High Ratio of Manure Substitution Enhanced Soil Organic Carbon Storage via Increasing Particulate Organic Carbon and Nutrient Availability
Abstract
1. Introduction
2. Results
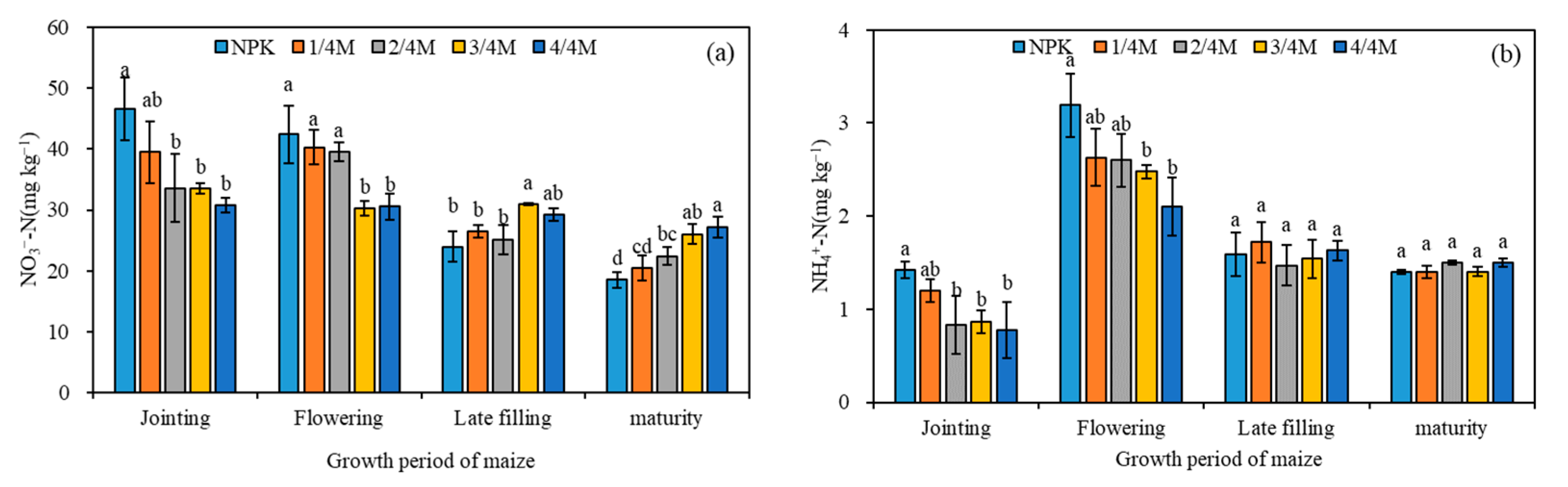
2.1. Soil Fertility
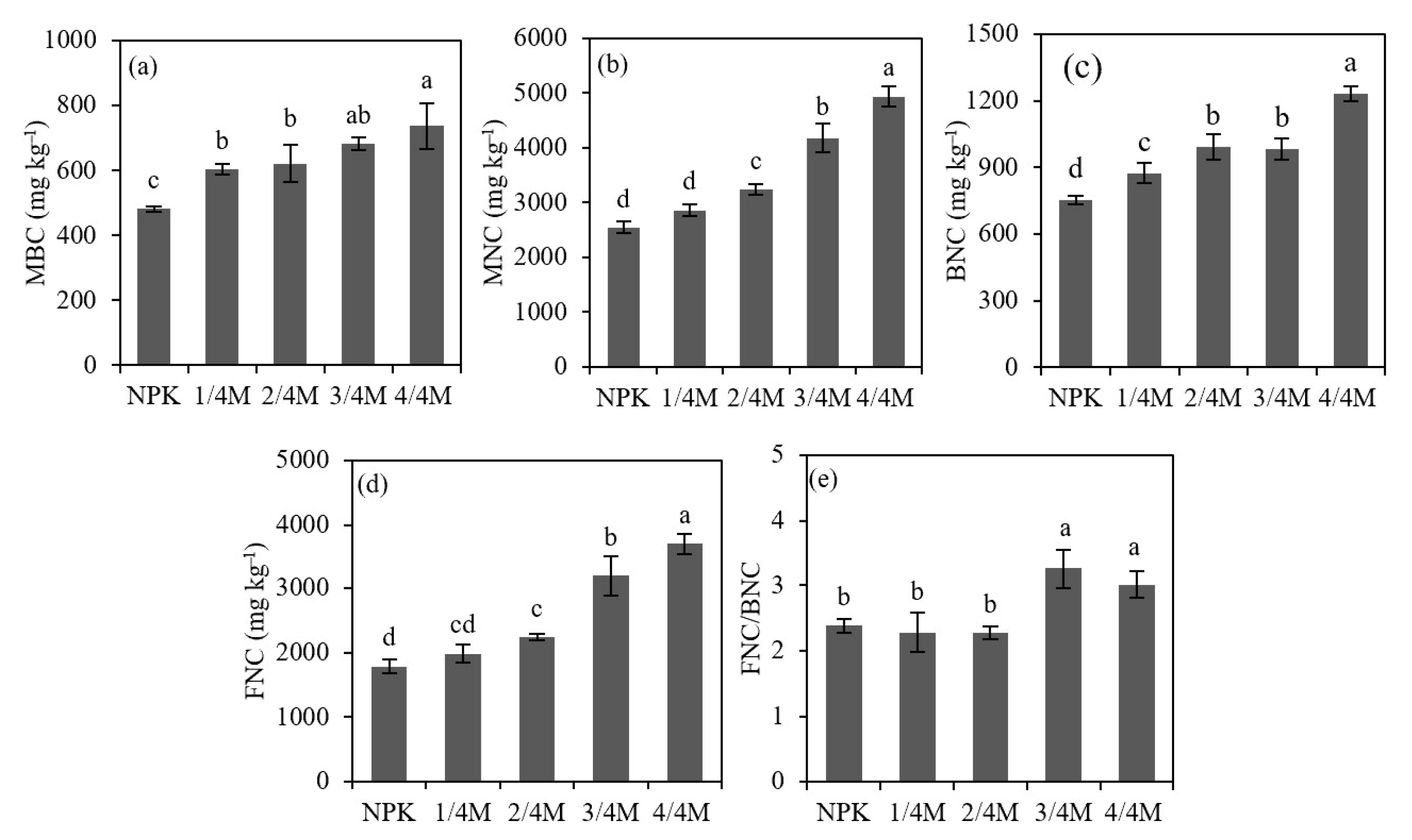
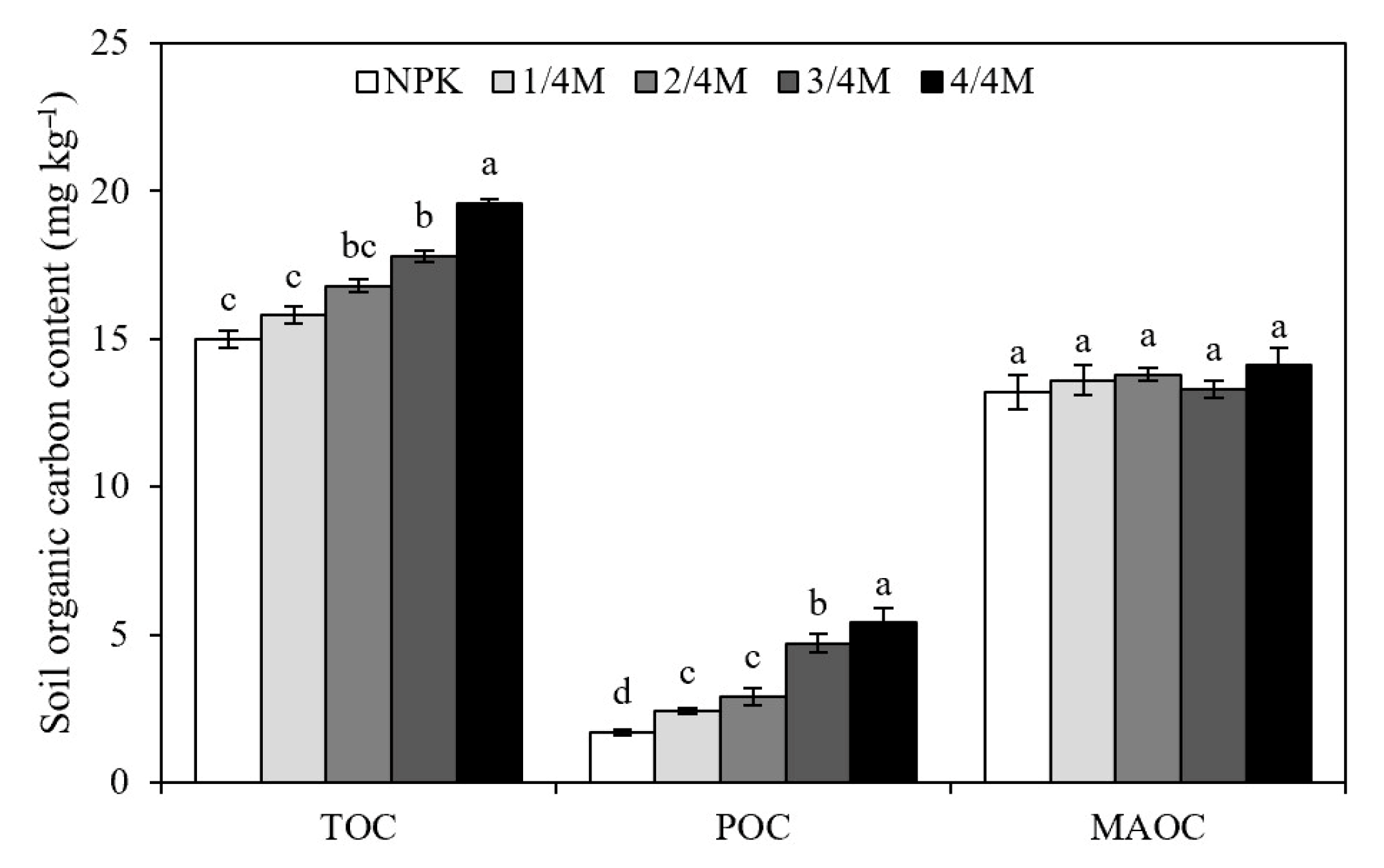
2.2. Soil Organic Carbon Fraction
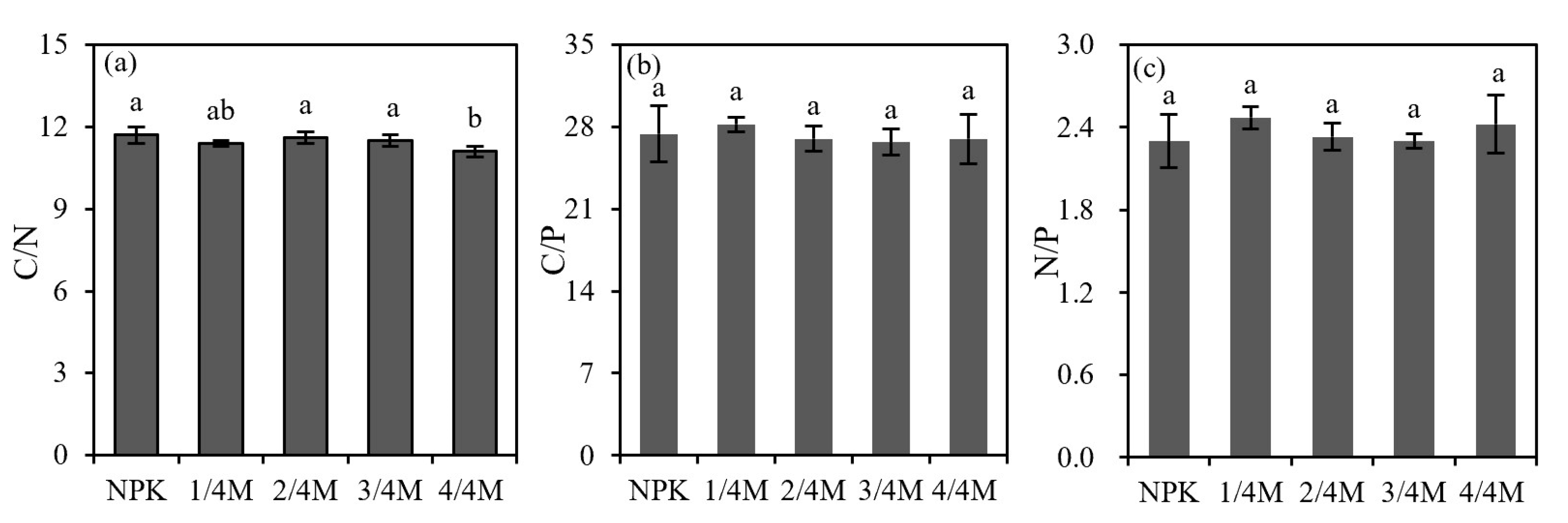
2.3. Soil Organic Carbon and Nutrient Stoichiometry
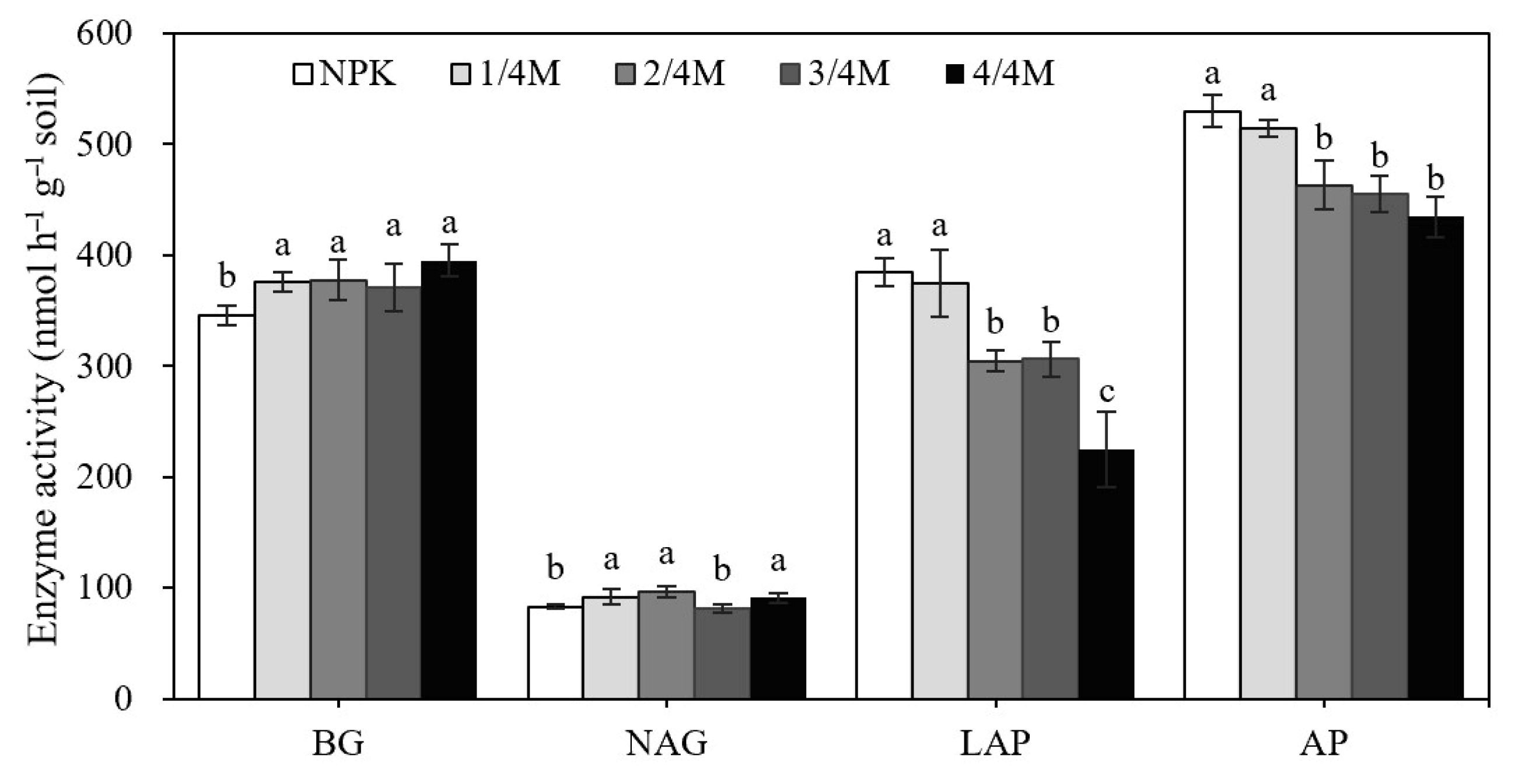
2.4. Soil Enzyme Activity and Enzymatic Stoichiometry
2.5. Partial Least Squares Path Modeling Analyses
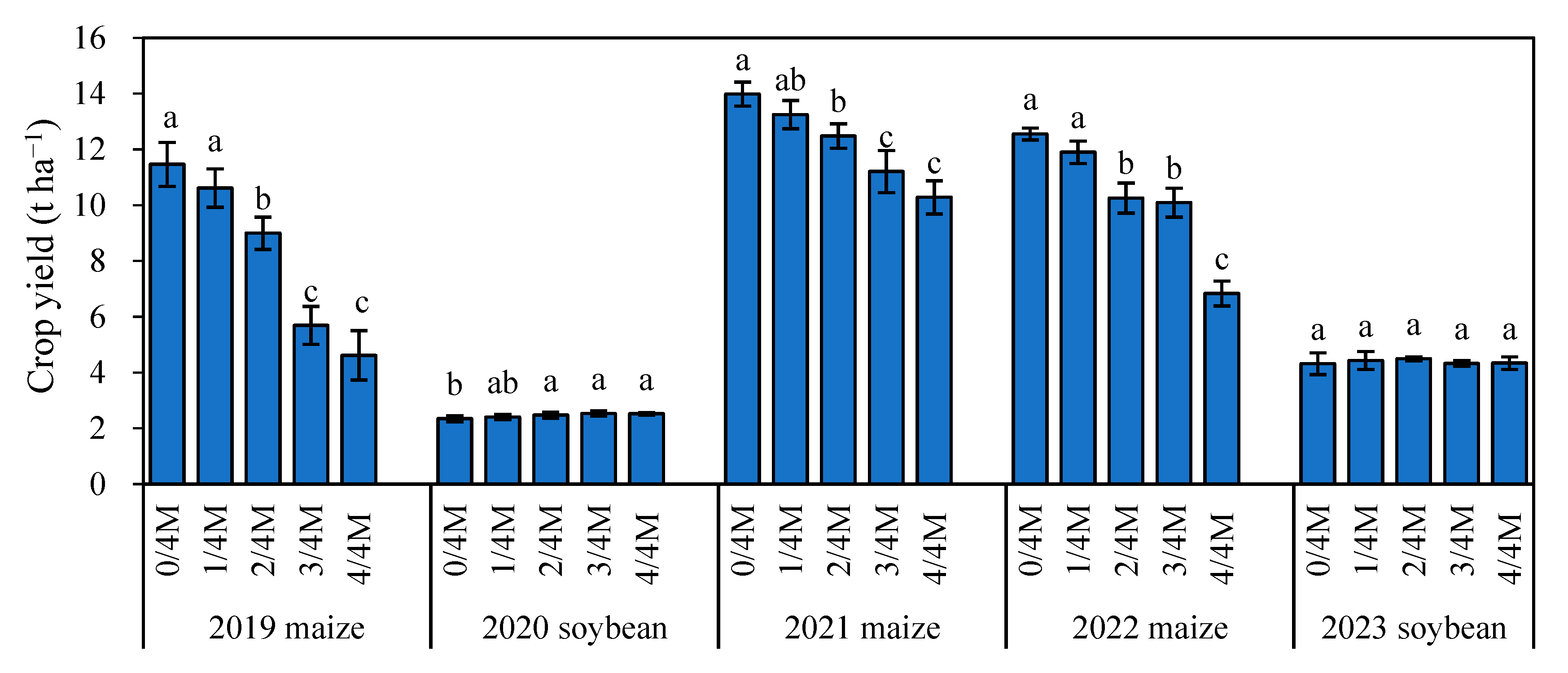
2.6. Annual Variation in Crop Yield
3. Discussion
3.1. Effect of Different Manure Substitution Treatments on Soil Fertility
3.2. Effect of Different Manure Substitution Treatments on Soil Organic Carbon Content and Fractions
3.3. Effect of Different Rates of Manure Substitution on Soil Nutrient Stoichiometry and Enzyme Activity
3.4. Effect of Different Rates of Manure Substitution on Crop Yield
4. Materials and Methods
4.1. Experimental Site Description
4.2. Experimental Design
4.3. Crop Harvest and Soil Sample Collection
4.4. Soil Physicochemical Property Analysis
4.5. Separation and Determination of Soil Organic Carbon Fractions
4.6. Soil Biological Property Analysis
4.7. Amino Sugar Analysis
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, G.Q. Research on the Development Characteristics and Sustainability of China’s Fertilizer Industry. Ph.D. Thesis, Agriculture University, Beijing, China, 2014. [Google Scholar]
- Song, D.L.; Hou, S.P.; Wang, X.B.; Liang, G.Q.; Zhou, W. Nutrient resource quantity of crop straw and its potential of substituting. J. Plant Nutri. Fertil. 2018, 24, 1–21, (In Chinese with English Abstract). [Google Scholar]
- Malhi, S.S.; Nyborg, M.; Solberg, E.D.; Dyck, M.R.; Puurveen, R. Improving crop yield and N uptake with long-term straw retention in two contrasting soil types. Field Crops Res. 2011, 124, 378–391. [Google Scholar] [CrossRef]
- Gu, L.M.; Liu, T.N.; Zhao, J.; Dong, S.T.; Liu, P.; Zhang, J.W.; Zhao, B. Nitrate leaching of winter wheat grown in lysimeters as affected grown in lysimeters as affected by fertilizers and irrigation in the North China Plain. J. Integr. Agric. 2015, 14, 374–388. [Google Scholar] [CrossRef]
- Sun, T.; Mao, X.L.; Ma, Q.X.; Han, K.F.; Wang, X.J.; Cheng, Q.; Sheng, X.C.; Mi, W.H.; Wu, L.H. Response of rice yield to organic amendments was regulated by soil chemical properties, microbial functional genes and bacterial community rather than fungal community. Appl. Soil Ecol. 2023, 188, 104923. [Google Scholar] [CrossRef]
- Chaudhary, S.; Dheri, G.S.; Brar, B.S. Long-term effects of NPK fertilizers and organic manures on carbon stabilization and management index under rice-wheat cropping system. Soil Till. Res. 2017, 166, 59–66. [Google Scholar] [CrossRef]
- Lv, F.L.; Song, J.S.; Giltrap, D.; Feng, Y.T.; Yang, X.Y.; Zhang, G.S.L. Crop yield and N2O emission affected by long-term organic manure substitution fertilizer under winter wheat- summer maize cropping system. Sci. Total Environ. 2020, 732, 11. [Google Scholar] [CrossRef]
- Wang, X.Y. Effects of Substituting Organic Fertilizer for Chemical Fertilizer on Potato Growth and Soil Microbial Properties. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2022. (In Chinese with English Abstract). [Google Scholar]
- Kundu, S.; Bhattacharyya, R.; Prakash, V.; Gupta, H.S.; Pathak, H.; Ladha, J.K. Long-term yield trend and sustainability of rainfed soybean–wheat system through farmyard manure application in a sandy loam soil of the Indian Himalayas. Biol. Fertil. Soils 2007, 43, 271–280. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, Q.; Zhang, T.; Ma, W.; Velthof, G.L.; Hou, Y.; Oenema, O.; Zhang, F. Benefits and trade-offs of replacing synthetic fertilizers by animal manures in crop production in China: A meta-analysis. Glob. Change Biol. 2020, 26, 888–900. [Google Scholar] [CrossRef]
- Hou, Q.; Ni, Y.M.; Zuo, T.; Wang, J.; Ni, W.Z. Effects of substituting chemical fertilizers with manure on rice yield and soil labile nitrogen in paddy fields of China: A meta-analysis. Pedosphere 2023, 33, 172–184. [Google Scholar] [CrossRef]
- Ma, B.L.; Liang, C.; Gregorich, E.G.; McLaughlin, N.B.; Morrison, M.J.; Dwyer, L.M. Nitrogen fertilizer replacement value of stockpiled and rotted dairy cattle manures for corn (Zea mays L.) from a long-term study. Field Crops Res. 2024, 315, 109455. [Google Scholar] [CrossRef]
- Gong, W.; Yan, X.Y.; Wang, J.Y.; Hu, T.X.; Gong, Y.B. Long-term manuring and fertilization effects on soil organic carbon pools under a wheat–maize cropping system in North China Plain. Plant Soil 2009, 314, 67–76. [Google Scholar] [CrossRef]
- Diatta, A.A.; Bassène, C.; Manga, A.G.; Abaye, O.; Thomason, W.; Battaglia, M.; Emre, B.; Uslu, Ö.; Min, D.; Seleiman, M.; et al. Integrated use of organic amendments increased mungbean (Vigna radiata (L.) Wilczek) yield and its components compared to inorganic fertilizers. Urban Agric. Reg. Food Syst. 2023, 8, e20048. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Beare, M.H.; McKim, U.F.; Skjemstad, J.O. Chemical and biological characteristics of physically uncomplexed organic matter. Soil Sci. Soc. Am. J. 2006, 70, 975–985. [Google Scholar] [CrossRef]
- Liang, C.; Fujinuma, R.; Balser, T.C. Comparing PLFA and amino sugars for microbial analysis in an Upper Michigan old growth forest. Soil Biol. Biochem. 2008, 40, 2063–2065. [Google Scholar] [CrossRef]
- Ozlu, E.; Gozukara, G.; Acar, M.; Bilen, S.; Babur, E. Field-scale evaluation of the soil quality index as influenced by dairy manure and inorganic fertilizers. Sustainability 2022, 14, 7593. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ranalli, M.G.; Haddix, M.L.; Six, J.; Lugato, E. Soil carbon storage informed by particulate and mineral associated organic matter. Nat. Geosci. 2019, 12, 989–994. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kastner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Change Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef]
- Yang, Y.L.; Xie, H.T. Fungi determine increased SOC more than bacteria through their necromass inputs in conservation tillage croplands. Soil Biol. Biochem. 2022, 167, 108587. [Google Scholar] [CrossRef]
- Paterson, E.; Sim, A.; Osborne, S.M.; Murray, P. Long-term exclusion of plant inputs to soil reduces the functional capacity of microbial communities to mineralize recalcitrant root- derived carbon sources. Soil Biol. Biochem. 2011, 43, 1873–1880. [Google Scholar] [CrossRef]
- Deng, F.B.; Liang, C. Revisiting the quantitative contribution of microbial necromass to soil C pool: Stoichiometric control by microbes and soil. Soil Biol. Biochem. 2022, 165, 108486. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, Y.; Dungait, J.A.J.; Kumar, A.; Wang, J.; Tiemann, L.K.; Zhang, F.; Kuzyakov, Y.; Tian, J. Microbial necromass in cropland soils: A global meta-analysis of management effects. Glob. Change Biol. 2023, 29, 1998–2014. [Google Scholar] [CrossRef]
- Li, Y.L.; Wu, L.; Tang, L.Y.; Ren, F.L.; Wang, X.H.; Zhu, P.; Sun, N.; Xu, M.G. Manure application decreases soil organic carbon priming by increasing mineral protection and nitrogen availability. Geoderma 2023, 439, 116676. [Google Scholar] [CrossRef]
- Ma, Z.B.; Liang, T.; Fu, H.R.; Ma, Q.X.; Chang, A.N.; Zhang, J.D.; Che, Z.X.; Zhou, G.P.; Cao, W.D. Long-term green manuring increases soil C sequestration via decreasing qCO2 caused by lower microbial P limitation in a dry land field. Agric. Ecosyst. Environ. 2024, 374, 109142. [Google Scholar] [CrossRef]
- Hill, B.H.; Elonen, C.M.; Seifert, L.R.; May, A.A.; Tarquinio, E. Microbial enzyme stoichiometry and nutrient limitation in US streams and rivers. Ecol. Indic. 2012, 18, 540–551. [Google Scholar] [CrossRef]
- Tiemann, L.K.; Billings, S.A. Indirect effects of N amendments on organic substrate quality increase enzymatic activity driving decomposition in a Mesic Grassland. Ecosystems 2011, 14, 234–247. [Google Scholar] [CrossRef]
- Li, P.; Lu, J.; Hou, W.; Pan, Y.; Wang, Y.; Khan, M.K.; Ren, T.; Cong, R.; Li, X. Reducing N losses through NH3 volatilization and surface runoff to improve apparent N recovery of double cropping of late rice using controlled release urea. Environ. Sci. Pollut. Res. 2017, 24, 11722–11733. [Google Scholar] [CrossRef]
- Guo, Y.J.; Qiu, H.Z.; Zhang, Y.J.; Zhang, J.B.; Wang, Y.L.; Zhang, C.H.; Kwadwo, A.D. Effects of four fertilization regimes on soil organic carbon fractions and carbon pool management index of potato farmland. Chin. J. Soil Sci. 2021, 52, 912–919. [Google Scholar]
- Liu, Y.; Ma, M.H.; Wu, S.J.; Ran, Y.G.; Wang, X.X.; Huang, P. Soil aggregates as affected by wetting- drying cycle: A review. Soil. 2018, 50, 853–865. [Google Scholar]
- Li, J.; Zhang, X.C.; Luo, J.F.; Lindsey, S.; Zhou, F.; Xie, H.T.; Li, Y.; Zhu, P.; Wang, L.C.; Shi, Y.L.; et al. Differential accumulation of microbial necromass and plant lignin in synthetic versus organic fertilizer-amended soil. Soil Biol. Biochem. 2020, 149, 107967. [Google Scholar] [CrossRef]
- Song, X.J.; Wu, H.J.; Li, S.P.; He, P.; Wu, X.P. The need to update and refine concepts relating to mineral-associated organic matter saturation in soil. Soil Biol. Biochem. 2025, 202, 109672. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, X.; Zheng, B.; Yue, S.; Zhang, X.; Zhai, B.; Wang, Z.; Zheng, W.; Li, Z.; Zamanian, K.; et al. Effects of plastic and straw mulching on soil microbial P limitations in maize fields: Dependency on soil organic carbon demonstrated by ecoenzymatic stoichiometry. Geoderma 2021, 388, 114928. [Google Scholar] [CrossRef]
- Chapin, F.S.; Matson, P.A.; Mooney, H.A.; Vitousek, P.M. Principles of Terrestrial Ecosystem Ecology, 1st ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Gai, X.; Liu, H.B.; Liu, J.; Zhai, L.M.; Yang, B.; Wu, S.X.; Ren, T.Z.; Wang, H.Y. Long-term benefits of combining chemical fertilizer and manure applications on crop yields and soil carbon and nitrogen stocks in North China Plain. Agric. Water Manag. 2018, 208, 384–392. [Google Scholar] [CrossRef]
- Hou, P.; Li, B.W.; Cao, E.K.; Jian, S.Q.; Liu, Z.H.; Li, Y.; Sun, Z.Q.; Ma, C.J. Optimizing maize yield and mitigating salinization in the Yellow River Delta through organic fertilizer substitution for chemical fertilizers. Soil Till. Res. 2025, 249, 106498. [Google Scholar] [CrossRef]
- Panwar, N.R.; Ramesh, P.; Singh, A.B.; Ramana, S. Influence of organic, chemical, and integrated management practices on soil organic carbon and soil nutrient status under semi-arid tropical conditions in central India. Commun. Soil Sci. Plant Anal. 2010, 41, 1073–1083. [Google Scholar] [CrossRef]
- Wei, W.L.; Yan, Y.; Cao, J.; Christie, P.; Zhang, F.S.; Fan, M.S. Effects of combined application of organic amendments and fertilizers on crop yield and soil organic matter: An integrated analysis of long-term experiments. Agric. Ecosyst. Environ. 2016, 225, 86–92. [Google Scholar] [CrossRef]
- Sun, C.; Zheng, H.; He, S.; Zhao, Q.; Liu, Y.; Liu, H. Partial substitution of chemical fertilizer by organic fertilizer increases yield, quality and nitrogen utilization of Dioscorea polystachya. PLoS ONE 2024, 19, e0301108. [Google Scholar] [CrossRef]
- Shu, X.; He, J.; Zhou, Z.; Xia, L.; Hu, Y.; Zhang, Y.; Luo, Y.; Chu, H.; Liu, W.; Yuan, S.; et al. Organic amendments enhance soil microbial diversity, microbial functionality and crop yields: A meta-analysis. Sci. Total Environ. 2022, 829, 154627. [Google Scholar] [CrossRef]
- Seufert, V.; Ramankutty, N.; Foley, J.A. Comparing the yields of organic and conventional agriculture. Nature 2012, 485, 229–232. [Google Scholar] [CrossRef]
- Li, H.; Feng, W.T.; He, X.H.; Zhu, P.; Gao, H.J.; Sun, N.; Xu, M.G. Chemical fertilizers could be completely replaced by manure to maintain high maize yield and soil organic carbon (SOC) when SOC reaches a threshold in the Northeast China Plain. J. Integr. Agric. 2017, 16, 937–946. [Google Scholar] [CrossRef]
- Yang, J.; Gao, W.; Ren, S.R. Long-term effects of combined application of chemical nitrogen with organic materials on crop yields, soil organic carbon and total nitrogen in fluvo-aquic soil. Soil Till. Res. 2015, 151, 67–74. [Google Scholar] [CrossRef]
- Choi, W.J.; Ro, H.M.; Chang, S.X. Recovery of fertilizer-derived inorganic-15N in vegetable field soil as affected by application of an organic amendment. Plant Soil. 2004, 263, 191–201. [Google Scholar] [CrossRef]
- Zhai, L.P. The Dynamic Study on the Organism Yield and Nutrient Absorb of Corn in Different Yield Treatments. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2006. (In Chinese with English Abstract). [Google Scholar]
- Lu, R.K. Methods of Agricultural Chemical Analysis; China agricultural Science and Technology Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Cheng, X.; Luo, Y.; Chen, J.Q.; Lin, G.; Chen, J.K.; Li, B. Short-term C4 plant Spartina alterniflora invasions change the soil carbon in C3 plant-dominated tidal wetlands on a growing estuarine island. Soil Biol. Biochem. 2006, 38, 3380–3386. [Google Scholar] [CrossRef]
- Sokol, N.W.; Kuebbing, S.E.; Karlsen-Ayala, E.; Bradford, M.A. Evidence for the primacy of living root inputs, not root or shoot litter, in forming soil organic Carbon. New Phytol. 2019, 221, 233–246. [Google Scholar] [CrossRef]
- Jilling, A.; Keiluweit, M.; Gutknecht, J.L.M.; Grandy, A.S. Priming mechanisms providing plants and microbes access to mineral-associated organic matter. Soil Biol. Biochem. 2021, 158, 108265. [Google Scholar] [CrossRef]
- Wu, J.S.; Joergensen, R.G.; Pommerening, B.; Chaussod, R.; Brooks, P.C. Measurement of soil microbial biomass C by fumigation–extraction-An automated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long-term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- DeForest, J. The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and L-DOPA. Soil Biol. Biochem. 2009, 41, 1180–1186. [Google Scholar] [CrossRef]
- Zhang, X.D.; Amelung, W. Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biol. Biochem. 1996, 28, 1201–1206. [Google Scholar] [CrossRef]
- Appuhn, A.; Joergensen, R.G.; Raubuch, M.; Scheller, E.; Wilke, B. The automated determination of glucosamine, galactosamine, muramic acid, and mannosamine in soil and root hydrolysates by HPLC. J. Plant Nutr. Soil Sci. 2004, 167, 17–21. [Google Scholar] [CrossRef]
- Tenenhaus, M.; Vinzi, V.E.; Chatelin, Y.M.; Lauro, C. PLS path modeling. Comput. Stat. Data Anal. 2005, 48, 159–205. [Google Scholar] [CrossRef]


| Treatment | pH | TN (g kg−1) | NH4+-N (mg kg−1) | NO3−-N (mg kg−1) | TP (g kg−1) | TK (g kg−1) | AVP (mg kg−1) | AVK (mg kg−1) | DOC (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| NPK | 6.36 ± 0.18 c | 1.3 ± 0.01 c | 1.4 ± 0.02 a | 18.5 ± 1.3 d | 0.56 ± 0.03 c | 23.2 ± 0.1 c | 29.2 ± 1.4 cd | 190.3 ± 5.7 c | 7.4 ± 0.2 c |
| 1/4M | 6.62 ± 0.1 c | 1.4 ± 0.04 c | 1.4 ± 0.07 a | 20.4 ± 2.1 cd | 0.56 ± 0.02 c | 23.6 ± 0.2 c | 28.2 ± 1.3 d | 198.2 ± 5.7 bc | 8.1 ± 0.6 c |
| 2/4M | 6.93 ± 0.14 b | 1.5 ± 0.04 bc | 1.5 ± 0.02 a | 22.4 ± 1.5 bc | 0.62 ± 0.01 b | 24.2 ± 0.1 bc | 32.8 ± 2.4 bc | 199.1 ± 9.2 bc | 9.8 ± 0.7 bc |
| 3/4M | 7.05 ± 0.27 ab | 1.5 ± 0.05 b | 1.4 ± 0.05 a | 26.0 ± 1.6 ab | 0.64 ± 0.02 b | 25.6 ± 0.2 ab | 36.1 ± 1.7 b | 206.7 ± 6.6 b | 10.4 ± 1.1 b |
| 4/4M | 7.29 ± 0.1 a | 1.7 ± 0.07 a | 1.5 ± 0.04 a | 27.2 ± 1.7 a | 0.71 ± 0.05 a | 27.4 ± 0.2 a | 60.3 ± 1.8 a | 238.9 ± 5.6 a | 13.6 ± 1.3 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, X.; Ma, X.; Sun, L.; Liu, S.; Ji, J.; Zhou, B.; Zhao, Y.; Zheng, Y.; Kuang, E.; Liu, Y.; et al. High Ratio of Manure Substitution Enhanced Soil Organic Carbon Storage via Increasing Particulate Organic Carbon and Nutrient Availability. Plants 2025, 14, 2045. https://doi.org/10.3390/plants14132045
Hao X, Ma X, Sun L, Liu S, Ji J, Zhou B, Zhao Y, Zheng Y, Kuang E, Liu Y, et al. High Ratio of Manure Substitution Enhanced Soil Organic Carbon Storage via Increasing Particulate Organic Carbon and Nutrient Availability. Plants. 2025; 14(13):2045. https://doi.org/10.3390/plants14132045
Chicago/Turabian StyleHao, Xiaoyu, Xingzhu Ma, Lei Sun, Shuangquan Liu, Jinghong Ji, Baoku Zhou, Yue Zhao, Yu Zheng, Enjun Kuang, Yitian Liu, and et al. 2025. "High Ratio of Manure Substitution Enhanced Soil Organic Carbon Storage via Increasing Particulate Organic Carbon and Nutrient Availability" Plants 14, no. 13: 2045. https://doi.org/10.3390/plants14132045
APA StyleHao, X., Ma, X., Sun, L., Liu, S., Ji, J., Zhou, B., Zhao, Y., Zheng, Y., Kuang, E., Liu, Y., & Zhao, S. (2025). High Ratio of Manure Substitution Enhanced Soil Organic Carbon Storage via Increasing Particulate Organic Carbon and Nutrient Availability. Plants, 14(13), 2045. https://doi.org/10.3390/plants14132045







