Quality of Wild Passion Fruit at Different Ripening Stages Under Irrigated and Rainfed Cultivation Systems
Abstract
1. Introduction
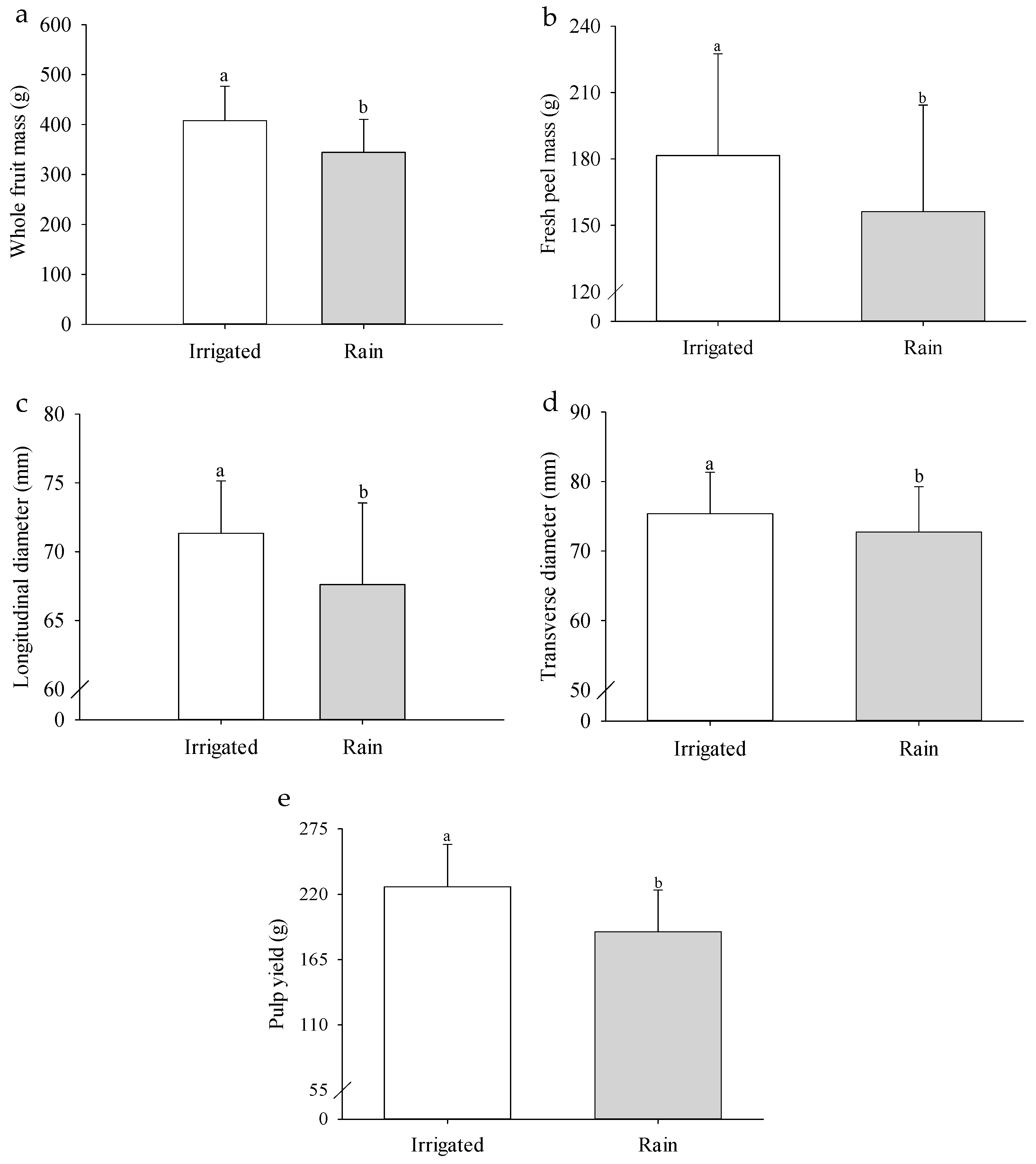
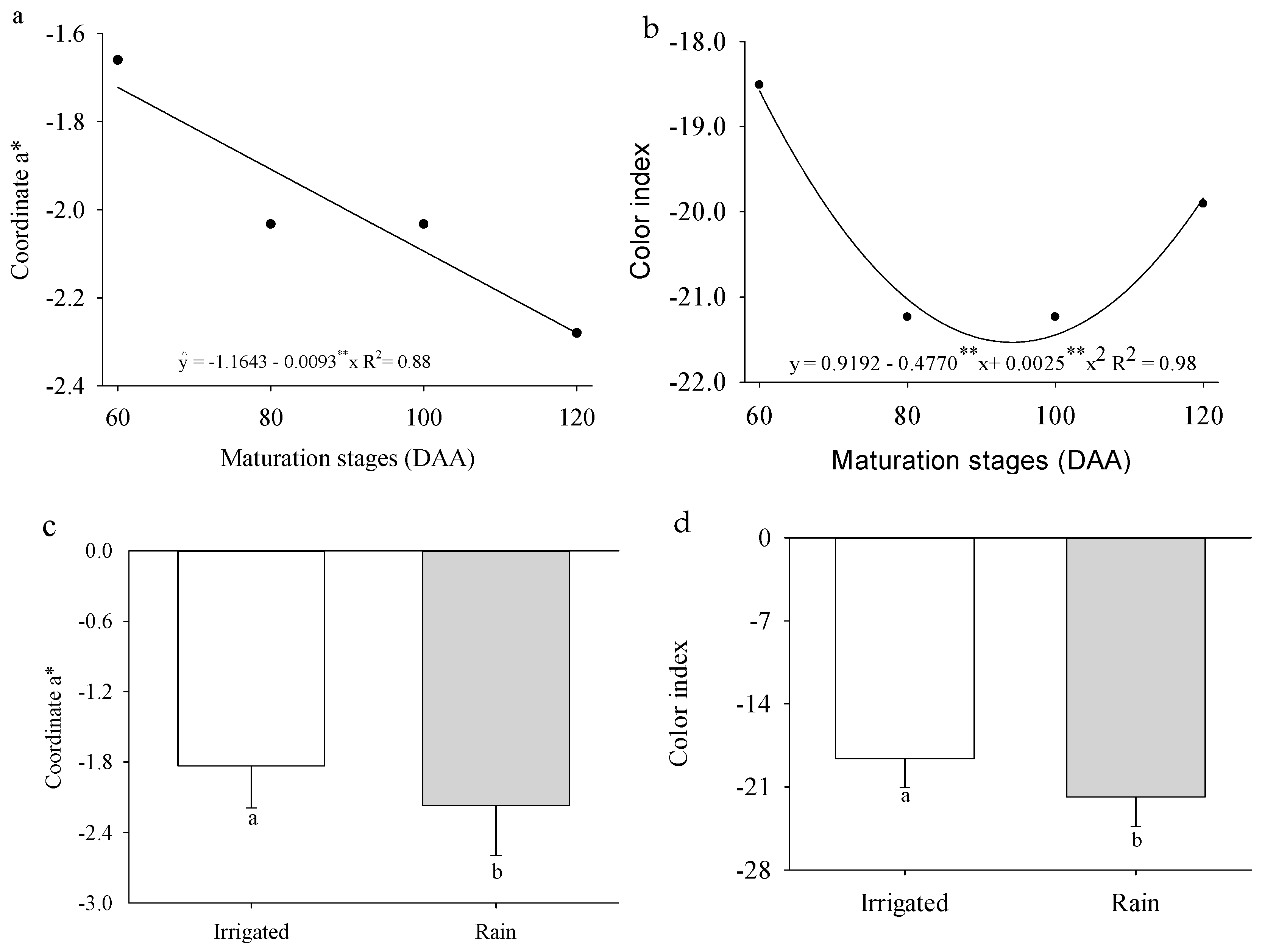
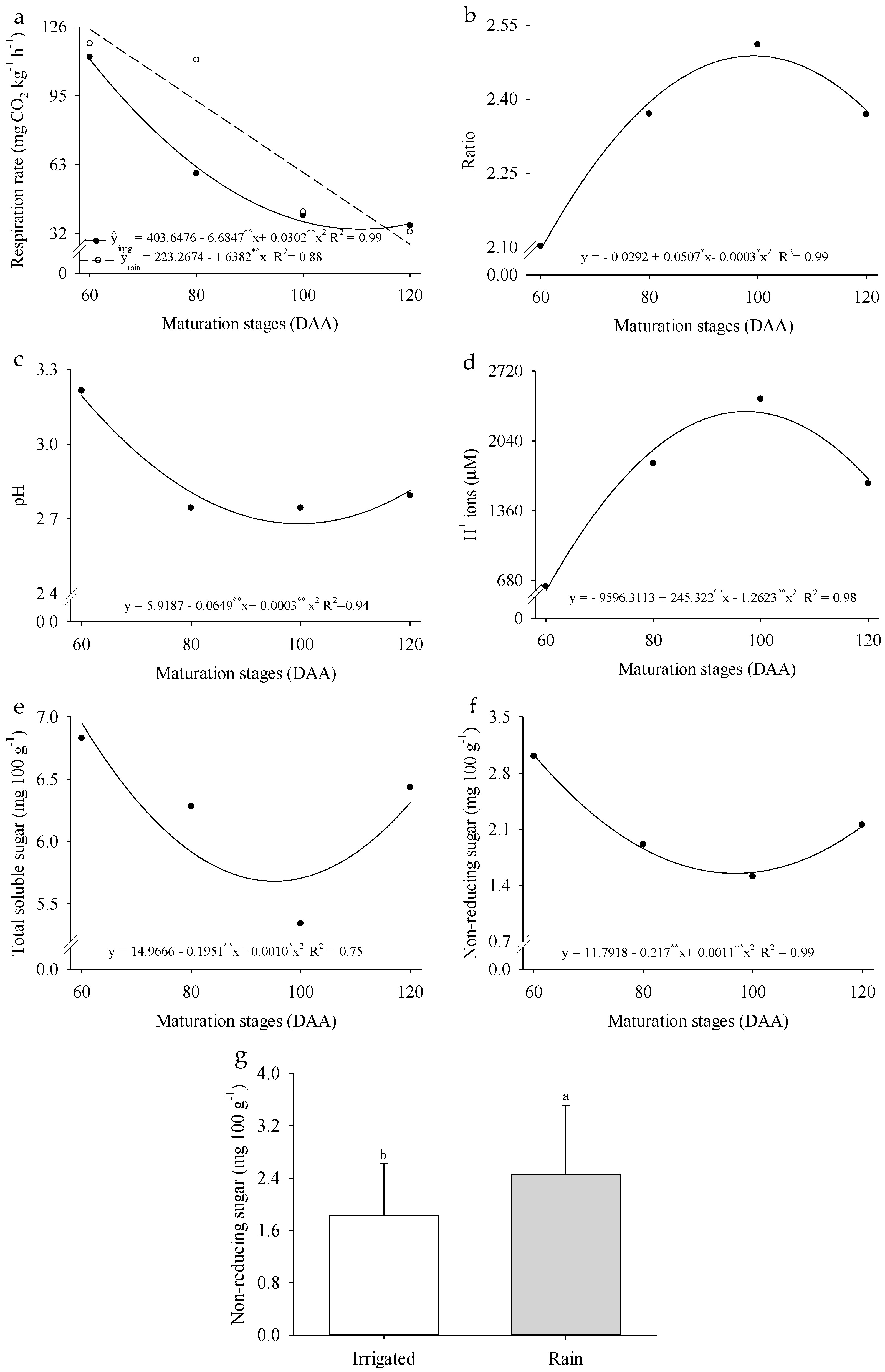
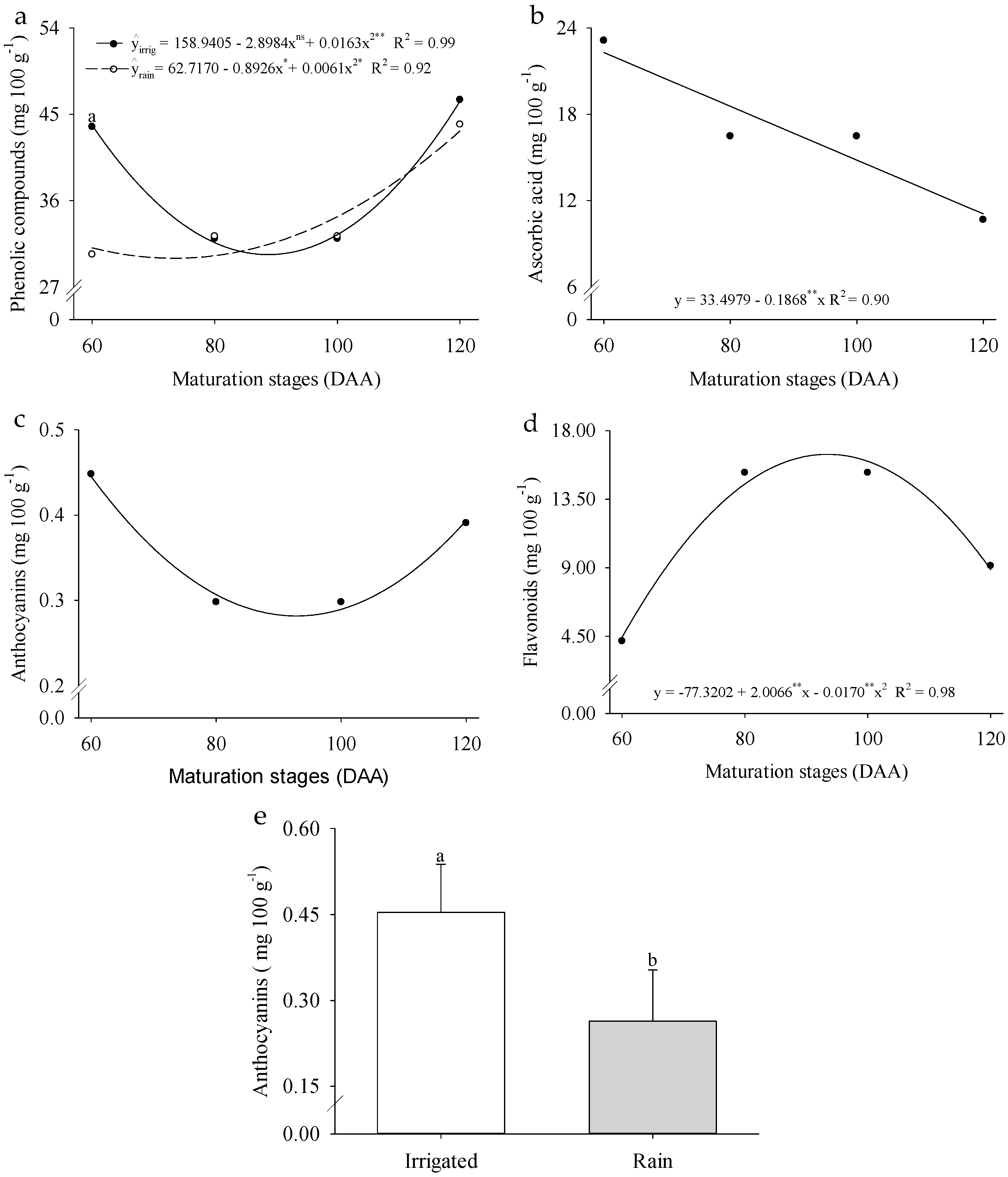
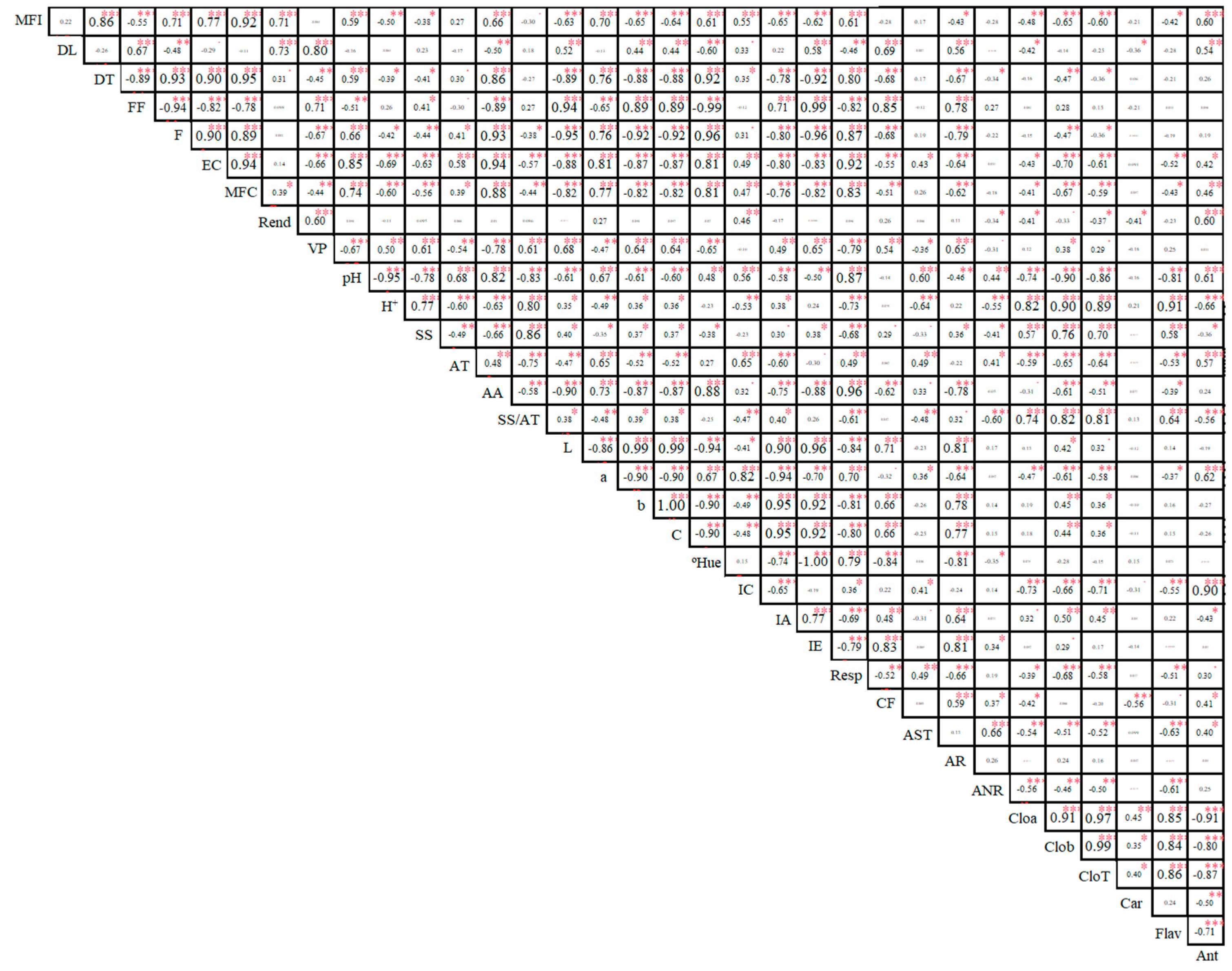
2. Results
3. Discussion
4. Materials and Methods
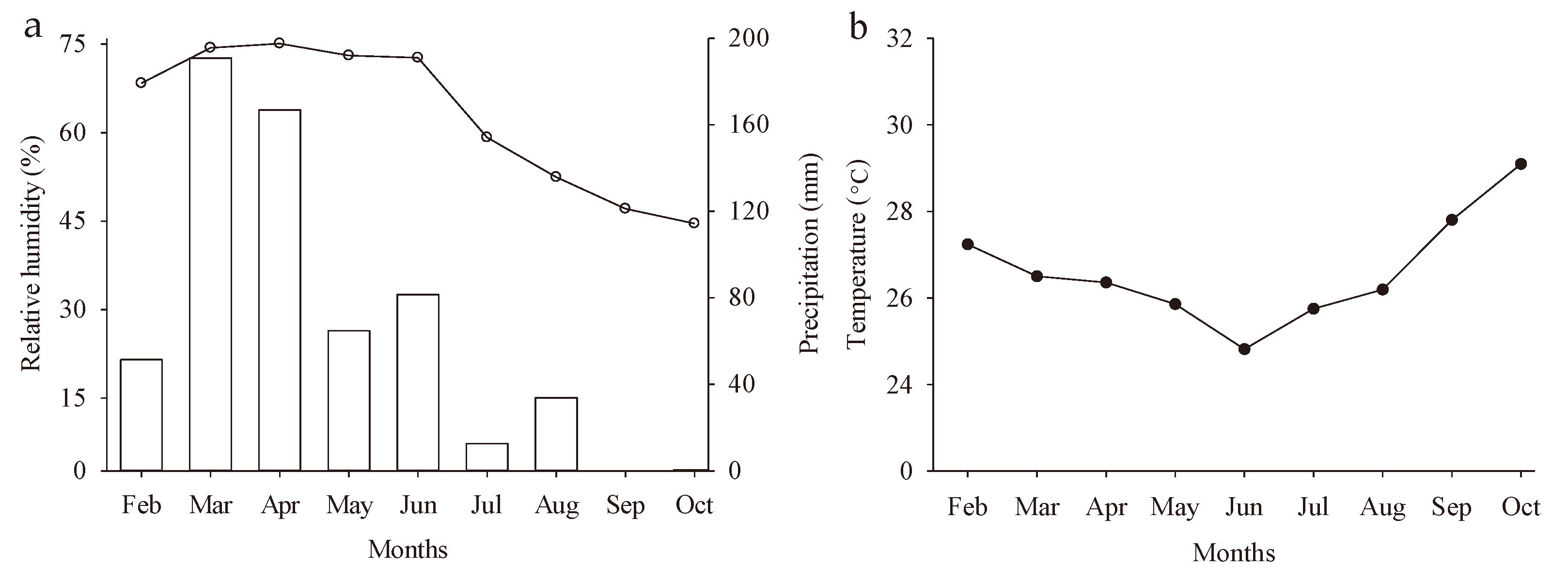
4.1. Experimental Place
4.2. Experimental Structure and Design
4.3. Experimental Procedures
4.4. Variables Analyzed
4.4.1. Respiratory Rate
4.4.2. Physical Analysis of the Fruit
4.4.3. Colorimetry CIE/Lab (L*, a*, and b*)
4.4.4. Physicochemical Analysis of the Pulp
4.4.5. pH and Concentration of H+ ions
4.4.6. Soluble Solids, Titratable Acidity, and SS/TA Ratio
4.4.7. Soluble, Reducing, and Non-Reducing Sugars
4.4.8. Analysis of Bioactive Compounds
Ascorbic Acid
Total Chlorophyll and Carotenoids
Flavonoids and Anthocyanins
Phenolic Compounds
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zárate, V.; Hernández, D.C. Simplified Deep Learning for Accessible Fruit Quality Assessment in Small Agricultural Operations. Appl. Sci. 2024, 14, 8243. [Google Scholar] [CrossRef]
- Souza, P.U.; Lima, L.K.S.; Soares, T.L.; Jesus, O.N.; Coelho Filho, M.A.; Girardi, E.A. Biometric, physiological and anatomical responses of Passiflora spp. to controlled water deficit. Sci. Hortic. 2018, 229, 77–90. [Google Scholar] [CrossRef]
- Mendes, R.M.L.; Andrade, R.H.C.; Marques, M.F.F.; Andrade, E.R. Potential use of the passion fruit from caatinga in kefir. Food Biosci. 2021, 39, 100809. [Google Scholar] [CrossRef]
- EMBRAPA—Empresa Brasileira de Pesquisa Agropecuária. Cultivar de Maracujazeiro Silvestre (Passiflora Cincinnata Mast.) para a Caatinga e para o Cerrado BRS Sertão Forte; Embrapa: Petrolina, Brazil, 2016. [Google Scholar]
- Gonçalves, Z.S.; Lima, L.K.S.; Soares, T.L.; Abreu, E.F.M.; Barbosa, C.J.; Cerqueira-Silva, C.B.M.; Jesus, O.N.; Oliveira, E.J. Identification of Passiflora spp. genotypes resistant to Cowpea aphid-borne mosaic virus and leaf anatomical response under controlled conditions. Sci. Hortic. Biotechnol. 2018, 231, 166–178. [Google Scholar] [CrossRef]
- Moura, R.S.; Soares, T.L.; Lima, L.K.S.; Gheyi, H.R.; Dias, E.A.; Jesus, O.N.; Coelho Filho, M.A. Effects of salinity on growth, physiological and anatomical traits of Passiflora species propagated from seeds and cuttings. Braz. J. Bot. 2021, 44, 17–32. [Google Scholar] [CrossRef]
- Francisco, W.D.M.; Araújo Neto, S.E.D.; Uchôa, T.L.; Souza, L.G.S.; Silva, N.M. Productivity and quality of irrigated organic yellow passion fruits in deep planting in Southeastern Amazon. Rev. Bras. Frutic. 2020, 42, e-584. [Google Scholar] [CrossRef]
- Yang, C.; Wang, H.; Chen, J.; Zhang, Y.; Huang, J.; Chen, J. The key metabolite of fruit flavor change in different ripening stages of Baccaure ramiflora. Food Chem. 2024, 24, 101894. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Gao, J.; Sui, X.; Weng, T.; Kong, A. Effect of fruit ripeness on electrical impedance spectrum parameters. LWT Food Sci. Technol. 2024, 208, 116751. [Google Scholar] [CrossRef]
- Quamruzzaman, A.K.M.; Islam, F.; Akter, L.; Mallick, S.R. Effect of maturity indices on growth and quality of high value vegetables. Am. J. Plant Sci. 2022, 13, 1042–1062. [Google Scholar] [CrossRef]
- Rizzo, M.; Marcuzzo, M.; Zangari, A.; Gasparetto, A.; Albarelli, A. Fruit ripeness classification: A survey. Artif. Intell. Agric. 2023, 7, 44–57. [Google Scholar] [CrossRef]
- Gunny, A.A.N.; Gopinath, S.C.; Ali, A.; Wongs-Aree, C.; Salleh, N.H.M. Challenges of postharvest water loss in fruits: Mechanisms, influencing factors, and effective control strategies—A comprehensive review. J. Agric. Food Res. 2024, 17, 101249. [Google Scholar]
- Li, S.; Zhao, Y.; Wu, P.; Grierson, D.; Gao, L. Ripening and rot: How ripening processes influence disease susceptibility in fleshy fruits. J. Integr. Plant Biol. 2024, 66, 1831–1863. [Google Scholar] [CrossRef]
- D’Abadia, A.C.A.; Costa, A.M.; Faleiro, F.G.; Rinaldi, M.M.; Oliveira, L.L.; Malaquias, J.V. Determination of the maturations stage and characteristics of the fruits of two populations of Passiflora cincinnata Mast. Rev. Caatinga 2020, 33, 349–360. [Google Scholar] [CrossRef]
- Veras, M.C.M.; Pinto, A.C.Q.; Meneses, J.B. Influência da época de produção e dos estádios de maturação nos maracujás doce e ácido nas condições de cerrado. Pesqui. Agropecu. Bras. 2000, 35, 959–966. [Google Scholar] [CrossRef]
- Ritzinger, R.; Manica, I.; Riboldi, J. Efeito do espaçamento e da época de colheita sobre a qualidade do maracujá amarelo. Pesqui. Agropecuária Bras. 1989, 24, 241–245. [Google Scholar]
- Silva, T.V.; Resende, E.D.; Viana, A.P.; Pereira, S.M.F.; Carlos, L.A.; Vitorazi, L. Determinação da escala de coloração da casca e do rendimento em suco do maracujá-amarelo em diferentes épocas de colheita. Rev. Bras. De Frutic. 2008, 30, 880–884. [Google Scholar] [CrossRef]
- Wagg, C.; Hann, S.; Kupriyanovich, Y.; Li, S. Timing of short period water stress determines potato plant growth, yield and tuber quality. Agric. Water Manag. 2021, 247, 106731. [Google Scholar] [CrossRef]
- Ghannem, A.; Ben Aissa, I.; Majdoub, R. Effects of regulated deficit irrigation applied at different growth stages of greenhouse grown tomato on substrate moisture, yield, fruit quality, and physiological traits. Environ. Sci. Pollut. Res. 2021, 28, 46553–46564. [Google Scholar] [CrossRef]
- Vaz, A.F.S.; Martelleto, L.A.P.; Antunes, L.F.S.; Rosa, R.C.C.; Andrade, G.S.; Carvalho, D.F. Desempenho produtivo e qualidade dos frutos do maracujazeiro cultivado em manejo orgânico sob mulching e sistema automatizado de irrigação. Res. Soc. Dev. 2022, 11, 1–14. [Google Scholar] [CrossRef]
- Dubois, M.; Inzé, D. Plant growth under suboptimal water conditions: Early responses and methods to study them. J. Exp. Bot. 2020, 71, 1706–1722. [Google Scholar] [CrossRef]
- Jeena, G.S.; Phukan, U.J.; Shukla, R.K. Drought-Tolerant Plants. In Current Developments in Biotechnology and Bioengineering: Crop Modification, Nutrition, and Food Production; Elsevier: Amsterdam, The Netherlands, 2017; pp. 101–123. [Google Scholar]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 22, 151. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, J.; Urban, L.; Staudt, M.; Lopez-Lauri, F.; Bidel, L.P.R.; Bertin, N. Water shortage and quality of fleshy fruits—Making the most of the unavoidable. J. Exp. Bot. 2014, 65, 4097–4117. [Google Scholar] [CrossRef]
- Medyouni, I.; Zouaoui, R.; Rubio, E.; Serino, S.; Ahmed, H.B.; Bertin, N. Effects of water deficit on leaves and fruit quality during the development period in tomato plant. Food Sci. Nutr. 2021, 9, 1949–1960. [Google Scholar] [CrossRef]
- Mansinhos, I.; Gonçalves, S.; Romano, A. How climate change-related abiotic factors affect the production of industrial valuable compounds in Lamiaceae plant species: A review. Front. Plant Sci. 2024, 15, 1370810. [Google Scholar] [CrossRef] [PubMed]
- D’Abadia, A.C.A.; Costa, A.M.; Faleiro, F.G.; Malaquias, J.V.; Araújo, F.P. Yield and physical characterization of Passiflora cincinnata in the Brazilian Savanna. Pesqui. Agropecu. Trop. 2021, 51, e65795. [Google Scholar] [CrossRef]
- Enamorado, H.E.P.; Finger, F.L.; Barros, R.S.; Puschmann, R. Development and ripening of yellow passion fruit. J. Hortic. Sci. 1995, 70, 573–576. [Google Scholar] [CrossRef]
- Pongener, A.; Sagar, V.; Pal, R.K.; Asrey, R.; Sharma, R.R.; Singh, S.K. Physiological and quality changes during postharvest ripening of purple passion fruit (Passiflora edulis Sims). Fruits 2014, 69, 19–30. [Google Scholar] [CrossRef]
- Tessmer, M.A.; Kluge, R.A.; Appezzato-Daglória, B. The accumulation of tannins during the development of “Giombo” and “Fuyu” persimmon fruits. Sci. Hortic. 2014, 172, 292–299. [Google Scholar] [CrossRef]
- Martinez, H.E.; Bohorquez, C.A.A.; Cecon, P.R. Efficiency of absorption, translocation, and use of nitrogen by water-stressed coffee. Acta Scientiarum. Agron. 2024, 46, e62923. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Fisiologia Vegetal; Artmed: Porto Alegre, Brazil, 2017. [Google Scholar]
- Li, Y.; He, H.; Hou, Y.; Kelimu, A.; Wu, F.; Zhao, Y.; Shi, L.; Zhu, X. Salicylic acid treatment delays apricot (Prunus armeniaca L.) fruit softening by inhibiting ethylene biosynthesis and cell wall degradation. Sci. Hortic. 2022, 300, 111061. [Google Scholar] [CrossRef]
- Shiga, T.M.; Fabi, J.P.; Nascimento, J.R.O.; Petkowicz, C.L.D.; Vriesmann, L.C.; Lajolo, F.M. Changes in cell wall composition associated to the softening of ripening papaya: Evidence of extensive solubilization of large molecular mass galactouronides. J. Agric. Food Chem. 2009, 57, 7064–7071. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, B.; Ma, Y.; Xu, W.; Wu, H.; Wang, S. Carotenoid accumulation and expression of carotenoid biosynthesis genes in mango flesh during fruit development and ripening. Sci. Hortic. 2018, 237, 201–206. [Google Scholar] [CrossRef]
- Conesa, A.; Manera, F.C.; Brotons, J.M.; Fernandez-Zapata, J.C.; Simon, I.; Simon-Grao, S.; Alfosea-Simon, M.; Martínez Nicolas, J.J.; Valverde, J.M.; García-Sanchez, F. Changes in the content of chlorophylls and carotenoids in the rind of Fino 49 lemons during maturation and their relationship with parameters from the CIELAB color space. Sci. Hortic. 2019, 243, 252–260. [Google Scholar] [CrossRef]
- Song, Z.; Xu, X.; Chen, X.; Chang, J.; Li, J.; Cheng, J.; Zhang, B. Multi-omics analysis provides insights into the mechanism underlying fruit color formation in Capsicum. Front. Plant Sci. 2024, 15, 1448060. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, R.; Abbasi, N.A.; Ahmad, I.; Khalid, A. Impacto f climate variables on fruit internal quality of kinnow mandarin (Citrus nobilis Lour x Citrus deliciosa Tenora) in ripening phase grown under varying environmental conditions. Sci. Hortic. 2020, 265, 109235. [Google Scholar] [CrossRef]
- Espley, R.V.; Jaakola, L. The role of environmental stress in fruit pigmentation. Plant Cell Environ. 2023, 46, 3663–3679. [Google Scholar] [CrossRef]
- Sun, C.; Aernouts, B.; Saeys, W. Effects of harvest time, fruit size and cultivar on the bulk optical properties of Satsuma mandarin. Postharvest Biol. Technol. 2020, 175, 111412. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Alquézar, B.; Alós, E.; Lado, J.; Zacarías, L. Bicochemical bases and molecular regulation of pigmentartion in the peel of citrus fruti. Sci. Hortic. 2013, 163, 46–62. [Google Scholar] [CrossRef]
- Rinaldi, M.M.; Dianese, A.C.; Costa, A.M. Avaliação do uso de cera de carnaúba na conservação pós-colheita de frutos de Passiflora cincinnata cv BRS Sertão Forte. Agrotópica 2021, 33, 29–38. [Google Scholar] [CrossRef]
- You, M.; Duan, X.Y.; Li, X.; Luo, L.; Zhao, Y.; Huahong, P.; Gong, W.; Yang, L.R.; Xiang, Z.; Li, G. Effect of 1-methylcyclopropene combined whit chitosan-coated film on storage quality of passion fruit. Sustain. Chem. Pharm. 2022, 27, 100679. [Google Scholar] [CrossRef]
- Tokatli, K.; Demirdöven, A. Effects of chitosan edible film coatings on the physicochemical and microbiological qualities of sweet cherry (Prunus avium L.). Sci. Hortic. 2020, 259, 108656. [Google Scholar] [CrossRef]
- Lado, J.; Gambetta, G.; Zacarias, L. Key determinants of citrus fruit quality: Metabolites and main changes during maturation. Sci. Hortic. 2018, 233, 238–248. [Google Scholar] [CrossRef]
- Menzel, C.M. Effect of temperature on soluble solids content in strawberry in Queensland, Australia. Horticulturae 2022, 8, 367. [Google Scholar] [CrossRef]
- Cavichioli, J.C.; Ruggiero, C.; Volpe, C.A. Caracterização físico-química de frutos de Maracujazeiro-amarelo submetidos a iluminação artificial, irrigação e sombreamento. Rev. Bras. Frutic. 2008, 30, 649–656. [Google Scholar] [CrossRef]
- Rasmussen, G.K.; Peynado, A.; Hilgeman, R. The organic acid content of Valencia oranges from four location in the United States. J. Am. Soc. Hortic. Sci. 1966, 206–210. [Google Scholar]
- Chitarra, I.M.F.; Chitarra, A.B. Pós-Colheita de Frutas e Hortaliças: Fisiologia e Manuseio, 2nd ed.; UFLA: Lavras, Brazil, 2005. [Google Scholar]
- Santos, J.L.V.; Resende, E.D.; Martins, D.R.; Gravina, G.A.; Cenci, S.A.; Maldonado, J.F.M. Determinação do ponto de colheita de diferentes cultivares de maracujá. Rev. Bras. Eng. Agric. Ambient. 2013, 17, 750–755. [Google Scholar] [CrossRef][Green Version]
- Costa, C.A.R.; Machado, G.G.L.; Rodrigues, L.J.; Barros, H.E.A.; Natarelli, C.V.; Barros, E.V. Phenolic compounds profile and antioxidante activity of purple passion fruit’s pulp, peel and seed at diferente maturation stages. Sci. Hortic. 2023, 231, 112244. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and disease: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar]
- Erol, Ü.H. Pepper fruits at different ripening periods have potential phyto-biochemical and enzymatic responses to irrigation levels. J. Food Qual. 2024, 2024, 9082436. [Google Scholar] [CrossRef]
- Rotta, E.M.; Rodrigues, C.A.; Jardim, I.C.S.F.; Maldaner, L.; Visentainer, J.V. Determination of phenolic compounds and antioxidante activity in passion fruit pulp (Passiflora spp.) using a modified QuEChERS method and UHPLC-MS/MS. LWT Food Sci. Technol. 2018, 100, 397–403. [Google Scholar] [CrossRef]
- Emmanouilidou, M.G.; Kyriacou, M.C. Rootstock-modulated yield performance, fruit maturation and phytochemical quality of ‘Lane Late’ and ‘Delta’ sweet Orange. Sci. Hortic. 2017, 225, 112–121. [Google Scholar] [CrossRef]
- Santos, R.T.S.; Biasoto, A.C.T.; Rybka, A.C.P.; Castro, C.D.P.C.; Aidar, S.T.; Borges, G.S.C.; Silva, F.L.H. Physicochemical characterization, bioactive compounds, in vitro antioxidante activity, sensory profile and consumer acceptability of fermented alcoholic beverage obtained from Caatinga passion fruit (Passiflora cincinnata Mast.). LTW Food Sci. Technol. 2021, 148, 111714. [Google Scholar]
- Ammar, I.; Ennouri, M.; Khemakhem, B.; Yangui, T.; Attia, H. Variation in chemical composition and biological activities of two species of Opuntia flowers at four stage of flowering. Ind. Crops Prod. 2012, 37, 34–40. [Google Scholar] [CrossRef]
- Kuskoski, E.M.; Asuero, A.G.; Morales, M.T.; Fett, R. Frutos tropicais silvestres e polpas de frutas congeladas: Atividade antioxidante, polifenóis e antocianinas. Ciênc. Rural 2006, 36, 1283–1287. [Google Scholar] [CrossRef]
- Macrae, E.; Quick, W.P.; Benker, C.; Stitt, M. Carhydrate metabolism during postharvest ripening in kiwi-fruit. Planta 1992, 188, 314–323. [Google Scholar] [CrossRef]
- Paula, J.T.; Resende, J.T.V.; Farias, M.V.; Figueiredo, A.S.T.; Schwarz, K.; Neumann, E.R. Características físico-químicas e compostos bioativos em frutos de tomateiro colhidos em diferentes estádios de maturação. Hortic. Bras. 2015, 33, 434–440. [Google Scholar] [CrossRef]
- Rotili, M.C.C.; Coutro, S.; Celant, V.M.; Vorpagel, J.A.; Barp, F.K.; Salibe, A.B.; Braga, G.C. Composição, atividade antioxidante e qualidade do maracujá amarelo durante armazenamento. Semin. Cienc. Agrár. 2013, 34, 227–240. [Google Scholar] [CrossRef]
- Do, D.T.; Harbourne, N.; Ellis, A. Anthocyanins: Anthocyanidins, berries, colorants, and copigmentation. In Handbook of Food Bioactive Ingredients: Properties and Applications; Springer International Publishing: Cham, Switzerland, 2023; pp. 341–364. [Google Scholar]
- Liu, Y.; Liu, J.; Tang, C.; Uyanga, V.A.; Xu, L.; Zhang, F.; Chen, Y. Flavonoids targeted metabolomic analysis following rice yellowing. Food Chem. 2024, 430, 136984. [Google Scholar] [CrossRef]
- Sun, T.; Wang, P.; Rao, S.; Zhou, X.; Wrightstone, E.; Lu, S.; Yuang, H.; Yang, Y.; Fish, T.; Thannhauser, T.; et al. Co-chaperoning of chlorophyll and carotenoid biosynthesis by ORANGE family proteins in plants. Mol. Plant 2023, 16, 1048–1065. [Google Scholar] [CrossRef]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Marwal, A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Josse, E.M.; Simkin, A.J.; Gaffé, J.; Labouré, A.M.; Kuntz, M.; Carol, P. A plastid terminal oxidase associated with carotenoid desaturation during chromoplast differentiation. Plant Physiol. 2000, 123, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Rissler, H.M.; Pogson, B.J. Antisense inhibition of the beta-carotene hydroxylase enzyme in Arabidopsis and implications for carotenoid accumulation, photo-protection and antena assembly. Photosynth. Res. 2001, 67, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; Moreau, H.; Kuntz, M.; Pagny, G.; Lin, C.; Tanksley, S.; McCarthy, J. An investigation of carotenoid biosynthesis in coffea canephora and coffea arábica. J. Plant Physiol. 2008, 165, 1087–1106. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Du, T. Coupled mechanisms of water deficit and soil salinity affecting tomato fruit growth. Agric. Water Manag. 2024, 295, 108747. [Google Scholar] [CrossRef]
- Rosa, C.I.L.F.; Moribe, A.M.; Yamamot, L.Y.; Sperandio, D. Pós-colheita e comercialização. In Hortaliças-Fruto; Eduem: Maringá, Brazil, 2018; pp. 489–526. [Google Scholar]
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing climate-resilient crops: Improving plant tolerance to stress combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef]
- Köppen, W.; Geiger, R. Klimate der Erde; Verlag Justus Perthes: Gotha, Germany, 1928. [Google Scholar]
- Sousa, V.F.; Borges, A.L. Irrigação e fertirrigação na cultura do maracujá. In Irrigação e Fertirrigação em Frutíferas e Hortaliças; Sousa, V.F., Marouelli, W.A., Coelho, E.F., Pinto, J.M., Coelho Filho, M.A., Eds.; Embrapa Informação Tecnológica: Brasília, Brazil, 2011; pp. 499–522. [Google Scholar]
- Crispim, J.E.; Martins, J.C.; Pires, J.C.; Rosolem, C.A. Determinação da taxa de respiração em sementes de soja pelo método da titulação. Pesqui. Agropecu. Bras. 1994, 29, 1517–1521. [Google Scholar]
- Costa, F.B.; Pereira, M.M.D.; Silva, J.L.; Nascimento, A.M.; Silva, B.R.S.; Sales, G.N.B. Determinação da atividade respiratória (CO2) em frutos de Juazeiro colhidos em cinco estádios de maturação. Rev. Principia 2020, 1, 202–208. [Google Scholar] [CrossRef]
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Agricultural Chemists, 18th ed.; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Ferreira, M.D.; Spricigo, P.C. Parte 4. Análises não destrutivas: Calorimetria—Princípios e aplicações na agricultura. Embrapa Instrumentação 2017, 4, 209–220. [Google Scholar]
- IAL—Instituto Adolfo Lutz. Normas Analíticas do Instituto Adolfo Lutz, 4th ed.; IAL: São Paulo, Brazil, 2008; pp. 103–104. [Google Scholar]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in planta extracts by anthrone. J. Biochem. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Strohecker, R.; Henning, H.M. Analisis de Vitaminas: Métodos Comprobados; Paz Montalvo: Madrid, Spain, 1967; p. 428. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Packer, L., Douce, R., Eds.; Academic Press: London, UK, 1987; pp. 426–428. [Google Scholar]
- Francis, F.J. Analysis of anthocyanins in foods. In Anthocyanins as Food Colors; Markakis, P., Ed.; Academic Press: New York, NY, USA, 1982; pp. 181–207. [Google Scholar]
- Waterhouse, A. Folin-ciocalteau micro method for total phenol in wine. Am. J. Enol. Vitic. 2006, 3–5. [Google Scholar]
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A. ExpDes: Experimental Designs Package; Version 1.2.1; CRAN—R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Version 4.1.0; R Foundation for Statistical Computing: Viena, Austria, 2021. [Google Scholar]
- Peterson, B.G.; Carl, C. Performance Analytics: Econometric Tools for Performance and Risk Analysis; R Package Version 2.0.4; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]





| Attributes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Depth | pH | P | K+ | Na+ | H+ + Al3+ | Al3+ | Ca2+ | Mg2+ | SB | CEC | V | OM |
| (cm) | H2O | -- mg dm−3-- | ------------------------------ cmol dm−3 ------------------------ | % | g dm−3 | |||||||
| 00–20 cm | 8.94 | 46.18 | 7.89 | 6.52 | 0.00 | 0.00 | 15.25 | 24.55 | 53.91 | 53.91 | 100 | 1.74 |
| 20–40 cm | 6.78 | 13.77 | 0.37 | 0.91 | 0.48 | 0.00 | 7.41 | 2.44 | 11.13 | 11.61 | 95.86 | 1.74 |
| Manure | 6.71 | 16.87 | 0.51 | 0.74 | 0.40 | 0.00 | 6.18 | 1.92 | 9.35 | 9.75 | 95.89 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sales, G.N.B.; Rodrigues, M.H.B.S.; da Silva, T.I.; Lacerda, R.R.d.A.; Medeiros, B.L.; Macedo, L.F.; Dias, T.J.; Pereira, W.E.; Faleiro, F.G.; de Sousa Queiroga Lacerda, I.; et al. Quality of Wild Passion Fruit at Different Ripening Stages Under Irrigated and Rainfed Cultivation Systems. Plants 2025, 14, 2147. https://doi.org/10.3390/plants14142147
Sales GNB, Rodrigues MHBS, da Silva TI, Lacerda RRdA, Medeiros BL, Macedo LF, Dias TJ, Pereira WE, Faleiro FG, de Sousa Queiroga Lacerda I, et al. Quality of Wild Passion Fruit at Different Ripening Stages Under Irrigated and Rainfed Cultivation Systems. Plants. 2025; 14(14):2147. https://doi.org/10.3390/plants14142147
Chicago/Turabian StyleSales, Giuliana Naiara Barros, Marília Hortência Batista Silva Rodrigues, Toshik Iarley da Silva, Rodolfo Rodrigo de Almeida Lacerda, Brencarla Lima Medeiros, Larissa Felix Macedo, Thiago Jardelino Dias, Walter Esfrain Pereira, Fabio Gelape Faleiro, Ivislanne de Sousa Queiroga Lacerda, and et al. 2025. "Quality of Wild Passion Fruit at Different Ripening Stages Under Irrigated and Rainfed Cultivation Systems" Plants 14, no. 14: 2147. https://doi.org/10.3390/plants14142147
APA StyleSales, G. N. B., Rodrigues, M. H. B. S., da Silva, T. I., Lacerda, R. R. d. A., Medeiros, B. L., Macedo, L. F., Dias, T. J., Pereira, W. E., Faleiro, F. G., de Sousa Queiroga Lacerda, I., & da Costa, F. B. (2025). Quality of Wild Passion Fruit at Different Ripening Stages Under Irrigated and Rainfed Cultivation Systems. Plants, 14(14), 2147. https://doi.org/10.3390/plants14142147






