Abstract
The primary worldwide variables limiting plant development and agricultural output are the ever-present threat that environmental stressors such as salt (may trigger osmotic stress plus ions toxicity, which impact on growth and yield of the plants), drought (provokes water stress, resulting in lowering photosynthesis process and growth rate), heavy metals (induced toxicity, hindering physiological processes also lowering crop quantity and quality), and pathogens (induce diseases that may significantly affect plant health beside productivity). This review explores the integrated effects of these stressors on plant productivity and growth rate, emphasizing how each stressor exceptionally plays a role in physiological responses. Owing to developments in technology that outclass traditional breeding methods and genetic engineering techniques, powerful alleviation strategies are vital. New findings have demonstrated the remarkable role of nanoparticles in regulating responses to these environmental stressors. In this review, we summarize the roles and various applications of nanomaterials in regulating abiotic and biotic stress responses. This review discusses and explores the relationship between various types of nanoparticles (metal, carbon-based, and biogenic) and their impact on plant physiology. Furthermore, we assess how nanoparticle technology may play a role in practices of sustainable agriculture by reducing the amount of compounds used, providing them with a larger surface area, highly efficient mass transfer abilities, and controlled, targeted delivery of lower nutrient or pesticide amounts. A review of data from several published studies leads to the conclusion that nanoparticles may act as a synergistic effect, which can effectively increase plant stress tolerance and their nutritional role.
1. Introduction
Recently, horticulture crops have suffered deterioration and production losses due to adverse environmental conditions [,,,]. Environmental stressors, including biotic and abiotic stresses, are identified as any external factors that negatively affect plant growth and development [,]. Water shortage is the primary source of drought and salinity, which is indirectly leading to heavy metal toxicity in contemporary agriculture. In addition, because of global warming, climate change is causing heat waves, droughts, and other abiotic stressors, raising the salt content of the soil in several agricultural areas worldwide [,,,,].
The different stressor conditions have led to different environmental and biological limitations that may negatively impact plant growth, yield, and quality [,,,]. These stresses impact a variety of horticulture crops, causing osmotic stress, water stress, oxidative stress, and ionic strength imbalances that impact several plant physiological, biochemical, and metabolic processes []. Different adverse effects of climate change, such as chilling and high temperatures, are experienced by horticulture crops []. These effects include oxidative stress, cell membrane breakdown, protein denaturation, and nucleotide damage [].
Depending on the kind of crops, the stressor, and the duration of exposure, abiotic stress causes several morphological, metabolic, and gene expression alterations in plants [,,,]. In response to abiotic stress, plant cells produce more free radicals. Roughly 2% of the oxygen taken in is transformed into reactive oxygen species (ROS), which are reactive byproducts of aerobic metabolism that compromise plant metabolism and negatively impact plant production [,]. Excessive ROS levels from abiotic stress may harm plant proteins, membranes, and other structural components, inevitably impeding vital physiological functions. Irrespective of stress, some plants have been shown to exhibit membrane peroxidation and damage to their photosynthetic systems. Therefore, scavenging ROS by activating antioxidant molecules is the primary target of plant defense systems [,,,,].
One technological advancement crucial in addressing global issues like the depletion of natural resources and the acceleration of climate change is nanotechnology. Enhancing fertilizer use efficiency, reducing plant production losses, and increasing water usage efficiency are critical. Nanotechnology has the potential to improve water and nutrient uptake, antioxidant activity, and photosynthesis, thereby increasing the resilience of horticultural crops to environmental challenges [,,].
Furthermore, it could also control genes that respond to stress and initiate signaling pathways that help plants adapt to stress [,]. There has been a rise in the utilization of goods containing nanomaterials, which might mean higher yield quality and productivity. It has been demonstrated that using nanoparticles (NPs) increases plant tolerance to abiotic stress conditions significantly faster than using strategies like genetic improvement [,]. NPs offer a better and more comprehensive approach to reducing stress in horticultural crops because of their rapid results and low cost. They also help protect the environment when used carefully, unlike other methods such as genetic improvement, which is time-consuming, or genetic engineering, which is expensive and requires expensive equipment.
Recently, using nanotechnology in agriculture as a substitute for the traditional application of bulk chemical pesticides and fertilizers [] has the potential to revolutionize sustainable agricultural practices []. NPs might provide numerous benefits over conventional agricultural practices, including a large surface area, highly efficient mass transfer abilities, and controlled and targeted delivery of lower nutrient or pesticide amounts, which could improve crop productivity []. Nanofertilizers can deliver nutrients more efficiently. Improved uptake by plants due to nanoscale size and surface properties, saving resources and labor. Including a large surface area, highly efficient mass transfer abilities, and controlled and targeted delivery of lower nutrient or pesticide amounts. NPs can be engineered to deliver pesticides in a targeted and controlled-release manner. This means lower quantities of chemicals are needed. Some NPs even help plants tolerate drought stress by modulating physiological responses. Under stress conditions, nanotechnology can improve the efficiency and performance of physio-biochemical and molecular systems in plants [,]. Numerous hormone pathways may be triggered or downregulated due to NPs-plant interactions, which can affect plant metabolism and stress responses and control plant growth, development, and stress tolerance []. By improving plant tolerance to stress, NPs can enhance crop yields in sustainable agriculture, addressing current and upcoming production challenges [,,]. This can be performed by suppressing plant infections, increasing chlorophyll concentration and photosynthesis’s effectiveness, and activating certain enzymes [].
This article stresses the necessity for responsible and sustainable usage while examining NPs possible adverse effects and environmental ramifications. This study explores NPs’ role and potential in stress management in horticultural plant species. Furthermore, we look at the influences of NPs on scavenging ROS by activating anti-oxidative enzymes, and how NPs affect fruit quality and production.
2. Different Approaches for Nanoparticle Preparation
NPs are molecules engineered typically range in diameter and dimensions from 1 to 100 nanometers (nm). NPs can be classified into the following categories: metallic NPs are zero-dimension nanosized metals (e.g., Zn, B, Cu, Ag, and Se), polymeric NPs are prepared from biocompatible colloidal polymers (e.g., nanospheres or nano-capsules), and non-metallic NPs are prepared from environmentally friendly materials (e.g., CNTs, fullerenes, and graphene) [,,].
Owing to their enormous surface area and nanoscale size, NPs have particular physicochemical characteristics. Although many different approaches to prepare nanostructures may be broadly divided into top-down and bottom-up methods (Figure 1), metal NPs can generally be synthesized through various methods; chemical ones are the most popular and successful. The materials produced have the benefit of being able to be stored for long periods without exhibiting a discernible loss of stability []. It may also be made physically as a metallic nanoparticle by employing ultrasound, microwaves, and irradiation—all of which need substantial energy []. Using various biological systems as potential ecological substitutes for physical and chemical methods is another method of synthesis known as biological methods. These methods involve using microorganisms, natural plant extracts, bacterial extracts, enzymes, and plants or plant extracts that can transform metal ions into metal NPs [,,].
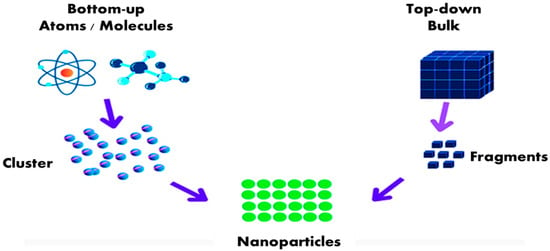
Figure 1.
Different ways of preparing nanostructures.
In particular, let us briefly discuss some chemical methods used to synthesize metal NPs. For example, we can synthesize magnetite (Fe3O4) or maghemite (-Fe2O3) using hydrothermal methods that reduce ferric chloride with ascorbic acid. The resulting product is a black powder with a spherical shape of about 20 nm and a particle size range of 50 ± 5 nm to [,].
Ahamed et al. [] created CuO NPs via the co-precipitation approach, employing NaOH as a reducing agent and copper (II) acetate as a precursor salt. The black precipitate result of the CuO NPs was around 23 nm in size. Additionally, Ganeshan et al. [] utilized 0.2 M manganese (II) sulfate diluted in distilled water and added NaOH solution until the pH became alkaline, resulting in particles 40–50 nm in size and spherical, crystalline.
Similarly, zinc nitrate hexahydrate reduction by urea at alkaline pH was reported by Abou El-Nasr et al. [], yielding diameters of spherical NPs at 97.31 nm. Kshirsagar et al. [] employed the sol–gel technique to dissolve copper (II) chloride in an aqueous solution (0.2 M). They added 1 mL of glacial acetic acid to the solution while stirring continuously and heating it to boil. Next, they added 8 M NaOH to the mixture until the pH reached 7, at which point the size of the copper nanoparticle was 20.4 nm.
It is crucial to understand the distribution, stability, and structure of NPs during the characterization process. Understanding and researching the absorption and transport of NPs in plants is made feasible by tools such as Zeta sizers, X-ray diffraction (XRD), transmission electron microscope (TEM), scanning electron microscope (SEM), and dynamic light scattering (DLS) []. Furthermore, absorption efficiency is influenced by several variables, including the kind of plant, its growth stages, the features of the nanomaterial (such as its size, shape, and stability), concentration, and application techniques [,,].
3. Nanomaterials’ Uptake in Plants
Plant absorption, or the transport of materials into plants, is an essential process for the growth and development of plants. Plant transport involves the transfer of gases, water, nutrients, and exogenous substances as different NPs []. In order to develop the best possible NPs for agricultural usage, it is essential to comprehend how NPs are absorbed and transported by plants [,]. Simultaneously, knowing NPs mechanism behind their’ action and their accumulation in plants might assist in elucidating their biological safety and offer recommendations for their safe application [].
By administering direct treatment to seeds that have been absorbed during the germination stage, NPs can infiltrate plant tissues. It is immediately absorbed in the subsequent phases through various root tissues, passing through root barriers such as the cortex, endodermis, and epidermis before reaching the xylem and being carried to the vegetative system []. Additionally, NPs can boost plant canopy penetration by foliar spray, which avoids the epidermis even if polar compounds are resisted by cuticle, or through stomatal pores [,], and diverse plant components []. They can enter the vascular tissue via the apoplastic or symplastic pathway. The researchers suggested that the stomatal pathway might be a method by which NPs are absorbed (Figure 2).
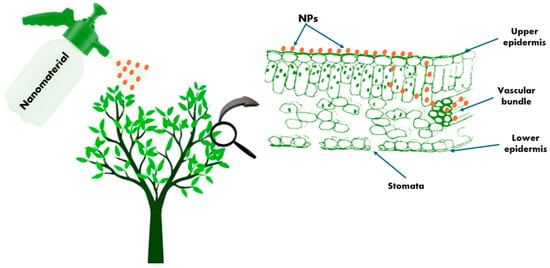
Figure 2.
Plant’s absorption and translocation of NPs through foliar spraying.
Indeed, the particle size and zeta potential of NPs are closely related to their efficiency in penetrating cell membranes. The effect of NPs increases with smaller particle sizes and higher zeta potentials. The size and zeta potential of the resulting particles vary depending on the method of preparing the NPs and the chemicals used.
Materials greater than 100 µm3 can only pass through leaves with open stomata when applied topically (foliar) []. However, several studies have demonstrated that water-suspended NPs may naturally penetrate via stomata holes with a diameter of less than 100 nm [] or a pore size of less than 1.1 µm []. NPs of 40–50 nm size may readily pass through the cell wall []. Osmotic pressure and capillary action aid in the entry of NPs into the cell, and NPs contact with the outermost layer modify membrane proteins as transporters and receptors []. Numerous variables, including the NPs’ size, shape, surface characteristics, and the pH and presence of other ions or chemicals in the solution, might affect the NPs’ transportation [].
4. Innovative Solutions in Managing Stress in Horticulture
Several effective strategies are followed to manage stress in horticulture crops (Figure 3) as follows:
- −
- Plant materials: Plants have adopted multiple strategies to survive under different stressors used for plant improvement, like vigorous growth, osmotic adjustment, higher water use efficiency (WUE), etc. Rootstocks and improved varieties play an important role in adaptation to stress with different tolerance levels. Grafting is used as an additional tool to alleviate environmental stresses, and this technique is applied to many high-yielding fruits and vegetables such as cucurbits and eggplant to enhance tolerance to saline soil, water shortage, heavy metals, etc. [,,].
- −
- Exogenously applied phytohormones: They are chemical compounds that play an important role in plant extrinsic and intrinsic factors, essential in regulating many signal transduction pathways under stress conditions [,] and increasingly accepted by horticultural plants to tolerate stress diversity. Their action is regulated in their metabolism as they are extracted in small amounts from chemical systems []. For example, salicylic acid (SA) is one of the hormones that positively influence many developmental processes such as seed germination, seedling growth, photosynthesis, cell reproduction, changes in stomatal aperture, respiration, antioxidant defense system, delaying plant senescence, etc. []. SA exposure alleviated chilling injury on the seed germination of muskmelon plants [] and enhanced the total soluble sugars and cold-response gene expression in peach fruit [].
- −
- Utilize of biological treatments: Biofertilizers are one of the strategies for agricultural sustainability, especially under stress conditions, as they do not cause any harm or manipulation to the soil microflora, but rather enhance the association between soil, arbuscular mycorrhizal fungi (AMF), and plant growth-promoting rhizobacteria (PGPR) and are effective in developing tolerance to various abiotic stresses or improving nutrient cycling in the soil, thereby enhancing plant productivity [].
- −
- Use of biotechnological tools: Advances in various biotechnological tools and the development of transgenic lines have been made possible by the introduction of modern molecular or biotechnological tools such as miRNA identification and signaling pathway analysis [], CRISPR/Cas-mediated genome editing [], quantitative trait loci (QTL) mapping, and genomic selection (GS), which have made it possible to identify and position the desired gene in the new genome with greater precision, which is involved in different metabolic activities, signaling pathways, and the expression of different genes. This technology helps understand the molecular and physiological mechanisms, stress responses, and improve plant productivity [].
- −
- Utilization of nanotechnology: It is one of the most promising technologies in mitigating the effects of climate change and enhancing stress management techniques. The application of nanofertilizers via various techniques (e.g., soil irrigation, foliar spray, and seed coating), nanosensors to track the health status of plants in real time, and genetic engineering of plants to boost defense-related phytohormones and photosynthetic efficiency are examples of nano-enabled technologies that have been developed to support plant growth. Several studies have reported using NPs as nanofertilizers to improve plant production under stress conditions [,].
Natural resources generally deteriorate, and agricultural production is reduced due to various abiotic pressures caused by climate change, global warming, human activity, and other unavoidable causes []. Innovative methods such as using NPs and other technologies are being explored to lessen the adverse effects of these stresses on horticultural plants, as shown in Figure 3. Therefore, understanding these abiotic stresses is essential for creating efficient management strategies and enhancing plant resilience.
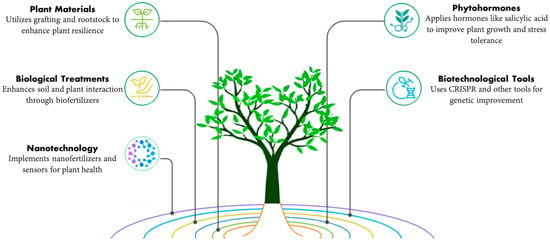
Figure 3.
Strategies for stress management in horticulture.
5. Role of Nanoparticles on Abiotic Stresses
Environmental stressors mitigate the growth, development, and production of plants. Several studies show that plants subjected to various stressors produce ROS, which leads to oxidative damage [,,]. Drought causes wilting, decreased leaf area, decreased photosynthetic activity, and eventually poor development or plant mortality, among other physiological changes in plants. Additionally, it might cause oxidative stress, which damages chloroplasts and mitochondria, causing cellular degeneration. Salinity can result in ionic toxicity, impair cellular processes, and osmotic stress, which restricts plants’ ability to absorb water, with reduced growth rates, necrosis, and chlorosis of the leaves. High temperatures can lead to heat stress, causing protein denaturation, increased respiration rates, and loss of photosynthetic efficiency. Conversely, low temperatures can cause frost damage, impair cellular functions, and reduce metabolic activity. Heavy metals can disrupt plant metabolism by generating ROS, leading to oxidative stress, and impairing enzymatic activities essential for growth and development. ROS are highly reactive oxygen-based molecular intermediates whose metabolism is altered by these unfavorable environments []. Antioxidant defense mechanisms guard against oxidative stress, but too much can be detrimental. High concentrations of ROS can damage macromolecules like proteins, nucleic acids, and membrane lipids. This imbalance can lead to nitro-oxidative stress and plant cell death [,,].
NPs are an effective rolling tool for enhancing plant resilience against abiotic stresses. They facilitate various physiological and biochemical responses that contribute to stress alleviation through three primary mechanisms. (I) Improving nutrient uptake and water retention: NPs increase nutrient availability and absorption by enhancing root interaction and improving soil structure, which boosts water retention. For instance, silicon dioxide (SiO2) NPs improved the growth of banana plants under drought stress by elevating chlorophyll content and shoot growth [], while titanium dioxide (TiO2) NPs enhanced nutrient uptake and drought tolerance in grape plants []. (II) Enhancing antioxidant activity in plants, NPs can induce the expression of antioxidant enzymes, enabling plants to scavenge ROS and mitigate oxidative damage []. Research showed that selenium NPs (Se NPs) increased antioxidant enzymes and phenolic compounds in drought-stressed pomegranate plants [], while iron nanoparticles (Fe NPs) similarly enhanced antioxidant enzyme activity in grape plants []. (III) As carriers for stress-mitigating compounds, NPs effectively deliver growth regulators and nutrients, improving their bioavailability and effectiveness [].
Nanotechnology has great promises for mitigating abiotic pressures and securing the future of agriculture worldwide. It has been shown that NPs can assist plants in withstanding various challenges by improving plant development, growth, and productivity. It is examined here in the ensuing major areas:
5.1. Water Stress
Water stressors affected fruit and vegetable crops, in addition to cutting flowers, reduced plant life, productivity, and quality by altering the levels and functions of photosynthetic pigments, osmolyte content, and enzyme activity [,,]. Nanotechnology has recently been incorporated into agricultural systems. Several NP types have been developed for decreased economic and nutritional failures during drought stress, improved yield, and balanced crop production [].
When applied exogenously to micro-propagated banana cv. “Grand Nain” under drought stress conditions, SiO2 NPs at a rate of 50 mg L−1 have been shown to increase shoot growth and chlorophyll content, protect cellular membranes by lowering malondialdehyde (MDA) levels, and lessen oxidative damage to plants []. Furthermore, when applied to pomegranate plants under drought stress, selenium nanoparticles (Se-NPs) at a dosage of 20 mg L−1 and a size of 10 nm increase levels of photosynthetic pigment in leaves, phenolic content, and antioxidant enzymes while decreasing stress-induced lipid peroxidation and H2O2 content. In contrast to other treatments, severe drought stress also increased the amount of phytohormone accumulated as abscisic acid [].
Research on vegetables has demonstrated that when ZnO-NPs were applied topically to eggplant (Solanum melongena L.) for thirty to forty-five days following transplanting, the plants increased their relative water content (RWC), membrane stability index, and photosynthetic effectiveness []. It has been highlighted that exogenous TiO2 NPs treatment at 100 mg L−1 on tomato plants under drought stress increased stress tolerance, leading to an increase in photosynthesis-related and intrinsic proteins in the plasma membrane, an increase in RWC, and a decrease in MDA, proline, and lipid peroxidation [,,].
When treated with a solution containing 10 mg L−1 of silver NPs, some ornamental plants exposed to drought stress, such as cut chrysanthemum flowers, showed an increase in vase life of 3.21 days above the control treatment [] and prevented vascular occlusion. Additionally, Moringa seedlings treated with 0.1% and 0.05% ZnO-NPs by foliar spray demonstrated a substantial reduction in chlorophyll degradation and increased antioxidant activities and phenolic contents [].
Table 1 provides an overview of the nanomaterials that improve horticultural crops’ resistance to drought stress by causing biochemical and physiological changes and altering gene expression.

Table 1.
Nanomaterials and their impact on horticulture crops under drought stress conditions.
5.2. Temperature Stress
Low or high temperatures throughout the growing season can cause a variety of physiological abnormalities in the plant cells, which can negatively impact the quality of the fruit and reduce the rate of photosynthesis and damage to the cell membranes [].
Severe alterations might harm the intermolecular bonds needed for healthy growth in the summer heat, impeding plant growth and fruit set []. Heat stress often shortens the life cycle of plants and lowers output by decreasing the efficacy of photosynthetic activities []. Heat stress may become a significant problem limiting productivity in tropical and subtropical regions. When plants suffer heat stress, they produce heat shock proteins and molecular chaperones. A heat shock protein contributes to heat stress resistance and helps other proteins remain stable under stressful conditions. Moreover, multiwall carbon nanotubes promote heat shock protein production and upregulate the gene expression of heat shock proteins [,].
Excess energy can be released through plants’ stomatal conductance and increasing transpiration rate, which is positively impacted by NPs when under heat stress []. Spraying of titanium dioxide nanoparticles (TiO2 NPs) was found to be effective in enhancing the physiological and metabolic activities of tomato plants under heat stress by using a low concentration of 50–100 mg L−1 []. CH-NPs administered topically at lower concentrations could successfully boost banana tolerance to cold stress by reducing oxidative stress through activating the antioxidant [] as shown in Figure 4.
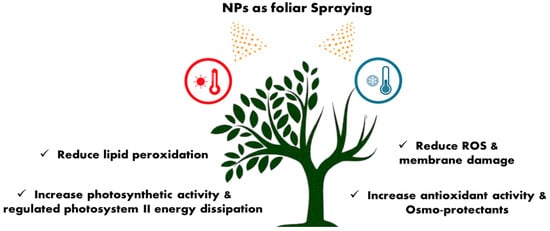
Figure 4.
Alleviation of heat stress in plants by nanoparticles.
5.3. Salinity and/or Alkalinity Stress
Salinity significantly inhibits plant development and productivity and changes the activities of enzymes [,,,]. It also severely affects cellular structure by causing Na+ and chloride Cl− ions to accumulate in the cytosol []. Salt accumulation in the mesophyll cells causes carbon dioxide to be absorbed and the concentration of CO2 inside the leaf to rise, which lowers stomatal conductance []. By improving antioxidant activities, osmolyte synthesis, and stress-related gene expression, an exogenous application of NPs can mitigate the detrimental effects of salt stress. It can also improve growth-related traits and productivity [,].
Furthermore, when subjected to salt stress, the plants adjust and survive by controlling signal transduction pathways and turning on stress-responsive genes []. It causes an imbalance in the amounts of nutrients, which causes plants to have severe nutritional shortages []. Cu NPs have been demonstrated to support tomato plant development and maintain a balanced Na+/K+ ratio in the face of salt stress [].
Based on the current body of research, certain NPs may improve plant development and growth when exposed to saline stress. Applying selenium on lemon verbena “Lippia citriodora Kunth” showed improved salinity tolerance by lowering leaf electrolyte leakage, malondialdehyde, and H2O2 accumulation, enhancing secondary metabolites’ biosynthesis []. Graphene oxide nanoparticles (GO-NPs) can somewhat mitigate the effects of salinity stress. Another study [] suggested that exposure concentrations of 5 and 10 mg/L of GO-NPs could moderately alleviate the harmful effects of salinity or alkalinity stress in strawberries. GO-NPs improve the morphology and physiology of growth and development in stressed plants by affecting the photosynthetic system and the electron transport chain. Table 2 provides an overview of the nanomaterials that improve horticultural crops’ resistance to salinity stress by causing biochemical and physiological changes.

Table 2.
Nanomaterials and their effects on horticulture crops under salinity stress conditions.
5.4. Heavy Metal Stress
One of the harmful elements of reducing plant production in the modern era is heavy metal stress. Human activities, including industrialization and urbanization, are the global source of heavy metal contamination []. The use of chemical fertilizers and pesticides, which are agricultural instruments that negatively impact plants with heavy metals like cadmium (Cd), lead (Pb), cobalt (Co), nickel (Ni), and silver (Ag) [], has also increased the stress caused by heavy metals in plants. By causing physiological and morphological anomalies as well as metabolic pathway malfunction, heavy metals directly disrupt plant growth performance. This affects the amount and quality of plant production, particularly in vegetable crops and medicinal plants [].
A few research works have examined how well NPs work to lower heavy metal stress. By absorbing and changing, newly introduced NPs can affect heavy metals in soil by reducing their mobility and bioaccumulation. By releasing phosphate ions, hydroxyapatite NPs can impact the reduction of copper and lead to heavy metal bioavailability while maintaining the pH of the soil at []. The quantity of Cd metal in soil has decreased due to Fe3O4 NPs treatment []. By triggering the antioxidant system, NPs can lower ROS by [].
For instance, using chitosan NPs as a putrescine carrier, grapevines under Cd stress conditions demonstrated, in unambiguous terms, how NP treatments mitigated the negative effects of stress by increasing antioxidant enzymatic activities, total phenolic compounds, anthocyanins, and lowering Cd content in leaf and root []. Similarly, applying 10 mg L−1 of Fe3O4 NPs to coriander plants resulted in a 97.37% and 98.55% reduction in the bioavailability of Cd and Pb, respectively []. Furthermore, ref. [] showed that SiO2 NPs increase the activity of the antioxidant enzymes SOD, CAT, and APX, which in turn reduces translocation and partially mitigates arsenic toxicity in tomato plants (Figure 5).
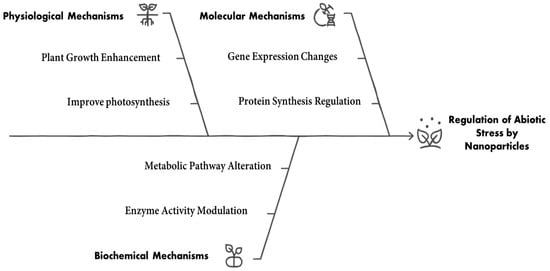
Figure 5.
Regulation of abiotic stress by nanoparticles.
6. Role of Nanoparticles on Biotic Stress
Biotic stress refers to the negative impacts on plants caused by living organisms. These stresses can significantly affect plant growth, development, and yield. Key examples of biotic stress include
- −
- Pests: Such as insects and nematodes, that feed on plants and can cause direct damage to plant tissues by chewing leaves, stems, or roots. Common pests include aphids, beetles, and caterpillars. They can also act as vectors for diseases.
- −
- Pathogens: These include fungi, bacteria, viruses, and other microorganisms that can infect plants, leading to diseases. Pathogenic infections can cause various symptoms, including wilting, leaf spots, and root rot. Examples include downy mildew (fungal), bacterial blight, and viral infections like mosaics.
- −
- Weeds: Weeds are unwanted plants that compete with cultivated crops for resources such as light, water, nutrients, and space. They can reduce crop yields and quality by overshadowing crops, harboring pests, and acting as reservoirs for diseases.
It is critical to remember that the NPs’ reactions are dose-dependent and that, when applied improperly, NPs can be phytotoxic to crops, due to the increased concentration of compounds inside the cell and the occurrence of cell toxicity, which results in an increase in free radicals in the cells []. They may significantly impact photosynthetic efficiency, apical growth, plant biomass, and seed germination. NPs enhance plant defense by increasing levels of phenolics, activity of antioxidant enzymes in plants, stimulating hormone production like salicylic acid and jasmonic acid, which are key in plant immune responses, enhancing nutrient uptake, improving root architecture, making plants more robust overall, and improving plant resistance against pathogens []. NPs demonstrate significant potential in enhancing agricultural practices, particularly pest and disease management. Here is an elaboration on their roles in specific applications:
- (a)
- Delivering pesticides or fungicides in a controlled manner, NPs can encapsulate pesticides or fungicides, allowing for controlled and sustained release []. This reduces the need for frequent applications and minimizes the risk of environmental contamination. By improving solubility and stability, NPs can enhance the efficacy of pesticides [].
- (b)
- Inducing resistance in plants against pathogens, certain NPs can act as elicitors, prompting physiological responses that enhance stress tolerance. Silica or copper-based NPs can stimulate the plant’s innate immune system, activating defense pathways that enhance resistance against pathogens. This may involve increased production of ROS and the accumulation of phenolic compounds, which bolster plant defense mechanisms [].
- (c)
- Targeting specific pests without harming non-target organisms, NPs can be engineered to be selectively toxic to specific pests while minimizing effects on beneficial and non-target organisms []. For instance, targeted delivery using lipid-based NPs can affect pest behavior without adversely impacting pollinators like bees [].
Through NPs’ ion release, which damages DNA by interacting with bases in DNA, they can directly impact plant homeostasis. Alternatively, they can have indirect impacts by producing ROS, which alters the activity of antioxidant enzymes. By improving plant nutrition, reducing pathogen infections (bacterial, fungal, and viral), and raising crop output and nutrition quality, many NPs can directly combat pathogens or strengthen defenses against illnesses []. They primarily suppress the plant’s ROS response and promote plant growth and development by regulating the antioxidant systems, endogenous plant hormones, and the transcriptional regulation of stress-related genes [,]. In terms of direct pathogen-attacking activity, distinct NPs employ various antibacterial mechanisms. Agriculture may use a variety of nanomaterials, with metal or metal oxide NPs like Ag NPs and CuO2 NPs, as well as lipid or polymer-based NPs, such as micelles and chitosan nanoparticles (CS NPs), being particularly useful [].
There is a significant distinction between the two primary kinds of NPs (organic and inorganic NPs) in terms of applications that center on biotic stress: (i) Inorganic NPs often have inherent action against the pathogen (Ag NPs, for example, directly exhibit antifungal and antibacterial activity against bacteria, fungi, and nematodes that infect plants) []. (ii) The primary use of polymeric nanoparticles (PNPs) is as nanocarriers, which facilitate the effective release of the active drug [] and improve bioavailability. In recent years, PNPs have been the subject of much research due to their potential use in controlled release and environmental protection of active substances. There is much interest in using PNPs in agriculture because of their excellent stability and capacity to release active chemicals in a specific plant target zone. Moreover, PNPs have minimal toxicity due to their biodegradability and biocompatibility. Furthermore, these NPs can contain many active substances with minimal environmental influence because of their delayed release. The usefulness of PNPs is therefore frequently dependent on their combination with other active compounds, such as antibiotics, herbicides, insecticides, or even micronutrients (Table 3) [].

Table 3.
Nanomaterials and their effects on horticulture crops under biotic stress conditions.
6.1. A Polymeric Nanoparticle
With lower quantities of the active ingredient, PNPs often possess the ability to transport molecules efficiently in agriculture. Consequently, there is less soil leaching, better thermal and photostability, and higher adhesion and absorption []. Given these factors, commonly produced PNPs for agricultural applications are best composed of low-cost, biocompatible, and biodegradable polymers with low toxicity. Because of its cost-effectiveness, biocompatibility, and biodegradability, chitosan is the most often used biopolymer in agricultural applications [].
ROS generation, elevated antioxidant enzyme activity (catalase, glutathione peroxidase, peroxidase, and superoxide dismutase), and antioxidant biomolecules (flavonoids) all support plant defense against biotic stress []. An inventive technique for reducing the severity of leaf rust disease included misting salicylic acid and chitosan NPs before and during plant leaf inoculation with Puccinia striiformis, an essential fungal parasite [].
In addition to chitosan, atrazine, a pesticide that works against the weed species Bidens pilosa, was included in nano-capsules made of the natural polymer poly []. Encasing the herbicide in PCL NPs reduced the genotoxicity of atrazine and its soil mobility. PCL containing atrazine was useful in suppressing agricultural weeds while lessening the herbicide’s toxicity [].
It has been possible to produce new and efficient nanocarriers to lessen biotic stress in agriculture by employing various ingenious and intriguing techniques. Sustainable development principles guided the creation of pesticides (such as the model insecticide Azox) utilizing renewable plant oil-based polymers []. Promoting the sustainable release of agrochemicals is another use for NPs based on alginate [].
6.2. Metal Nanoparticles
Silver NPs are effective nanopesticides against a variety of phytopathogens, including Fusarium oxysporum, Cladosporium cucumerinum, Pyricularia oryzae, Alternaria alternata, and Sclerotinia sclerotiorum [,,]. They work by various methods, such as ion release, creating pits and holes in bacterial membranes, and interacting with enzymes’ disulfide or sulfhydryl groups, impairing metabolic processes [].
Besides their antibacterial properties, silver nanoparticles (Ag NPs) enhanced the germination of seeds and altered the biochemical composition of pearl millet []. This included increased phenol, flavonoid, and protein content, and peroxidase and superoxide dismutase activity []. Cu NPs are also highly effective nanopesticides that improve plant nutrition and development. Their antibacterial action includes disrupting essential enzymes and removing NPs from the bacterial cell membrane []. Important phytopathogens like Fusarium oxysporum, Aspergillus niger, Rhizoctonia solani, Xanthomonas axonopodis, Fusarium destructiva, Curvularia lunata, Alternaria alternata, Clavibacter michiganensis, Gibberella fujikuroi, and Drechslera sorghicola are among those against which they exhibit antimicrobial activity [].
Cu NPs applied topically to foliage enhanced growth and chlorophyll content while reducing Fusarium wilt incidence and severity by 68 and 66.5%, respectively []. Another study found that applying Cu NPs topically to plants improved the quality of their fruit by encouraging the build-up of bioactive substances such as flavonoids, lycopene, vitamin C, and total phenols []. Apart from these two NPs, TiO2 NP, a metal-based nanoparticle, exhibits potential uses in reducing biotic stress in various plants. Producing ROS and lipid peroxidation can induce oxidative stress, damaging pathogen cell integrity and increasing membrane fluidity [].
Because silica NPs directly affect plant development, they offer enormous agricultural potential []. The increased absorption and translocation of silica by silica-based nanomaterials decreases the production and build-up of ROS and lipid peroxidation, enhancing resilience to biotic and abiotic stressors []. In addition to serving as nanocarriers for proteins, nucleotides, and other active molecules in agriculture, empty silica NPs can be employed as biostimulants, nanofertilizers, herbicides, and insecticides []. By reducing the amount of sodium ions and heavy metals that enter plants, nanosilica reduces salinity and the toxicity of heavy metals.
The accumulation of nanosilica in plant leaves significantly strengthens the plants’ resistance to infections. Not only can nanosilica bio-stimulate plant cells, but it also possesses antibacterial and antifungal characteristics. Furthermore, it is known that nanosilica stimulates the production of genes involved in defense. Nanosilica can induce acquired resistance in plants, a plant immunological response that greatly enhances plant defense against biotic stress [].
According to a study by [], silicon-based NPs have been created and employed as pesticide agents and nanocarriers in plants to guard against diseases. Lately, spherical silicon nanoparticles (NPs) with a size of 45 nm and a negative zeta potential of −26 mV, produced by Fusarium oxysporum SM5, showed nematicide effects due to the presence of meloxydone []. When sprayed in a concentration of 100 mg/L, the silica NPs significantly reduced the damage caused by Alternaria solani and demonstrated in vitro antifungal activity against the fungus. Furthermore, a notable increase in the content of antioxidant metabolites and enzymes was noted. The authors found a notable rise in antioxidant metabolism, a notable drop in infection damage indices, and a more noteworthy production of chemicals linked to defense against biotic stress [,].
However, research showed that silicon NPs prevented egg hatching, and after 72 h of exposure to NPs at 100 and 200 ppm, the death rate of second-stage juvenile root-knot nematodes varied from 87% to 98.5%. Interestingly, commercial nematicides at half-recommended doses combined with silicon NPs with 100 ppm further reduced egg hatching and second-stage juvenile root-knot nematode mortality. Based on these findings, it may be possible to combine silicon NPs with conventional nematodes to control pathogens in agricultural production []. Additionally, the therapy enhanced the nematode-invaded plants’ growth and ability to absorb vital nutrients.
Selenium nanoparticles (Se NPs), in addition to nanosilica, are recognized as an environmentally and ecologically benign method of boosting plant yield by reducing abiotic stressors since Se triggers plant defense systems []. Because biosynthesized Se NPs may produce ROS, which destroy pathogen cell walls, degrade the integrity of pathogen cell membranes, and limit ATP synthetase activity, they have antibacterial effects against phytopathogens such as fungi and bacteria []. By breaking down the cell wall and changing the cycle of food metabolism, protein synthesis, modification, and deoxyribonucleic acid replication, Se NPs can also directly prevent the growth of pathogens, ultimately leading to the death of the microbes [,].
6.3. Metal Oxide-Based Nanoparticles
Another metallic NP to be aware of is oxide-based NPs. In this regard, Botrytis cinerea, which causes gray mold, was 80% stopped from growing by photoactivated ZnO NPs. ZnO NP spraying on strawberries improved plant yield by 28.5%, prevented harvested fruit deterioration during storage by 8 days, and decreased the incidence of Botrytis cinerea by 43% [].
An alternative method involved inoculating the soil with ZnO NPs generated by Matricaria chamomilla, which reduced the number of Ralstonia solanacearum that causes bacterial wilt and the severity of the illness while promoting better plant development. Because of the likely release of Zn+2 ions, the bacterial cells exhibited morphological deformation, including breakage of the cell wall and membrane and leaking of cell contents [,]. Free radicals can accumulate due to a change in the redox balance of plant cells. These radicals can alter cell signaling pathways and oxidative damage macromolecules []. In addition to metal-based and polymeric NPs, several notable NPs have been successfully studied in agricultural applications to alleviate plant stress.
SiO2 NPs, or nanosilica, have become important in plant defense against abiotic stressors. Nanosilica’s excellent surface-to-volume ratio and biocompatibility contribute to its widespread biological use []. Because of its nanoscale size, which enables quick absorption by the apoplastic route and translocation in plant tissues and as a nanocarrier for active chemicals, nanosilica exhibits better plant effects than bulk silica []. It is commonly known that nanosilica has a positive effect on helping plants develop stress tolerance. Although the beneficial effects of nano silicon have been well-documented, ref. [] noted that the effect varies depending on many factors, including the type of application (usually foliar or to the substrate), the concentration and bioavailability of nano silicon, and the plant species used as a biological model.
The rust-causing bacterium Ustilago tritici was shown to be susceptible to antimicrobial action by biogenic TiO2 NPs made from Chenopodium quinoa leaf extracts, which suppressed up to 75% of mycelial growth []. Additionally, Satti and associates demonstrated that TiO2 NPs made from Moringa oleifera leaf aqueous extract exhibit antimicrobial activity against Bipolaris sorokiniana. This pathogen causes spot blotch disease, stabilizing the plant’s chlorophyll content, soluble sugar, protein, proline, flavonoid, and phenolic contents to induce disease tolerance in wheat plants [].
6.4. Carbon Nanomaterials
Carbon-based NPs are a significant type of nanomaterial that can help plants under biotic stress. Atoms of carbon (C) may arrange themselves into various configurations. A stack of two-dimensional single sheets called graphite is created when C atoms are arranged as a honeycomb lattice. The atomic structure of graphene is composed of a single graphite layer []. Carbon nanotubes (CNTs) are another carbon-based structure that may be considered a single graphene sheet rolled down an axis parallel to the graphene crystallographic orientations [].
Nanomaterials based on carbon have also shown practical antiviral, antifungal, and antibacterial qualities. Reference [] reported that a carbon-based nanomaterial exhibited significant antibacterial activity while preventing the development of biofilms. Plants can employ CNTs as antibacterial agents. For example, CNTs lessened Alternaria solani harmful impact on plants. Through both direct antibacterial action and induction of the plant antioxidant defense system, this illness results in plant losses []. Giving plants CNTs increased their flavonoid concentration, ascorbic acid content, and glutathione peroxidase activity. Based on the production of ROS following nanomaterial absorption by plant tissue, CNTs have antibacterial action that lessens the severity of A. solani.
7. Nanoparticle Interaction with Plants
The role of NPs in mitigating abiotic or biotic stress of plants is related to the composition and structure of cell walls. Some elements are involved in the structure and functioning of the cell wall and membrane. Therefore, it affects the metabolite transport reactions. It can influence the signaling pathway that regulates cell expansion, which is associated with stress resistance [,]. The interaction of NPs with plants is complex and can have both beneficial and detrimental effects. These interactions are influenced by the NPs’ properties and the plants’ physiological status. The interactions occur at a molecular level through various mechanisms that can significantly affect their physiology and biochemistry as follows:
(a) Uptake Mechanisms: NPs can be absorbed by either endocytosis or passive diffusion by plant roots. For instance, Ref. [] found that gold nanoparticles (Au NPs) could penetrate root cells through various mechanisms, including apoplastic and simplistic pathways. Negatively charged NPs were observed to gradually accumulate in protoplasts and roots, eventually becoming noticeable in the root xylem, while positively charged particles accumulated on root surfaces and were not absorbed by either []. (b) Cellular Interactions: NPs interact with the composition of the cell wall, which consists of cellulose, hemicellulose, and pectin. Their small size allows for easy penetration of the cell wall. Several literature data indicate that the cell wall pore size ranges between 2 and 20 nanometers. It is noted that NPs smaller than 20 nanometers easily pass through the cell wall []. The cell wall’s composition can influence nanoparticle attachment and penetration []. (c) Biochemical effects: Certain NPs can induce stress by generating ROS in plant cells, leading to alterations in growth and metabolism, and confer stress tolerance by modulating oxidative stress responses []. (d) Gene expression: NPs can affect gene expression related to stress response pathways, growth regulation, and metabolism [,]. NPs strengthen antioxidant activities and shield plants from oxidative stress by causing the accumulation of antioxidant genes, osmolytes, nutrients, and amino acids []. (e) Toxicological impacts: In some cases, NPs can be toxic to plants, leading to cell damage or death [], it has been discovered that NPs immediately interact to the cell membrane and organelles, causing harmful ions to dissolve and be released, ROS to be produced, and oxidative stress to follow []. (f) Transport and Distribution: NPs can be translocated to various plant parts (leaves, stems), affecting overall plant health and physiological processes [].
Also, NPs play a multifaceted role in modulating various physiological and biochemical processes in plants. Such as growth promotion, improving nutrient uptake and bioavailability, facilitating better absorption by plants [], bolstering plants’ resistance to abiotic stresses [], enhancing chlorophyll content and photosynthetic efficiency [], regulating phytohormone signaling [], and modulating responses to environmental stimuli [].
On the contrary, excessive concentrations of NPs can cause phytotoxicity, which affects seed germination and biomass production, interferes with the photosynthetic system, produces oxidative stress, damages cell membrane integrity, changes gene expression, damages DNA, and results in epigenetic alterations in plants [].
8. Biosafety of Nanomaterials
The expanding use of nanotechnology in agriculture prompts enquiries and concerns about its possible impacts on human and environmental health [,]. Therefore, it is imperative to study the residual effect of nanomaterials in fruits on experimental animals as a mammalian model to determine the degree of potential harm that these items may cause. Follow ethical and scientific considerations during animal studies to avoid undue suffering of animals []. Nanoparticle toxicity is assessed by long exposure; for acute/sub-chronic toxicity, exposure times vary from 28 to 90 days []. Oral feeding or injection are the treatment methods [], and a histopathological analysis is conducted to ascertain the level of toxicity as shown in Figure 6.
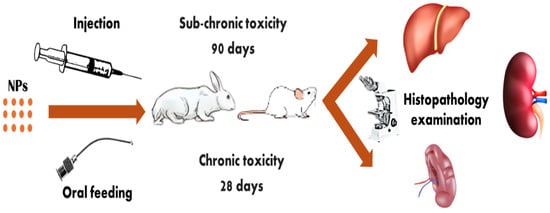
Figure 6.
Chronic or sub-chronic toxicity of nanoparticles on experimental animals.
With little or almost no research on this point, we review the most important potential effects of nanomaterials on experimental animals’ growth, development, and histological alteration. At low dosages, NPs have much potential that impacts animal nutrition, growth performance, and overall health []. NPs help animals achieve their nutritional needs, increase their production, boost their immune system and microbial profile, and lower their risk of illness, but may increase cellular absorption and translocation inside an animal’s body, which might impact their toxicity []. Inflammation or cell death may result from NPs at the cellular level and can cause pathological alterations in the liver, pancreas, kidney, intestine, brain, and adrenal glands [].
According to [], selenium NPs have positive effects on liver function and lower blood sugar levels and alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), and alkaline phosphatase (ALP) levels. Furthermore, in people with diabetes, selenium NPs may lower blood levels of renal components such as albumin, urea, and creatinine.
According to [], the effects of prolonged exposure to gold nanoparticles (Au NPs) on the structure and function of the liver, spleen, and kidneys have been investigated to determine the main characteristics of Au NP accumulation in the tissues that may contribute to their toxicity. The function of the liver and spleen was unaffected by the long-term buildup of Au NPs in these organs. However, modifications in these organs’ structure, such as higher macrophage infiltration and higher fibronectin levels, indicated a toxic reaction linked to long-term exposure to Au NPs. It is necessary to clarify whether these alterations are temporary and will go away with time, or if they are the first of a progressive inflammatory disease.
9. Real-World Applications of Nanoparticles in Horticultural Plants
NPs have found numerous applications in horticultural plants, primarily aimed at enhancing growth, improving pest and disease resistance, aiding nutrient delivery, and promoting sustainable agricultural practices. Key practical uses of NPs in horticulture crops include the following:
- (a)
- Improvement of Nutrient Uptake: NPs can formulate slow or controlled-release fertilizers. Nanostructured fertilizers enhance nutrient absorption and reduce loss due to leaching.
- (b)
- Disease and Pest Management: NPs can enhance the efficacy of pesticides by improving their delivery to the target site while reducing the required dosage. For instance, chitosan NPs encapsulating pesticides have shown effective control of pests in crops like cucumbers [].
- (c)
- Stress Mitigation: NPs can mitigate abiotic stress effects in horticultural crops, such as drought and salinity. For instance, silica NPs enhance drought tolerance in plants like cucumber, improving overall plant health and yield [].
- (d)
- Growth Enhancement: NPs can be used in seed priming to improve germination rates and seedling vigor. For example, zinc oxide NPs have enhanced germination rate and early growth in crops [].
- (e)
- Improved Photosynthesis: Certain NPs, such as titanium dioxide (TiO2), can enhance photosynthetic efficiency and chlorophyll content in horticultural crops. For example, TiO2 NPs have been shown to improve growth and increase net photosynthetic rate in Mentha piperita L. [].
- (f)
- Disease Resistance: NPs can induce systemic plant resistance against diseases. For example, selenium NPs have been found to induce defense responses against fungal pathogens in crops like strawberries (Fragaria × ananassa) [].
The application of NPs in horticultural crops shows great promise for enhancing agricultural practices by improving nutrient uptake, pest and disease management, stress tolerance, and overall crop yield. As research advances, these applications can lead to more efficient and sustainable horticultural systems.
10. Advancements in Nanotechnology-Enabled Stress Management Techniques
Advancements in nanotechnology have introduced innovative stress management techniques that enhance the resilience and productivity of crops under various stresses. Here are some notable advancements in nanotechnology-enabled stress management techniques:
- (a)
- High dispersibility, heavy metal-free nanomaterials
Despite the potential benefits of nano-enabled plant stress tolerance, heavy metal-containing NPs, such as quantum dots Ag and with Cd2+ may cause environmental harm and biosafety issues []; the potential for NPs to aggregate on plants, which might hinder their ability to respond to stress, is an additional problem []. Aggregated NPs have less surface area and cellular absorption than dispersed NPs, frequently leading to toxicity. Therefore, the development of nano-enabled agriculture requires the usage of suitable nanomaterials []. The adoption of NPs for agricultural practices and consistent biological activity would be supported by improving the dispersion quality of the materials to prevent agglomeration after application [].
- (b)
- Nanosensors for detecting stress
Stress sensing and signaling are two of the many complex defense systems plants have developed to withstand stress. Plant signaling molecules include H2O2, Ca2+, gas molecules (nitric oxide, carbon monoxide, and hydrogen sulfide), plant hormones (ethylene, methyl salicylate, jasmonic acid, and abscisic acid), sugars (glucose and sucrose), and volatile organic compounds (isoprenes) [,]. In plants under salt stress, signal transduction from root to shoot involves Ca2+ signaling [,]. The ratiometric quantum dot sensor for glucose [], hemin-complexed DNA aptamer-coated single-walled carbon nanotubes for H2O2 [], are examples of plant nanobiotechnology’s effectiveness in monitoring signaling molecules in different plant species.
- (c)
- Nano-encapsulated
Developing nano-encapsulated fertilizers and nutrients allows for targeted delivery, mitigating stress effects due to nutrient deficiencies. Nano-encapsulation is an innovative and promising nanotechnology that allows the active ingredients to be released from capsules or particles in a controlled and progressive way []. Studies have shown that the exogenous application of chitosan NPs encapsulated spermine alleviated the adverse effect of salinity on chili pepper plants and enhanced growth, yield, chlorophyll content, and antioxidant enzymes [].
11. Future Perspectives
Ongoing research in nanotechnology applied to agriculture is rapidly advancing, focusing on various aspects such as enhanced crop protection, precision agriculture, efficient resource usage, sustainability, disease resistance, plant immunity, and soil remediation. Nanotechnology is expected to be a transformative technology that enhances many aspects, with increasing uses of nanomaterials in agricultural applications and consumer products. In addition, it has become a fascinating way to enhance plant development under biotic and abiotic stress situations, especially in light of sustainability. Using NPs can greatly enhance plant development and, in the event of biotic stress, might lessen the detrimental effects of phytopathogens. In agriculture, biotic stress remains a major contributor to yield loss, accounting for 20–40% of losses due to pests and diseases []. In addition to carrying compounds with long-lasting antibacterial properties, nanomaterials can be directly harmful to plant pathogens [].
Despite significant advancements in the application of nanomaterials in agriculture, specific crucial points still require clarification. NMs have been the subject of in-depth experimental or pilot research. To confirm their possible long-term effects on the soil and its microbiome, plants, and animals, further research at the scale of hectares of agricultural fields and the scale of several years of treatments is still required []. Further data is also required on the safe and viable commercial application of NMs and the safety of plants grown with their help []. One way to emphasize the difficulties in using nanomaterials to counteract biotic stress is the need for more thorough assessment of the absorption, transport, and alteration of nanomaterials in plant tissue. Numerous factors, including the chemical makeup of the material, size distribution, surface charge and chemical surface, form, dose, concentration, application method, length of treatment, interactions between the material and the target plant, and environmental microbiota, all have a significant impact on how nanomaterials affect plants []. As anticipated, nanomaterials have beneficial effects at low concentrations while becoming harmful at higher dosages or concentrations. NPs can serve several purposes because of their well-researched capacity to break down hazardous substances found in the environment [].
Areas needing more investigation to assess how NPs influence the diversity and functioning of microbial soil communities over prolonged periods. Changes in microbial populations can affect nutrient cycling and soil fertility []. Also, understand how plants take up NPs over time and their accumulation in edible plant tissues. The long-term implications for food safety and human health remain largely unexplored. In addition, understanding the persistence of NPs in soil and water is crucial for evaluating their long-term environmental risks.
Despite the obvious advantages of nanomaterials, there are still unanswered problems regarding the potential environmental effects of the NPs utilized in daily life []. A significant additional obstacle is the public’s acceptance of this technology, as people and/or animals are the ultimate users of plants treated with nanomaterials, and it is necessary to assess the impact of these technologies on human and animal health. NPs may accumulate inside cells and cause intracellular alterations, including gene mutations or organelle damage []. Therefore, it is necessary to evaluate nanomaterials’ toxicity and biological activity on experimental animals as a mammalian model. Ecotoxicological research on nanomaterials and establishing a regulatory framework for their use in large-scale agricultural production are crucial steps in this approach. We welcome more research to improve the safe application of nanomaterials on a large scale in the agricultural industry.
12. Conclusions
Nano-bioengineering is a significant future research interest that could improve food security and crop production under biotic stress. The main challenge to developing horticultural crops today is climate change. This has resulted in significant losses in horticultural crops, which are an important source of income for those employed in the agricultural sector because they are a fundamental component of food security, as well as in the pharmaceutical and many other industries. In this work, we have reviewed the types of NPs and the different methods of preparation (top-down and bottom-up approaches), emphasizing their potential application in agriculture to improve stress resilience in horticultural plants. Also, the mechanism of action and interaction between NPs and plants at the molecular level facilitates uptake and translocation. They can penetrate plant tissues through root systems or foliar application, enhancing nutrient absorption and providing protective effects against ROS generated during stress. The article explores how NPs can alleviate various biotic and abiotic stresses. They achieve this by enhancing antioxidant defenses, regulating osmotic adjustment, and promoting healthy growth through improved nutrient delivery and signaling pathways. The review underscores the necessity for responsible and sustainable usage of NPs, highlighting potential ecological impacts and the importance of ecotoxicological studies in promoting safe agricultural applications. Ultimately, the review concludes that nanotechnology offers innovative solutions to some of the most pressing challenges facing food production, sustainability, and environmental health. Their unique properties at the nanoscale can enhance crop yields, improve pest and disease resistance, optimize resource use, and contribute to efficient soil and water management, with the need for further research and responsible application.
Funding
This paper has been supported by the RUDN University Strategic Academic Leadership Program.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors have no competing interests to declare relevant to this work. The present study has no conflicts of interest.
References
- Mantri, N.; Patade, V.; Pang, E.C.K. Recent Advances in Rapid and Sensitive Screening for Abiotic Stress. In Improvement of Crops in the Era of Climatic Changes; Ahmad, P., Wani, M., Azooz, M., Phan Tran, L.S., Eds.; Springer: New York, NY, USA, 2014; pp. 37–47. [Google Scholar] [CrossRef]
- Nejat, N.; Mantri, N. Plant Immune System: Crosstalk Between Responses to Biotic and Abiotic Stresses the Missing Link in Understanding Plant Defence. Curr. Issues Mol. Biol. 2017, 23, 1–16. [Google Scholar] [CrossRef]
- Francini, A.; Sebastiani, L. Abiotic Stress Effects on Performance of Horticultural Crops. Horticulturae 2019, 5, 67. [Google Scholar] [CrossRef]
- Nasser, M.A.; El-Mogy, M.M.; Samaan, M.S.F.; Hassan, K.M.; El-Sayed, S.M.; Alsubeie, M.S.; Darwish, D.B.E.; Mahmoud, S.F.; Al-Harbi, N.A.; Al-Qahtani, S.M.; et al. Postharvest exogenous melatonin treatment of table grape berry enhances quality and maintains bioactive compounds during refrigerated storage. Horticulturae 2022, 8, 860. [Google Scholar] [CrossRef]
- Mareri, L.; Parrotta, L.; Cai, G. Environmental Stress and Plants. Int. J. Mol. Sci. 2022, 23, 5416. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, M.; Geioushy, R.A.; Fouad, O.A.; Khaled, A.G. Investigation the activities of photosynthetic pigments, antioxidant enzymes and inducing genotoxicity of cucumber seedling exposed to copper oxides nanoparticles stress. Sci. Hortic. 2022, 305, 111364. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Zandalinas, I.S.; Fritschi, F.B.; Mittler, R. Global Warming, Climate Change, and Environmental Pollution: Recipe for a Multifactorial Stress Combination Disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Fawzy, M.; Al-zahrani, M.; Abdelkader, M. Enhancing Metabolic Processes and Water Deficit Stress Tolerance of Maize Plants through Biochar Addition and Foliar Application of Zinc Nanoparticles. Russ. J. Plant Physiol. 2025, 72, 1–12. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Rashmi, R.; Ulhas, R.S.; Sudheer, W.N.; Banadka, A.; Nagella, P.; Aldaej, M.I.; Rezk, A.A.S.; Shehata, W.F.; Almaghasla, M.I. The Role of Nanoparticles in Response of Plants to Abiotic Stress at Physiological, Biochemical, and Molecular Levels. Plants 2023, 12, 292. [Google Scholar] [CrossRef]
- Hassan, K.M.; Ajaj, R.; Abdelhamid, A.N.; Ebrahim, M.; Hassan, I.F.; Alam-Eldein, S.M.; Ali, M.A. Silicon: A powerful aid for medicinal and aromatic plants against abiotic and biotic stresses for sustainable agriculture. Horticulturae 2024, 10, 806. [Google Scholar] [CrossRef]
- Kumar, R.; Tiwari, R.K.; Jeevalatha, A.; Siddappa, S.; Shah, M.A.; Sharma, S.; Sagar, V.; Kumar, M.; Chakrabarti, S.K. Potato apical leaf curl disease: Current status and perspectives on a disease caused by tomato leaf curl new Delhi virus. J. Plant Dis. Prot. 2021, 128, 897–911. [Google Scholar] [CrossRef]
- Devi, R.; Behera, B.; Raza, M.B.; Mangal, V.; Altaf, M.A.; Kumar, R.; Kumar, A.; Tiwari, R.K.; Lal, M.K.; Singh, B. An insight into microbes mediated heavy metal detoxification in plants: A review. J. Soil Sci. Plant Nutr. 2022, 22, 914–936. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, C.-B.; Ren, R.-M.; Jiang, J.-H. Salicylic acid had the potential to enhance tolerance in horticultural crops against abiotic stress. Front. Plant Sci. 2023, 14, 1141918. [Google Scholar] [CrossRef] [PubMed]
- Samal, I.; Bhoi, T.K.; Majhi, P.K.; Murmu, S.; Pradhan, A.K.; Kumar, D.; Saini, V.; Paschapur, A.U.; Raj, M.N.; Ankur Manik, S. Combatting insects mediated biotic stress through plant associated endophytic entomopathogenic fungi in horticultural crops. Front. Plant Sci. 2023, 13, 1098673. [Google Scholar] [CrossRef]
- Sinha, R.K.; Verma, S.S. Proteomics approach in horticultural crops for abiotic-stress tolerance. In Stress Tolerance in Horticultural Crops; Woodhead Publishing: Sawston, UK, 2021; pp. 371–385. [Google Scholar] [CrossRef]
- Singh, A.; Tiwari, S.; Pandey, J.; Lata, C.; Singh, I.K. Role of nanoparticles in crop improvement and abiotic stress management. J. Biotechnol. 2021, 337, 57–70. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.A.; Ali, E.F. Foliar Application of Zinc Oxide Nanoparticles Promotes Drought Stress Tolerance in Eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M. Role of Nanoparticles in Enhancing Crop Tolerance to Abiotic Stress: A Comprehensive Review. Front. Plant Sci. 2022, 13, 946717. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antiox 2021, 10, 277. [Google Scholar] [CrossRef]
- Ali, M.A.A.; Nasser, M.A.; Abdelhamid, A.N.; Ali, I.A.A.; Saudy, H.S.; Hassan, K.M. Melatonin as a Key Factor for Regulating and Relieving Abiotic Stresses in Harmony with Phytohormones in Horticultural Plants—A Review. J. Soil Sci. Plant Nutr. 2023, 24, 54–73. [Google Scholar] [CrossRef]
- Bidi, H.; Fallah, H.; Niknejad, Y.; Tari, D.B. Iron oxide nanoparticles alleviate arsenic phytotoxicity in rice by improving iron uptake, oxidative stress tolerance and diminishing arsenic accumulation. Plant Physiol. Biochem. 2021, 163, 348–357. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.M.; Hassan, K.M.; Abdelhamid, A.N.; Yousef, E.E.; Abdellatif, Y.M.R.; Abu-Hussien, S.H.; Nasser, M.A.; Elshalakany, W.A.; Darwish, D.B.E.; Abdulmajeed, A.M.; et al. Exogenous paclobutrazol reinforces the antioxidant and antimicrobial properties of lavender (Lavandula officinalis L.) oil through modulating its composition of oxygenated terpenes. Plants 2022, 11, 1607. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; El-Sayed, S.M.; Abdelhamid, A.N.; Hassan, K.M.; Elshalakany, W.A.; Nossier, M.I.; Alabdallah, N.M.; Al-Harbi, N.A.; Al-Qahtani, S.M.; Darwish, D.B.E.; et al. Potentiating biosynthesis of alkaloids and polyphenolic substances in Catharanthus roseus plant using ĸ-carrageenan. Molecules 2023, 28, 3642. [Google Scholar] [CrossRef]
- Zuverza-Mena, N.; Martínez-Fernández, D.; Du, W.; Hernandez-Viezcas, J.A.; Bonilla-Bird, N.; López-Moreno, M.L.; Komárek, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses-A review. Plant Physiol. Biochem. 2017, 110, 236–264. [Google Scholar] [CrossRef]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef]
- Babu, S.; Singh, R.; Yadav, D.; Rathore, S.S.; Raj, R.; Avasthe, R.; Yadav, S.K.; Das, A.; Yadav, V.; Yadav, B.; et al. Nanofertilizers for agricultural and environmental sustainability. Chemosphere 2022, 292, 133451. [Google Scholar] [CrossRef]
- Dilnawaz, F.; Misra, A.N.; Apostolova, E. Involvement of nanoparticles in mitigating plant’s abiotic stress. Plant Stress 2023, 10, 100280. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Karimi, M.; da Silva, J.A.T. The use of nanotechnology to increase quality and yield of fruit crops. J. Sci. Food Agric. 2019, 100, 25–31. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, L.; Wang, A.; Zhang, H.; Huang, M.; Wu, H.; Xing, B.; Wang, Z.; Ji, R. Nano-biotechnology in agriculture: Use of nanomaterials to promote plant growth and stress tolerance. J. Agric. Food Chem. 2020, 68, 1935–1947. [Google Scholar] [CrossRef]
- Kalwani, M.; Chakdar, H.; Srivastava, A.; Pabbi, S.; Shukla, P. Effects of nanofertilizers on soil and plant-associated microbial communities: Emerging trends and perspectives. Chemosphere 2022, 287, 132107. [Google Scholar] [CrossRef]
- Gupta, A.; Rayeen, F.; Mishra, R.; Tripathi, M.; Pathak, N. Nanotechnology applications in sustainable agriculture: An emerging eco-friendly approach. Plant Nano Biol. 2023, 4, 100033. [Google Scholar] [CrossRef]
- Hussain, M.; Zahra, N.; Lang, T.; Zain, M.; Raza, M.; Shakoor, N.; Adeel, M.; Zhou, H. Integrating nanotechnology with plant microbiome for next-generation crop health. Plant Physiol. Biochem. 2023, 196, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, M.D.; Surendhar, G.J.; Natrayan, L.; Patil, P.P.; Ram, P.B.; Paramasivam, P. Evolution and Recent Scenario of Nanotechnology in Agriculture and Food Industries. J. Nanomater. 2022, 2022, 1280411. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Salehi, H.; Abbas, S.; Saeed, F.; Poinern, G.E.J.; Siddique, K.H.M.; Varshney, R.K. Nano-enabled stress-smart agriculture: Can nanotechnology deliver drought and salinity-smart crops? J. Sustain. Agric. Environ. 2023, 2, 189–214. [Google Scholar] [CrossRef]
- Zulfiqar, F.H.; Afroze, B.; Shakoor, S.; Bhutta, S.M.; Ahmed, M.; Hassan, S.; Batool, F.; Rashid, B. Nanoparticles in Agriculture: Enhancing Crop Resilience and Productivity against Abiotic Stresses. In Abiotic Stress in Crop Plants—Ecophysiological Responses and Molecular Approaches; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Kandhol, N.; Jain, M.; Tripathi, D.K. Nanoparticles as potential hallmarks of drought stress tolerance in plants. Physiol. Plant 2022, 174, e13665. [Google Scholar] [CrossRef]
- Khan, Y.; Sadia, H.; Ali Shah, S.Z.; Khan, M.N.; Shah, A.A.; Ullah, N.; Ullah, M.F.; Bibi, H.; Bafakeeh, O.T.; Khedher, N.B.; et al. Classification, Synthetic, and Characterization Approaches to Nanoparticles, and Their Applications in Various Fields of Nanotechnology: A Review. Catalysts 2022, 12, 1386. [Google Scholar] [CrossRef]
- Shahid, M.; Javed, H.M.A.; Ahmad, M.I.; Qureshi, A.A.; Khan, M.I.; Alnuwaiser, M.A.; Ahmed, A.; Khan, M.A.; Tag-ElDin, E.S.M.; Shahid, A.; et al. A Brief Assessment on Recent Developments in Efficient Electrocatalytic Nitrogen Reduction with 2D Non-Metallic Nanomaterials. Nanomaterials 2022, 12, 3413. [Google Scholar] [CrossRef]
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef]
- Iravani, S. Methods for Preparation of Metal Nanoparticles. In Metal Nanoparticles; Thota, S., Crans, D.C., Eds.; Wiley-VCH: Weinheim, Germany, 2017; pp. 15–31. [Google Scholar] [CrossRef]
- Szczyglewska, P.; Feliczak-Guzik, A.; Nowak, I. Nanotechnology–General Aspects: A Chemical Reduction Approach to the Synthesis of Nanoparticles. Molecules 2023, 28, 4932. [Google Scholar] [CrossRef]
- Stanley, S. Biological nanoparticles and their influence on organisms. Curr. Opin. Biotechnol. 2014, 28, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Saallah, S.; Lenggoro, I.W. Nanoparticles carrying biological molecules: Recent advances and applications. KONA Powder Part. J 2018, 35, 89–111. [Google Scholar] [CrossRef]
- Abou El-Nasr, M.K.; El-Hennawy, H.M.; El-Kereamy, A.M.H.; Abou El-Yazied, A.; Salah Eldin, T.A. Effect of Magnetite Nanoparticles (Fe3O4) as Nutritive Supplement on Pear Saplings. Middle East J. Appl. Sci. 2015, 5, 777–785. Available online: https://www.curresweb.com/mejas/mejas/2015/777-785.pdf (accessed on 21 May 2025).
- Hassan, N.S.; Salah El Din, T.A.; Hendawey, M.H.; Borai, I.H.; Mahdi, A.A. Magnetite and Zinc Oxide Nanoparticles Alleviated Heat Stress in Wheat Plants. Curr. Nanomater. 2018, 3, 32–43. [Google Scholar] [CrossRef]
- Ahamed, M.; Alhadlaq, H.A.; Khan, M.A.M.; Karuppiah, P.; Al-Dhabi, N.A. Synthesis, Characterization, and Antimicrobial Activity of Copper Oxide Nanoparticles. J. Nanomater. 2014, 2014, 637858. [Google Scholar] [CrossRef]
- Ganeshan, S.; Ramasundari, P.; Elangovan, A.; Arivazhagan, G.; Vijayalakshmi, R. Synthesis and Characterization of MnO2 Nanoparticles: Study of Structural and Optical Properties. Int. J. Sci. Res. Phys. Appl. Sci. 2017, 5, 5–8. [Google Scholar] [CrossRef]
- Abou El-Nasr, M.K.; El-Hennawy, H.M.; Samaan, M.S.F.; Salaheldin, T.A.; Abou El-Yazied, A.; El-Kereamy, A. Using Zinc Oxide Nanoparticles to Improve the Color and Berry Quality of Table Grapes Cv. Crimson Seedless. Plants 2021, 10, 1285. [Google Scholar] [CrossRef]
- Kshirsagar, J.; Shrivastava, R.; Adwani, P. Preparation and characterization of copper oxide nanoparticles and determination of enhancement in critical heat flux. Therm. Sci. 2017, 21, 233–242. [Google Scholar] [CrossRef]
- Gao, M.; Chang, J.; Wang, Z.; Zhang, H.; Wang, T. Advances in transport and toxicity of nanoparticles in plants. J. Nanobiotechnol. 2023, 21, 75. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef] [PubMed]
- Sembada, A.A.; Lenggoro, I.W. Transport of Nanoparticles into Plants and Their Detection Methods. Nanomaterials 2024, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale Drug Delivery Systems: From Medicine to Agriculture. Front. Bioeng. Biotechnol. 2020, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Grillo, R.; Mattos, B.D.; Antunes, D.R.; Forini, M.M.L.; Monikh, F.A.; Rojas, O.J. Foliage adhesion and interactions with particulate delivery systems for plant nanobionics and intelligent agriculture. Nano Today 2021, 37, 101078. [Google Scholar] [CrossRef]
- Wang, W.-N.; Tarafdar, J.C.; Biswas, P. Nanoparticle synthesis and delivery by an aerosol route for watermelon plant foliar uptake. J. Nanopart. Res. 2013, 15, 1413–1417. [Google Scholar] [CrossRef]
- Schwab, F.; Zhai, G.; Kern, M.; Turner, A.; Schnoor, J.L.; Wiesner, M.R. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants—Critical review. Nanotoxicology 2015, 10, 257–278. [Google Scholar] [CrossRef]
- Avellan, A.; Yun, J.; Morais, B.P.; Clement, E.T.; Rodrigues, S.M.; Lowry, G.V. Critical review: Role of inorganic nanoparticle properties on their foliar uptake and in planta translocation Environ. Sci. Technol. 2021, 55, 13417–13431. [Google Scholar] [CrossRef]
- Kaiser, H. Stomatal uptake of mineral particles from a sprayed suspension containing an organosilicone surfactant. J. Plant Nutr. Soil Sci. 2014, 177, 869–874. [Google Scholar] [CrossRef]
- Wohlmuth, J.; Tekielska, D.; Čechová, J.; Baránek, M. Interaction of the Nanoparticles and Plants in Selective Growth Stages-Usual Effects and Resulting Impact on Usage Perspectives. Plants 2022, 11, 2405. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaka, M.; Abbasi, B.H.; Rahman, L.; Shah, A. Seed germination and biochemical profile of Silybum marianum exposed to monometallic and bimetallic alloy nanoparticles. IET Nanobiotechnol. 2022, 10, 359–366. [Google Scholar] [CrossRef]
- Kumar, P.; Rouphael, Y.; Cardarelli, M.; Colla, G. Vegetable Grafting as a Tool to Improve Drought Resistance and Water Use Efficiency. Front. Plant Sci. 2017, 8, 1130. [Google Scholar] [CrossRef]
- Al-Harbi, A.R.; Al-Omran, A.M.; Alharbi, K. Grafting improves cucumber water stress tolerance in Saudi Arabia. Saudi J. Biol. Sci. 2018, 25, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Razi, K.; Suresh, P.; Mahapatra, P.P.; Al Murad, M.; Venkat, A.; Notaguchi, M.; Bae, D.W.; Prakash, M.A.S.; Muneer, S. Exploring the role of grafting in abiotic stress management: Contemporary insights and automation trends. Plant Direct 2024, 8, e70021. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Kharal, M.A. Role of Phytohormones in Stress Tolerance of Plants. In Plant, Soil and Microbes; Springer: Berlin/Heidelberg, Germany, 2016; pp. 385–421. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Naz, S.; Altaf, M.M.; Khan, L.U.; Lal, M.K.; Tiwari, R.K.; Shakoor, A. Melatonin mitigates cadmium toxicity by promoting root architecture and mineral homeostasis of tomato genotypes. J. Soil Sci. Plant Nutr. 2022, 22, 1112–1128. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, G.; Liu, C.; Li, H.; Chen, X.; Fu, Q.; Lin, Y.; Guo, B. Salicylic Acid, a Multifaceted Hormone, Combats Abiotic Stresses in Plants. Life 2022, 12, 886. [Google Scholar] [CrossRef]
- Kaur, S.; Gupta, N. Effect of proline and salicylic acid on germination and antioxidant enzymes at different temperatures in Muskmelon (Cucumis melo L.) seeds. J. Appl. Nat. Sci. 2017, 9, 2165–2169. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, C.; Brummell, D.A.; Qi, S.; Lin, Q.; Bi, J.; Duan, Y. Salicylic acid treatment mitigates chilling injury in peach fruit by regulation of sucrose metabolism and soluble sugar content. Food Chem. 2021, 358, 129867. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact. 2014, 13, 66. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Cai, X.; Zeng, X.; Zou, J.-J.; Xing, W. A systematic review on the role of miRNAs in plant response to stresses under the changing climatic conditions. Plant Stress 2024, 14, 100674. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Zhang, Y.; Zhang, Q. CRISPR/Cas genome editing improves abiotic and biotic stress tolerance of crops. Front. Genome Ed. 2022, 4, 987817. [Google Scholar] [CrossRef]
- Anwar, A.; Kim, J.K. Transgenic breeding approaches for improving abiotic stress tolerance: Recent progress and future perspectives. Int. J. Mol. Sci. 2020, 21, 2695. [Google Scholar] [CrossRef]
- Ahmed, T.; Noman, M.; Manzoor, N.; Shahid, M.; Abdullah, M.; Ali, L.; Wang, G.; Hashem, A.; Al-Arjani, A.B.F.; Alqarawi, A.A.; et al. Nanoparticle-based amelioration of drought stress and cadmium toxicity in rice via triggering the stress responsive genetic mechanisms and nutrient acquisition. Ecotoxicol Environ. Saf. 2021, 209, 111829. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, N.; Ali, L.; Ahmed, T.; Noman, M.; Adrees, M.; Shahid, M.S.; Ogunyemi, S.O.; Radwan, K.S.; Wang, G.; Zaki, H.E. Recent Advancements and Development in Nano-Enabled Agriculture for Improving Abiotic Stress Tolerance in Plants. Front. Plant Sci. 2022, 13, 951752. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, M.; Shabala, S. Adapting crops for climate change: Regaining lost abiotic stress tolerance in crops. Front. Sci. 2024, 2, 1416023. [Google Scholar] [CrossRef]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS Regulation During Abiotic Stress Responses in Crop Plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali, A.; Kour, N.; Bornhorst, J.; AlHarbi, K.; Rinklebe, J.; Abd El Moneim, D.; Ahmad, P.; Chung, Y.S. Heavy Metal Induced Oxidative Stress Mitigation and ROS Scavenging in Plants. Plants 2023, 12, 3003. [Google Scholar] [CrossRef]
- Gagné, F. Chapter 6—Oxidative Stress. In Biochemical Ecotoxicology; Gagné, F., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 103–115. [Google Scholar] [CrossRef]
- Raliya, R.; Franke, C.; Chavalmane, S.; Nair, R.; Reed, N.; Biswas, P. Quantitative Understanding of Nanoparticle Uptake in Watermelon Plants. Front. Plant Sci. 2016, 7, 1288. [Google Scholar] [CrossRef]
- Chaki, M.; Begara-Morales, J.C.; Barroso, J.B. Oxidative Stress in Plants. Antioxidants 2020, 9, 481. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Dutt, M.; Shalana, A.M.; El-Kadya, M.E.; El-Boraya, M.S.; Shabana, Y.M.; Grosser, J.W. Silicon nanoparticles mitigate oxidative stress of in vitro-derived banana (Musa acuminata ‘Grand Nain’) under simulated water deficit or salinity stress. S. Afr. J. Bot. 2020, 132, 155–163. [Google Scholar] [CrossRef]
- Daler, S.; Kaya, O.; Korkmaz, N.; Kılıç, T.; Karadağ, A.; Hatterman-Valenti, H. Titanium Nanoparticles (TiO2-NPs) as Catalysts for Enhancing Drought Tolerance in Grapevine Saplings. Horticulturae 2024, 10, 1103. [Google Scholar] [CrossRef]
- Fallah, S.; Yusefi-Tanha, E.; Peralta-Videa, J.R. Interaction of nanoparticles and reactive oxygen species and their impact on macromolecules and plant production. Plant Nano Biol. 2024, 10, 100105. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Daneshvar Hakimi Meybodi, N.; Peijnenburg, W. Mitigation of the effect of drought on growth and yield of pomegranates by foliar spraying of different sizes of selenium nanoparticles. J. Sci. Food Agric. 2021, 101, 5202–5213. [Google Scholar] [CrossRef]
- Mozafari, A.A.; Ghadakchi Asl, A.; Ghaderi, N. Grape response to salinity stress and role of iron nanoparticle and potassium silicate to mitigate salt induced damage under in vitro conditions. Physiol. Mol. Biol. Plants 2018, 24, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Arshad, R.; Gulshad, L.; Haq, I.U.; Farooq, M.A.; Al-Farga, A.; Siddique, R.; Manzoor, M.F.; Karrar, E. Nanotechnology: A novel tool to enhance the bioavailability of micronutrients. Food Sci. Nutr. 2021, 9, 3354–3361. [Google Scholar] [CrossRef] [PubMed]
- Cevik, S. TiO2 nanoparticles alleviates the effects of drought stress in tomato seedlings. Bragantia 2022, 82, e20220203. [Google Scholar] [CrossRef]
- Alsadon, A.; Dewir, Y.H.; Ibrahim, A.; Alenazi, M.; Osman, M.; Al-Selwey, W.A.; Ali, M.A.; Shady, M.; Alsughayyir, A.; Hakiman, M. Compost Amendment Enhances Leaf Gas Exchange, Growth, and Yield in Water-challenged ‘Crimson Giant’ Red Radish (Raphanus sativus L.). HortScience 2024, 59, 84–91. [Google Scholar] [CrossRef]
- Alkhateeb, O.A.; Mahmoud, A.A.; Abdou, A.H.; Abdelaal, K.; EL-Azm, N.A. Integrating soil mulching and subsurface irrigation for optimizing deficit irrigation effectiveness as a water-rationing strategy in tomato production. Not. Bot. Horti Agrobot. 2024, 52, 13514. [Google Scholar] [CrossRef]
- Kazemipour, S.; Hashemabadi, F.; Kaviani, B. Effect of silver nanoparticles on the vase life and quality of cut chrysanthemum (Chrysanthemum morifolium L.) flower. Eur. J. Exp. Biol. 2013, 3, 298–302. [Google Scholar] [CrossRef]
- Foroutan, L.; Solouki, M.; Abdossi, V.; Fakheri, B.A.; Mahdinezhad, N.; Gholamipourfard, K.; Safarzaei, A. The effects of zinc oxide nanoparticles on drought stress in Moringa peregrina populations. Int. J. Basic Sci. Med. 2019, 4, 119–127. [Google Scholar] [CrossRef]
- Almutairi, K.F.; Górnik, K.; Awad, R.M.; Ayoub, A.; Abada, H.S.; Mosa, W.F. Influence of Selenium, Titanium, and Silicon Nanoparticles on the Growth, Yield, and Fruit Quality of Mango under Drought Conditions. Horticulturae 2023, 9, 1231. [Google Scholar] [CrossRef]
- Bidabadi, S.S.; Sabbatini, P.; VanderWeide, J. Iron oxide (Fe2O3) nanoparticles alleviate PEG-simulated drought stress in grape (Vitis vinifera L.) plants by regulating leaf antioxidants. Sci. Hortic. 2023, 312, 111847. [Google Scholar] [CrossRef]
- Mosa, W.F.A.; Behiry, S.I.; Ali, H.M.; Abdelkhalek, A.; Sas-Paszt, L.; Al-Huqail, A.A.; Ali, M.M.; Salem, M.Z. Pomegranate trees quality under drought conditions using potassium silicate, nanosilver, and selenium spray with valorization of peels as fungicide extracts. Sci. Rep. 2022, 12, 6363. [Google Scholar] [CrossRef] [PubMed]
- Javan, M.; Selahvarzi, Y.; Sayyad-Amin, P.; Rastegar, S. Potential application of TiO2 nanoparticles to improve the nutritional quality of strawberry cv. Camarosa under drought stress. Sci. Hortic. 2024, 330, 113055. [Google Scholar] [CrossRef]
- Şener, S.; Sayğı, H.; Duran, C.N. Responses of In Vitro Strawberry Plants to Drought Stress under the Influence of Nano-Silicon Dioxide. Sustainability 2023, 15, 15569. [Google Scholar] [CrossRef]
- Mozafari, A.A.; Havas, F.; Ghaderi, N. Application of iron nanoparticles and salicylic acid in in vitro culture of strawberries (Fragaria × ananassa Duch.) to cope with drought stress. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 132, 511–523. [Google Scholar] [CrossRef]
- Mubashir, A.; Nisa Z-u Shah, A.A.; Kiran, M.; Hussain, I.; Ali, N.; Zhang, L.; Madnay, M.M.; Alsiary, W.A.; Korany, S.M.; Ashraf, M. Effect of foliar application of nano-nutrients solution on growth and biochemical attributes of tomato (Solanum lycopersicum) under drought stress. Front. Plant Sci. 2023, 13, 1066790. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Qi, M.; Huang, A.; Xu, X.; Begum, N.; Qin, C.; Zhang, C.; Ahmad, N.; Mustafa, N.S.; Ashraf, M.; et al. Improving growth and photosynthetic performance of drought stressed tomato by application of nano-organic fertilizer involves up-regulation of nitrogen, antioxidant and osmolyte metabolism. Ecotoxicol. Environ. Saf. 2021, 216, 112195. [Google Scholar] [CrossRef]
- Sallam, A.R.; Mahdi, A.A.; Farroh, K.Y. Improving Drought Stress Tolerance in Potato (Solanum tuberosum L.) Using Magnetite and Zinc Oxide Nanoparticles. Plant Cell Biotechnol. Mol. Biol. 2022, 23, 1–16. [Google Scholar] [CrossRef]
- Kobdani, A.; Piri, I.; Tavassoli, A. Effect of iron nano-chelate fertilizer on quantity and quality yield of Okra (Abelmoschus esculentus L.) in condition of drought stress. J. Veg. Sci. 2021, 5, 109–123. [Google Scholar] [CrossRef]
- Afshari, M.; Pazoki, A.; Sadeghipour, O. Foliar-applied Silicon and its Nanoparticles Stimulate Physio-chemical Changes to Improve Growth, Yield and Active Constituents of Coriander (Coriandrum Sativum, L.) Essential Oil Under Different Irrigation Regimes. Silicon 2021, 13, 4177–4188. [Google Scholar] [CrossRef]
- Hatami, M. Toxicity assessment of multi-walled carbon nanotubes on Cucurbita pepo L. under well-watered and water-stressed conditions. Ecotoxicol. Environ. Saf. 2017, 142, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Gerdini, F.S. Effect of nano potassium fertilizer on some parchment pumpkin (Cucurbita pepo) morphological and physiological characteristics under drought conditions. Int. J. Farm. Alli Sci. 2016, 5, 367–371. Available online: http://ijfas.com/wp-content/uploads/2016/07/367-371.pdf (accessed on 21 May 2025).
- Dowom, S.A.; Karimian, Z.; Dehnavi, M.M.; Samiei, L. Chitosan nanoparticles improve physiological and biochemical responses of Salvia abrotanoides (Kar.) under drought stress. BMC Plant Biol. 2022, 22, 364. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.F.; El-Shehawi, A.M.; Ibrahim, O.H.M.; Abdul-Hafeez, E.Y.; Moussa, M.M.; Hassan, F.A.S. A vital role of chitosan nanoparticles in improvisation the drought stress tolerance in Catharanthus roseus (L.) through biochemical and gene expression modulation. Plant Physiol. Biochem. 2021, 161, 166–175. [Google Scholar] [CrossRef]
- Fischer, G.; Ramírez, F.; Casierra-Posada, F. Ecophysiological aspects of fruit crops in the era of climate change. A Review. Agron. Colomb. 2016, 34, 190–199. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Wang, L.; Jin, B. Plant responses to heat stress: Physiology, transcription, noncoding RNAs, and epigenetics. Int. J. Mol. Sci. 2021, 22, 117. [Google Scholar] [CrossRef]
- Shafqat, W.; Jaskani, M.J.; Maqbool, R.; Chattha, W.S.; Ali, Z.; Naqvi, S.A.; Haider, M.S.; Khan, I.A.; Vincent, C.I. Heat shock protein and aquaporin expression enhance water conserving behavior of citrus under water deficits and high temperature conditions. Environ. Exp. Bot. 2021, 181, 104270. [Google Scholar] [CrossRef]
- Khalid, M.F.; Iqbal Khan, R.; Jawaid, M.Z.; Shafqat, W.; Hussain, S.; Ahmed, T.; Rizwan, M.; Ercisli, S.; Pop, O.L.; Alina Marc, R. Nanoparticles: The Plant Saviour under Abiotic Stresses. Nanomaterials 2022, 12, 3915. [Google Scholar] [CrossRef]
- Qi, M.; Liu, Y.; Li, T. Nano-TiO2 Improve the Photosynthesis of Tomato Leaves under Mild Heat Stress. Biol. Trace Elem. Res. 2013, 156, 323–328. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; AL-Huqail, A.A.; AL-Harbi, M.S.; Ali, E.F.; Wang, J.; Ding, Z.; Rekaby, S.A.; Ghoneim, A.M.; Eissa, M.A. Mechanisms of Chitosan Nanoparticles in the Regulation of Cold Stress Resistance in Banana Plants. Nanomaterials 2021, 11, 2670. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Petropoulos, S.A.; Rouphael, Y. Response and defence mechanisms of vegetable crops against drought, heat and salinity stress. Agriculture 2021, 11, 463. [Google Scholar] [CrossRef]
- Lasheen, F.F.; Hewidy, M.; Abdelhamid, A.N.; Thabet, R.S.; Abass, M.M.M.; Fahmy, A.A.; Saudy, H.S.; Hassan, K.M. Exogenous application of humic acid mitigates salinity stress on pittosporum (Pittosporum tobira) plant by adjusting the osmolytes and nutrient homeostasis. Gesun. Pflanz. 2023, 76, 317–325. [Google Scholar] [CrossRef]
- Alharbi, B.M.; Ali, M.A.; Abdelaal, K.; Dessoky, E.S.; Al-Harbi, N.A.; Al-Balawi, S.M.; Anazi, H.K.; Darwish, D.B.E.; Kashgry, N.A.T.A.; Alayafi, A.A.; et al. Spermidine (Spd) as a modulator of osmotic, redox and ion homeostasis in common bean seedlings under salinity stress: Physiological, biochemical and molecular aspects. Chil. J. Agric. Res. 2025, 85, 98–111. [Google Scholar] [CrossRef]
- Ranjan, A.; Rajput, V.D.; Minkina, T.; Bauer, T.; Chauhan, A.; Jindal, T. Nanoparticles induced stress and toxicity in plants. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100457. [Google Scholar] [CrossRef]
- Malekzadeh, M.R.; Roosta, H.R.; Kalaji, H.M. GO nanoparticles mitigate the negative effects of salt and alkalinity stress by enhancing gas exchange and photosynthetic efficiency of strawberry plants. Sci. Rep. 2023, 13, 8457. [Google Scholar] [CrossRef]
- Rehman, A.; Khan, S.; Sun, F.; Peng, Z.; Feng, K.; Wang, N.; Jia, Y.; Pan, Z.; He, S.; Wang, L.; et al. Exploring the nano-wonders: Unveiling the role of Nanoparticles in enhancing salinity and drought tolerance in plants. Front. Plant Sci. 2024, 14, 1324176. [Google Scholar] [CrossRef]
- Rasheed, A.; Li, H.; Tahir, M.M.; Mahmood, A.; Nawaz, M.; Shah, A.N.; Aslam, M.T.; Negm, S.; Moustafa, M.; Hassan, M.U.; et al. The role of nanoparticles in plant biochemical, physiological, and molecular responses under drought stress: A review. Front. Plant Sci. 2022, 13, 976179. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Pérez-De-Luque, A. Interaction of nanomaterials with plants: What do we need for real applications in agriculture? Front. Environ. Sci. 2017, 5, 12. [Google Scholar] [CrossRef]
- Ghanbari, F.; Bag-Nazari, M.; Azizi, A. Exogenous application of selenium and nano-selenium alleviates salt stress and improves secondary metabolites in lemon verbena under salinity stress. Sci. Rep. 2023, 13, 5352. [Google Scholar] [CrossRef]
- Aazami, M.A.; Maleki, M.; Rasouli, F.; Gohari, G. Protective effects of chitosan based salicylic acid nanocomposite (CS-SA NCs) in grape (Vitis vinifera cv. ‘Sultana’) under salinity stress. Sci. Rep. 2023, 13, 883. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Helaly, M.N.; El-Hoseiny, H.M.; Alam-Eldein, S.M. Zinc Oxide and Silicone Nanoparticles to Improve the Resistance Mechanism and Annual Productivity of Salt-Stressed Mango Trees. Agronomy 2020, 10, 558. [Google Scholar] [CrossRef]
- Abu Zeid, I.M.; Mohamed, F.H.; Metwali, E.M.R. Responses of two strawberry cultivars to NaCl-induced salt stress under the influence of ZnO nanoparticles. Saudi J. Biol. Sci. 2023, 30, 103623. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Abdelrahman, M.; Hosseini, M.S.; Hoveizeh, N.F.; Tran, L.-S.P. Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium nanoparticles. Environ. Pollut. 2019, 253, 246e258. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.; González-Morales, S.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Effects of Chitosan–PVA and Cu Nanoparticles on the Growth and Antioxidant Capacity of Tomato under Saline Stress. Molecules 2018, 23, 178. [Google Scholar] [CrossRef]
- Lu, L.; Huang, M.; Huang, Y.; Corvini, P.F.; Ji, R.; Zhao, L. Mn3O4 nanozymes boost endogenous antioxidant metabolites in cucumber (Cucumis sativus) plant and enhance resistance to salinity stress. Environ. Sci. Nano 2020, 7, 1692–1703. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Tiwari, S.; Lata, C. Heavy Metal Stress, Signaling, and Tolerance Due to Plant-Associated Microbes: An Overview. Front. Plant Sci. 2018, 9, 452. [Google Scholar] [CrossRef]
- Cui, H.; Shi, Y.; Zhou, J.; Chu, H.; Cang, L.; Zhou, D. Effect of different grain sizes of hydroxyapatite on soil heavy metal bioavailability and microbial community composition. Agric. Ecosyst. Environ. 2018, 267, 165–173. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhan, W.; Zheng, K.; Lian, M.; Zhang, C.; Ruan, X.; Li, T. Long-term stabilization of Cd in agricultural soil using mercapto-functionalized nano-silica (MPTS/nano-silica): A three-year field study. Ecotoxicol. Environ. Saf. 2020, 197, 110600. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tito, N.; Giraldo, J.P. Anionic Cerium Oxide Nanoparticles Protect Plant Photosynthesis from Abiotic Stress by Scavenging Reactive Oxygen Species. ACS Nano 2017, 11, 11283–11297. [Google Scholar] [CrossRef] [PubMed]
- Panahirad, S.; Gohari, G.; Mahdavinia, G.; Jafari, H.; Kulak, M.; Fotopoulos, V.; Alcázar, R.; Dadpour, M. Foliar application of chitosan-putrescine nanoparticles (CTS-Put NPs) alleviates cadmium toxicity in grapevine (Vitis vinifera L.) cv. Sultana: Modulation of antioxidant and photosynthetic status. BMC Plant Biol. 2023, 23, 411. [Google Scholar] [CrossRef]
- Fahad, A.B.; Agheem, M.H.; Memon, S.A.; Baloch, A.; Tunio, A.; Abdullah; Pato, A.H.; Jagirani, M.S.; Panah, P.; Gabole, A.A. Efficient mitigation of cadmium and lead toxicity in coriander plant utilizing magnetite (Fe3O4) nanofertilizer as growth regulator and antimicrobial agent. Int. J. Environ. Anal. Chem. 2020, 102, 3868–3879. [Google Scholar] [CrossRef]
- González-Moscoso, M.; Martínez-Villegas, N.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Effect of Silicon Nanoparticles on Tomato Plants Exposed to Two Forms of Inorganic Arsenic. Agronomy 2022, 12, 2366. [Google Scholar] [CrossRef]
- Abou El-Nasr, M.K.; Nasser, M.A.; Ebrahim, M.; Samaan, M.S.F. Alleviating biotic stress of Powdery Mildew in mango cv. Keitt by Sulfur nanoparticles and assessing their effect on productivity and disease severity. Sci. Rep. 2025, 15, 5537. [Google Scholar] [CrossRef]
- Pascoli, M.; Lopes-Oliveira, P.J.; Fraceto, L.F.; Seabra, A.B.; Oliveira, H.C. State of the art of polymeric nanoparticles as carrier systems with agricultural applications: A minireview. Energy Ecol. Environ. 2018, 3, 137–148. [Google Scholar] [CrossRef]
- Paradva, K.C.; Kalla, S. Nanopesticides: A review on current research and future perspective. Chem. Sel. 2023, 8, e202300756. [Google Scholar] [CrossRef]
- Yin, J.; Su, X.; Yan, S.; Shen, J. Multifunctional Nanoparticles and Nanopesticides in Agricultural Application. Nanomaterials 2023, 13, 1255. [Google Scholar] [CrossRef] [PubMed]
- Tortella, G.; Rubilar, O.; Pieretti, J.C.; Fincheira, P.; de Melo Santana, B.; Fernández-Baldo, M.A.; Benavides-Mendoza, A.; Seabra, A.B. Nanoparticles as a Promising Strategy to Mitigate Biotic Stress in Agriculture. Antibiotics 2023, 12, 338. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Ahmad, N.; Dar, M.A.; Manan, S.; Rani, A.; Alghanem, S.M.S.; Khan, K.A.; Sethupathy, S.; Elboughdiri, N.; Mostafa, Y.S.; et al. Nano-Agrochemicals as Substitutes for Pesticides: Prospects and Risks. Plants 2023, 13, 109. [Google Scholar] [CrossRef]
- Hooven, L.A.; Chakrabarti, P.; Harper, B.J.; Sagili, R.R.; Harper, S.L. Potential Risk to Pollinators from Nanotechnology-Based Pesticides. Molecules 2019, 24, 4458. [Google Scholar] [CrossRef]
- Shakiba, S.; Astete, C.E.; Paudel, S.; Sabliov, C.M.; Rodrigues, D.F.; Louie, S.M. Emerging investigator series: Polymeric nanocarriers for agricultural applications: Synthesis, characterization, and environmental and biological interactions. Environ. Sci. Nano 2020, 7, 37–67. [Google Scholar] [CrossRef]
- Khan, M.; Khan, A.U.; Bogdanchikova, N.; Garibo, D. Antibacterial and antifungal studies of biosynthesized silver nanoparticles against plant parasitic nematode Meloidogyne incognita, plant pathogens Ralstonia solanacearum and Fusarium oxysporum. Molecules 2021, 26, 2462. [Google Scholar] [CrossRef]
- Solis Vizcaino, I.; Rubio Rosas, E.; Águila Almanza, E.; Marín Castro, M.; Hernández Cocoletzi, H. Ultrasonic Production of Chitosan Nanoparticles and Their Application Against Colletotrichum gloeosporioides Present in the Ataulfo Mango. Polymers 2024, 16, 3058. [Google Scholar] [CrossRef]
- Haq, I.U.; Cai, X.; Ali, H.; Akhtar, M.R.; Ghafar, M.A.; Hyder, M.; Hou, Y. Interactions Between Nanoparticles and Tomato Plants: Influencing Host Physiology and the Tomato Leafminer’s Molecular Response. Nanomaterials 2024, 14, 1788. [Google Scholar] [CrossRef]
- Lamsal, K.; Kim, S.W.; Jung, J.H.; Kim, Y.S.; Kim, K.S.; Lee, Y.S. Inhibition Effects of Silver Nanoparticles against Powdery Mildews on Cucumber and Pumpkin. Mycobiology 2011, 39, 26–32. [Google Scholar] [CrossRef]
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Soroush, F.; Varma, R.S. Nano/microencapsulation of plant biocontrol agents by chitosan, alginate, and other important biopolymers as a novel strategy for alleviating plant biotic stresses. Int. J. Biol. Macromol. 2022, 222, 1589–1604. [Google Scholar] [CrossRef] [PubMed]
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 2006, 28, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.E.S.; Grillo, R.; Mello, N.F.S.; Rosa, A.H.; Fraceto, L.F. Application of poly (epsilon-caprolactone) nanoparticles containing atrazine herbicide as an alternative technique to control weeds and reduce damage to the environment. J. Hazard. Mater. 2014, 268, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Artusio, F.; Casà, D.; Granetto, M.; Tosco, T.; Pisano, R. Alginate Nanohydrogels as a Biocompatible Platform for the Controlled Release of a Hydrophilic Herbicide. Processes 2021, 9, 1641. [Google Scholar] [CrossRef]
- Ahmed, T.; Shahid, M.; Noman, M.; Niazi, M.B.K.; Mahmood, F.; Manzoor, I.; Zhang, Y.; Li, B.; Yang, Y.; Yan, C.; et al. Silver Nanoparticles Synthesized by Using Bacillus cereus SZT1 Ameliorated the Damage of Bacterial Leaf Blight Pathogen in Rice. Pathogens 2020, 9, 160. [Google Scholar] [CrossRef]
- Satti, S.H.; Raja, N.I.; Ikram, M.; Oraby, H.F.; Mashwani, Z.-U.; Mohamed, A.H.; Singh, A.; Omar, A.A. Plant-Based Titanium Dioxide Nanoparticles Trigger Biochemical and Proteome Modifications in Triticum aestivum L. under Biotic Stress of Puccinia striiformis. Molecules 2022, 27, 4274. [Google Scholar] [CrossRef]
- Consolo, V.F.; Torres-Nicolini, A.; Alvarez, V.A. Mycosinthetized Ag, CuO and ZnO nanoparticles from a promising Trichoderma harzianum strain and their antifungal potential against important phytopathogens. Sci. Rep. 2020, 10, 20499. [Google Scholar] [CrossRef]
- Khan, I.; Raza, M.A.; Awan, S.A.; Khalid, M.H.B.; Raja, N.; Min, S.; Zhang, A.; Naeem, M.; Meraj, T.A.; Iqbal, N.; et al. In vitro effect of metallic silver nanoparticles (AgNPs): A novel approach toward the feasible production of biomass and natural antioxidants in pearl millet (Pennisetum glaucum L.). Appl. Ecol. Environ. Res. 2020, 17, 12877–12892. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A. Modulation of phytoremediation and plant growth by the treatment with PGPR, Ag nanoparticle and untreated municipal wastewater. Int. J. Phytoremediat. 2016, 18, 1258–1269. [Google Scholar] [CrossRef] [PubMed]
- Kanhed, P.; Birla, S.; Gaikwad, S.; Gade, A.; Seabra, A.B.; Rubilar, O.; Duran, N.; Rai, M. In vitro antifungal efficacy of copper nanoparticles against selected crop pathogenic fungi. Mater. Lett. 2014, 115, 13–17. [Google Scholar] [CrossRef]
- Lopez-Lima, D.; Mtz-Enriquez, A.I.; Carrión, G.; Basurto-Cereceda, S.; Pariona, N. The bifunctional role of copper nanoparticles in tomato: Effective treatment for Fusarium wilt and plant growth promoter. Sci. Hortic. 2021, 277, 109810. [Google Scholar] [CrossRef]
- El-Ashry, R.M.; El-Saadony, M.T.; El-Sobki, A.E.A.; El-Tahan, A.M.; Al-Otaibi, S.; El-Shehawi, A.M.; Saad, A.M.; Elshaer, N. Biological silicon nanoparticles maximize the efficiency of nematicides against biotic stress induced by Meloidogyne incognita in eggplant. Saudi J. Biol. Sci. 2022, 29, 920–932. [Google Scholar] [CrossRef]
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted application of silica nanoparticles. A review. Silicon 2020, 12, 1337–1354. [Google Scholar] [CrossRef]
- Bapat, G.; Zinjarde, S.; Tamhane, V. Evaluation of silica nanoparticle mediated delivery of protease inhibitor in tomato plants and its effect on insect pest Helicoverpa armigera. Colloids Surf. B Biointerfaces 2020, 193, 111079. [Google Scholar] [CrossRef]
- Zhang, J.; Kothalawala, S.; Yu, C. Engineered silica nanomaterials in pesticide delivery: Challenges and perspectives. Environ. Pollut. 2023, 320, 121045. [Google Scholar] [CrossRef]
- Zohra, E.; Ikram, M.; Omar, A.A.; Hussain, M.; Satti, S.H.; Raja, N.I.; Mashwani, Z.U.R.; Ehsan, M. Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives. Green Process. Synth 2021, 10, 456–475. [Google Scholar] [CrossRef]
- Hu, D.; Yu, S.; Yu, D.; Liu, N.; Tang, Y.; Fan, Y.; Wang, C.; Wu, A. Biogenic Trichoderma harzianum-derived selenium nanoparticles with control functionalities originating from diverse recognition metabolites against phytopathogens and mycotoxins. Food Control 2019, 106, 106748. [Google Scholar] [CrossRef]
- Luksiene, Z.; Rasiukeviciute, N.; Zudyte, B.; Uselis, N. Innovative approach to sunlight activated biofungicides for strawberry crop protection: ZnO nanoparticles. J. Photochem. Photobiol. B Biol. 2020, 203, 111656. [Google Scholar] [CrossRef]
- Giorgetti, L. Chapter 4—Effects of Nanoparticles in Plants: Phytotoxicity and Genotoxicity Assessment. In Nanomaterials in Plants, Algae and Microorganisms; Academic Press: Cambridge, MA, USA, 2018; Volume 2, pp. 65–87. [Google Scholar] [CrossRef]
- González-García, Y.; Cadenas-Pliego, G.; Alpuche-Solís, Á.; Cabrera, R.; Juárez-Maldonado, A. Carbon Nanotubes Decrease the Negative Impact of Alternaria solani in Tomato Crop. Nanomaterials 2021, 11, 1080. [Google Scholar] [CrossRef]
- Mathur, P.; Roy, S. Nanosilica facilitates silica uptake, growth and stress tolerance in plants. Plant Physiol. Biochem. 2020, 157, 114–127. [Google Scholar] [CrossRef]
- Fan, N.; Zhao, C.; Yue, L.; Ji, H.; Wang, X.; Xiao, Z.; Rasmann, S.; Wang, Z. Nanosilicon alters oxidative stress and defence reactions in plants: A meta-analysis, mechanism and perspective. Environ. Sci. Nano 2022, 9, 3742–3755. [Google Scholar] [CrossRef]
- Satti, S.H.; Raja, N.I.; Javed, B.; Akram, A.; Mashwani, Z.-U.; Ahmad, M.S.; Ikram, M. Titanium dioxide nanoparticles elicited agro-morphological and physicochemical modifications in wheat plants to control Bipolaris sorokiniana. PLoS ONE 2021, 16, e0246880. [Google Scholar] [CrossRef] [PubMed]
- Grasseschi, D.; Silva, W.C.; Souza Paiva, R.; Starke, L.D.; Nascimento, A.S. Surface coordination chemistry of graphene: Understanding the coordination of single transition metal atoms. Coord. Chem. Rev. 2020, 422, 213469. [Google Scholar] [CrossRef]
- Hassanpour, S.; Behnam, B.; Baradaran, B.; Hashemzaei, M.; Oroojalian, F.; Mokhtarzadeh, A.; De la Guardia, M. Carbon based nanomaterials for the detection of narrow therapeutic index pharmaceuticals. Talanta 2021, 221, 121610. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Briso, A.L.; Aachmann, F.L.; Moreno-Manzano, V.; Serrano-Aroca, Á. Graphene oxide nanosheets versus carbon nanofibers: Enhancement of physical and biological properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) films for biomedical applications. Int. J. Biol. Macromol. 2020, 143, 1000–1008. [Google Scholar] [CrossRef]
- Srivastava, N.; Mukhopadhyay, M. Green synthesis and structural characterization of selenium nanoparticles and assessment of their antimicrobial property. Bioprocess Biosyst. Eng. 2015, 38, 1723–1730. [Google Scholar] [CrossRef]
- Huang, B.; Liao, Q.; Fu, H.; Ye, Z.; Mao, Y.; Luo, J.; Wang, Y.; Yuan, H.; Xin, J. Effect of potassium intake on cadmium transporters and root cell wall biosynthesis in sweet potato. Ecotoxicol. Environ. Saf. 2023, 250, 114501. [Google Scholar] [CrossRef]
- Huang, B.; Huang, Y.; Shen, C.; Fan, L.; Fu, H.; Liu, Z.; Sun, Y.; Wu, B.; Zhang, J.; Xin, J. Roles of boron in preventing cadmium uptake by Capsicum annuum root tips: Novel insights from ultrastructural investigation and single-cell RNA sequencing. Sci. Total Environ. 2024, 957, 177858. [Google Scholar] [CrossRef]
- Parkinson, S.J.; Tungsirisurp, S.; Joshi, C.; Richmond, B.L.; Gifford, M.L.; Sikder, A.; Lynch, I.; O’Reilly, R.K.; Napier, R.M. Polymer nanoparticles pass the plant interface. Nat. Commun. 2022, 13, 7385. [Google Scholar] [CrossRef] [PubMed]
- Kurczyńska, E.; Godel-Jędrychowska, K.; Sala, K.; Milewska-Hendel, A. Nanoparticles—Plant Interaction: What We Know, Where We Are? Appl. Sci. 2021, 11, 5473. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Primavera, R.; Wilson, R.J.; Thakor, A.S.; Kevadiya, B.D. Cellular uptake and retention of nanoparticles: Insights on particle properties and interaction with cellular components. Mater. Today Commun. 2020, 25, 101692. [Google Scholar] [CrossRef]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles Alter Secondary Metabolism in Plants via ROS Burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef]
- Javaid, A.; Munir, N.; Abideen, Z.; Duarte, B.; Siddiqui, Z.S.; Haq, R.; Naz, S. The potential effects of nanoparticles in gene regulation and expression in mammalian, bacterial and plant cells-A comprehensive review. Plant Nano Biol. 2025, 11, 100145. [Google Scholar] [CrossRef]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotech. 2020, 2, 10. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Anbazhagan, V.; Dhankher, O.P.; Prasad, P.V.V. Uptake, Translocation, Toxicity, and Impact of Nanoparticles on Plant Physiological Processes. Plants 2024, 13, 3137. [Google Scholar] [CrossRef]
- Samynathan, R.; Venkidasamy, B.; Ramya, K.; Muthuramalingam, P.; Shin, H.; Kumari, P.S.; Thangavel, S.; Sivanesan, I. Recent Update on the Impact of Nano-Selenium on Plant Growth, Metabolism, and Stress Tolerance. Plants 2023, 12, 853. [Google Scholar] [CrossRef]
- Ochoa, L.; Shrivastava, M.; Srivastava, S.; Cota-Ruiz, K.; Zhao, L.; White, J.C.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L. Nanomaterials for managing abiotic and biotic stress in the soil–plant system for sustainable agriculture. Environ. Sci. Nano 2025, 12, 1037–1058. [Google Scholar] [CrossRef]
- Li, P.; Xia, Y.; Song, K.; Liu, D. The Impact of Nanomaterials on Photosynthesis and Antioxidant Mechanisms in Gramineae Plants: Research Progress and Future Prospects. Plants 2024, 13, 984. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Singh, M.; Pandey-Rai, S. Crosstalk of nanoparticles and phytohormones regulate plant growth and metabolism under abiotic and biotic stress. Plant Stress 2022, 6, 100107. [Google Scholar] [CrossRef]
- Shen, M.; Liu, S.; Jiang, C.; Zhang, T.; Chen, W. Recent advances in stimuli-response mechanisms of nano-enabled controlled-release fertilizers and pesticides. Eco-Environ. Health 2023, 2, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Kah, M. Nanopesticides and nanofertilizers: Emerging contaminants or opportunities for risk mitigation? Front. Chem. 2015, 3, 64. [Google Scholar] [CrossRef]
- Iavicoli, I.; Leso, V.; Beezhold, D.H.; Shvedova, A.A. Nanotechnology in agriculture: Opportunities, toxicological implications, and occupational risks. Toxicol. Appl. Pharmacol. 2017, 329, 96–111. [Google Scholar] [CrossRef]
- Kiani, A.K.; Pheby, D.; Henehan, G.; Brown, R.; Sieving, P.; Sykora, P.; Marks, R.; Falsini, B.; Capodicasa, N.; Miertus, S.; et al. Ethical considerations regarding animal experimentation. J. Prev. Med. Hyg. 2022, 63 (Suppl. S3), E255–E266. [Google Scholar] [CrossRef]
- Warheit, D.B.; Brown, S.C.; Donner, E.M. Acute and subchronic oral toxicity studies in rats with nanoscale and pigment grade titanium dioxide particles. Food Chem. Toxicol. 2015, 84, 208–224. [Google Scholar] [CrossRef]
- Grodzicki, W.; Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Wilczak, J.; Oczkowski, M.; Kopiasz, Ł.; Sapierzyński, R.; Kruszewski, M.; Grzelak, A. In Vivo Pro-Inflammatory Effects of Silver Nanoparticles on the Colon Depend on Time and Route of Exposure. Int. J. Mol. Sci. 2024, 25, 4879. [Google Scholar] [CrossRef]
- Michalak, I.; Dziergowska, K.; Alagawany, M.; Farag, M.R.; El-Shall, N.A.; Tuli, H.S.; Emran, T.B.; Dhama, K. The effect of metal-containing nanoparticles on the health, performance and production of livestock animals and poultry. Vet. Q. 2022, 42, 68–94. [Google Scholar] [CrossRef]
- Gatoo, M.A.; Naseem, S.; Arfat, M.Y.; Mahmood Dar, A.; Qasim, K.; Zubair, S. Physicochemical properties of nanomaterials: Implication in associated toxic manifestations. BioMed Res. Int. 2014, 2014, 498420. [Google Scholar] [CrossRef]
- Bąkowski, M.; Kiczorowska, B.; Samolińska, W.; Klebaniuk, R.; Lipiec, A. Silver and zinc nanoparticles in animal nutrition—A review. Ann. Anim. Sci. 2018, 18, 879–898. [Google Scholar] [CrossRef]
- Rezaei-Kelishadi, M.; Ghasemi, A.; Abdolyosefi, N.N.; Zamani-Doabi, S.; Ramezani, M.; Changizi-Ashtiyani, S.; Rahimi, A. Effects of selenium nanoparticles on kidney and liver functional disorders in streptozotocin-induced diabetic rats. Physiol. Pharmacol. 2017, 21, 155–162. Available online: http://ppj.phypha.ir/article-1-1229-en.html (accessed on 21 May 2025).
- Jakic, K.; Selc, M.; Razga, F.; Nemethova, V.; Mazancova, P.; Havel, F.; Sramek, M.; Zarska, M.; Proska, J.; Masanova, V.; et al. Long-Term Accumulation, Biological Effects and Toxicity of BSA-Coated Gold Nanoparticles in the Mouse Liver, Spleen, and Kidneys. Int. J. Nanomed. 2024, 19, 4103–4120. [Google Scholar] [CrossRef] [PubMed]
- Ingle, P.U.; Shende, S.S.; Shingote, P.R.; Mishra, S.S.; Sarda, V.; Wasule, D.L.; Rajput, V.D.; Minkina, T.; Rai, M.; Sushkova, S.; et al. Chitosan nanoparticles (ChNPs): A versatile growth promoter in modern agricultural production. Heliyon 2022, 8, e11893. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, B.; Chitdeshwari, T.; Mohanapriya, G. Fascinating role of nanosilica in mitigating drought and nutrient stress—A review. Plant Stress 2024, 14, 100672. [Google Scholar] [CrossRef]
- Nile, S.H.; Thiruvengadam, M.; Wang, Y.; Samynathan, R.; Shariati, M.A.; Rebezov, M.; Nile, A.; Sun, M.; Venkidasamy, B.; Xiao, J.; et al. Nano-priming as emerging seed priming technology for sustainable agriculture—Recent developments and future perspectives. J. Nanobiotechnol. 2022, 20, 254. [Google Scholar] [CrossRef]
- Ahmad, B.; Shabbir, A.; Jaleel, H.; Khan, M.M.A.; Sadiq, Y. Efficacy of titanium dioxide nanoparticles in modulating photosynthesis, peltate glandular trichomes and essential oil production and quality in Mentha piperita L. Curr. Plant Biol. 2018, 13, 6–15. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Santana, I.; Fahlgren, M.; Giraldo, J.P. Standoff Optical Glucose Sensing in Photosynthetic Organisms by a Quantum Dot Fluorescent Probe. ACS Appl. Mater. Interfaces 2018, 10, 28279–28289. [Google Scholar] [CrossRef]
- Tan, W.; Du, W.; Barrios, A.C.; Armendariz, R.; Zuverza-Mena, N.; Ji, Z.; Chang, C.H.; Zink, J.I.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; et al. Surface coating changes the physiological and biochemical impacts of nano-TiO2 in basil (Ocimum basilicum) plants. Environ. Pollut. 2017, 222, 64–72. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z. Recent advances in nano-enabled agriculture for improving plant performance. Crop J. 2022, 10, 1–12. [Google Scholar] [CrossRef]
- Kobayashi, K.; Wei, J.; Iida, R.; Ijiro, K.; Niikura, K. Surface engineering of nanoparticles for therapeutic applications. Polym. J. 2014, 46, 460–468. [Google Scholar] [CrossRef]
- Gilroy, S.; Białasek, M.; Suzuki, N.; Górecka, M.; Devireddy, A.R.; Karpiński, S.; Mittler, R. ROS, Calcium, and Electric Signals: Key Mediators of Rapid Systemic Signaling in Plants. Plant Physiol. 2016, 171, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.-G.; Toyota, M.; Kim, S.-H.; Gilroy, S. Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Biol. Sci. 2014, 111, 6497–6502. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Shomali, A.; Azad, N.; Hassani, B.; Lastochkina, O.; Li, T. Calcium signaling and salt tolerance are diversely entwined in plants. Plant Signal. Behav. 2019, 14, 1665455. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Nißler, R.; Morris, V.; Herrmann, N.; Hu, P.; Jeon, S.J.; Kruss, S.; Giraldo, J.P. Monitoring Plant Health with Near-Infrared Fluorescent H2O2 Nanosensors. Nano Lett. 2020, 20, 2432–2442. [Google Scholar] [CrossRef] [PubMed]
- Saifullah, M.; Shishir, I.M.R.; Ferdowsi, R.; Rahman, T.M.R.; Van Vuong, Q. Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: A critical review. Trends Food Sci. Technol. 2019, 86, 230–251. [Google Scholar] [CrossRef]
- Ramadan, M.E.; El-Saber, M.M.; Abdelhamid, A.E.; El-Sayed, A.A. Effect of nano chitosan encapsulated spermine on growth, productivity and bioactive compounds of chili pepper (Capsicum annuum L.) under salinity stress. Egypt. J. Chem. 2022, 65, 197–207. [Google Scholar] [CrossRef]
- Gupta, R.; Xie, H. Nanoparticles in Daily Life: Applications, Toxicity and Regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef]
- Egbuna, C.; Parmar, V.K.; Jeevanandam, J.; Ezzat, S.M.; Patrick-Iwuanyanwu, K.C.; Adetunji, C.O.; Khan, J.; Onyeike, E.N.; Uche, C.Z.; Akram, M.; et al. Toxicity of Nanoparticles in Biomedical Application: Nanotoxicology. J. Toxicol. 2021, 30, 9954443. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).