Phenolic Compounds in Nectar of Crataegus monogyna Jacq. and Prunus spinosa L.
Abstract
1. Introduction
2. Results
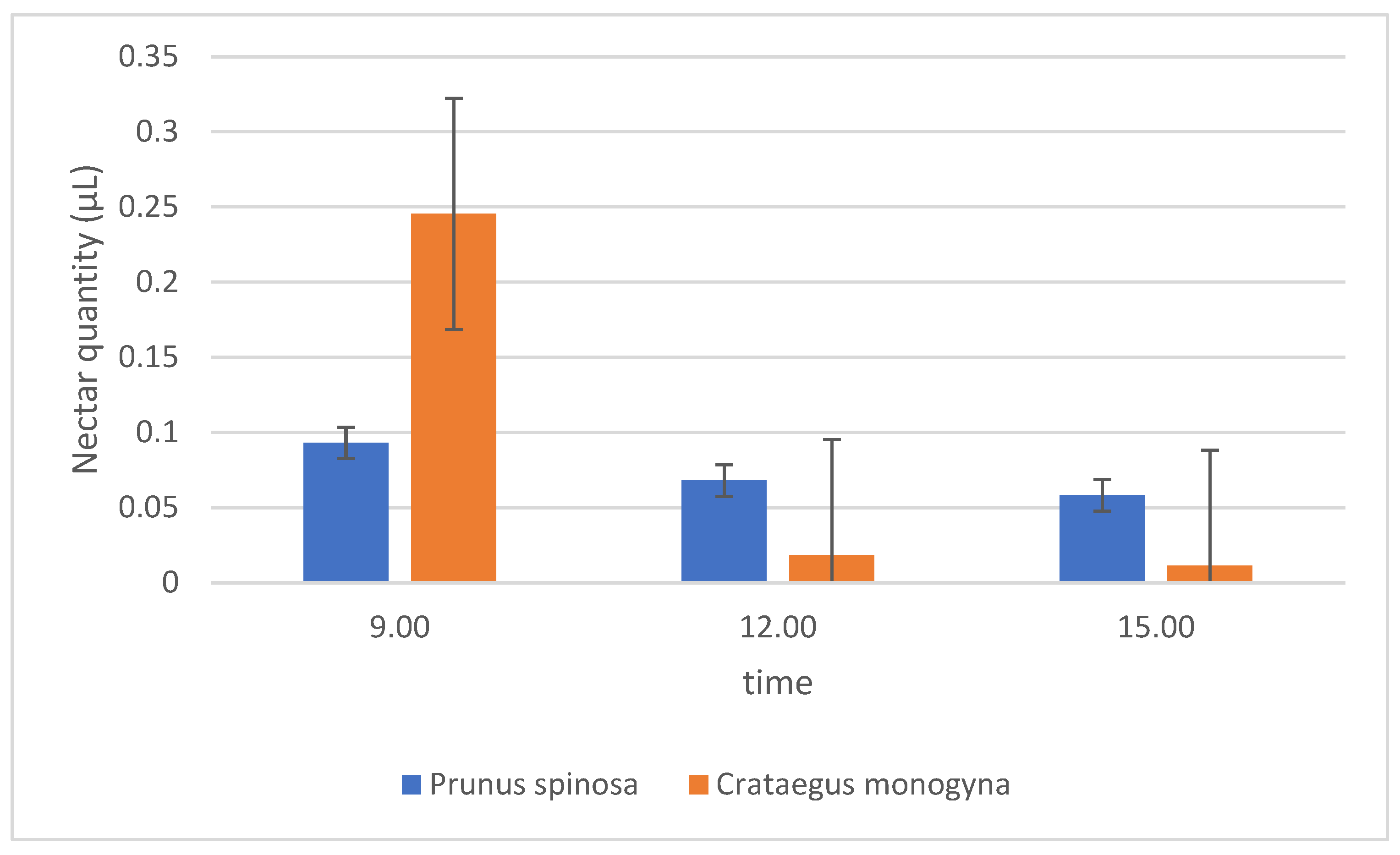
2.1. Quantity of Nectar in the Plant Species Studied
2.2. Content of Phenolic Compounds Studied in the Nectar of P. spinosa and C. monogyna
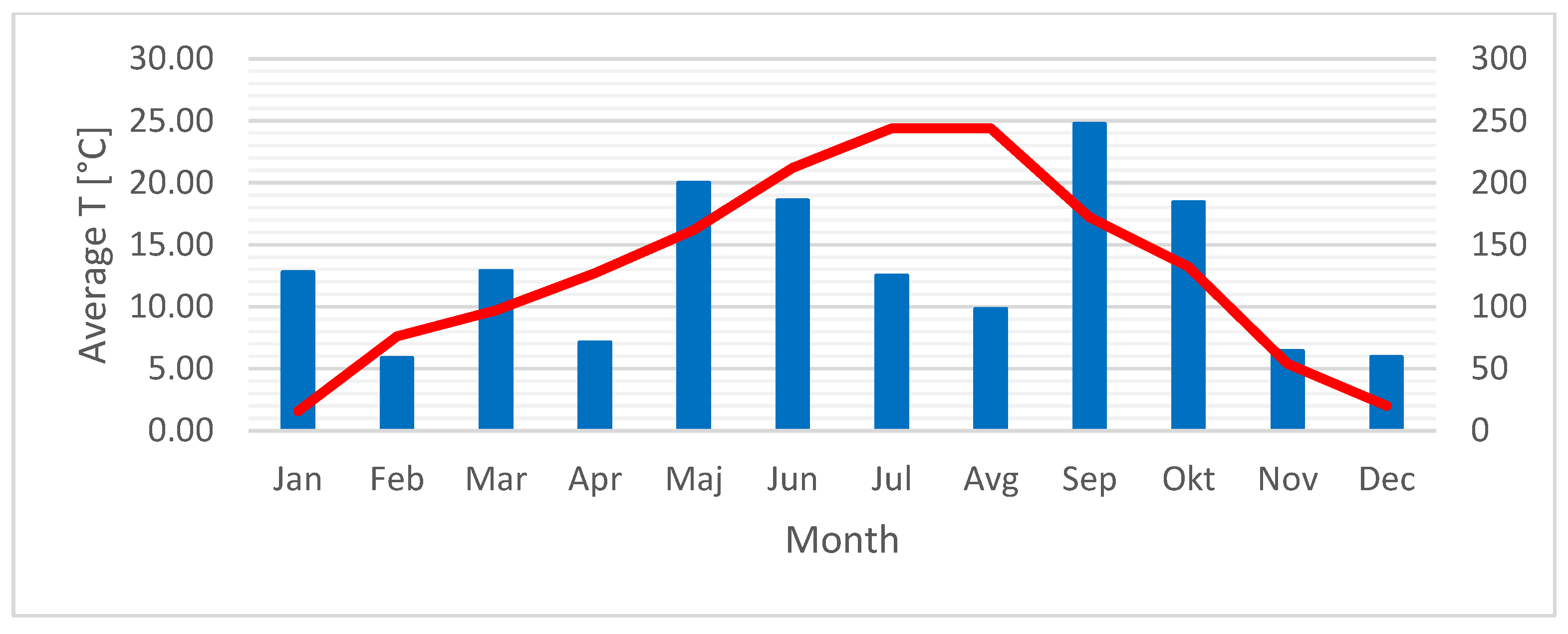
2.3. Effect of Environmental Factors on Phenolic Compounds
2.3.1. Effect of Environmental Factors on Phenolic Compounds in P. spinosa Nectar
2.3.2. Effect of Environmental Factors on Phenolic Compounds in C. monogyna Nectar
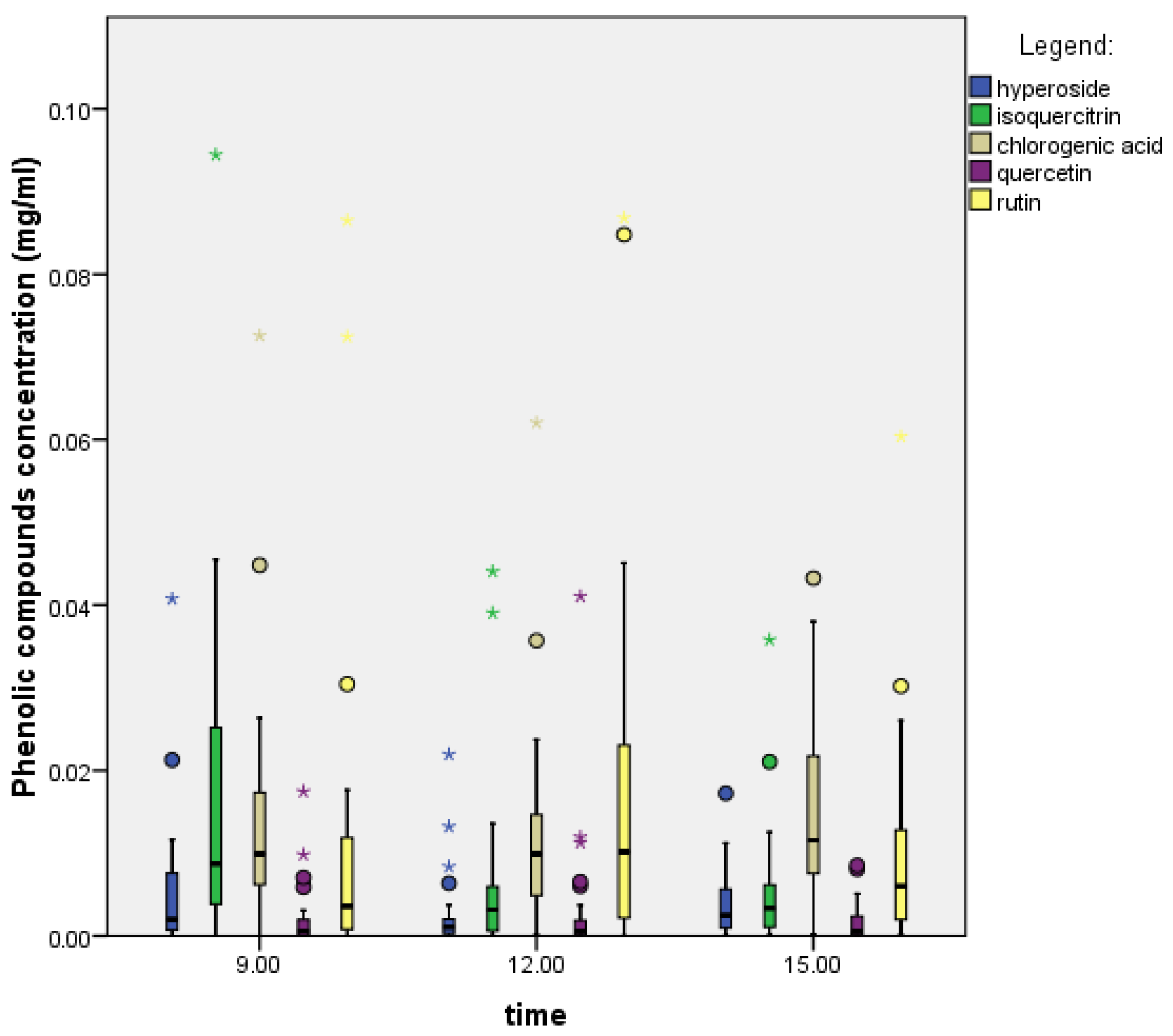
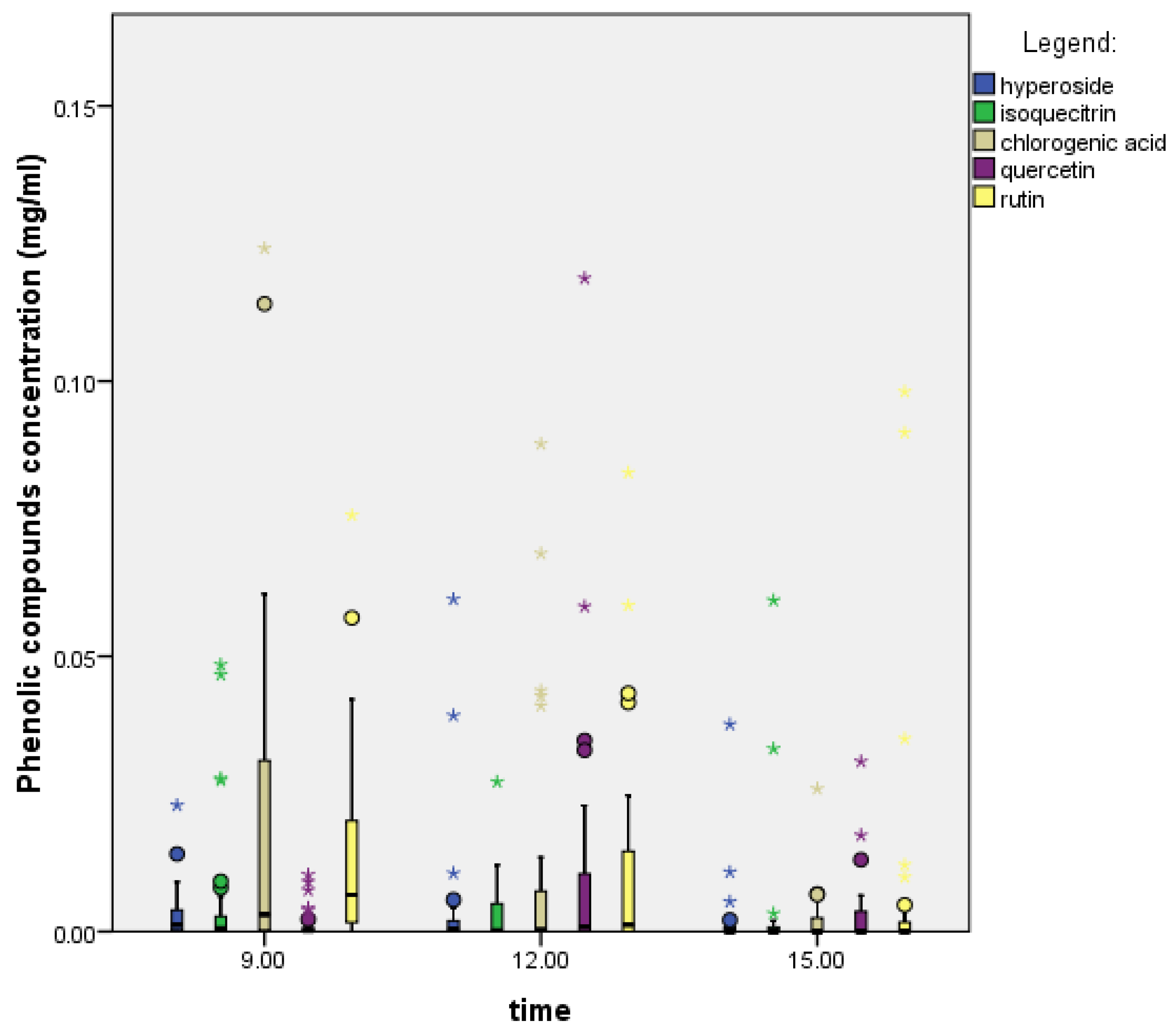
2.4. Effect of the Hour (Time of Day) on Phenolic Compounds
2.4.1. Prunus Spinosa
2.4.2. Crataegus Monogyna
3. Discussion
4. Materials and Methods
4.1. Selected Species
4.2. Sampling Locations
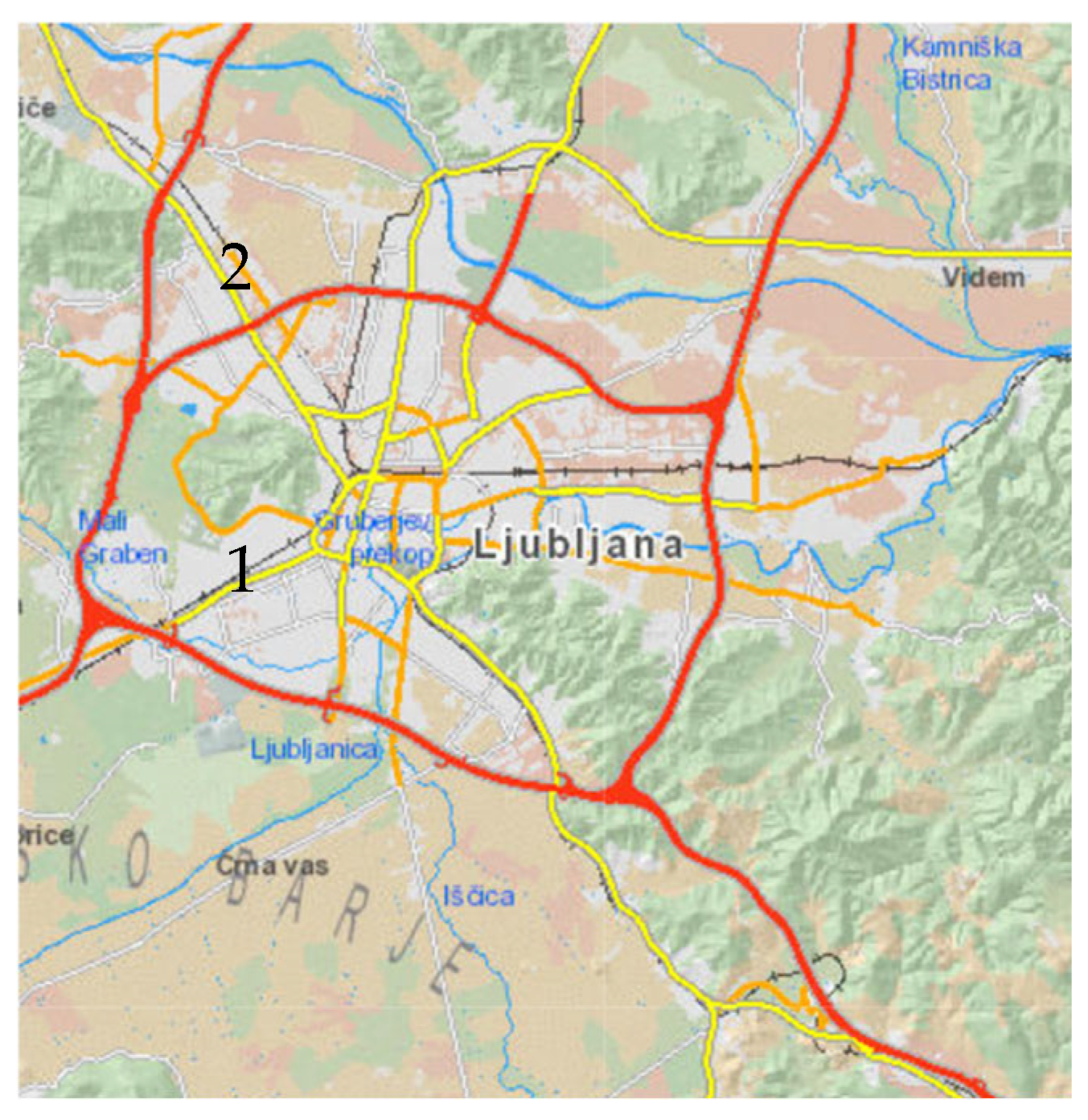
- Location 1: Večna pot Biological Centre in Ljubljana (Figure 7)
- Location 2: Meadow in Roje (Figure 7)
4.3. Nectar Sampling
4.4. Analysis of Phenolic Compounds
4.5. Data Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Knuth, P. Handbook of Flower Pollination Based upon Herman’s Muller Work, The Fertilisation of Flowers by Insects; The Clarendon Press: Oxford, UK, 1906; 383p. [Google Scholar]
- Kulloli, S.K.; Chandore, A.; Aitawade, M.M. Nectar dynamics and pollination studies in three species of Lamiaceae. Curr. Sci. 2011, 100, 509–516. [Google Scholar]
- Roy, R.; Schmitt, A.J.; Thomas, J.B.; Carter, C.J. Rewiew: Nectar biology: From molecules to ecosystems. Plant Sci. 2017, 262, 148–164. [Google Scholar] [CrossRef]
- Pyke, G.H. Floral Nectar: Pollinator Attraction or Manipulation? Trends Ecol. Evol. 2016, 31, 339–341. [Google Scholar] [CrossRef]
- Nepi, M.; Grasso, D.A.; Mancuso, S. Nectar in plant–insect mutualistic relationships: From food reward to partner manipulation. Front. Plant Sci. 2018, 9, 1063. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Thornburg, R.W. Nectar chemistry. In Nectaries and Nectar; Nicolson, S., Nepi, M., Pacini, E., Eds.; Springer: New York, NY, USA, 2007; pp. 215–263. [Google Scholar]
- Pozo, M.I.; Lievens, B.; Jacquemyn, H. Impact of microorganisms on nectar chemistry, pollinator attraction and plant fitness. In Nectar: Production, Chemical Composition and Benefits to Animals and Plants; Peck, R.L., Ed.; Nova Science Publishers Inc: New York, NY, USA, 2015; pp. 1–45. [Google Scholar]
- Adler, L.S. The ecological significance of toxic nectar. Oikos 2000, 91, 409–420. [Google Scholar] [CrossRef]
- Hagler, J.R.; Buchmann, S.L. Honeybee (Hymenoptera: Apidae) foraging responses to phenolic-rich nectars. J. Kans. Entomol. Soc. 1993, 66, 223–230. [Google Scholar]
- Schoonhoven, L.M.; van Loon, J.J.A.; Dicke, M. Plant Chemistry: Endless Variety. Insect-Plant Biology; Oxford University Press: Oxford, UK, 2005; pp. 3–82. [Google Scholar]
- Irwin, R.E.; Cook, D.; Richardson, L.L.; Manson, J.S.; Gardner, D.R. Secondary compounds in floral rewards of toxic rangeland plants: Impacts on pollinators. J. Agric. Food Chem. 2014, 62, 7335–7344. [Google Scholar] [CrossRef]
- Nešović, M.; Gašić, U.; Tosti, T.; Horvacki, N.; Šikoparija, B.; Nedić, N.; Blagojević, S.; Ignjatović, L.; Tešić, Ž. Polyphenol profile of buckwheat honey, nectar and pollen. R. Soc. Open Sci. 2020, 7, 201576. [Google Scholar] [CrossRef]
- Riveros, A.J.; Gronenberg, W. The flavonoid rutin protects the bumble bee Bombus impatiens against cognitive impairment by imidacloprid and fipronil. J. Exp. Biol. 2022, 225, jeb244526. [Google Scholar] [CrossRef]
- Kaurinovic, B.; Vastag, D. Flavonoids and Phenolic Acids as Potential Natural Antioxidants. In Antioxidants; Shalaby, E., Ed.; Intech Open: London, UK, 2019. [Google Scholar]
- Gao, J.; Zhao, G.; Yu, Y.; Liu, F. High Concentration of Nectar Quercetin Enhances Worker Resistance to Queen’s Signals in Bees. Chem. Ecol. 2010, 36, 1241–1243. [Google Scholar] [CrossRef]
- Nicolson, S. Sweet solutions: Nectar chemistry and quality. Phil. Trans. R. Soc. B 2022, 377, 20210163. [Google Scholar] [CrossRef] [PubMed]
- Soto, V.C.; Fernandez, M.; Galmarini, C.R.; Silva, M.F. Analysis of phenolic compounds in onion nectar by miniaturized off-line solid phase extraction-capillary zone electrophoresis. Anal. Methods 2014, 6, 4878. [Google Scholar]
- Nguyen, V.; Taine, E.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Heil, M.; Rattke, J.; Boland, W. Postsecretory hydrolysis of nectar sucrose and specialization in ant/plant mutualism. Science 2005, 308, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, H.; Yuan, L.; Li, X.; Cai, Y. Potential Implications of Hyperoside on Oxidative Stress-Induced Human Diseases: A Comprehensive Review. J. Inflamm. Res. 2023, 16, 4503–4526. [Google Scholar] [CrossRef]
- Ravnjak, B.; Bavcon, J.; Božič, J. Avtohtone Medovite Rastline; Botanični vrt Univerze v Ljubljani, Oddelek za biologijo, Biotehniška fakulteta = University Botanic Gardens Ljubljana, Department of Biology, Biotechnical Faculty: Ljubljana, Slovenia, 2020; 285p. [Google Scholar]
- Crane, E.; Walker, P.; Day, R. Directory of Important World Honey Sources; International Bee Research Association: London, UK, 1984; 384p. [Google Scholar]
- Gregori, J. Medonosne rastline. In Enciklopedija Slovenije; zv. 7; Voglar, D., Ed.; Mladinska knjiga: Ljubljana, Slovenia, 1993; pp. 49–50. [Google Scholar]
- Kradolfer, U.; Erhardt, A. Nectar secretion patterns in Salvia pratensis L. (Lamiaceae). Flora 1995, 190, 229–235. [Google Scholar] [CrossRef]
- Mačukanović-Jocić, M.; Djurdjević, L. Influence of microclimatic conditions on nectar exudation in Glechoma hirsuta. Arch. Biol. Sci. 2005, 57, 119–126. [Google Scholar] [CrossRef]
- Mačukanović Jocić, M.; Duletić-Laušević, S.; Jocic, G. Nectar production in three melliferous species of Lamiaceae in natural and experimental conditions. Acta Vet. 2004, 54, 475–487. [Google Scholar]
- Cardoza, Y.J.; Harris, G.K.; Grozinger, C.M. Effects of soil quality enhancement on pollinator-plant interactions. Psyche A J. Entomol. 2012, 2012, 581458. [Google Scholar] [CrossRef]
- Kostryco, M.; Chwil, M. Nectar abundance and nectar composition in selected Rubus idaeus L. varieties. Agriculture 2022, 12, 1132. [Google Scholar] [CrossRef]
- Meheriuk, M.; Lane, W.D.; Hall, J.W. Influence of cultivar on nectar sugar content in several species of tree fruits. HortScience 1987, 22, 448–450. [Google Scholar] [CrossRef]
- Percival, M.S. Types of nectar in angiosperms. New Phytol. 1961, 60, 235–281. [Google Scholar] [CrossRef]
- Chalcoff, V.R.; Aizen, M.A.; Galetto, L. Nectar concentration and composition of 26 species from the temperate forest of South America. Ann. Bot. 2006, 97, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Lanza, J.; Smith, G.C.; Sack, S.; Cash, A. Variation in nectar volume and composition of Impatiens capensis at the individual, plant, and population levels. Oecologia 1995, 102, 113–119. [Google Scholar] [CrossRef]
- Palmer-Young, E.C.; Farrell, I.W.; Adler, L.S.; Milano, N.J.; Egan, P.A.; Junker, R.R.; Irwin, R.E.; Stevenson, P.C. Chemistry of floral rewards: Intra-and interspecific variability of nectar and pollen secondary metabolites across taxa. Ecol. Monogr. 2019, 89, e01335. [Google Scholar] [CrossRef]
- Herrera, C.M.; Perez, R.; Alonso, C. Extreme interplant variation in nectar sugar composition in an insect pollinated perennial herb. Am. J. Bot. 2006, 93, 575–581. [Google Scholar] [CrossRef]
- Leiss, K.A.; Klinkhamer, P.G.L. Genotype by environment interactions in the nectar production of Echium vulgare. Funct. Ecol. 2005, 19, 454–459. [Google Scholar] [CrossRef]
- Buchmann, S.L.; Naphan, G.P. The Forgotten Pollinators; Island Press: Washington, DC, USA, 1997; 292p. [Google Scholar]
- Saunders, M.E. Insect pollinators collect pollen from wind-pollinated plants: Implications for pollination ecology and sustainable agriculture. Insect Conserv. Divers. 2017, 11, 13–31. [Google Scholar] [CrossRef]
- Baker, G.; Baker, I. Amino-acids in nectar and their evolutionary significance. Nature 1973, 241, 543–545. [Google Scholar] [CrossRef]
- Petanidou, T.; Goethals, V.; Smets, E. Nectary structure of Labiatae in relation to their nectar secretion and characteristics in a Mediterranean shrub community: Does flowering time matter? Plant Syst. Evol. 2000, 225, 103–118. [Google Scholar] [CrossRef]
- Heywood, V. Cvetnice: Kritosemenke Sveta; DZS: Ljubljana, Slovenia, 1995; 335p. [Google Scholar]
- Weryszko-Chmielewska, E.; Masierowska, M.L.; Konarska, A. Characteristics of floral nectaries and nectar in two species of Crataegus (Rosaceae). Plant Syst. Evol. 2003, 238, 33–41. [Google Scholar] [CrossRef]
- Vesprini, J.L.; Nepi, M.; Pacini, E. Nectary structure, nectar secretion patterns and nectar composition in two Helleborus species. Plant Biol. 1999, 1, 560–568. [Google Scholar] [CrossRef]
- Erbar, C.; Leins, P. Nectar production in the pollen flower of Anemone nemorosa in comparison with other Ranunculaceae and Magnolia (Magnoliaceae). Org. Divers. Evol. 2013, 13, 287–300. [Google Scholar] [CrossRef]
- Ballantyne, G.; Wilmer, P. Nectar theft and floral ant-repellence: A link between nectar volume and ant-repellent traits? PLoS ONE 2012, 7, e43869. [Google Scholar] [CrossRef] [PubMed]
- Corbet, S.A. Nectar sugar content: Estimating standing crop and secretion rate in the field. Apidologie 2003, 34, 1–10. [Google Scholar] [CrossRef]
- Morrant, D.S.; Schumann, R.; Petit, S. Field methods for sampling and storing nectar from flowers with low nectar volumes. Ann. Bot. 2008, 103, 533–542. [Google Scholar] [CrossRef]
- Corbet, S.A. Pollination and the weather. Isr. J. Bot. 1990, 39, 13–30. [Google Scholar]
- Corbet, S.A.; Willmer, P.G.; Beament, J.W.L.; Unwin, D.M.; Prys-Jones, O.E. Post-secretory determinants of sugar concentration in nectar. Plant Cell Environ. 1979, 2, 293–308. [Google Scholar] [CrossRef]
- Petanidou, T. Sugars in Mediterranean Floral Nectars: An Ecological and Evolutionary Approach. J. Chem. Ecol. 2005, 31, 1065–1088. [Google Scholar] [CrossRef]
- Karbassioon, A.; Yearlsey, J.; Dirilgen, T.; Hodge, S.; Stour, J.; Stanley, D.A. Responses in honeybee and bumblebee activity to changes in weather conditions. Oecologia 2023, 201, 689–701. [Google Scholar] [CrossRef]
- Gimeno, C.E. Compuestos Fenólicos. Un Análisis de Sus Beneficios Para La Salud. Offarm 2004, 23, 80–84. [Google Scholar]
- Sambangi, P. Phenolic Compounds in the Plant Development and Defense: An Overview. In Plant Stress Physiology—Perspectives in Agriculture; Hasanuzzaman, M., Nahar, K., Eds.; InTech: London, UK, 2022. [Google Scholar]
- Minatel, I.O.; Vanz, C.; Ferreira, M.; Gomez, H.A.; Chen, C.-Y.; Pereira, G. Phenolic compounds: Functional properties, impact of processing and bioavailability. In Phenolic Compounds. Biological Activity; Soto-Hernández, M., Palma-Tenango, M., García-Mateos, R., Eds.; InTech: London, UK, 2017. [Google Scholar]
- Slavković, F.; Bendahmane, A. Floral Phytochemistry: Impact of Volatile Organic Compounds and Nectar Secondary Metabolites on Pollinator Behavior and Health. Chem. Biodivers. 2023, 20, e202201139. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo, L.L.; Martínez, F.S.; Agudo, P.T.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ji, C.; Wang, R.; Gao, L.; Luo, W.; Jualin, L. Dietary Quercetin Regulates Gut Microbiome Diversity and Abundance in Apis cerana (Hymenoptera Apidae). Insects 2024, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef] [PubMed]
- ARSO. Available online: https://meteo.arso.gov.si/met/sl/climate/ (accessed on 29 March 2025).
- Guffa, B.; Nedić, N.M.; Dabić Zagorac, D.Č.; Tosti, T.B.; Gašić, U.M.; Natić, M.M.; Fotirić Akšić, M.M. Characterization of Sugar and Polyphenolic Diversity in Floral Nectar of Different “Oblačinska” Sour Cherry Clones. Chem. Biodivers. 2017, 14, e1700061. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Mačukanović-Jocić, M.; Radođević, R.; Nedić, N.; Gašić, U.; Tosti, T.; Tešić, Ž.; Meland, M. The Morpho-Anatomy of Nectaries and Chemical Composition of Nectar in Pear Cultivars with Different Susceptibility to Erwinia amlylovora. Horticulturae 2023, 9, 424. [Google Scholar] [CrossRef]
- Hoffmann, J.F.; Zandoná, G.P.; dos Santos, P.S.; Dallmann, C.M.; Madruga, F.B.; Rombaldi, C.V.; Chaves, F.C. Stability of bioactive compounds in butiá (Butia odorata) fruit pulp and nectar. Food Chem. 2017, 237, 638–644. [Google Scholar] [CrossRef]
- Becerril-Sanchez, A.L.; Quintero-Salazar, B.; Dublan-Garcia, O.; Escalona-Buendia, H. Phenolic Compounds in Honey and Their Relationship with Antioxidant Activity, Botanical Origin, and Color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Heil, M. Nectar: Generation, regulation and ecological functions. Trends Plant Sci. 2011, 16, 191–200. [Google Scholar] [CrossRef]
- McArt, S.H.; Koch, H.; Irwin, R.E.; Adler, L.S. Arranging the bouquet of disease: Floral traits and the transmission of plant and animal pathogens. Ecol. Lett. 2014, 17, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Kadakal, C.; Duman, T. Thermal degradation kinetics of rutin and total phenolic compounds in rosehip (Rosa canina L.) nectar. Pamukkale Univ. Muh. Bilim. Derg. 2018, 24, 1370–1375. [Google Scholar] [CrossRef]
- Martinčič, A.; Wraber, T.; Jogan, N.; Podobnik, A.; Turk, B.; Vreš, B.; Ravnik, V.; Frajman, B.; Strgulc Krajšek, S.; Trčak, B.; et al. Mala Flora Slovenije: Ključ Za Določanje Praprotnic in Semenk; Tehniška založba Slovenije: Ljubljana, Slovenia, 2007; 967p. [Google Scholar]
- Jogan, N.; Bačič, T.; Frajman, B.; Leskovar, I.; Naglič, D.; Podobnik, A.; Rozman, B.; Strgulc-Krajšek, S.; Trčak, B. Gradivo Za Atlas Flore Slovenije [Materials for the Atlas of Flora of Slovenia]; Center za kartografijo favne in flore: Miklavž na Dravskem polju, Slovenia, 2001; 443p. [Google Scholar]
- Wraber, M. Pflanzengeographische Stellung und Gliederung Sloweniens. Vegetatio; Acta Geobotanica: Ljubljana, Slovenia, 1969; pp. 176–199. [Google Scholar]
- Tomažič, G. 1. Bazofilni borovi gozdovi. In Asociacije Borovih Gozdov v Sloveniji; Razprave matematično-prirodoslovnega razreda Akademije znanosti in umetnosti 1; Slovenska Akademija Znanosti in Umetnosti: Ljubljana, Slovenia, 1940; Volume 1, pp. 77–120. [Google Scholar]
- Collins, B.G.; Newland, C. Honeyeater population changes in relation to food availability in the jarrah forest of Western Australia. Aust. J. Ecol. 1986, 11, 63–76. [Google Scholar] [CrossRef]


| chl. Acid (μg/mL) | Rutin (μg/mL) | (iso)quercitrin (μg/mL) | Hyperoside (μg/mL) | Quercetin (μg/mL) | |
|---|---|---|---|---|---|
| P. spinosa (mean) | 14.6 | 17.2 | 13.8 | 6.1 | 2.22 |
| P. spinosa (median ± SD) | 10.3 ± 17.2 | 6 ± 33.6 | 4.7 ± 46.5 | 1.8 ± 23.2 | 0.6 ± 5 |
| C. monogyna (mean) | 22.0 | 11.0 | 4.0 | 3.0 | 21.0 |
| C. monogyna (median ± SD) | 0.5 ± 86.7 | 1.4 ± 20.4 | 0.19 ± 10.6 | 0.4 ± 8.6 | 0.09 ± 136 |
| Air T (°C) | Soil T (°C) | Soil Humidity (%) | Absolute Air Humidity (g/kg) | UVB (μW/cm2) | |
|---|---|---|---|---|---|
| chloro. acid | x | x | x | x | x |
| rutin | 0.257 p = 0.012 | 0.201 p = 0.049 | 0.244 p = 0.017 | x | x |
| (iso)quercitrin | x | −0.315 p = 0.002 | x | x | x |
| hyperoside | x | x | x | x | x |
| quercetin | x | x | x | x | x |
| Air T (°C) | Soil T (°C) | Soil Humidity (%) | Absolute Air Humidity (g/kg) | UVB (μW/cm2) | |
|---|---|---|---|---|---|
| chloro. acid | −0.425 p = 0.000 | −0356 p = 0.000 | x | −0.236 p = 0.021 | −0.376 p = 0.000 |
| rutin | −0.366 p = 0.000 | −0.340 p = 0.001 | x | −0.202 p = 0.048 | −0.279 p = 0.006 |
| (iso)quercitrin | −0.326 p = 0.001 | −0.321 p = 0.001 | x | x | −0.260 p = 0.011 |
| hyperoside | −0.290 p = 0.004 | −0.309 p = 0.002 | x | x | x |
| quercetin | x | x | x | x | x |
| chl. Acid (μg/mL) | Rutin (μg/mL) | (iso)quercitrin (μg/mL) | Hyperoside (μg/mL) | Quercetin (μg/mL) | |
|---|---|---|---|---|---|
| 09:00 (mean) | 17 | 16 | 30 | 12 | 22 |
| 09:00 (median ± SD) | 9.9 ± 25.1 | 4.4 ± 38 | 8.7 ± 77.9 | 1.9 ± 39.5 | 0.6 ± 3.5 |
| 12:00 (mean) | 12 | 25 | 6 | 3 | 3 |
| 12:00 (median ± SD) | 9.9 ± 11.7 | 10 ± 41.5 | 3.1 ± 9.9 | 1.7 ± 4.5 | 0.6 ± 7.5 |
| 15:00 (mean) | 15 | 10 | 5 | 4 | 2 |
| 15:00 (median ± SD) | 11.6 ± 10.9 | 6 ± 12.2 | 3.4 ± 7.1 | 2.5 ± 4.1 | 0.6 ± 2.2 |
| chl. Acid (μg/mL) | Rutin (μg/mL) | (iso)quercitrin (μg/mL) | Hyperoside (μg/mL) | Quercetin (μg/mL) | |
|---|---|---|---|---|---|
| 09:00 | 30 | 14 | 6 | 3 | 1 |
| 3 ± 60.5 | 6 ± 17.9 | 0.6 ± 12.8 | 12 ± 4.8 | 0.1 ±2 | |
| 12:00 | 11 | 11 | 3 | 4 | 58 |
| 0.3 ± 21.9 | 1.2 ± 19.8 | 0.2 ± 5.8 | 0.3 ± 12.4 | 0.8 ± 233.2 | |
| 15:00 | 26 | 8 | 3 | 2 | 3 |
| 0 ± 135 | 0 ± 23.5 | 0 ± 11.9 | 0 ± 6.8 | 0 ± 6.5 |
| Analyte | Repeatability (% RSD; n = 5) | Reproducibility (% RSD; n = 3) | LOD (ng) * | LOQ (ng) * | Linearity Range (ng *; r) |
|---|---|---|---|---|---|
| Proline | 2.82 | 5.37 | 0.7 | 2.3 | 2.3–200; 0.9993 |
| Leucine/Isoleucine | 2.07 | 3.71 | 0.9 | 3.0 | 3.0–200; 0.9995 |
| Methionine | 4.74 | 6.39 | 1.9 | 6.4 | 6.4–200; 0.9988 |
| Alanine | 0.58 | 4.38 | 0.1 | 0.5 | 0.5–200; 0.9958 |
| Tyrosine | 1.96 | 2.53 | 0.2 | 0.5 | 0.5–200; 0.9992 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malovrh, K.; Ravnjak, B.; Križman, M.; Bavcon, J. Phenolic Compounds in Nectar of Crataegus monogyna Jacq. and Prunus spinosa L. Plants 2025, 14, 2064. https://doi.org/10.3390/plants14132064
Malovrh K, Ravnjak B, Križman M, Bavcon J. Phenolic Compounds in Nectar of Crataegus monogyna Jacq. and Prunus spinosa L. Plants. 2025; 14(13):2064. https://doi.org/10.3390/plants14132064
Chicago/Turabian StyleMalovrh, Katja, Blanka Ravnjak, Mitja Križman, and Jože Bavcon. 2025. "Phenolic Compounds in Nectar of Crataegus monogyna Jacq. and Prunus spinosa L." Plants 14, no. 13: 2064. https://doi.org/10.3390/plants14132064
APA StyleMalovrh, K., Ravnjak, B., Križman, M., & Bavcon, J. (2025). Phenolic Compounds in Nectar of Crataegus monogyna Jacq. and Prunus spinosa L. Plants, 14(13), 2064. https://doi.org/10.3390/plants14132064





