (accessed on 11 February 2025).
- Cooperation Centre for Scientific Research Relative to Tobacco (CORESTA). Available online: https://www.coresta.org/good-agricultural-practices-gap-guidelines-29207.html (accessed on 6 March 2025).
- Diana, N.E.; Jamil, A.H.; Yogi, Y.A.; Nugraheni, S.D.; Verona, L. Improving the production and quality of Virginia tobacco through topping and suckering: A Review. IOP Conf. Ser. Earth Environ. Sci. 2022, 974, 012020. [Google Scholar] [CrossRef]
- Cosmetic Ingredient Database (CosIng) of the European Commission. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022D0677 (accessed on 2 February 2025).
- Leffingwell, J.C. Leaf chemistry. In Tobacco: Production, Chemistry and Technology; Davis, D.L., Nielsen, M.T., Eds.; Blackwell Science Ltd.: Oxford, UK, 1999. [Google Scholar]
- Chen, Y.; Yu, Q.; Li, X.; Luo, Y.; Liu, H. Extraction and HPLC Characterization of Chlorogenic Acid from Tobacco Residuals. Sep. Sci. Technol. 2007, 42, 3481–3492. [Google Scholar] [CrossRef]
- Shifflett, J.R.; Watson, L.; McNally, D.J.; Bezabeh, D.Z. Analysis of the polyphenols of tobacco using pressurized liquid extraction (PLE) and ultra performance liquid chromatography with electrospray ionization–tandem mass spectrometric detection (UPLC-ESI-MS/MS). CTNR 2017, 27, 195–207. [Google Scholar] [CrossRef]
- Sifola, M.I.; Carrino, L.; Cozzolino, E.; del Piano, L.; Graziani, G.; Ritieni, A. Potential of Pre-Harvest Wastes of Tobacco (Nicotiana tabacum L.) Crops, Grown for Smoke Products, as Source of Bioactive Compounds (Phenols and Flavonoids). Sustainability 2021, 13, 2087. [Google Scholar] [CrossRef]
- Zou, X.; Bk, A.; Rauf, A.; Saeed, M.; Al-Awthan, Y.S.; Al-Duais, M.; Bahattab, O.; Hamayoon Khan, M.; Suleria, H.A. Screening of polyphenols in tobacco (Nicotiana tabacum) and determination of their antioxidant activity in different tobacco varieties. ACS Omega 2021, 6, 25361–25371. [Google Scholar] [CrossRef] [PubMed]
- Banožic, M.; Babi´c, J.; Joki´c, S. Recent advances in extraction of bio-active compounds from tobacco industrial waste-a review. Ind. Crops Prod. 2020, 144, 112009. [Google Scholar] [CrossRef]
- Severson, R.F.; Arrendale, R.F.; Chortyk, O.T.; Johnson, A.W.; Jackson, D.M.; Gwynn, G.R.; Chaplin, J.F.; Stephenson, M.G. Quantitation of the major cuticular components from green leaf of different tobacco types. J. Agric. Food Chem. 1984, 32, 566–570. [Google Scholar] [CrossRef]
- Sisson, V.A.; Severson, R.F. Alkaloid composition of the Nicotiana species. Beitr. Tab. Int. 1990, 14, 327–339. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlen-brock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Villa, C.; Caviglia, D.; Robustelli della Cuna, F.S.; Zuccari, G.; Russo, E. NaDES Application in Cosmetic and Pharmaceutical Fields: An Overview. Gels 2024, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Benoit, C.; Virginie, C.; Boris, V. Chapter Twelve -The Use of NADES to Support Innovation in the Cosmetic Industry. Adv. Bot. Res. 2021, 97, 309–332. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Kloskowski, A.; Namiesnik, J. Perspectives on the Replacement of Harmful Organic Solvents in Analytical Methodologies: A Framework toward the Implementation of a Generation of Eco-Friendly Alternatives. Green Chem. 2015, 17, 3687–3705. [Google Scholar] [CrossRef]
- Roccha, D.; Freitas, D.S.; Magalhães, J.; Fernandes, M.; Silva, S.; Noro, J.; Ribeiro, A.; Cavaco-Paulo, A.; Martins, M.; Silva, C. NADES-Based Cork Extractives as Green Ingredients for Cosmetics and Textiles. Processes 2023, 11, 309. [Google Scholar] [CrossRef]
- Leal, M.; Moreno, M.A.; Albornoz, P.L.; Mercado, M.I.; Zampini, I.C.; Isla, M.I. Nicotiana tabacum leaf waste: Morphological characterization and chemical-functional analysis of extracts obtained from powder leaves by Using Green Solvents. Molecules 2023, 28, 1396. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.; Moreno, M.A.; Albornoz, P.L.; Mercado, M.I.; Zampini, I.C.; Isla, M.I. Morphological Characterization of Nicotiana tabacum Inflorescences and Chemical-Functional Analysis of Extracts Obtained from Its Powder by Using Green Solvents (NaDESs). Plants 2023, 12, 1554. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Lee, Y.H.; Rho, N.K.; Park, K.Y. Skin aging from mechanisms to interventions: Focusing on dermal aging. Front. Physiol. 2023, 14, 1195272. [Google Scholar] [CrossRef] [PubMed]
- Banglao, W.; Thongmee, A.; Sukplang, P.; Wanakhachornkrai, O. Determination of antioxidant, anti-aging and cytotoxicity activity of the essential oils from Cinnamomum zeylanicum. J. Microbiol. Biotechnol. Food Sci. 2020, 10, 436–440. [Google Scholar] [CrossRef]
- Abd Razak, D.L.; Jamaluddin, A.; Abd Rashid, N.Y.; Sani, N.A.; Abdul Manan, M. Assessment of cosmeceutical potentials of selected mushroom fruitbody extracts through evaluation of antioxidant, anti-hyaluronidase and anti-tyrosinase activity. J 2020, 3, 329–342. [Google Scholar] [CrossRef]
- Nworu, C.S.; Akah, P.A. Anti-inflammatory medicinal plants and the molecular mechanisms underlaying their activities. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 52–61. [Google Scholar] [CrossRef]
- Perfetti, T.A.; Rodgman, A. The complexity of tobacco and tobacco smoke. Beitr. Tabakforsch. Int. 2011, 24, 215–232. [Google Scholar] [CrossRef]
- Ncube, E.N.; Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Dubery, I.A.; Madala, N.E. Analyses of chlorogenic acids and related cinnamic acid derivatives from Nicotiana tabacum tissues with the aid of UPLC-QTOF-MS/MS based on the in-source collision-induced dissociation method. Chem. Cent. J. 2014, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Mikami, Y.; Tomita, H. Isolation of 2 Isopropyl-4,4-dimethyl-5-vinylidene-2-cyclopenten-1-one and 2-Isopropyl-3-isopentyl-maleic Anhydride from the Pyrolytic Products of 2-Isopropylmalic acid. Agric. Biol. Chem. 1976, 40, 245–246. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, J.; Zhang, L.; Long, M.; Zuo, J. Optimization of microwave drying biomass material of stem granules from waste tobacco using response surface methodology. Dry. Technol. 2013, 31, 1234–1244. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Du, Y.; Chen, S.; Tang, H. Dynamic metabonomic responses of tobacco (Nicotiana tabacum) plants to salt stress. J. Proteome Res. 2011, 10, 1904–1914. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Zhang, B.; Yuan, X.; Gao, Y.; Lu, P.; Wang, W.; Xu, M. Determination of free amino acids in burley tobacco by high performance liquid chromatography. Saudi J. Biol. Sci. 2016, 23, S64–S68. [Google Scholar] [CrossRef] [PubMed]
- Wightman, F.; Lighty, D.L. Identification of phenylacetic acid as a natural auxin in the shoots of higher plants. Physiol. Plant. 1982, 55, 17–24. [Google Scholar] [CrossRef]
- Negrel, J.; Martin, C. The biosynthesis of feruloyltyramine in Nicotiana tabacum. Phytochemistry 1984, 23, 2797–2801. [Google Scholar] [CrossRef]
- Whenham, R.J.; Fraser, R.S.S.; Brown, L.P.; Payne, J.A. Tobacco-mosaic-virus-induced increase in abscisic-acid concentration in tobacco leaves: Intracellular location in light and dark-green areas, and relationship to symptom development. Planta 1986, 168, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.Á.; Kátay, G.; Gullner, G.; Király, L.; Ádám, A.L. Azelaic acid accumulates in phloem exudates of TMV-infected tobacco leaves, but its application does not induce local or systemic resistance against selected viral and bacterial pathogens. Acta Physiol. Plant. 2017, 39, 1–10. [Google Scholar] [CrossRef]
- Yang, C.; Xie, S.N.; Ni, L.; Du, Y.M.; Liu, S.; Li, M.Y.; Xu, K. Chemical constituents from Nicotiana tabacum L. and their antifungal activity. Nat. Prod. Commun. 2021, 16. [Google Scholar] [CrossRef]
- de Macêdo, I.S.V.; Cunha, K.G.; Alves, A.T.V.; Martins, R.M.; da Silva Simões, M.O. Atividade antioxidante da rutina: Uma revisão. BioFarm 2007, 13, 14–19. [Google Scholar]
- Girsang, E.; Lister, I.N.E.; Ginting, C.N.; Sholihah, I.A.; Raif, M.A.; Kunardi, S.; Million, H.; Widowati, W. Antioxidant and antiaging activity of rutin and caffeic acid. Pharmaciana 2020, 10, 147–156. [Google Scholar] [CrossRef]
- Dehaghani, Z.A.; Asghari, G.; Dinani, M.S. Isolation and identification of nicotiflorin and narcissin from the aerial parts of Peucedanum aucheri Boiss. J. Agric. Sci. Technol. A 2007, 7, 45–51. [Google Scholar] [CrossRef]
- Liana, L.; Rizal, R.; Widowati, W.; Fioni, F.; Akbar, K.; Fachrial, E.; Lister, I.N.E. Antioxidant and anti-hyaluronidase activities of dragon fruit peel extract and kaempferol-3-o-rutinoside. JKB 2019, 30, 247–252. [Google Scholar] [CrossRef]
- Singh, S.K.; Chaubey, S.; Bansal, A.; Kaur, G.; Malik, D.S. Cosmeceutical aptitudes of azelaic acid. Curr. Drug Res. Rev. 2021, 13, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Sieber, M.A.; Hegel, J.K.E. Azelaic acid: Properties and mode of action. Ski. Pharmacol. Physiol. 2013, 27, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Fitton, A.; Goa, K.L. Azelaic acid: A review of its pharmacological properties and therapeutic efficacy in acne and hyperpigmentary skin disorders. Drugs 1991, 41, 780–798. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, S.; André, V.; Antunes, A.M.; Vilela, S.M.; Amariei, G.; Arenas-Vivo, A.; Rosal, R.; Horcajada, P.; Duarte, M.T. Novel antibacterial azelaic acid BioMOFs. Cryst. Growth Des. 2019, 20, 370–382. [Google Scholar] [CrossRef]
- Ercan, L.; Doğru, M. Antioxidant and antimicrobial capacity of quinic acid. Bitlisfen 2022, 11, 1018–1025. [Google Scholar] [CrossRef]
- Zeng, K.; Thompson, K.E.; Yates, C.R.; Miller, D.D. Synthesis and biological evaluation of quinic acid derivatives as anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2009, 19, 5458–5460. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Lee, J.W.; Jang, H.; Kim, J.G.; Lee, M.K.; Hong, J.T.; Lee, M.S.; Hwang, B.Y. Quinic acid esters from Erycibe obtusifolia with antioxidant and tyrosinase inhibitory activities. Nat. Prod. Res. 2021, 35, 3026–3032. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Hadavi, E.; Hekmati, J. Effect of salicylic acid, malic acid, citric acid and sucrose on antioxidant activity, membrane stability and ACC-Oxidase activity in relation to vase life of carnation cut flowers. J. Agric. Technol. 2012, 8, 2053–2063. [Google Scholar] [CrossRef]
- Singh, S.K.; Kaldate, R.; Bisht, A. Citric acid, antioxidant effects in health. In Antioxidants Effects in Health. The Bright and the Dark Side; Elsevier: Amsterdam, The Netherlands, 2022; pp. 309–322. [Google Scholar] [CrossRef]
- Ramachandran, S.; Fontanille, P.; Pandey, A.; Larroche, C. Gluconic acid: Properties, applications and microbial production. FTB 2006, 44, 185–195. [Google Scholar]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial properties, sources, clinical, and traditional applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef] [PubMed]
- Rho, H.S.; Ghimeray, A.K.; Yoo, D.S.; Ahn, S.M.; Kwon, S.S.; Lee, K.H.; Cho, D.H.; Cho, J.Y. Kaempferol and kaempferol rhamnosides with depigmenting and anti-inflammatory properties. Molecules 2011, 16, 3338–3344. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, F.; Hadidi, M. Recent advances in potential health benefits of quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.S.; Abid, U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull. 2023, 80, 241–262. [Google Scholar] [CrossRef] [PubMed]
- Moon-Hee, C.; Shin, H.J. Anti-Melanogenesis Effect of Quercetin. Cosmetics 2016, 3, 18. [Google Scholar] [CrossRef]
- Mu, L.L.; Kou, J.P.; Zhu, D.N.; Yu, B.Y. Antioxidant activities of the chemical constituents isolated from the leaves of Ginkgo biloba. CJNM 2008, 6, 26–29. [Google Scholar] [CrossRef]
- Cai, H.; Sale, S.; Schmid, R.; Britton, R.G.; Brown, K.; Steward, W.P.; Gescher, A.J. Flavones as colorectal cancer chemopreventive agents—Phenol-O-methylation enhances efficacy. Cancer Prev. Res. 2009, 2, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.V.; Thien, D.D.; Khanh, T.D.; Tran, H.D.; Xuan, T.D. Momilactones A, B, and tricin in rice grain and by-products are potential skin aging inhibitors. Foods 2019, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current advances in naturally occurring caffeoylquinic acids: Structure, bioactivity, and synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef] [PubMed]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Zhou, H.T.; Hu, Y.H.; Tang, J.Y.; Su, M.X.; Guo, Y.J.; Chen, Q.L.; Liu, B. Antityrosinase and antimicrobial activities of 2-phenylethanol, 2-phenylacetaldehyde and 2-phenylacetic acid. Food Chem. 2011, 124, 298–302. [Google Scholar] [CrossRef]
- Rawlings, A.V.; Wandeler, E.; Bendik, I.; Fuchs, P.; Monneuse, J.M.; Imfeld, D.; Schütz, R. Effect of regioisomers of hydroxystearic acids as peroxisomal proliferator-activated receptor agonists to boost the anti-ageing potential of retinoids. Int. J. Cosmet. Sci. 2021, 43, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Mhiri, R.; Koubaa, I.; Chawech, R.; Auberon, F.; Allouche, N.; Michel, T. New isoflavones with antioxidant activity isolated from Cornulaca monacantha. Chem. Biodivers. 2020, 17, e2000758. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yu, L.; Wang, M.H. N-trans-feruloyltyramine inhibits LPS-induced NO and PGE2 production in RAW 264.7 macrophages: Involvement of AP-1 and MAP kinase signalling pathways. Chem. Biol. Interact. 2015, 235, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Ricciutelli, M.; Bartolucci, G.; Campana, R.; Salucci, S.; Benedetti, S.; Caprioli, G.; Maggi, F.; Sagratini, G.; Vittori, S.; Lucarini, S. Quantification of 2-and 3-isopropylmalic acids in forty Italian wines by UHPLC-MS/MS triple quadrupole and evaluation of their antimicrobial, antioxidant activities and biocompatibility. Food Chem. 2020, 321, 126726. [Google Scholar] [CrossRef] [PubMed]
- Union European. Regulation (EC) No 1223/2009 of the European Parliament and of the Council. Off. J. Eur. Union 2009, 342, 59. [Google Scholar]
- Agrawal, R.; Hu, A.; Bollag, W.B. The Skin and Inflamm-Aging. Biology 2023, 12, 1396. [Google Scholar] [CrossRef] [PubMed]
- Mangerich, A.; Dedon, P.C.; Fox, J.G.; Tannenbaum, S.R. Chemistry meets biology in colitis-associated carcinogenesis. Free Radic. Res. 2013, 47, 958–986. [Google Scholar] [CrossRef] [PubMed]
- Isla, M.I.; Ezquer, M.E.; Leal, M.; Moreno, M.A.; Zampini, I.C. Flower beverages of native medicinal plants from Argentina (Acacia caven, Geoffroea decorticans and Larrea divaricata) as antioxidant and anti-inflammatory. J. Ethnopharmacol. 2021, 281, 114490. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Asmi, K.S.; Lakshmi, T.; Balusamy, S.R.; Parameswari, R. Therapeutic aspects of taxifolin–An update. J. Adv. Pharm. Educ. Res. 2017, 7, 187–189. [Google Scholar]
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Il Fármaco 2001, 56, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Rengarajan, T.; Nandakumar, N.; Palaniswami, R.; Nishigaki, Y.; Nishigaki, I. Kaempferol, a potential cytostatic and cure for inflammatory disorders. Eur. J. Med. Chem. 2024, 86, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Naylor, E.C.; Watson, R.E.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-endocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Prommaban, A.; Kheawfu, K.; Chittasupho, C.; Sirilun, S.; Hemsuwimon, K.; Chaiyana, W. Phytochemical, antioxidant, antihyaluronidase, antityrosinase, and antimicrobial properties of Nicotiana tabacum L. leaf extracts. eCAM 2022, 2022, 5761764. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, G.; Guo, M. Potential Anti-aging Components from Moringa oleifera Leaves Explored by Affinity Ultrafiltration with Multiple Drug Targets. Front. Nutr. 2022, 9, 854882. [Google Scholar] [CrossRef] [PubMed]
- Kinari, S.; Girsang, E.; Nasution, A.N.; Lister, I.N.E. Antioxidant and anti-Collagenase effectivity of red dragon fruit peel and Kaempferol 3-0-Rutinoside. ASRJETS 2019, 59, 244–251. [Google Scholar]
- Kuzniewski, R.; Załuski, D.; Olech, M.; Banaszczak, P.; Nowak, R. LC-ESI-MS/MS profiling of phenolics in the leaves of Eleutherococcus senticosus cultivated in the West Europe and anti-hyaluronidase and anti-acetylcholinestarase activities. Nat. Prod. Res. 2018, 32, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Xinghua, L.; Yingying, H.; Shuai, W. Anti-aging Effect of Rutin in Caenorhabditis elegans and D-Gal-Induced Aging Mouse Model. Dokl. Biochem. Biophys. 2023, 513, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Matsunami, K. Frailty and Caenorhabditis elegans as a benchtop animal model for screening drugs including natural herbs. Front. Nutr. 2018, 5, 111. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Ahn, A.; Kim, S.; Won, C.W. Global prevalence of physical frailty by fried’s criteria in community-dwelling elderly with national population-based surveys. J. Am. Med. Dir. Assoc. 2015, 16, 548–550. [Google Scholar] [CrossRef] [PubMed]
- David, D.C.; Ollikainen, N.; Trinidad, J.C.; Cary, M.P.; Burlingame, A.L.; Kenyon, C. Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol. 2010, 8, e1000450. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Leal, M.; Zampini, C.; Mercado, M.I.; Moreno, M.A.; Simirgiotis, M.; Borquez, J.; Ponessa, G.; Isla, M.I. Flourensia fiebrigii S.F. blake: A medicinal plant from the Argentinean highlands with potential use as anti-rheumatic and anti-inflammatory. J. Ethnopharmacol. 2021, 264, 113296. [Google Scholar] [CrossRef] [PubMed]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.H.; Min, K.R.; Lee, K.S.; Ro, J.S.; Ryu, J.C.; Kim, Y. Inhibitory effects of hydrolyzable tannins on Ca2+-activated hyaluronidase. Planta Med. 1993, 59, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Osathanunkul, M.; Buddhachat, K.; Chomdej, S. A modified colorimetric method of gelatinolytic assay using bacterial collagenase type II as a model. Anal. Biochem. 2023, 433, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1983, 113, 173–215. [Google Scholar] [CrossRef] [PubMed]
- Orqueda, M.E.; Zampini, I.C.; Torres, S.; Isla, M.I. Functional Characterization and Toxicity of Pectin from Red Chilto Fruit Waste (Peels). Plants 2023, 12, 2603. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat, version 2015. Grupo InfoStat, FCA. Universidad Nacional de Córdoba: Córdoba, Argentina, 2015.
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 10 July 2025).

| DW | CU | FGS | |
|---|---|---|---|
| Phenolic compounds μg GAE/mL | 986.0 ± 19.6 a | 2106.0 ± 3.7 b | 2402.5 ± 3.7 b |
| Flavonoids μg QE/mL | 18.6 ± 1.8 a | 138.4 ± 3.2 c | 93.3 ± 7.5 b |
| RT (min) | Theoretical Mass (m/z) | Accuracy (ppm) | Measured Mass (m/z) | Name | [M-H]− | Metabolite Type | MS Ions (ppm) | DW-E | FGS-E | CU-E | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.37 | 113.9829 | 2.3 | 113.9842 | Na formiate (internal standard) | NaC2H2O4 | Standard | - | |||
| 2 | 1.83 | 191.05621 | 0.598 | 192.06349 | Quinic acid | C7H12O6 | Organic acid | 127.0399 | 33,683 | 54,892 | 0 |
| 3 | 1.88 | 133.01434 | 0.665 | 134.02163 | Malic acid | C4H6O5 | Organic acid | 115.003, 96.9525 | 56,926 | 56,472 | 47,285 |
| 4 | 1.91 | 290.08845 | 1.5 | 291.09573 | N-Fructosyl pyroglutamate | C11H17NO8 | Amino acid | 111.04517, 93.0354, | 8130 | 18,261 | 0 |
| 5 | 2.26 | 191.01999 | 1.479 | 192.02727 | Citric acid | C6H8O7 | Organic acid | 179.0217, | 2561 | 10,812 | 18,591 |
| 6 | 4.01 | 353.08878 | 1.928 | 354.09605 | Caffeoyl quinic acid | C16H18O9 | Phenolic acid | 163.0354, 145.0287 | 3742 | 0 | 0 |
| 7 | 4.75 | 609.14728 | 1.505 | 610.15455 | Rutin | C27H30O16 | Flavonoid | 301.0491, | 0 | 31,265 | 89,709 |
| 8 | 6.27 | 175.06133 | 0.136 | 176.06862 | 2-Isopropylmalic acid | C7H12O5 | Fatty acid | 133.0142, 115.003, 96.9525 | 49,278 | 19,727 | 4222 |
| 9 | 6.58 | 337.09372 | 3.045 | 338.101 | Coumaroyl quinic acid | C16H18O8 | Phenolic acid | 155.0351, 145.0287 | 7504 | 4203 | 0 |
| 10 | 7.12 | 250.0724 | 1.007 | 251.07967 | Phenylacetyl aspartic acid | C12H13NO5 | Amino acid | 177.0404 | 22,344 | 13,372 | 14,175 |
| 11 | 7.12 | 135.0296 | −2.168 | 136.03688 | Threonic acid | C4H8O5 | Organic acid | 96.1281, 81.6734 | 1411 | 9836 | 0 |
| 12 | 7.28 | 195.05139 | 1.86 | 196.05867 | Gluconic acid | C6H12O7 | Organic acid | 195.0510, 129.0191 | 34,223 | 34,047 | 1875 |
| 13 | 7,72 | 341.10971 | 4.914 | 342.11699 | Melibiose | C12H22O11 | Glycoside | 101.0187, 89.0198, | 0 | 2117 | 0 |
| 14 | 8.12 | 132.03085 | 4.658 | 133.03812 | Aspartate | C4H7NO4 | Amino acid | 88.0392, 74.0241 | 2247 | 33,604 | 947 |
| 15 | 8.28 | 117.01954 | 1.77 | 118.02682 | Succinic acid | C4H6O4 | Organic acid | 99.0083, 73.0293 | 2991 | 59,406 | 882 |
| 16 | 8.34 | 147.03117 | 7.562 | 148.03845 | Citramalic acid | C5H8O5 | Organic acid | 87.0089 | 5456 | 4094 | 824 |
| 17 | 8.58 | 179.05343 | −22.439 | 180.06071 | Theophylline | C7H8N4O2 | Alkaloid | 124.0325 | 7662 | 0 | 2008 |
| 18 | 8.82 | 593.15128 | 0.018 | 594.15856 | Nicotiflorin | C27H30O15 | Flavonoid | 287.0544, 129.0546 | 0 | 4283 | 9171 |
| 19 | 9.37 | 187.09756 | −0.122 | 188.10484 | Azelaic acid | C9H16O4 | Organic acid | 95.0481, 57.0356 | 13,719 | 7994 | 15,655 |
| 20 | 9.52 | 135.04317 | −14.716 | 136.05044 | Phenylacetic acid | C8H8O2 | Phenolic acid | 91.1034 | 1213 | 9938 | 1457 |
| 21 | 10.57 | 312.12439 | 1.636 | 313.13167 | Feruloyltyramine | C18H19NO4 | Phenolic amide | 180–9076, 147.0723 | 349 | 0 | 0 |
| 22 | 10.07 | 263.12873 | −0.852 | 264.13601 | Abscisic acid | C15H20O4 | Organic acid | 194.0970, 156.0863, 133.0965 | 3074 | 3320 | 3270 |
| 23 | 11.51 | 201.11302 | −1.033 | 202.1203 | Sebacic acid | C10H18O4 | Organic acid | 183.1033, 139.1138 | 1236 | 718 | 1258 |
| 24 | 12.23 | 301.03553 | 0.518 | 302.04281 | Quercetin | C15H10O7 | Flavonoid | 257.0432, 155.0477, | 0 | 4878 | 0 |
| 25 | 12.65 | 285.0408 | 1.18 | 286.04807 | Kaempferol | C15H10O6 | Flavonoid | 153.0182, 121.0287 | 41,899 | 1462 | 0 |
| 26 | 11.56 | 329.06666 | 0.059 | 330.07393 | Tricin | C17H14O7 | Flavonoid | 299.0205, 271.0257, 227.0345 | 701 | 1356 | 0 |
| 27 | 13.94 | 299.2594 | −0.85 | 300.2665 | 10-Hydroxystearic acid | C18H36O3 | Fatty acid | 241.0232, 57.0347 | 1087 | 1017 | 3306 |
| XOD | LOX | |
|---|---|---|
| N. tabacum Apical Leaves Waste Extracts | IC50 (µg GAE/mL) | IC50 (µg GAE/mL) |
| DW-E | ND | 15.20 ± 1.05 b |
| FGS-E | 85.50 ± 2.00 c | 17.50 ± 0.83 b |
| CU-E | 63.50 ± 1.10 b | 8.0 ± 1.19 a |
| Naproxen | - | 14.0 ± 0.70 b |
| Allopurinol | 50.0 ± 2.0 a | - |
| C. elegans Assay | S. Typhimurium Assay | ||
|---|---|---|---|
| % Survival | Mutagenicity Relation | ||
| TA100 | TA98 | ||
| DW | 87.5 ± 2 | 0.12 ± 0.01 | 0.12 ± 0.01 |
| DW-E | 89.5 ± 5 | 1.24 ± 0.2 | 1.02 ± 0.1 |
| FGS | 89.4 ± 5 | 0.16 ± 0.01 | 0.16 ± 0.01 |
| FGS-E | 91.3 ± 3 | 0.99 ± 0.01 | 1.42 ± 0.1 |
| CU | 81.2 ± 5 | 0.17 ± 0.01 | 0.17 ± 0.01 |
| CU-E | 95.0 ± 6 | 1.02 ± 0.1 | 1.01 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leal, M.; Moreno, M.A.; Orqueda, M.E.; Simirgiotis, M.; Isla, M.I.; Zampini, I.C. Green Extract from Pre-Harvest Tobacco Waste as a Non-Conventional Source of Anti-Aging Ingredients for Cosmetic Applications. Plants 2025, 14, 2189. https://doi.org/10.3390/plants14142189
Leal M, Moreno MA, Orqueda ME, Simirgiotis M, Isla MI, Zampini IC. Green Extract from Pre-Harvest Tobacco Waste as a Non-Conventional Source of Anti-Aging Ingredients for Cosmetic Applications. Plants. 2025; 14(14):2189. https://doi.org/10.3390/plants14142189
Chicago/Turabian StyleLeal, Mariana, María A. Moreno, María E. Orqueda, Mario Simirgiotis, María I. Isla, and Iris C. Zampini. 2025. "Green Extract from Pre-Harvest Tobacco Waste as a Non-Conventional Source of Anti-Aging Ingredients for Cosmetic Applications" Plants 14, no. 14: 2189. https://doi.org/10.3390/plants14142189
APA StyleLeal, M., Moreno, M. A., Orqueda, M. E., Simirgiotis, M., Isla, M. I., & Zampini, I. C. (2025). Green Extract from Pre-Harvest Tobacco Waste as a Non-Conventional Source of Anti-Aging Ingredients for Cosmetic Applications. Plants, 14(14), 2189. https://doi.org/10.3390/plants14142189









