Silicon Protects Rice Plants Against Striped Stem Borer by Disturbing Herbivory-Induced Putrescine Accumulation
Abstract
1. Introduction
2. Results
2.1. Effects of Si on Rice Resistance to SSB
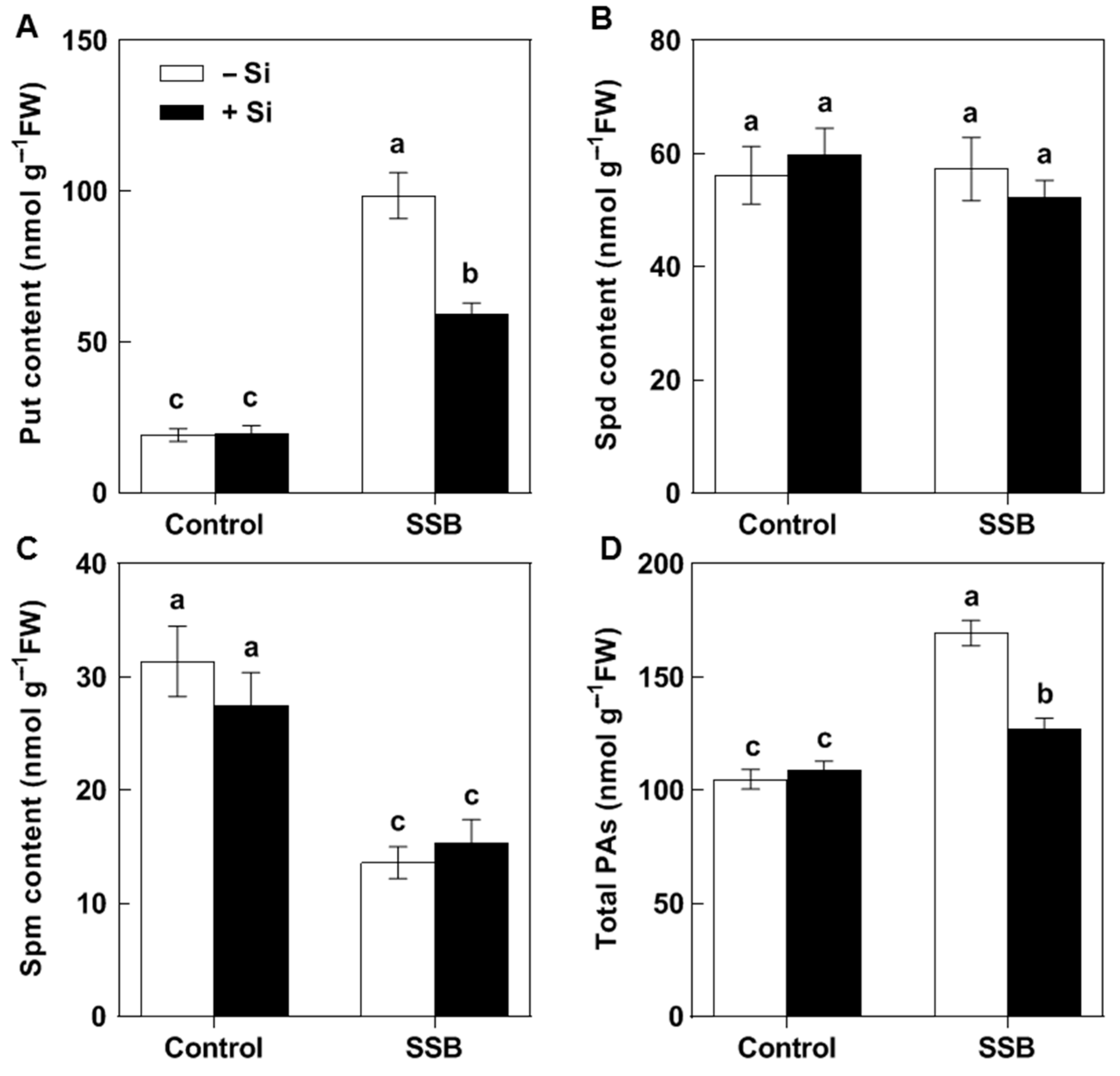
2.2. Effects of Si on SSB Attack-Induced Put Accumulation in Rice
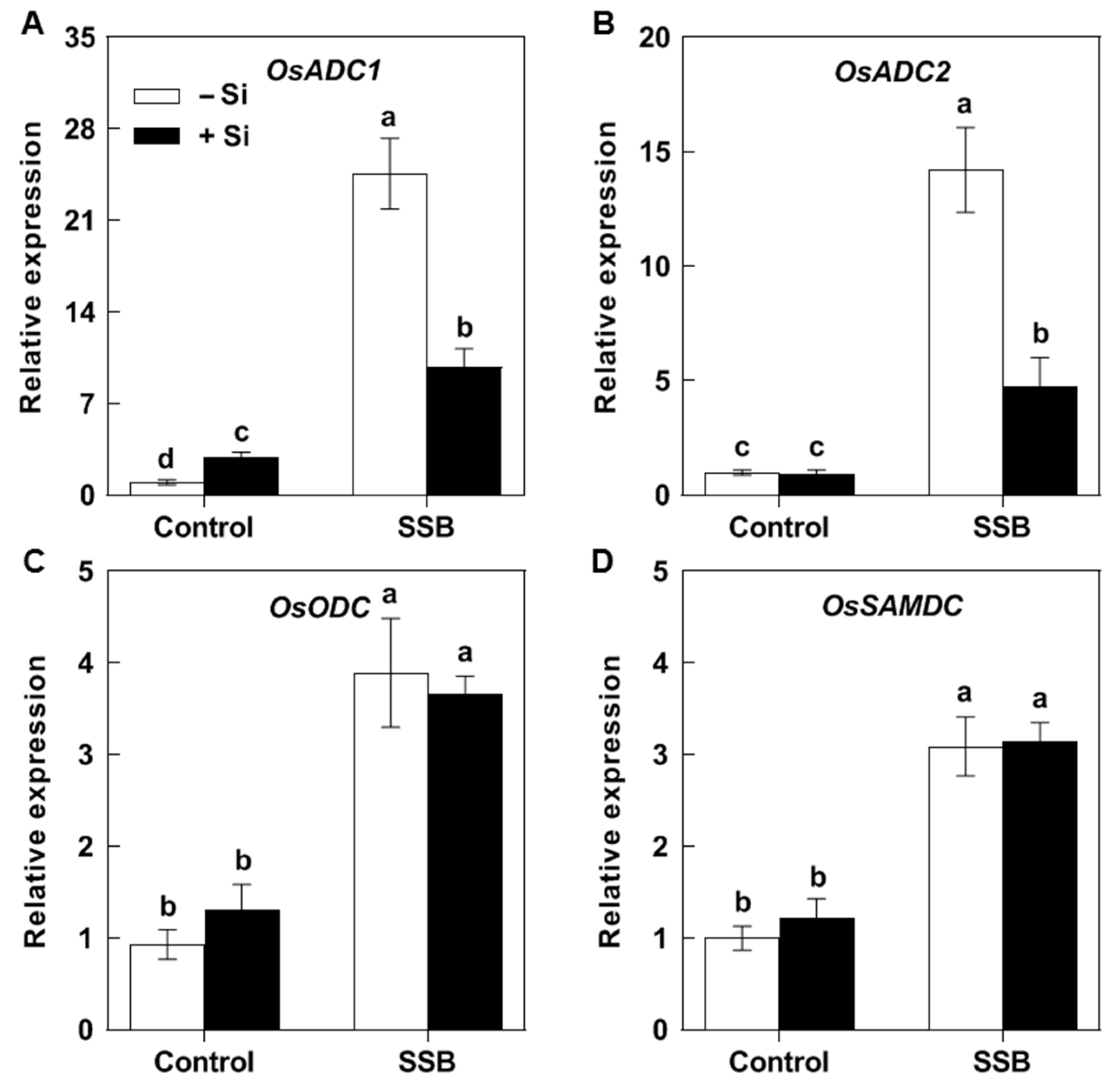
2.3. Effects of Si on SSB Attack-Induced Expression of Rice Put Biosynthesis Genes
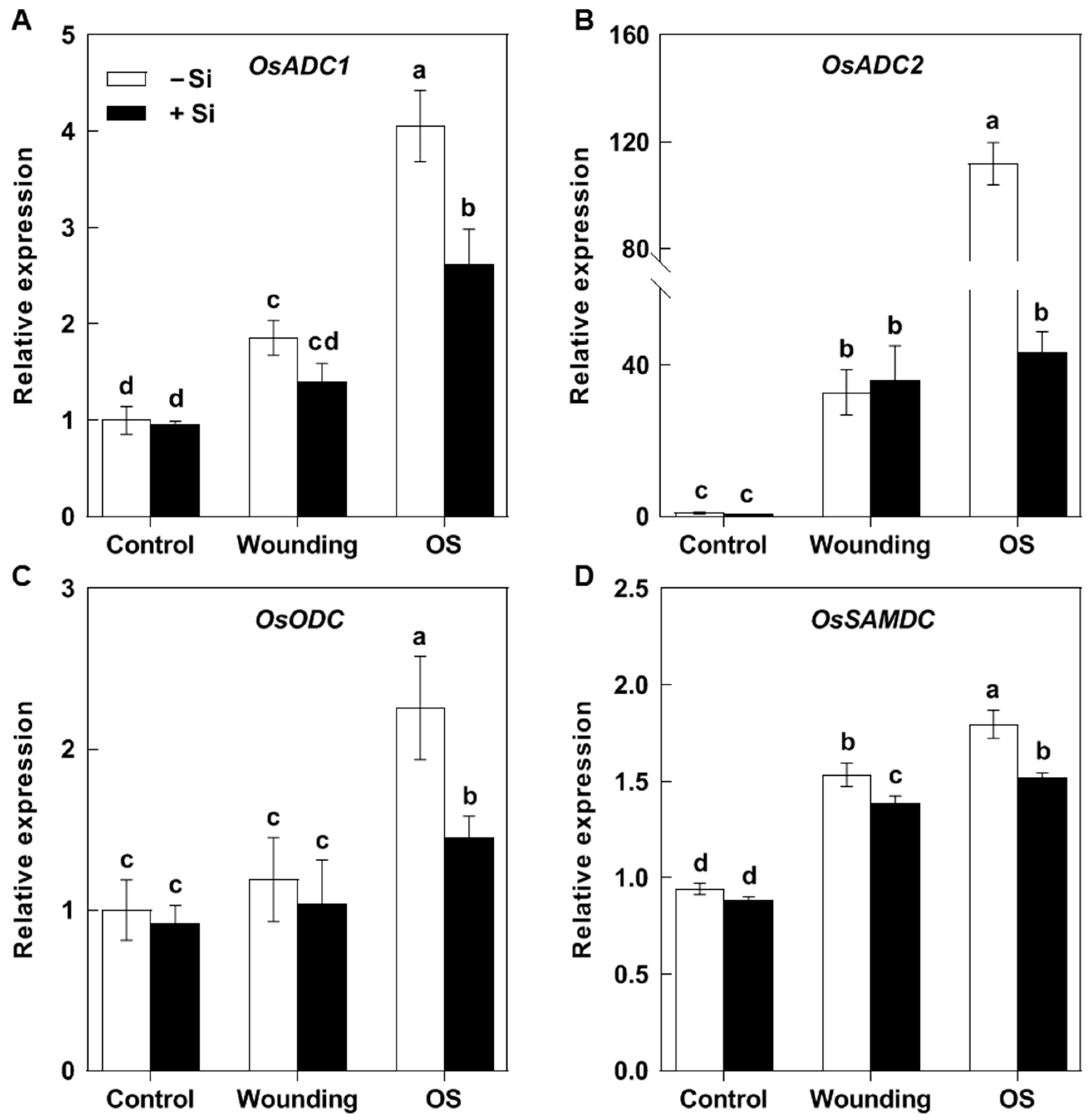
2.4. The Effects of Si on the Induction of Put Biosynthesis Genes by SSB Larval Oral Secretion
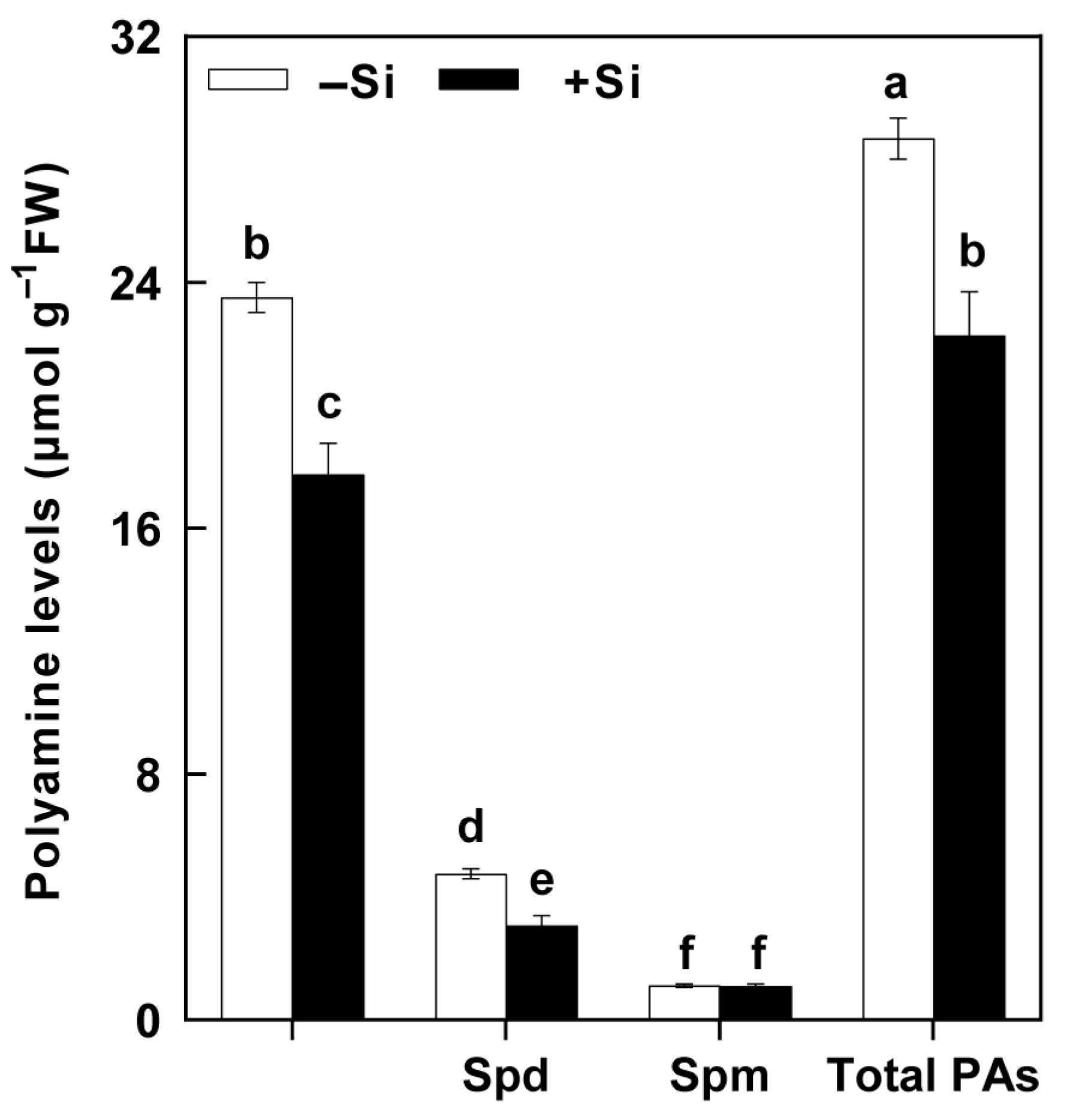
2.5. The Effects of Si on PA Levels in the SSB Larvae Fed on the Rice Plants
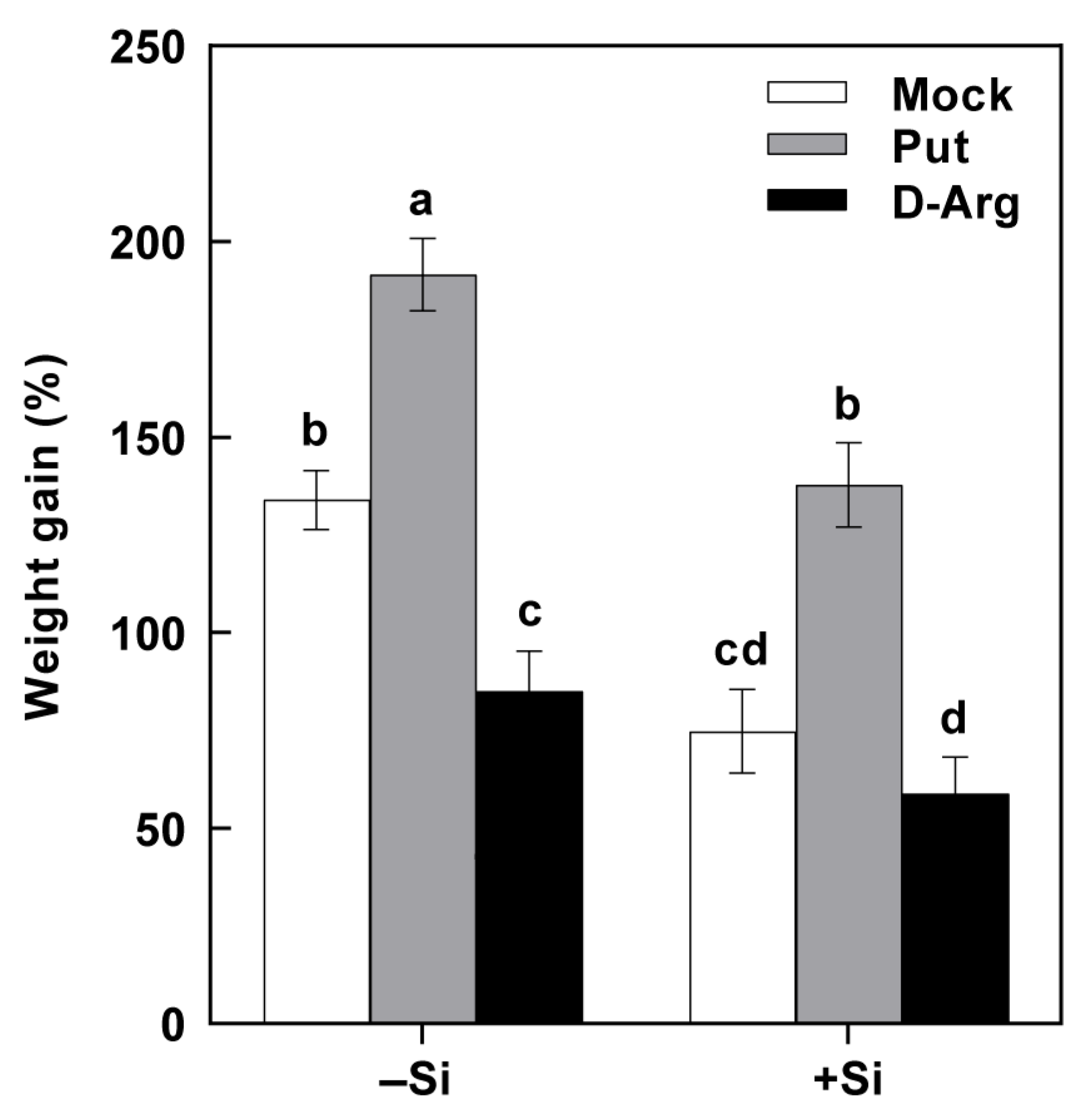
2.6. Effects of Exogenous Application of Put on Si-Enhanced Resistance to SSB
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. SSB Rearing and Infestation
4.3. Polyamine Quantification
4.4. Gene Expression Analysis
4.5. Oral Secretion Treatment
4.6. Exogenous Application of Put and D-Arginine
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAs | Polyamines |
| SSB | Striped stem borer |
| Si | Silicon |
| Put | Putrescine |
| Spd | Spermidine |
| Spm | Spermine |
| ADC | Arginine decarboxylase |
| ODC | Ornithine decarboxylase |
| SAMDC | S-adenosyl-methionine decarboxylase |
| OS | Oral secretion |
| D-Arg | D-arginine |
| HPLC | High-performance liquid chromatography |
References
- Zhang, H.; Li, Y.; Liu, H.; Kong, L.; Ding, X. Research Progress on Regulatory Genes of Important Agronomic Traits and Breeding Utilization in Rice. Biotechnol. Bull. 2020, 36, 155–169. [Google Scholar]
- Wang, L.; Lu, H.; Zhang, X.; He, Y.; Zhang, J.; Guo, X.; Fu, H.; Ye, G.; Shu, Q. Disruption of Serotonin Biosynthesis Increases Resistance to Striped Stem Borer without Changing Innate Defense Response in Rice. J. Pineal Res. 2023, 75, e12895. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Xiao, H.; Sun, Y.; Ding, S.; Situ, G.; Li, F. Transgenic Micro RNA-14 Rice Shows High Resistance to Rice Stem Borer. Plant Biotechnol. J. 2019, 17, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.-F.; Yang, L.; Shen, C.; Li, J.-C.; Hoffmann, A.A.; Huang, Y.-X.; Zhu, F.; Ji, R.; Luo, G.-H.; Fang, J.-C. Defence and Nutrition Synergistically Contribute to the Distinct Tolerance of Rice Subspecies to the Stem Borer, Chilo suppressalis. Plant Cell Environ. 2024, 47, 2426–2442. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Liu, S.; Li, H.; Danso Ofori, A.; Yi, X.; Zheng, A. Defense Strategies of Rice in Response to the Attack of the Herbivorous Insect, Chilo suppressalis. Int. J. Mol. Sci. 2023, 24, 14361. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and Plants: Current Knowledge and Technological Perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef]
- Bhoi, T.K.; Samal, I.; Mahanta, D.K.; Komal, J.; Jinger, D.; Sahoo, M.R.; Achary, G.C.; Nayak, P.; Sunani, S.K.; Saini, V.; et al. Understanding How Silicon Fertilization Impacts Chemical Ecology and Multitrophic Interactions among Plants, Insects and Beneficial Arthropods. Silicon 2023, 15, 2529–2549. [Google Scholar] [CrossRef]
- Islam, W.; Tayyab, M.; Khalil, F.; Hua, Z.; Huang, Z.; Chen, H.Y.H. Silicon-Mediated Plant Defense against Pathogens and Insect Pests. Pestic. Biochem. Physiol. 2020, 168, 104641. [Google Scholar] [CrossRef]
- Jinger, D.; Devi, M.T.; Dhar, S.; Dass, A.; Sharma, V.K.; S, V.K.; Joshi, E.; Jatav, H.S.; Singh, N. Silicon Application Mitigates Abiotic Stresses in Rice: A Review. Indian J. Agric. Sci. 2020, 90, 2043–2050. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Isayenkov, S.V. The Physiological and Molecular Mechanisms of Silicon Action in Salt Stress Amelioration. Plants 2024, 13, 525. [Google Scholar] [CrossRef]
- Pang, Z.; Qiu, L.-X.; Guan, D.-X.; Zeng, X.; Wang, Y.; Peng, H.; Song, A.; Liang, Y. A Novel Layered Culture Device Reveals Spatial Dynamics of Root Element Uptake and Optimal Silicon Application Site for Mitigating Chromium Uptake by Rice. J. Environ. Manag. 2025, 373, 123488. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Han, Y. Silicon-Mediated Rice Plant Resistance to the Asiatic Rice Borer (Lepidoptera: Crambidae): Effects of Silicon Amendment and Rice Varietal Resistance. J. Econ. Entomol. 2010, 103, 1412–1419. [Google Scholar] [CrossRef]
- Ye, M.; Song, Y.; Long, J.; Wang, R.; Baerson, S.R.; Pan, Z.; Zhu-Salzman, K.; Xie, J.; Cai, K.; Luo, S.; et al. Priming of Jasmonate-Mediated Antiherbivore Defense Responses in Rice by Silicon. Proc. Natl. Acad. Sci. USA 2013, 110, E3631–E3639. [Google Scholar] [CrossRef]
- Rasoolizadeh, A.; Labbé, C.; Sonah, H.; Deshmukh, R.K.; Belzile, F.; Menzies, J.G.; Bélanger, R.R. Silicon Protects Soybean Plants against Phytophthora sojae by Interfering with Effector-Receptor Expression. BMC Plant Biol. 2018, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The Controversies of Silicon’s Role in Plant Biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef]
- Majumdar, R.; Minocha, R.; Lebar, M.D.; Rajasekaran, K.; Long, S.; Carter-Wientjes, C.; Minocha, S.; Cary, J.W. Contribution of Maize Polyamine and Amino Acid Metabolism toward Resistance against Aspergillus flavus Infection and Aflatoxin Production. Front. Plant Sci. 2019, 10, 692. [Google Scholar] [CrossRef] [PubMed]
- Ramazan, S.; Nazir, I.; Yousuf, W.; John, R. Environmental Stress Tolerance in Maize (Zea mays): Role of Polyamine Metabolism. Funct. Plant Biol. 2022, 50, 85–96. [Google Scholar] [CrossRef]
- Wang, W.; Paschalidis, K.; Feng, J.-C.; Song, J.; Liu, J.-H. Polyamine Catabolism in Plants: A Universal Process with Diverse Functions. Front. Plant Sci. 2019, 10, 561. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine Function in Plants: Metabolism, Regulation on Development, and Roles in Abiotic Stress Responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Gerlin, L.; Baroukh, C.; Genin, S. Polyamines: Double Agents in Disease and Plant Immunity. Trends Plant Sci. 2021, 26, 1061–1071. [Google Scholar] [CrossRef]
- Gonzalez, M.E.; Jasso-Robles, F.I.; Flores-Hernández, E.; Rodríguez-Kessler, M.; Pieckenstain, F.L. Current Status and Perspectives on the Role of Polyamines in Plant Immunity. Ann. Appl. Biol. 2021, 178, 244–255. [Google Scholar] [CrossRef]
- Mo, H.; Wang, X.; Zhang, Y.; Zhang, G.; Zhang, J.; Ma, Z. Cotton Polyamine Oxidase Is Required for Spermine and Camalexin Signalling in the Defence Response to Verticillium Dahliae. Plant J. 2015, 83, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Tebayashi, S.; Horibata, Y.; Mikagi, E.; Kashiwagi, T.; Mekuria, D.B.; Dekebo, A.; Ishihara, A.; Kim, C.-S. Induction of Resistance against the Leafminer, Liriomyza trifolii, by Jasmonic Acid in Sweet Pepper. Biosci. Biotechnol. Biochem. 2007, 71, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Klose, M.; Atkinson, J.; Mercier, A. Effects of a Hydroxy-Cinnamoyl Conjugate of Spermidine on Arthropod Neuromuscular Junctions. J. Comp. Physiol. A 2002, 187, 945–952. [Google Scholar]
- Subramanyam, S.; Sardesai, N.; Minocha, S.C.; Zheng, C.; Shukle, R.H.; Williams, C.E. Hessian Fly Larval Feeding Triggers Enhanced Polyamine Levels in Susceptible but Not Resistant Wheat. BMC Plant Biol. 2015, 15, 3. [Google Scholar] [CrossRef]
- Cezary, S.; Weronika, M.; Bogumił, L.; Paweł, C. Participation of amino acid decarboxylases in biochemical interactions between triticale (Triticosecale; Poaceae) and bird cherry-oat aphid (Rhopalosiphum padi; Aphididae). Biochem. Syst. Ecology. 2013, 51, 349–356. [Google Scholar]
- Zu, H.; Zhang, J.; Bai, W.; Kuai, P.; Cheng, J.; Lu, J.; Lou, Y.; Li, R. Jasmonate-Mediated Polyamine Oxidase 6 Drives Herbivore-Induced Polyamine Catabolism in Rice. Plant J. 2024, 120, 2000–2013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gai, C.; Shao, M.; Fang, L.; Li, X.; Song, Y.; Zeng, R.; Chen, D. Herbivory by Striped Stem Borer Triggers Polyamine Accumulation in Host Rice Plants to Promote Its Larval Growth. Plants 2023, 12, 3249. [Google Scholar] [CrossRef]
- Chen, D.; Cao, B.; Qi, L.; Yin, L.; Wang, S.; Deng, X. Silicon-Moderated K-Deficiency-Induced Leaf Chlorosis by Decreasing Putrescine Accumulation in Sorghum. Ann. Bot. 2016, 118, 305–315. [Google Scholar] [CrossRef]
- Yin, L.; Wang, S.; Tanaka, K.; Fujihara, S.; Itai, A.; Den, X.; Zhang, S. Silicon-Mediated Changes in Polyamines Participate in Silicon-Induced Salt Tolerance in Sorghum bicolor L. Plant Cell Environ. 2016, 39, 245–258. [Google Scholar] [CrossRef]
- Gao, H.; Wu, X.; Yang, X.; Sun, M.; Liang, J.; Xiao, Y.; Peng, F. Silicon Inhibits Gummosis by Promoting Polyamine Synthesis and Repressing Ethylene Biosynthesis in Peach. Front. Plant Sci. 2022, 13, 986688. [Google Scholar] [CrossRef]
- Shi, X.; Xue, X.; Xu, H.; Yang, Y.; Kuang, Z.; Hou, X. Amelioration of Salt-Induced Damage on Paeonia ostii ‘Fengdan’by Exogenous Application of Silicon. Agronomy 2023, 13, 1349. [Google Scholar] [CrossRef]
- Xue, R.; Li, Q.; Guo, R.; Yan, H.; Ju, X.; Liao, L.; Zeng, R.; Song, Y.; Wang, J. Rice Defense Responses Orchestrated by Oral Bacteria of the Rice Striped Stem Borer, Chilo suppressalis. Rice 2023, 16, 1. [Google Scholar] [CrossRef]
- Asija, S.; Seth, T.; Umar, S.; Gupta, R. Polyamines and Their Crosstalk with Phytohormones in the Regulation of Plant Defense Responses. J. Plant Growth Regul. 2023, 42, 5224–5246. [Google Scholar] [CrossRef]
- Huang, S.; Ma, J.F. Silicon Transport and Its “Homeostasis” in Rice. Quant. Plant Biol. 2024, 5, e15. [Google Scholar] [CrossRef]
- Ma, J.F.; Mitani, N.; Nagao, S.; Konishi, S.; Tamai, K.; Iwashita, T.; Yano, M. Characterization of the Silicon Uptake System and Molecular Mapping of the Silicon Transporter Gene in Rice. Plant Physiol. 2004, 136, 3284–3289. [Google Scholar] [CrossRef]
- Srivani, G.; Kumar, G.S.; Janaguiraman, M.; Arthanari, P.M.; Malathi, P.; Priya, R.S.; Jagathjothi, N.; Yuvaraj, M.; Parasuraman, P. Potency of Silicon for Enhanced Rice Productivity: A Revelation for Global Food Security. Silicon 2024, 16, 5501–5523. [Google Scholar] [CrossRef]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef]
- Pál, M.; Szalai, G.; Janda, T. Speculation: Polyamines Are Important in Abiotic Stress Signaling. Plant Sci. 2015, 237, 16–23. [Google Scholar] [CrossRef]
- Birnbaum, M.J.; Whelan, T.M.; Gilbert, L.I. Temporal Alterations in Polyamine Content and Ornithine Decarboxylase Activity during the Larval-Pupal Development of Manduca sexta. Insect Biochem. 1988, 18, 853–859. [Google Scholar] [CrossRef]
- Birnbaum, M.J.; Gilbert, L.I. Juvenile Hormone Stimulation of Ornithine Decarboxylase Activity during Vitellogenesis in Drosophila melanogaster. J. Comp. Physiol. B 1990, 160, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Kogan, P.H.; Hagedorn, H.H. Polyamines, and Effects from Reducing Their Synthesis during Egg Development in the Yellow Fever Mosquito, Aedes Aegypti. J. Insect Physiol. 2000, 46, 1079–1095. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Nikolic, M.; Bélanger, R.; Gong, H.; Song, A. Silicon and Insect Pest Resistance. In Silicon in Agriculture; Springer: Dordrecht, The Netherlands, 2015; pp. 197–207. ISBN 978-94-017-9977-5. [Google Scholar]
- Reynolds, O.L.; Padula, M.P.; Zeng, R.; Gurr, G.M. Silicon: Potential to Promote Direct and Indirect Effects on Plant Defense against Arthropod Pests in Agriculture. Front. Plant Sci. 2016, 7, 744. [Google Scholar] [CrossRef]
- Sakr, N. The Role of Silicon (Si) in Increasing Plant Resistance against Insect Pests Review Article. Acta Phytopathol. Entomol. Hung. 2017, 52, 185–204. [Google Scholar] [CrossRef]
- Prajapati, V.K.; Vijayan, V.; Vadassery, J. Secret Weapon of Insects: The Oral Secretion Cocktail and Its Modulation of Host Immunity. Plant Cell Physiol. 2024, 65, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yin, Z.; Li, H.; Guo, J. Roles of Herbivorous Insects Salivary Proteins. Heliyon 2024, 10, e29201. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y. Protein Dynamics in Plant Immunity: Insights into Plant–Pest Interactions. Int. J. Mol. Sci. 2024, 25, 12951. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Liu, X.; Li, C.; Fang, L.; Gai, C.; Zeng, R.; Wang, Q.; Song, Y.; Chen, D. Silicon Protects Rice Plants Against Striped Stem Borer by Disturbing Herbivory-Induced Putrescine Accumulation. Plants 2025, 14, 2066. https://doi.org/10.3390/plants14132066
Zhang H, Liu X, Li C, Fang L, Gai C, Zeng R, Wang Q, Song Y, Chen D. Silicon Protects Rice Plants Against Striped Stem Borer by Disturbing Herbivory-Induced Putrescine Accumulation. Plants. 2025; 14(13):2066. https://doi.org/10.3390/plants14132066
Chicago/Turabian StyleZhang, Hao, Xiaodong Liu, Cunyan Li, Linzhi Fang, Chaoyue Gai, Rensen Zeng, Qiongli Wang, Yuanyuan Song, and Daoqian Chen. 2025. "Silicon Protects Rice Plants Against Striped Stem Borer by Disturbing Herbivory-Induced Putrescine Accumulation" Plants 14, no. 13: 2066. https://doi.org/10.3390/plants14132066
APA StyleZhang, H., Liu, X., Li, C., Fang, L., Gai, C., Zeng, R., Wang, Q., Song, Y., & Chen, D. (2025). Silicon Protects Rice Plants Against Striped Stem Borer by Disturbing Herbivory-Induced Putrescine Accumulation. Plants, 14(13), 2066. https://doi.org/10.3390/plants14132066








