Brassinosteroid-Mediated Resistance to Cobalt-Induced Toxicity by Regulating Hormonal Balance, Cellular Metabolism, and Antioxidant Defense in Maize
Abstract
1. Introduction
2. Results and Discussions
2.1. Effect of BRs on Plant Growth and Co and BR Accumulation
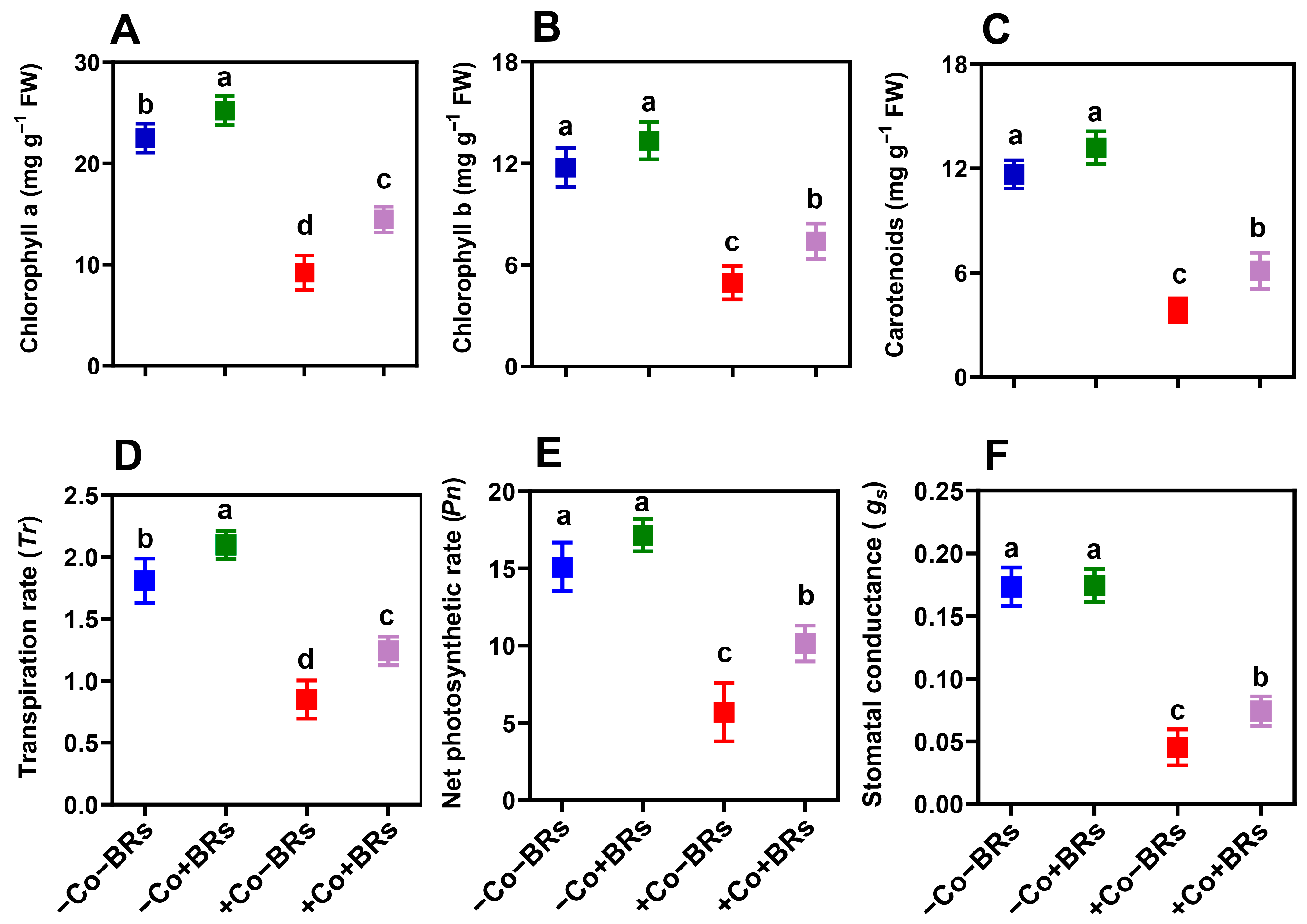
2.2. Effect of BRs on Light Harvesting and Gas Exchange Attributes
2.3. Effect of BRs on Endogenous Phytohormones
2.4. Effect of BRs on Polyphenol, Total Protein, and Soluble Sugar
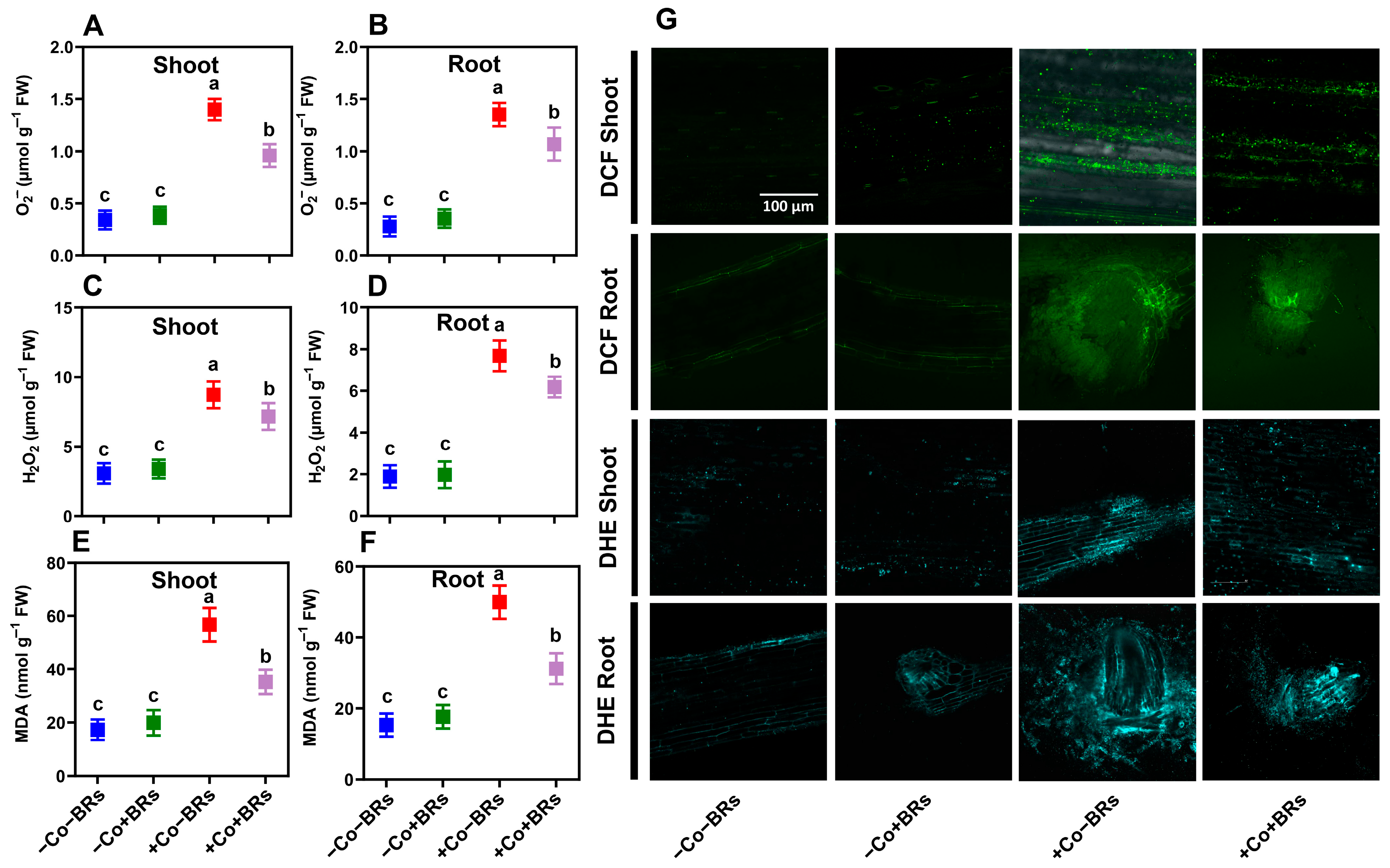
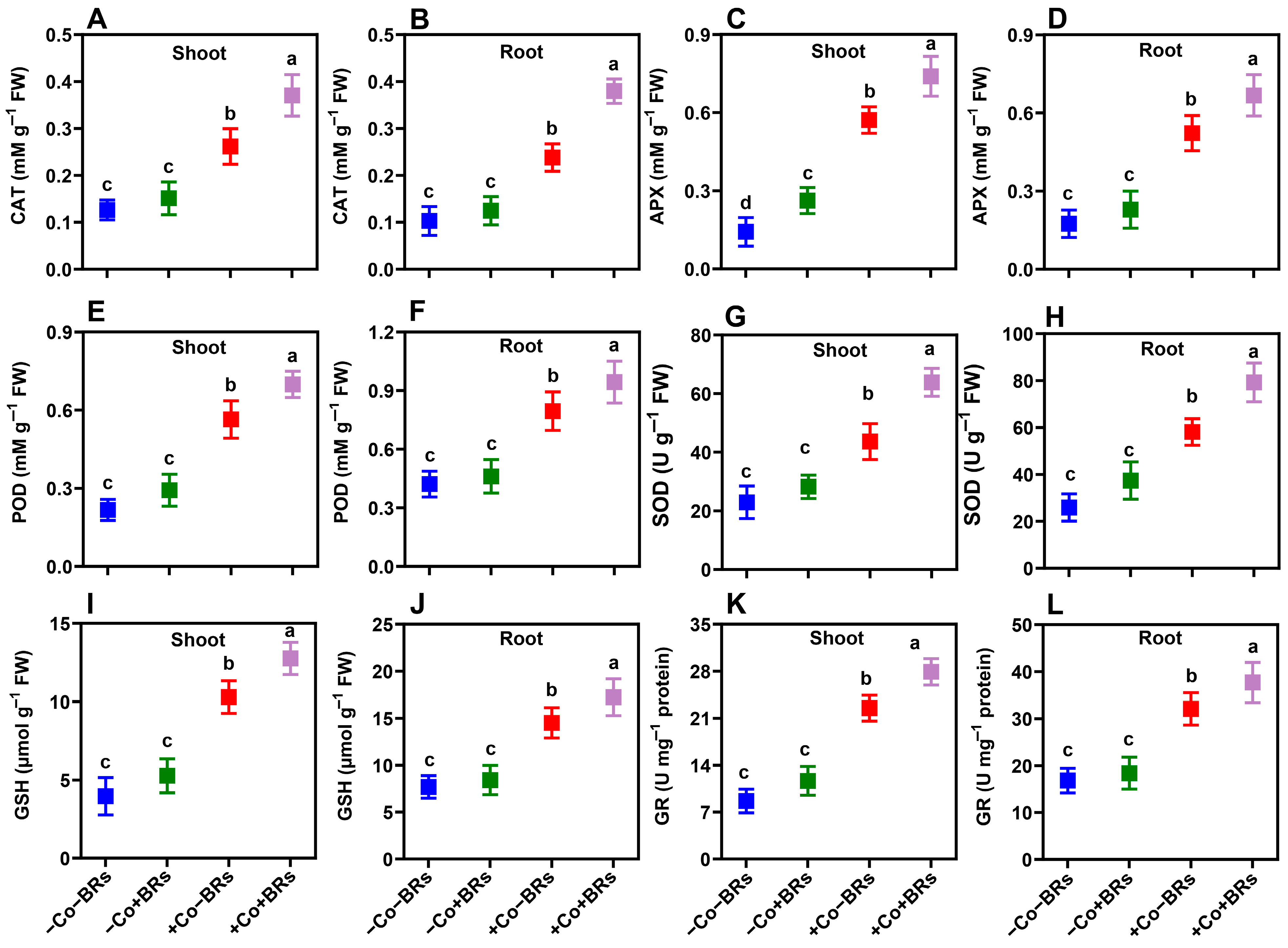
2.5. Effect of BRs on Oxidative Stress Markers and Antioxidant Defense Enzymes
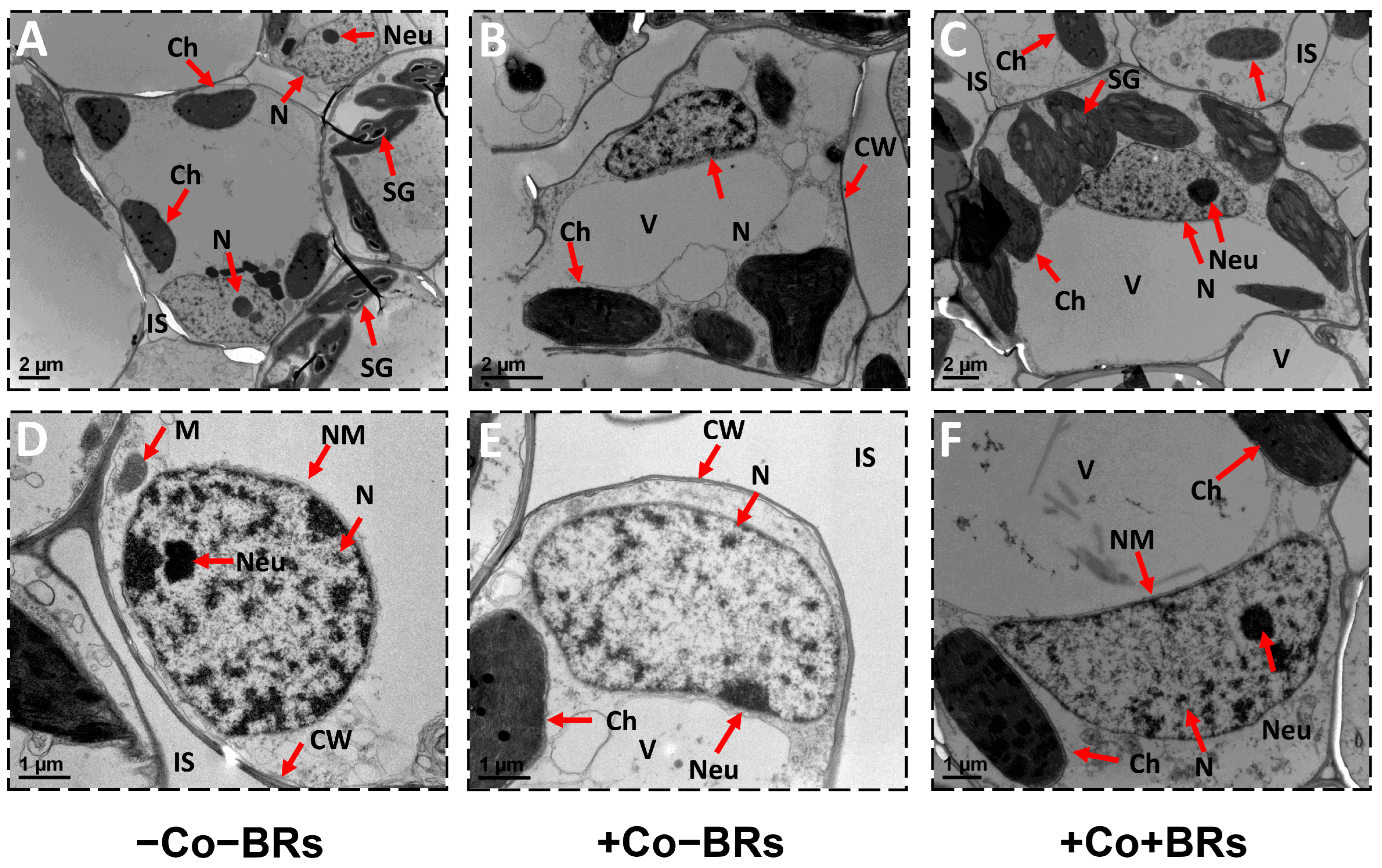
2.6. Effect of BRs on Plant Ultrastructure
3. Materials and Methods
3.1. Plant Materials and Growth Conditions
3.2. Experimental Setup and Treatment Induction
3.3. Plant Growth and Photosynthetic Parameters
3.4. Determination of Cobalt Contents
3.5. Measurements of Endogenous Phytohormones
3.6. Polyphenols, Soluble Sugar, and Total Protein
3.7. Hydrogen Peroxide (H2O2) and Superoxide Anion (O2•−) Quantification and Imaging
3.8. Lipid Peroxidation Assay and Antioxidant Enzymatic Activities
3.9. Determination of Reduced Glutathione and Glutathione Reductase Contents
3.10. Plant Ultrastructure Analysis
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant Hormone Regulation of Abiotic Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Oh, M.-H.; Honey, S.H.; Tax, F.E. The Control of Cell Expansion, Cell Division, and Vascular Development by Brassinosteroids: A Historical Perspective. Int. J. Mol. Sci. 2020, 21, 1743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, L.; Yuan, C.; Zhai, K.; Xia, W.; Duan, Y.; Zhao, B.; Chu, J.; Yao, X. Brassinolide as Potential Rescue Agent for Pinellia Ternata Grown under Microplastic Condition: Insights into Their Modulatory Role on Photosynthesis, Redox Homeostasis, and AsA-GSH Cycling. J. Hazard. Mater. 2024, 470, 134116. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Guo, R.; Jiang, Q.; Chen, C.Z.; Gao, Y.Q.; Jiang, M.M.; Shen, R.F.; Zhu, X.F.; Huang, J. Brassinosteroid Decreases Cadmium Accumulation via Regulating Gibberellic Acid Accumulation and Cd Fixation Capacity of Root Cell Wall in Rice (Oryza sativa). J. Hazard. Mater. 2024, 469, 133862. [Google Scholar] [CrossRef]
- Sehrish, A.K.; Ahmad, S.; Ali, S.; Tabssam, R.; Ai, F.; Du, W.; Guo, H. Alleviation of Cadmium Toxicity by Improving Antioxidant Defense Mechanism and Nutrient Uptake in Wheat (Triticum aestivum L.) through Foliar Application of 24-Epibrassinolide under Elevated CO2. J. Hazard. Mater. 2024, 480, 136209. [Google Scholar] [CrossRef]
- Maia, C.F.; Pereira, Y.C.; da Silva, B.R.S.; Batista, B.L.; Lobato, A.K. da S. Exogenously Applied 24-Epibrassinolide Favours Stomatal Performance, ROS Detoxification and Nutritional Balance, Alleviating Oxidative Damage Against the Photosynthetic Apparatus in Tomato Leaves Under Nickel Stress. J. Plant Growth Regul. 2023, 42, 2196–2211. [Google Scholar] [CrossRef]
- Basit, F.; Tao, J.; An, J.; Song, X.; Sheteiwy, M.S.; Holford, P.; Hu, J.; Jośko, I.; Guan, Y. Nitric Oxide and Brassinosteroids Enhance Chromium Stress Tolerance in Glycine max L. (Merr.) by Modulating Antioxidative Defense and Glyoxalase Systems. Environ. Sci. Pollut. Res. 2023, 30, 51638–51653. [Google Scholar] [CrossRef]
- Siddiqui, H.; Hayat, S.; Bajguz, A. Regulation of Photosynthesis by Brassinosteroids in Plants. Acta Physiol. Plant 2018, 40, 50. [Google Scholar] [CrossRef]
- Zafari, M.; Ebadi, A.; Sedghi, M.; Jahanbakhsh, S. Alleviating Effect of 24-Epibrassinolide on Seed Oil Content and Fatty Acid Composition under Drought Stress in Safflower. J. Food Compos. Anal. 2020, 92, 103544. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) Role in Plant Development and Coping with Different Stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Ashraf, M.; Bajguz, A.; Ahmad, P. Brassinosteroids Regulate Growth in Plants Under Stressful Environments and Crosstalk with Other Potential Phytohormones. J. Plant Growth Regul. 2018, 37, 1007–1024. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Barker, J.; Liu, G.; Hasanuzzaman, M.; Li, Y.; Ramakrishnan, M.; Mokhberdoran, F. Co-Application of 24-Epibrassinolide and Titanium Oxide Nanoparticles Promotes Pleioblastus pygmaeus Plant Tolerance to Cu and Cd Toxicity by Increasing Antioxidant Activity and Photosynthetic Capacity and Reducing Heavy Metal Accumulation and Translocation. Antioxidants 2022, 11, 451. [Google Scholar] [CrossRef] [PubMed]
- Afridi, M.S.; Van Hamme, J.; Bundschuh, J.; Sumaira; Khan, M.N.; Salam, A.; Waqar, M.; Munis, M.F.H.; Chaudhary, H.J. Biotechnological Approaches in Agriculture and Environmental Management—Bacterium Kocuria rhizophila 14ASP as Heavy Metal and Salt- Tolerant Plant Growth- Promoting Strain. Biologia 2021, 76, 3091–3105. [Google Scholar] [CrossRef]
- Angon, P.B.; Islam, M.S.; Kc, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, Effects and Present Perspectives of Heavy Metals Contamination: Soil, Plants and Human Food Chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, H.; Sun, J.; Cai, B.; Tang, R.; Song, X.; Huang, X.; Liu, Y.; Fan, Z. Human Health Risks of Heavy Metal(Loid)s Mediated through Crop Ingestion in a Coal Mining Area in Eastern China. Ecotoxicol. Environ. Saf. 2024, 276, 116305. [Google Scholar] [CrossRef]
- Kumar, V.; Bhatti, S.S.; Nagpal, A.K. Assessment of Metal (Loid) Contamination and Genotoxic Potential of Agricultural Soils. Arch. Environ. Contam. Toxicol. 2021, 81, 272–284. [Google Scholar] [CrossRef]
- Mahey, S.; Kumar, R.; Sharma, M.; Kumar, V.; Bhardwaj, R. A Critical Review on Toxicity of Cobalt and Its Bioremediation Strategies. SN Appl. Sci. 2020, 2, 1279. [Google Scholar] [CrossRef]
- Salam, A.; Rehman, M.; Qi, J.; Khan, A.R.; Yang, S.; Zeeshan, M.; Ulhassan, Z.; Afridi, M.S.; Yang, C.; Chen, N.; et al. Cobalt Stress Induces Photosynthetic and Ultrastructural Distortion by Disrupting Cellular Redox Homeostasis in Maize. Environ. Exp. Bot. 2024, 217, 105562. [Google Scholar] [CrossRef]
- Karuppanapandian, T.; Kim, W. Cobalt-Induced Oxidative Stress Causes Growth Inhibition Associated with Enhanced Lipid Peroxidation and Activates Antioxidant Responses in Indian Mustard (Brassica juncea L.) Leaves. Acta Physiol. Plant 2013, 35, 2429–2443. [Google Scholar] [CrossRef]
- Reshma; Agnihotri, R.; Vamil, R.; Singh, G.; Ahmad, M.; Sharma, R. Effect of Molybdenum and Cobalt Induced Heavy Metal Stress on Seedling Growth Stage of Vigna Radiata. Acta Bot. Hung. 2014, 56, 227–241. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A Review of Soil Heavy Metal Pollution from Industrial and Agricultural Regions in China: Pollution and Risk Assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lin, C.; Qiu, P.; Song, Y.; Yang, W.; Xu, G.; Feng, X.; Yang, Q.; Yang, X.; Niu, A. Tungsten- and Cobalt-Dominated Heavy Metal Contamination of Mangrove Sediments in Shenzhen, China. Mar. Pollut. Bull. 2015, 100, 562–566. [Google Scholar] [CrossRef]
- Ali, S.; Gill, R.A.; Ulhassan, Z.; Najeeb, U.; Kanwar, M.K.; Abid, M.; Mwamba, T.M.; Huang, Q.; Zhou, W. Insights on the Responses of Brassica napus Cultivars against the Cobalt-Stress as Revealed by Carbon Assimilation, Anatomical Changes and Secondary Metabolites. Environ. Exp. Bot. 2018, 156, 183–196. [Google Scholar] [CrossRef]
- Wa Lwalaba, J.L.; Zvogbo, G.; Mulembo, M.; Mundende, M.; Zhang, G. The Effect of Cobalt Stress on Growth and Physiological Traits and Its Association with Cobalt Accumulation in Barley Genotypes Differing in Cobalt Tolerance. J. Plant Nutr. 2017, 40, 2192–2199. [Google Scholar] [CrossRef]
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food Security and the Dynamics of Wheat and Maize Value Chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 617009. [Google Scholar] [CrossRef]
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops That Feed the World 6. Past Successes and Future Challenges to the Role Played by Maize in Global Food Security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Erenstein, O. The Evolving Maize Sector in Asia: Challenges and Opportunities. J. New Seeds 2010, 11, 1–15. [Google Scholar] [CrossRef]
- Akeel, A.; Jahan, A. Role of Cobalt in Plants: Its Stress and Alleviation. In Contaminants in Agriculture; Springer International Publishing: Cham, Swaziland, 2020; pp. 339–357. [Google Scholar]
- Piacentini, D.; Della Rovere, F.; Lanni, F.; Cittadini, M.; Palombi, M.; Fattorini, L.; Cecchetti, V.; Altamura, M.M.; Falasca, G. Brassinosteroids Interact with Nitric Oxide in the Response of Rice Root Systems to Arsenic Stress. Environ. Exp. Bot. 2023, 209, 105287. [Google Scholar] [CrossRef]
- Pandey, A.; Devi, L.L.; Singh, A.P. Review: Emerging Roles of Brassinosteroid in Nutrient Foraging. Plant Sci. 2020, 296, 110474. [Google Scholar] [CrossRef]
- Kir, G.; Ye, H.; Nelissen, H.; Neelakandan, A.K.; Kusnandar, A.S.; Luo, A.; Inzé, D.; Sylvester, A.W.; Yin, Y.; Becraft, P.W. RNA Interference Knockdown of BRASSINOSTEROID INSENSITIVE1 in Maize Reveals Novel Functions for Brassinosteroid Signaling in Controlling Plant Architecture. Plant Physiol. 2015, 169, 826–839. [Google Scholar] [CrossRef]
- Yusuf, M.; Khan, M.T.A.; Faizan, M.; Khalil, R.; Qazi, F. Role of Brassinosteroids and Its Cross Talk with Other Phytohormone in Plant Responses to Heavy Metal Stress. In Brassinosteroids Signalling; Springer: Singapore, 2022; pp. 179–201. [Google Scholar]
- Soares, T.F.S.N.; Dias, D.C.F.D.S.; Oliveira, A.M.S.; Ribeiro, D.M.; Dias, L.A.D.S. Exogenous Brassinosteroids Increase Lead Stress Tolerance in Seed Germination and Seedling Growth of Brassica juncea L. Ecotoxicol. Environ. Saf. 2020, 193, 110296. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, J.; Wang, L.; Wang, H.; Long, H.; Yang, L.; Li, G.; Guo, J.; Wang, Y.; Li, Y.; et al. Water Deficit Aggravated the Inhibition of Photosynthetic Performance of Maize under Mercury Stress but Is Alleviated by Brassinosteroids. J. Hazard. Mater. 2023, 443, 130365. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Choudhary, S.P.; Chen, S.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Role of Brassinosteroids in Alleviation of Phenanthrene–Cadmium Co-Contamination-Induced Photosynthetic Inhibition and Oxidative Stress in Tomato. J. Exp. Bot. 2013, 64, 199–213. [Google Scholar] [CrossRef]
- Collins, R.N.; Bakkaus, E.; Carrière, M.; Khodja, H.; Proux, O.; Morel, J.-L.; Gouget, B. Uptake, Localization, and Speciation of Cobalt in Triticum aestivum L. (Wheat) and Lycopersicon esculentum M. (Tomato). Environ. Sci. Technol. 2010, 44, 2904–2910. [Google Scholar] [CrossRef]
- Wu, X.; Li, L.; Hannan, F.; Qin, T.; Ayyaz, A.; Ma, J.; Athar, H.U.R.; Zafar, Z.U.; Farooq, M.A.; Zhou, W. Brassinosteroid-Induced Transcriptomic Rearrangements Unveiled the Physiological Mechanism of Chromium Stress Tolerance in Brassica napus. Curr. Plant Biol. 2024, 39, 100360. [Google Scholar] [CrossRef]
- Begović, L.; Mlinarić, S.; Antunović Dunić, J.; Katanić, Z.; Lončarić, Z.; Lepeduš, H.; Cesar, V. Response of Lemna minor L. to Short-Term Cobalt Exposure: The Effect on Photosynthetic Electron Transport Chain and Induction of Oxidative Damage. Aquat. Toxicol. 2016, 175, 117–126. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Mbarki, S.; Skalicky, M.; Noedoost, F.; Raeisi, M.; Brestic, M. Effect of Wastewater Irrigation on Photosynthesis, Growth, and Anatomical Features of Two Wheat Cultivars (Triticum aestivum L.). Water 2020, 12, 607. [Google Scholar] [CrossRef]
- Xia, X.-J.; Huang, L.-F.; Zhou, Y.-H.; Mao, W.-H.; Shi, K.; Wu, J.-X.; Asami, T.; Chen, Z.; Yu, J.-Q. Brassinosteroids Promote Photosynthesis and Growth by Enhancing Activation of Rubisco and Expression of Photosynthetic Genes in Cucumis sativus. Planta 2009, 230, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Ahmad, Z. Mechanisms of Selected Plant Hormones under Heavy Metal Stress. Pol. J. Environ. Stud. 2020, 30, 497–507. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Sesin, V.; Kisiala, A.; Emery, R.J.N. Phytohormonal Roles in Plant Responses to Heavy Metal Stress: Implications for Using Macrophytes in Phytoremediation of Aquatic Ecosystems. Environ. Toxicol. Chem. 2020, 40, 7–22. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, F.; Xie, Q. Balancing Growth and Adaptation to Stress: Crosstalk between Brassinosteroid and Abscisic Acid Signaling. Plant Cell Environ. 2020, 43, 2325–2335. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Xu, Z.-S.; He, G.-Y.; Yang, G.-X.; Chen, M.; Li, L.-C.; Ma, Y.-Z. A Mutation in Arabidopsis BSK5 Encoding a Brassinosteroid-Signaling Kinase Protein Affects Responses to Salinity and Abscisic Acid. Biochem. Biophys. Res. Commun. 2012, 426, 522–527. [Google Scholar] [CrossRef]
- Yang, T.; Zhuang, Z.; Bian, J.; Ren, Z.; Ta, W.; Peng, Y. Mechanisms of Exogenous Brassinosteroids and Abscisic Acid in Regulating Maize Cold Stress Tolerance. Int. J. Mol. Sci. 2025, 26, 3326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, T.; Lu, L.; Qiu, S.; Huang, Z.; Wang, Y.; Chen, X.; Li, L.; Sun, Y.; Zhang, R.; et al. Synergistic Interaction between Brassinosteroid and Jasmonate Pathways in Rice Response to Cadmium Toxicity. Sci. Total Environ. 2024, 954, 176369. [Google Scholar] [CrossRef] [PubMed]
- Zahid, A.; Ul Din, K.; Ahmad, M.; Hayat, U.; Zulfiqar, U.; Askri, S.M.H.; Anjum, M.Z.; Maqsood, M.F.; Aijaz, N.; Chaudhary, T.; et al. Exogenous Application of Sulfur-Rich Thiourea (STU) to Alleviate the Adverse Effects of Cobalt Stress in Wheat. BMC Plant Biol. 2024, 24, 126. [Google Scholar] [CrossRef]
- Stewart Lilley, J.L.; Gan, Y.; Graham, I.A.; Nemhauser, J.L. The Effects of DELLA on Growth Change with Developmental Stage and Brassinosteroid Levels. Plant J. 2013, 76, 165–173. [Google Scholar] [CrossRef]
- Bai, M.-Y.; Shang, J.-X.; Oh, E.; Fan, M.; Bai, Y.; Zentella, R.; Sun, T.; Wang, Z.-Y. Brassinosteroid, Gibberellin and Phytochrome Impinge on a Common Transcription Module in Arabidopsis. Nat. Cell. Biol. 2012, 14, 810–817. [Google Scholar] [CrossRef]
- Achard, P.; Renou, J.-P.; Berthomé, R.; Harberd, N.P.; Genschik, P. Plant DELLAs Restrain Growth and Promote Survival of Adversity by Reducing the Levels of Reactive Oxygen Species. Curr. Biol. 2008, 18, 656–660. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Ali, S.; Kaleem, Z.; Shahbaz, H.; He, D.; Khan, A.R.; Salam, A.; Hamid, Y.; Sheteiwy, M.S.; Zhou, W.; et al. Effects of Nanosilica Priming on Rapeseed (Brassica napus) Tolerance to Cadmium and Arsenic Stress by Regulating Cellular Metabolism and Antioxidant Defense. J. Agric. Food Chem. 2025, 73, 4518–4533. [Google Scholar] [CrossRef]
- Wu, J.; Lv, S.; Zhao, L.; Gao, T.; Yu, C.; Hu, J.; Ma, F. Advances in the Study of the Function and Mechanism of the Action of Flavonoids in Plants under Environmental Stresses. Planta 2023, 257, 108. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, V.; Alooparampil, S.; Pandya, R.V.; Tank, J.G. Physiological Function of Phenolic Compounds in Plant Defense. In Phenolic Compounds: Chemistry, Synthesis, Diversity, Non-Conventional Industrial, Pharmaceutical and Therapeutic Applications; Intechopen: London, UK, 2022; p. 185. [Google Scholar]
- Liu, X.; Zhu, Q.; Liu, W.; Zhang, J. Exogenous Brassinosteroid Enhances Zinc Tolerance by Activating the Phenylpropanoid Biosynthesis Pathway in Citrullus lanatus L. Plant Signal. Behav. 2023, 18, 2186640. [Google Scholar] [CrossRef]
- Zhang, L.; Ahammed, G.J.; Li, X.; Wei, J.-P.; Li, Y.; Yan, P.; Zhang, L.-P.; Han, W.-Y. Exogenous Brassinosteroid Enhances Plant Defense Against Colletotrichum Gloeosporioides by Activating Phenylpropanoid Pathway in Camellia sinensis L. J. Plant Growth Regul. 2018, 37, 1235–1243. [Google Scholar] [CrossRef]
- Niu, J.; Ahmad Anjum, S.; Wang, R.; Li, J.; Liu, M.; Song, J.; Zohaib, A.; Lv, J.; Wang, S.; Zong, X. Exogenous Application of Brassinolide Can Alter Morphological and Physiological Traits of Leymus chinensis (Trin.) Tzvelev under Room and High Temperatures. Chil. J. Agric. Res. 2016, 76, 27–33. [Google Scholar] [CrossRef]
- de Almeida, A.J.P.O.; de Oliveira, J.C.P.L.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and Its Implications in Aging Pathways. Oxid. Med. Cell Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef]
- Ahmad, P.; Ozturk, M.; Gucel, S. Oxidative Damage and Antioxidants Induced by Heavy Metal Stress in Two Cultivars of Mustard (Brassica juncea L.) Plants. Fresenius Environ. Bull. 2012, 21, 2953–2961. [Google Scholar]
- Ali, S.; Ali, B.; Sajid, I.A.; Ahmad, S.; Yousaf, M.A.; Ulhassan, Z.; Zhang, K.; Ali, S.; Zhou, W.; Mao, B. Synergistic Effects of Exogenous Melatonin and Zinc Oxide Nanoparticles in Alleviating Cobalt Stress in Brassica napus: Insights from Stress-Related Markers and Antioxidant Machinery. Environ. Sci. Nano 2025, 12, 368–387. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ghosh, P.K.; Akter, M.; Al Noor, M.M.; Rahman, M.A.; Keya, S.S.; Roni, M.S.; Biswas, A.; Bulle, M. Green Vanguards: Harnessing the Power of Plant Antioxidants, Signal Catalysts, and Genetic Engineering to Combat Reactive Oxygen Species under Multiple Abiotic Stresses. Plant Stress 2024, 13, 100547. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Hydrogen Peroxide Priming Modulates Abiotic Oxidative Stress Tolerance: Insights from ROS Detoxification and Scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef]
- Vardhini, B.V.; Anjum, N.A. Brassinosteroids Make Plant Life Easier under Abiotic Stresses Mainly by Modulating Major Components of Antioxidant Defense System. Front. Environ. Sci. 2015, 2. [Google Scholar] [CrossRef]
- Khan, A.R.; Fan, X.; Salam, A.; Azhar, W.; Ulhassan, Z.; Qi, J.; Liaquat, F.; Yang, S.; Gan, Y. Melatonin-Mediated Resistance to Copper Oxide Nanoparticles-Induced Toxicity by Regulating the Photosynthetic Apparatus, Cellular Damages and Antioxidant Defense System in Maize Seedlings. Environ. Pollut. 2023, 316, 120639. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Yang, S.; He, D.; Khan, A.R.; Salam, A.; Azhar, W.; Muhammad, S.; Ali, S.; Hamid, Y.; Khan, I.; et al. Seed Priming with Nano-Silica Effectively Ameliorates Chromium Toxicity in Brassica napus. J. Hazard. Mater. 2023, 458, 131906. [Google Scholar] [CrossRef]
- Rehman, M.; Salam, A.; Ali, B.; Ahmad, I.; Javaid, M.H.; Haider, Z.; Munir, R.; Yasin, M.U.; Ali, I.; Yang, C.; et al. Titanium Dioxide Nanoparticles Seed Priming as a Remedy for Nickel-Induced Stress in Maize through Antioxidant Enhancement and Ultrastructural Optimization. J. Environ. Manag. 2025, 373, 123487. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Hu, Y.X.; Guo, X.H.; Sun, C.Y.; Salam, A.; Ahmad, S.; Muhammad, I.; Nasar, J.; Jahan, M.S.; Fahad, S.; et al. Physiological and Transcriptomic Study Reveal SeNPs-Mediated AsIII Stress Detoxification Mechanisms Involved Modulation of Antioxidants, Metal Transporters, and Transcription Factors in Glycine max L. (Merr.) Roots. Environ. Pollut. 2023, 317, 120637. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Kim, C. Chloroplast ROS and Stress Signaling. Plant Commun. 2022, 3, 100264. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Nawaz, M.A.; Li, F.; Bai, L.; Li, J. Brassinosteroids Regulate Antioxidant System and Protect Chloroplast Ultrastructure of Autotoxicity-Stressed Cucumber (Cucumis sativus L.) Seedlings. Agronomy 2019, 9, 265. [Google Scholar] [CrossRef]
- Basit, F.; Chen, M.; Ahmed, T.; Shahid, M.; Noman, M.; Liu, J.; An, J.; Hashem, A.; Fahad Al-Arjani, A.-B.; Alqarawi, A.A.; et al. Seed Priming with Brassinosteroids Alleviates Chromium Stress in Rice Cultivars via Improving ROS Metabolism and Antioxidant Defense Response at Biochemical and Molecular Levels. Antioxidants 2021, 10, 1089. [Google Scholar] [CrossRef]
- Zhang, N.; Ali, S.; Huang, Q.; Yang, C.; Ali, B.; Chen, W.; Zhang, K.; Ali, S.; Ulhassan, Z.; Zhou, W. Seed Pretreatment with Brassinosteroids Stimulates Sunflower Immunity against Parasitic Weed (Orobanche cumana) Infection. Physiol. Plant. 2024, 176, e14324. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 39. [Google Scholar]
- Azhar, W.; Salam, A.; Khan, A.R.; Ahmad, I.; Gan, Y. Brassinosteroids Alleviate Ethylene-Induced Copper Oxide Nanoparticle Toxicity and Ultrastructural and Stomatal Damage in Rice Seedlings. Agriculture 2025, 15, 907. [Google Scholar] [CrossRef]
- Khan, A.R.; Azhar, W.; Wu, J.; Ulhassan, Z.; Salam, A.; Zaidi, S.H.R.; Yang, S.; Song, G.; Gan, Y. Ethylene Participates in Zinc Oxide Nanoparticles Induced Biochemical, Molecular and Ultrastructural Changes in Rice Seedlings. Ecotoxicol. Environ. Saf. 2021, 226, 112844. [Google Scholar] [CrossRef]
- Salam, A.; Khan, A.R.; Liu, L.; Yang, S.; Azhar, W.; Ulhassan, Z.; Zeeshan, M.; Wu, J.; Fan, X.; Gan, Y. Seed Priming with Zinc Oxide Nanoparticles Downplayed Ultrastructural Damage and Improved Photosynthetic Apparatus in Maize under Cobalt Stress. J. Hazard. Mater. 2022, 423, 127021. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Wu, J.; Xiong, H.; Zeb, A.; Yang, T.; Su, X.; Su, L.; Liu, W. Impact of Polystyrene Nanoplastics (PSNPs) on Seed Germination and Seedling Growth of Wheat (Triticum aestivum L.). J. Hazard. Mater. 2020, 385. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Welti, R.; Wang, X. Quantitative Analysis of Major Plant Hormones in Crude Plant Extracts by High-Performance Liquid Chromatography–Mass Spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef]
- Zhou, W.; Leul, M. Uniconazole-Induced Alleviation of Freezing Injury in Relation to Changes in Hormonal Balance, Enzyme Activities and Lipid Peroxidation in Winter Rape. Plant Growth Regul. 1998, 26, 41–47. [Google Scholar] [CrossRef]
- Zhang, W.F.; Zhang, F.; Raziuddin, R.; Gong, H.J.; Yang, Z.M.; Lu, L.; Ye, Q.F.; Zhou, W.J. Effects of 5-Aminolevulinic Acid on Oilseed Rape Seedling Growth under Herbicide Toxicity Stress. J. Plant Growth Regul. 2008, 27, 159–169. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of Glutathione and Glutathione Disulfide Using Glutathione Reductase and 2-Vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Foyer, C.H.; Souriau, N.; Perret, S.; Lelandais, M.; Kunert, K.J.; Pruvost, C.; Jouanin, L. Overexpression of Glutathione Reductase but Not Glutathione Synthetase Leads to Increases in Antioxidant Capacity and Resistance to Photoinhibition in Poplar Trees. Plant Physiol. 1995, 109, 1047–1057. [Google Scholar] [CrossRef]
- Azhar, W.; Khan, A.R.; Salam, A.; Ulhassan, Z.; Qi, J.; Shah, G.; Liu, Y.; Chunyan, Y.; Yang, S.; Gan, Y. Ethylene Accelerates Copper Oxide Nanoparticle-Induced Toxicity at Physiological, Biochemical, and Ultrastructural Levels in Rice Seedlings. Environ. Sci. Pollut. Res. 2022, 30, 26137–26149. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salam, A.; Chang, J.; Yang, L.; Zeeshan, M.; Iqbal, A.; Khan, A.R.; Afridi, M.S.; Ulhassan, Z.; Azhar, W.; Zhang, Z.; et al. Brassinosteroid-Mediated Resistance to Cobalt-Induced Toxicity by Regulating Hormonal Balance, Cellular Metabolism, and Antioxidant Defense in Maize. Plants 2025, 14, 2076. https://doi.org/10.3390/plants14132076
Salam A, Chang J, Yang L, Zeeshan M, Iqbal A, Khan AR, Afridi MS, Ulhassan Z, Azhar W, Zhang Z, et al. Brassinosteroid-Mediated Resistance to Cobalt-Induced Toxicity by Regulating Hormonal Balance, Cellular Metabolism, and Antioxidant Defense in Maize. Plants. 2025; 14(13):2076. https://doi.org/10.3390/plants14132076
Chicago/Turabian StyleSalam, Abdul, Jinzhe Chang, Liupeng Yang, Muhammad Zeeshan, Anas Iqbal, Ali Raza Khan, Muhammad Siddique Afridi, Zaid Ulhassan, Wardah Azhar, Zhixiang Zhang, and et al. 2025. "Brassinosteroid-Mediated Resistance to Cobalt-Induced Toxicity by Regulating Hormonal Balance, Cellular Metabolism, and Antioxidant Defense in Maize" Plants 14, no. 13: 2076. https://doi.org/10.3390/plants14132076
APA StyleSalam, A., Chang, J., Yang, L., Zeeshan, M., Iqbal, A., Khan, A. R., Afridi, M. S., Ulhassan, Z., Azhar, W., Zhang, Z., & Zhang, P. (2025). Brassinosteroid-Mediated Resistance to Cobalt-Induced Toxicity by Regulating Hormonal Balance, Cellular Metabolism, and Antioxidant Defense in Maize. Plants, 14(13), 2076. https://doi.org/10.3390/plants14132076












