Glucosinolate and Sugar Profiles in Space-Grown Radish
Abstract
1. Introduction
2. Results
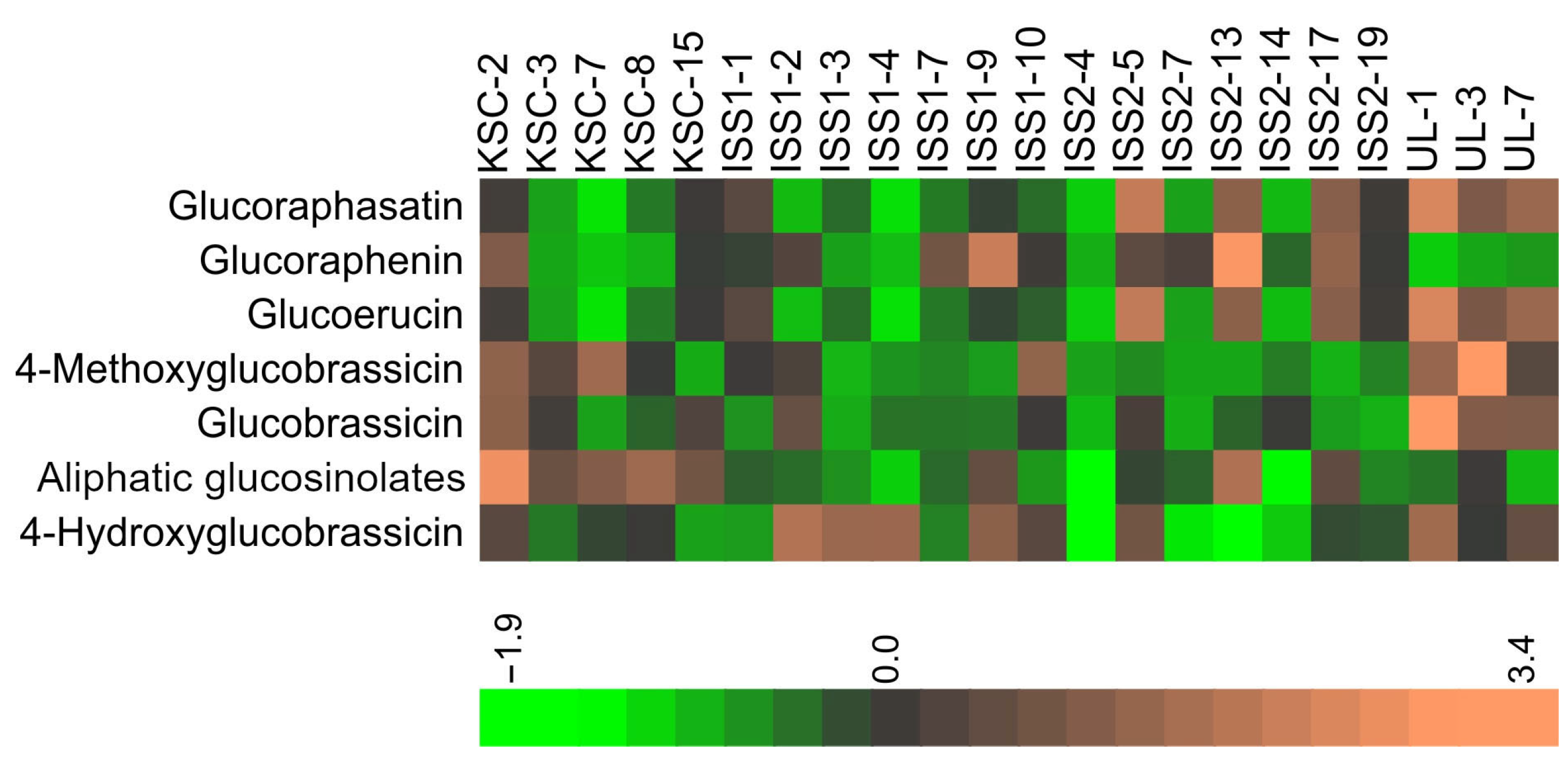
2.1. Glucosinolates
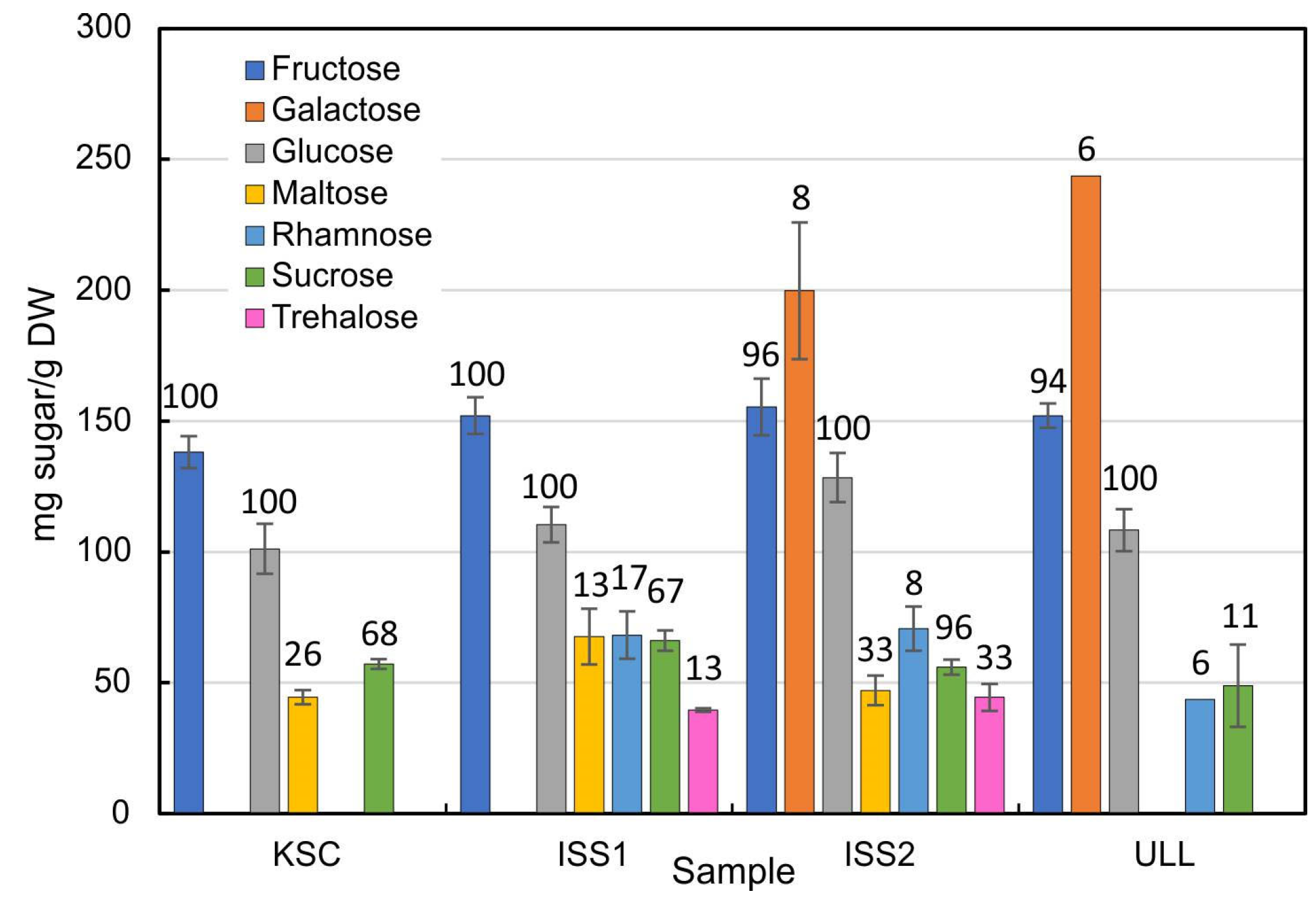
2.2. Sugar Analyses
3. Discussion
4. Materials and Methods
4.1. Plant Cultivation
4.2. Glucosinolate Analysis
4.3. UPLC-qTOF-MS Analysis
4.4. Sugar Quantification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APH | Advanced Plant Habitat |
| GSL | Glucosinolate |
| KSC | Kennedy Space Center |
| ISS | International Space Station |
| LC-MS | Liquid chromatography–mass spectrometry |
| UPLC-qTOF-MS | Ultra-high performance liquid chromatography with quadrupole time-of-flight mass spectrometry |
References
- Höhner, R.; Marques, J.V.; Ito, T.; Amakura, Y.; Budgeon, A.D.; Weitz, K.; Hixson, K.K.; Davin, L.B.; Kirchhoff, H.; Lewis, N.G. Reduced Arogenate Dehydratase Expression: Ramifications for Photosynthesis and Metabolism. Plant Physiol. 2018, 177, 115–131. [Google Scholar] [CrossRef]
- Kerwin, J.; Seddon, R. Eating in Space—From an Astronaut’s Perspective. Nutrition 2002, 18, 921–925. [Google Scholar] [CrossRef]
- Depree, J.A.; Howard, T.M.; Savage, G.P. Flavour and pharmaceutical properties of the volatile sulphur compounds of Wasabi (Wasabia japonica). Food Res. Int. 1999, 31, 329–337. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Klopsch, R.; Oliviero, T.; Schreiner, M.; Verkerk, R.; Dekker, M. Optimizing isothiocyanate formation during enzymatic glucosinolate breakdown by adjusting pH value, temperature and dilution in Brassica vegetables and Arabidopsis thaliana. Sci. Rep. 2017, 7, 40807. [Google Scholar] [CrossRef] [PubMed]
- Romeo, L.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Isothiocyanates: An overview of their antimicrobial activity against human infections. Molecules 2018, 23, 624. [Google Scholar] [CrossRef]
- Miekus, N.; Marszalek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Swiergiel, A.H. Health benefits of plant-derived sulfur compounds, glucosinolates, and organosulfur compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef]
- Hayes, J.D.; Kelleher, M.O.; Eggleston, I.M. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur. J. Nutr. 2008, 47, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Bian, Z.H.; Yuan, X.X.; Chen, X.; Lu, C.G. A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Ali, V.; Mandal, J.; Vyas, D. Insights into light-driven dynamics of phytochemicals in sprouts and microgreens. Plant Growth Regul. 2025, 105, 129–152. [Google Scholar] [CrossRef]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB light doses and harvesting time differentially tailor glucosinolate and phenolic profiles in broccoli sprouts. Molecules 2017, 22, 1065. [Google Scholar] [CrossRef]
- Matusheski, N.V.; Juvik, J.A.; Jeffery, E.H. Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochemistry 2004, 65, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Moyes, C.L.; Collin, H.A.; Raybould, A.F. Glucosinolates and differential herbivory in wild populations of Brassica oleracea. J. Chem. Ecol. 2000, 26, 2625–2641. [Google Scholar] [CrossRef]
- Velasco, P.; Cartea, M.E.; González, C.; Vilar, M.; Ordás, A. Factors affecting the glucosinolate content of kale (Brassica oleracea acephala group). J. Agric. Food Chem. 2007, 55, 955–962. [Google Scholar] [CrossRef]
- Kitainda, V.; Jez, J.M. Kinetic and catalytic mechanisms of the methionine-derived glucosinolate biosynthesis enzyme methylthioalkylmalate synthase. J. Biol. Chem. 2024, 300, 107814. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burcul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Abdelshafeek, K.A.; El-Shamy, A.M. Review on glucosinolates: Unveiling their potential applications as drug discovery leads in extraction, isolation, biosynthesis, biological activity, and corrosion protection. Food Biosci. 2023, 56, 103071. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef]
- Allen, J.; Bisbee, P.A.; Darnell, R.L.; Kuang, A.; Levine, L.H.; Musgrave, M.E.; van Loon, J. Gravity control of growth form in Brassica rapa and Arabidopsis thaliana (Brassicaceae): Consequences for secondary metabolism. Am. J. Bot. 2009, 96, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Hoson, T. Plant growth and morphogenesis under different gravity conditions: Relevance to plant life in space. Life 2014, 4, 205–216. [Google Scholar] [CrossRef]
- Mortley, D.G.; Bonsi, C.K.; Hill, W.A.; Morris, C.E.; Williams, C.S.; Davis, C.F.; Williams, J.W.; Levine, L.H.; Petersen, B.V.; Wheeler, R.M. Influence of microgravity environment on root growth, soluble sugars, and starch concentration of sweetpotato stem cuttings. J. Am. Soc. Hortic. Sci. 2008, 133, 327–332. [Google Scholar] [CrossRef]
- John, S.; Abou-Issa, F.; Hasenstein, K. Space flight cultivation for radish (Raphanus sativus) in the Advanced Plant Habitat. Gravitational Space Res. 2021, 9, 121–132. [Google Scholar] [CrossRef]
- Hasenstein, K.H.; John, S.P.; Vandenbrink, J.P. Assessing radish health during space cultivation by gene transcription. Plants 2023, 12, 3458. [Google Scholar] [CrossRef]
- Glauser, G.; Schweizer, F.; Turlings, T.C.J.; Reymond, P. Rapid Profiling of Intact Glucosinolates in Arabidopsis Leaves by UHPLC-QTOFMS Using a Charged Surface Hybrid Column. Phytochem. Anal. 2012, 23, 520–528. [Google Scholar] [CrossRef]
- Liang, X.; Lee, H.W.; Li, Z.F.; Lu, Y.H.; Zou, L.; Ong, C.N. Simultaneous Quantification of 22 Glucosinolates in 12 Brassicaceae Vegetables by Hydrophilic Interaction Chromatography-Tandem Mass Spectrometry. ACS Omega 2018, 3, 15546–15553. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Huang, Y.; Zhang, S.; Cui, L.; Jiao, Z.; Peng, Z.; Luo, X.; Liu, Y.; Qiu, Z. Dynamic profiling of intact glucosinolates in radish by combining UHPLC-HRMS/MS and UHPLC-QqQ-MS/MS. Front. Plant Sci. 2023, 14, 1216682. [Google Scholar] [CrossRef]
- Park, C.H.; Park, S.-Y.; Park, Y.J.; Kim, J.K.; Park, S.U. Metabolite profiling and comparative analysis of secondary metabolites in chinese cabbage, radish, and hybrid xBrassicoraphanus. J. Agric. Food Chem. 2020, 68, 13711–13719. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Sun, H.; Zhang, Z.; Qian, H.; Zhao, X.; He, H.; Zhang, L. Glucosinolate profiles in different organs of 111 radish accessions and candidate genes involved in converting glucobrassicin to 4-hydroxyglucobrassicin. J. Agric. Food Chem. 2022, 70, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Lim, S.; Chae, W.B.; Park, J.E.; Park, H.R.; Lee, E.J.; Huh, J.H. Root glucosinolate profiles for screening of radish (Raphanus sativus L.) genetic resources. J. Agric. Food Chem. 2016, 64, 61–70. [Google Scholar] [CrossRef]
- Hooshmand, K.; Fomsgaard, I.S. Analytical methods for quantification and identification of intact glucosinolates in Arabidopsis roots using LC-QqQ(LIT)-MS/MS. Metabolites 2021, 11, 47. [Google Scholar] [CrossRef]
- Castellaneta, A.; Losito, I.; Cisternino, G.; Leoni, B.; Santamaria, P.; Calvano, C.D.; Bianco, G.; Cataldi, T.R. All ion fragmentation analysis enhances the untargeted profiling of glucosinolates in Brassica microgreens by liquid chromatography and high-resolution mass spectrometry. J. Am. Soc. Mass Spectrom. 2022, 33, 2108–2119. [Google Scholar] [CrossRef]
- Kjær, A.; Ohashi, M.; Wilson, J.M.; Djerassi, C.; Munch-Petersen, J. Mass Spectra of Isothiocyanates. Acta Chem. Scand. 1963, 17, 2143–2154. [Google Scholar] [CrossRef]
- Kjær, A.; Øgaard Madsen, J.; Maeda, Y.; Ozawa, Y.; Uda, Y. Volatiles in distillates of fresh radish of Japanese and Kenyan origin. Agric. Biol. Chem. 1978, 42, 1715–1721. [Google Scholar][Green Version]
- Montaut, S.; Bleeker, R.S.; Jacques, C. Phytochemical constituents of Cardamine diphylla. Can. J. Chem. 2010, 88, 50–55. [Google Scholar] [CrossRef]
- Blažević, I.; Mastelić, J. Glucosinolate degradation products and other bound and free volatiles in the leaves and roots of radish (Raphanus sativus L.). Food Chem. 2009, 113, 96–102. [Google Scholar] [CrossRef]
- West, L.G.; Meyer, K.A.; Balch, B.A.; Rossi, F.J.; Schultz, M.R.; Haas, G.W. Glucoraphanin and 4-hydroxyglucobrassicin contents in seeds of 59 cultivars of broccoli, raab, kohlrabi, radish, cauliflower, brussels sprouts, kale, and cabbage. J. Agric. Food Chem. 2004, 52, 916–926. [Google Scholar] [CrossRef]
- Kim, S.-J.; Uddin, M.R.; Park, S.U. Glucosinolate accumulation in three important radish (Raphanus sativus) cultivars. Aust. J. Crop Sci. 2013, 7, 1843–1847. [Google Scholar]
- Mitreiter, S.; Gigolashvili, T. Regulation of glucosinolate biosynthesis. J. Exp. Bot. 2021, 72, 70–91. [Google Scholar] [CrossRef]
- Yatusevich, R.; Mugford, S.G.; Matthewman, C.; Gigolashvili, T.; Frerigmann, H.; Delaney, S.; Koprivova, A.; Flugge, U.-I.; Kopriva, S. Genes of primary sulfate assimilation are part of the glucosinolate biosynthetic network in Arabidopsis thaliana. Plant J. 2010, 62, 1–11. [Google Scholar] [CrossRef]
- Muthusamy, M.; Hwang, J.E.; Kim, S.H.; Kim, J.A.; Jeong, M.-J.; Park, H.C.; Lee, S.I. Elevated carbon dioxide significantly improves ascorbic acid content, antioxidative properties and restricted biomass production in cruciferous vegetable seedlings. Plant Biotechnol. Rep. 2019, 13, 293–304. [Google Scholar] [CrossRef]
- Stutte, G.W.; Monje, O.; Hatfield, R.D.; Paul, A.L.; Ferl, R.J.; Simone, C.G. Microgravity effects on leaf morphology, cell structure, carbon metabolism and mRNA expression of dwarf wheat. Planta 2006, 224, 1038–1049. [Google Scholar] [CrossRef]
- Kasahara, H.; Shiwa, M.; Takeuchi, Y.; Yamada, M. Effects of hypergravity on the elongation growth in radish and cucumber hypocotyls. J. Plant Res. 1995, 108, 59–64. [Google Scholar] [CrossRef]
- Seo, M.-S.; Kim, J.S.; Jeong, J.-h.; Park, B.-S. Analysis of sugars content by genotypes in 82 radish (Raphanus sativus L.). Korean J. Plant Reources 2018, 31, 453–465. [Google Scholar] [CrossRef]
- Porterfield, D.M.; Crispi, M.L.; Musgrave, M.E. Changes in soluble sugar, starch, and alcohol dehydrogenase in Arabidopsis thaliana exposed to N-2 diluted atmospheres. Plant Cell Physiol. 1997, 38, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Urbonaviciute, A.; Samuoliene, G.; Sakalauskaite, J.; Duchovskis, P.; Brazaityte, A.; Siksnianiene, J.B.; Ulinskaite, R.; Sabajeviene, G.; Baranauskis, K. The effect of elevated CO2 concentrations on leaf carbohydrate, chlorophyll contents and photosynthesis in radish. Pol. J. Environ. Stud. 2006, 15, 921–925. [Google Scholar]
- Barnes, J.D.; Pfirrmann, T. The influence of CO2 and O3, singly and in combination, on gas-exchange, growth and nutrient status of radish (Raphanus sativus L.). New Phytol. 1992, 121, 403–412. [Google Scholar] [CrossRef]
- Akram, N.A.; Waseem, M.; Ameen, R.; Ashraf, M. Trehalose pretreatment induces drought tolerance in radish (Raphanus sativus L.) plants: Some key physio-biochemical traits. Acta Physiol. Plant. 2016, 38, 3. [Google Scholar] [CrossRef]
- Raza, A.; Bhardwaj, S.; Rahman, M.A.; García-Caparrós, P.; Habib, M.; Saeed, F.; Charagh, S.; Foyer, C.H.; Siddique, K.H.M.; Varshney, R.K. Trehalose: A sugar molecule involved in temperature stress management in plants. Crop. J. 2024, 12, 1–16. [Google Scholar] [CrossRef]
- Musgrave, M.E.; Kuang, A.; Tuominen, L.K.; Levine, L.H.; Morrow, R.C. Seed storage reserves and glucosinolates in Brassica rapa L. grown on the international space station. J. Am. Soc. Hortic. Sci. 2005, 130, 848–856. [Google Scholar] [CrossRef]
- Zinkernagel, J.; Prince, M.; Koppel, M.; Rubo, S.; Schmidt, L. Nitrogen form mediates sink strength and resource allocation of a C3 root crop under elevated CO2. Environ. Exp. Bot. 2024, 226, 105892. [Google Scholar] [CrossRef]
- Prasad, B.; Richter, P.; Vadakedath, N.; Haag, F.W.M.; Strauch, S.M.; Mancinelli, R.; Schwarzwalder, A.; Etcheparre, E.; Gaume, N.; Lebert, M. How the space environment influences organisms: An astrobiological perspective and review. Int. J. Astrobiol. 2021, 20, 159–177. [Google Scholar] [CrossRef]
- Paul, A.-L.; Haveman, N.; Califar, B.; Ferl, R.J. Epigenomic regulators elongator complex subunit 2 and methyltransferase 1 differentially condition the spaceflight response in Arabidopsis. Front. Plant Sci. 2021, 12, 691790. [Google Scholar] [CrossRef] [PubMed]
- Medina, F.J. Space explorers need to be space farmers: What we know and what we need to know about plant growth in space. Metode Sci. Stud. J. 2021, 11, 55–62. [Google Scholar] [CrossRef]
- Manian, V.; Gangapuram, H.; Orozco, J.; Janwa, H.; Agrinsoni, C. Network analysis of local gene regulators in Arabidopsis thaliana under spaceflight stress. Computers 2021, 10, 18. [Google Scholar] [CrossRef]
- Maldini, M.; Foddai, M.; Natella, F.; Petretto, G.L.; Rourke, J.P.; Chessa, M.; Pintore, G. Identification and quantification of glucosinolates in different tissues of Raphanus raphanistrum by liquid chromatography tandem-mass spectrometry. J. Food Compos. Anal. 2017, 61, 20–27. [Google Scholar] [CrossRef]
- McKee, J.M.T. A Simple Method for the Extraction of reducing and non-reducing sugars from carrot and other storage root vegetables. J. Sci. Food Agric. 1985, 36, 55–58. [Google Scholar] [CrossRef]




| Annotation | Rt, min | Observed Mass ‡ [M–H]– | Calculated Mass [M–H]– | Error, ppm | Ion Fragments ** | Molecular Formulae |
|---|---|---|---|---|---|---|
| Glucoraphenin | 2.53 | 434.0248 | 434.0249 | 0.23 | 259, 97, 96 | C12H20NO10S3 |
| 4-Hydroxyglucobrassicin § | 4.12 | 463.0500 | 463.0481 | 4.10 | 259, 97, 96 | C16H19N2O10S2 |
| Glucoerucin | 4.59 | 420.0452 | 420.0457 | 1.19 | 259, 97, 96 | C12H22NO9S3 |
| Glucoraphasatin * | 4.60 | 418.0298 | 418.0300 | 0.48 | 259, 97, 96 | C12H20NO9S3 |
| Glucobrassicin | 4.80 | 447.0529 | 447.0532 | 0.67 | 259, 97, 96 | C16H19N2O9S2 |
| 4-Methoxyglucobrassicin | 5.19 | 477.0645 | 477.0638 | 1.47 | 259, 97, 96 | C17H21N2O10S2 |
| 3-Methylpentyl GSL | 5.50 | 402.0881 | 402.0861 | 4.97 | 259, 97, 96 | C13H24NO9S2 |
| 4-Methylpentyl GSL | 5.62 | 402.0877 | 402.0861 | 3.98 | 259, 97, 96 | C13H24NO9S2 |
| n-Hexyl GSL | 5.75 | 402.0877 | 402.0861 | 3.98 | 259, 97, 96 | C13H24NO9S2 |
| Carbohydrate | Rt, min |
|---|---|
| Rhamnose | 4.54 |
| Fucose | 5.08 |
| Psicose | 5.09 |
| Xylose | 5.11 |
| Arabinose | 5.55 |
| Fructose | 6.05 |
| Mannose | 6.67 |
| Sorbitol | 6.88 |
| Mannitol | 7.03 |
| Glucose | 7.05 |
| Galactose | 7.35 |
| Galactosamine | 9.21 |
| Sucrose | 10.19 |
| Myo-inositol | 11.35 |
| Maltose | 12.17 |
| Lactose | 13.93 |
| Trehalose | 14.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasenstein, K.H.; Moinuddin, S.G.A.; Berim, A.; Davin, L.B.; Lewis, N.G. Glucosinolate and Sugar Profiles in Space-Grown Radish. Plants 2025, 14, 2063. https://doi.org/10.3390/plants14132063
Hasenstein KH, Moinuddin SGA, Berim A, Davin LB, Lewis NG. Glucosinolate and Sugar Profiles in Space-Grown Radish. Plants. 2025; 14(13):2063. https://doi.org/10.3390/plants14132063
Chicago/Turabian StyleHasenstein, Karl H., Syed G. A. Moinuddin, Anna Berim, Laurence B. Davin, and Norman G. Lewis. 2025. "Glucosinolate and Sugar Profiles in Space-Grown Radish" Plants 14, no. 13: 2063. https://doi.org/10.3390/plants14132063
APA StyleHasenstein, K. H., Moinuddin, S. G. A., Berim, A., Davin, L. B., & Lewis, N. G. (2025). Glucosinolate and Sugar Profiles in Space-Grown Radish. Plants, 14(13), 2063. https://doi.org/10.3390/plants14132063








