Identification and Quantification of Anthocyanins in Various Organs of Potato Varieties (Solanum tuberosum L.) as Potential Visual Selection Markers During Breeding
Abstract
1. Introduction
2. Results and Discussion
2.1. Color Variation in Potato Tubers
2.2. HPLC-MS-Based Anthocyanin Profiles and Content in Potato Varieties
2.2.1. Anthocyanin Type and Content in Potato Inflorescences
2.2.2. Anthocyanin Type and Content in Potato Leaves
2.2.3. Anthocyanin Type and Content in Potato Tubers
2.2.4. Correlation Between the Anthocyanin Content in Potato Leaves and Tubers
2.2.5. Anthocyanin Type and Content in Potato Tubers (Flesh and Skin)
3. Material and Methods
3.1. Plant Material
3.2. Potato Specimen Preparation
3.3. Anthocyanin Separation by HPLC
3.4. Identification of Anthocyanins
3.5. Statistical Analysis and Visualization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolotov, V.M.; Nechaev, A.P.; Sarafanova, L.A. Food Colorants: Classification, Properties, Analysis, and Usage; GIORD: Saint-Petersburg, Russia, 2008. [Google Scholar]
- Yudina, R.S.; Gordeeva, E.I.; Shoeva, O.Y.; Tikhonova, M.A.; Khlestkina, E.K. Anthocyanins as Functional Food Components. Vavilov J. Genet. Breed. 2021, 25, 178–189. [Google Scholar] [CrossRef]
- Sunil, L.; Shetty, N.P. Biosynthesis and Regulation of Anthocyanin Pathway Genes. Appl. Microbiol. Biotechnol. 2022, 106, 1783–1798. [Google Scholar] [CrossRef]
- Tang, B.; Li, L.; Hu, Z.; Chen, Y.; Tan, T.; Jia, Y.; Xie, Q.; Chen, G. Anthocyanin Accumulation and Transcriptional Regulation of Anthocyanin Biosynthesis in Purple Pepper. J. Agric. Food Chem. 2020, 68, 12152–12163. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Holm, D.G.; Broeckling, C.D.; Prenni, J.E.; Heuberger, A.L. Metabolomics and Ionomics of Potato Tuber Reveals an Influence of Cultivar and Market Class on Human Nutrients and Bioactive Compounds. Front. Nutr. 2018, 5, 36. [Google Scholar] [CrossRef]
- Wu, J.; Ma, Y.; Xie, R.; Zhang, Z.; Zhang, S.; Wu, X.; Wang, P.; Wang, D.; Lu, C. Integrated Metabolomics and Transcriptomics Reveals Difference Involved in Flavonoid and Indole Alkaloids Biosynthesis in Potato Tuber Flesh. Sci. Hortic. 2024, 324, 112630. [Google Scholar] [CrossRef]
- Khalid, M.; Saeed-ur-Rahman; Bilal, M. Role of Flavonoids in Plant Interactions with the Environment and against Human Pathogens—A Review. J. Integr. Agric. 2019, 18, 211–230. [Google Scholar] [CrossRef]
- Li, Z.; Ahammed, G.J. Plant Stress Response and Adaptation via Anthocyanins: A Review. Plant Stress 2023, 10, 100230. [Google Scholar] [CrossRef]
- Upadhyaya, D.C.; Bagri, D.S.; Upadhyaya, C.P.; Kumar, A.; Thiruvengadam, M.; Jain, S.K. Genetic Engineering of Potato (Solanum tuberosum L.) for Enhanced A-tocopherols and Abiotic Stress Tolerance. Physiol. Plant. 2020, 173, 116–128. [Google Scholar] [CrossRef]
- Wen, H.; Kang, J.; Li, D.; Wen, W.; Yang, F.; Hu, H.; Liu, C. Antifungal Activities of Anthocyanins from Purple Sweet Potato in the Presence of Food Preservatives. Food Sci. Biotechnol. 2016, 25, 165–171. [Google Scholar] [CrossRef]
- Lee, W.; Yeo, Y.; Oh, S.; Cho, K.-S.; Park, Y.-E.; Park, S.K.; Lee, S.M.; Cho, H.S.; Park, S.-Y. Compositional Analyses of Diverse Phytochemicals and Polar Metabolites from Different-Colored Potato (Solanum tubersum L.) Tubers. Food Sci. Biotechnol. 2017, 26, 1379–1389. [Google Scholar] [CrossRef]
- Renita, A.A.; Gajaria, T.K.; Sathish, S.; Kumar, J.A.; Lakshmi, D.S.; Kujawa, J.; Kujawski, W. Progress and Prospective of the Industrial Development and Applications of Eco-Friendly Colorants: An Insight into Environmental Impact and Sustainability Issues. Foods 2023, 12, 1521. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Chai, Z.; Beta, T.; Feng, J.; Huang, W. Blueberry Anthocyanins: An Updated Review on Approaches to Enhancing Their Bioavailability. Trends Food Sci. Technol. 2021, 118, 808–821. [Google Scholar] [CrossRef]
- Bobrysheva, T.N.; Anisimov, G.S.; Zolotoreva, M.S.; Bobryshev, D.V.; Budkevich, R.O.; Moskalev, A.A. Polyphenols as Promising Bioactive Compounds. Probl. Nutr. 2023, 92, 92–107. [Google Scholar] [CrossRef]
- Calderaro, A.; Barreca, D.; Bellocco, E.; Smeriglio, A.; Trombetta, D.; Laganà, G. Colored Phytonutrients: Role and Applications in the Functional Foods of Anthocyanins. In Phytonutrients in Food; Elsevier: Amsterdam, The Netherlands, 2020; pp. 177–195. [Google Scholar]
- Beals, K.A. Potatoes, Nutrition and Health. Am. J. Potato Res. 2019, 96, 102–110. [Google Scholar] [CrossRef]
- Polivanova, O.B.; Gins, E.M. Antioxidant Activity of Potatoes (Solanum tuberosum L.) and Anthocyanin Content, Its Biosynthesis and Physiological Role. Veg. Crops Russ. 2019, 84–90. [Google Scholar] [CrossRef]
- Rabee, M.; Andersen, Ø.M.; Fossen, T.; Enerstvedt, K.H.; Abu Ali, H.; Rayyan, S. Acylated Flavone O-Glucuronides from the Aerial Parts of Nepeta Curviflora. Molecules 2020, 25, 3782. [Google Scholar] [CrossRef]
- Hellmann, H.; Goyer, A.; Navarre, D.A. Antioxidants in Potatoes: A Functional View on One of the Major Food Crops Worldwide. Molecules 2021, 26, 2446. [Google Scholar] [CrossRef]
- Bao, Y.; Nie, T.; Wang, D.; Chen, Q. Anthocyanin Regulatory Networks in Solanum tuberosum L. Leaves Elucidated via Integrated Metabolomics, Transcriptomics, and StAN1 Overexpression. BMC Plant Biol. 2022, 22, 228. [Google Scholar] [CrossRef]
- Koshechkina, A.S.; Tumolskaya, E.V.; Perova, I.B. Dietary Supplements as a Source of Anthocyanins. Probl. Nutr. 2023, 92, 87–92. [Google Scholar] [CrossRef]
- Bellumori, M.; Chasquibol Silva, N.; Vilca, L.; Andrenelli, L.; Cecchi, L.; Innocenti, M.; Balli, D.; Mulinacci, N. A Study on the Biodiversity of Pigmented Andean Potatoes: Nutritional Profile and Phenolic Composition. Molecules 2020, 25, 3169. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Zhang, L.; Duan, S.; Li, G.; Duan, Y. Fine Mapping and Candidate Genes Analysis for Regulatory Gene of Anthocyanin Synthesis in the Corolla, Shedding Light on Wild Potato Evolution. Int. J. Mol. Sci. 2025, 26, 1966. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Kaur, L.; Singh, J.; Zeng, F. Functional Food Based on Potato. Foods 2023, 12, 2145. [Google Scholar] [CrossRef]
- Hale, K.L.; Tufan, H.A.; Pickering, I.J.; George, G.N.; Terry, N.; Pilon, M.; Pilon-Smits, E.A.H. Anthocyanins Facilitate Tungsten Accumulation in Brassica. Physiol. Plant. 2002, 116, 351–358. [Google Scholar] [CrossRef]
- Górska-Warsewicz, H.; Rejman, K.; Kaczorowska, J.; Laskowski, W. Vegetables, Potatoes and Their Products as Sources of Energy and Nutrients to the Average Diet in Poland. Int. J. Environ. Res. Public Health 2021, 18, 3217. [Google Scholar] [CrossRef]
- Gins, E.M.; Moskalev, E.A.; Polivanova, O.B.; Mityushkin, A.V.; Simakov, E.A. Antioxidant Contents in Potato Cultivars from the Collection of Russian Potato Research Center. RUDN J. Agron. Anim. Ind. 2020, 15, 242–252. [Google Scholar] [CrossRef]
- Diep, T.; Pook, C.; Yoo, M. Phenolic and Anthocyanin Compounds and Antioxidant Activity of Tamarillo (Solanum betaceum Cav.). Antioxidants 2020, 9, 169. [Google Scholar] [CrossRef]
- Kim, I.; Klykov, A. Studying Anthocyanins in Potato Tubers (Solanum tuberosum L.) under the Conditions of the Primorsky Region. Bull. KSAU 2023, 4, 12–20. [Google Scholar] [CrossRef]
- Laimbeer, F.P.E.; Bargmann, B.O.; Holt, S.H.; Pratt, T.; Peterson, B.; Doulis, A.G.; Buell, C.R.; Veilleux, R.E. Characterization of the F Locus Responsible for Floral Anthocyanin Production in Potato. G3 Genes|Genomes|Genet. 2020, 10, 3871–3879. [Google Scholar] [CrossRef]
- Strygina, K.V.; Kochetov, A.V.; Khlestkina, E.K. Genetic Control of Anthocyanin Pigmentation of Potato Tissues. BMC Genet. 2019, 20, 27. [Google Scholar] [CrossRef]
- Zhao, D.; Zheng, Y.; Yang, L.; Yao, Z.; Cheng, J.; Zhang, F.; Jiang, H.; Liu, D. The Transcription Factor AtGLK1 Acts Upstream of MYBL2 to Genetically Regulate Sucrose-Induced Anthocyanin Biosynthesis in Arabidopsis. BMC Plant Biol. 2021, 21, 242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Duan, Y.; Zhang, Z.; Zhang, L.; Chen, S.; Cai, C.; Duan, S.; Zhang, K.; Li, G.; Cheng, F. OcBSA: An NGS-Based Bulk Segregant Analysis Tool for Outcross Populations. Mol. Plant 2024, 17, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Su, H.-Y.; Li, M.-L.; Yang, D.-L.; Yao, R.-Y.; Li, M.-F.; Wei, J.-H. Effect of UV-B Radiation on Growth, Flavonoid and Podophyllotoxin Accumulation, and Related Gene Expression in Sinopodophyllum hexandrum. Plant Biol. 2021, 23, 202–209. [Google Scholar] [CrossRef]
- Cai, C.; Zhou, F.; Li, W.; Yu, Y.; Guan, Z.; Zhang, B.; Guo, W. The R2R3-MYB Transcription Factor GaPC Controls Petal Coloration in Cotton. Crop J. 2023, 11, 1319–1330. [Google Scholar] [CrossRef]
- Tang, D.; Jia, Y.; Zhang, J.; Li, H.; Cheng, L.; Wang, P.; Bao, Z.; Liu, Z.; Feng, S.; Zhu, X.; et al. Genome Evolution and Diversity of Wild and Cultivated Potatoes. Nature 2022, 606, 535–541. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.-J.; Wang, Y.; Geng, Z.; Ding, B.; Jiang, J.; Chen, S.; Chen, F. An R2R3-MYB Transcription Factor CmMYB21 Represses Anthocyanin Biosynthesis in Color Fading Petals of Chrysanthemum. Sci. Hortic. 2022, 293, 110674. [Google Scholar] [CrossRef]
- Shi, S.; Liu, Y.; He, Y.; Li, L.; Li, D.; Chen, H. R2R3-MYB Transcription Factor SmMYB75 Promotes Anthocyanin Biosynthesis in Eggplant (Solanum melongena L.). Sci. Hortic. 2021, 282, 110020. [Google Scholar] [CrossRef]
- Simakov, E.A.; Mityushkin, A.V.; Zhuravlev, A.A.; Mityushkin, A.V.; Gayzatulin, A.S. Comparative Evaluation of Potatoes Basic Material in Breeding to Increase Tubers Nutrient Quality. Potato Grow. 2020, 27, 30–36. [Google Scholar]
- Rasheed, H.; Ahmad, D.; Bao, J. Genetic Diversity and Health Properties of Polyphenols in Potato. Antioxidants 2022, 11, 603. [Google Scholar] [CrossRef]
- de los Angeles Bohórquez-Quintero, M.; Galvis-Tarazona, D.Y.; Arias-Moreno, D.M.; Ojeda-Peréz, Z.Z.; Ochatt, S.; Rodríguez-Molano, L.E. Morphological and Anatomical Characterization of Yellow Diploid Potato Flower for Effective Breeding Program. Sci. Rep. 2022, 12, 16402. [Google Scholar] [CrossRef]
- Arslanoglu, F.; Autac, S.; Oner, K. Morphological Characterization of the Local Potato (Solanum tuberosum L.) Genotypes Collected from the Eastern Black Sea Region of Turkey. Afr. J. Biotechnol. 2011, 10, 922–932. [Google Scholar]
- Hayati, M.; Sabaruddin; Efendi; Anhar, A. Morphological Characteristics and Yields of Several Sweet Potato (Ipomoea batatas L.) Tubers. IOP Conf. Ser. Earth Environ. Sci. 2020, 425, 012055. [Google Scholar] [CrossRef]
- Zarzecka, K.; Ginter, A.; Gugała, M.; Durakiewicz, W. Nutritional Value of Coloured Flesh Potato Tubers in Terms of Their Micronutrient Content. Agronomy 2024, 14, 1537. [Google Scholar] [CrossRef]
- Singh, G.; Papoutsoglou, E.A.; Keijts-Lalleman, F.; Vencheva, B.; Rice, M.; Visser, R.G.F.; Bachem, C.W.B.; Finkers, R. Extracting Knowledge Networks from Plant Scientific Literature: Potato Tuber Flesh Color as an Exemplary Trait. BMC Plant Biol. 2021, 21, 198. [Google Scholar] [CrossRef]
- Hejtmánková, K.; Kotíková, Z.; Hamouz, K.; Pivec, V.; Vacek, J.; Lachman, J. Influence of Flesh Colour, Year and Growing Area on Carotenoid and Anthocyanin Content in Potato Tubers. J. Food Compos. Anal. 2013, 32, 20–27. [Google Scholar] [CrossRef]
- Anandan, A.; Mahender, A.; Sah, R.P.; Bose, L.K.; Subudhi, H.; Meher, J.; Reddy, J.N.; Ali, J. Non-Destructive Phenotyping for Early Seedling Vigor in Direct-Seeded Rice. Plant Methods 2020, 16, 127. [Google Scholar] [CrossRef]
- Ginzburg, D.N.; Cox, J.A.; Rhee, S.Y. Non-destructive, Whole-plant Phenotyping Reveals Dynamic Changes in Water Use Efficiency, Photosynthesis, and Rhizosphere Acidification of Sorghum Accessions under Osmotic Stress. Plant Direct 2024, 8, e571. [Google Scholar] [CrossRef]
- Elanskii, S.; Anisimov, B.V.; Zeiruk, G.N.; Kuznetsova, M.A.; Simakov, E.A.; Sklyarova, N.P.; Fillipov, S.N.; Yashina, I.M. Potato Varieties Grown in Russia; AGROSPAS: Moscow, Russia, 2013. [Google Scholar]
- de Villiers, A.; Venter, P.; Pasch, H. Recent Advances and Trends in the Liquid-Chromatography–Mass Spectrometry Analysis of Flavonoids. J. Chromatogr. A 2016, 1430, 16–78. [Google Scholar] [CrossRef]
- Eichhorn, S.; Winterhalter, P. Anthocyanins from Pigmented Potato (Solanum tuberosum L.) Varieties. Food Res. Int. 2005, 38, 943–948. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, W.; Liu, Z.; Niu, N.; Li, Z.; Chen, L.; Zhu, J.; Li, D.; Liu, Y. Anthocyanin Profiles in Colored Potato Tubers at Different Altitudes by HPLC–MS Analysis with Optimized Ultrasound-Assisted Extraction. Foods 2023, 12, 4175. [Google Scholar] [CrossRef]
- Nabais, P.; Oliveira, J.; Pina, F.; Teixeira, N.; de Freitas, V.; Brás, N.F.; Clemente, A.; Rangel, M.; Silva, A.M.S.; Melo, M.J. A 1000-Year-Old Mystery Solved: Unlocking the Molecular Structure for the Medieval Blue from Chrozophora Tinctoria, Also Known as Folium. Sci. Adv. 2020, 6, eaaz7772. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Q.; Fu, N.; Song, M.; Han, X.; Yang, Q.; Zhang, Y.; Tong, Z.; Zhang, J. Cyanidin-3-O-Glucoside Contributes to Leaf Color Change by Regulating Two BHLH Transcription Factors in Phoebe Bournei. Int. J. Mol. Sci. 2023, 24, 3829. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.; Salas, A.; Chavez, O.; Gomez, R.; Anglin, N. Ex Situ Conservation of Potato [Solanum Section Petota (Solanaceae)] Genetic Resources in Genebanks. In The Potato Crop; Springer International Publishing: Cham, Switzerland, 2020; pp. 109–138. [Google Scholar]
- Kacheyo, O.C.; van Dijk, L.C.M.; de Vries, M.E.; Struik, P.C. Augmented Descriptions of Growth and Development Stages of Potato (Solanum tuberosum L.) Grown from Different Types of Planting Material. Ann. Appl. Biol. 2021, 178, 549–566. [Google Scholar] [CrossRef]
- Taghavi, T.; Patel, H.; Rafie, R. Anthocyanin Extraction Method and Sample Preparation Affect Anthocyanin Yield of Strawberries. Nat. Prod. Commun. 2022, 17, 1934578X221099970. [Google Scholar] [CrossRef]
- Su, X.; Griffin, J.; Xu, J.; Ouyang, P.; Zhao, Z.; Wang, W. Identification and Quantification of Anthocyanins in Purple-Fleshed Sweet Potato Leaves. Heliyon 2019, 5, e01964. [Google Scholar] [CrossRef]
- Jyothi, A.N.; Moorthy, S.N.; Eswariamma, C.S. Anthocyanins in Sweet Potato Leaves-Varietal Screening, Growth Phase Studies and Stability in a Model System. Int. J. Food Prop. 2005, 8, 221–232. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Wang, H.; Race, E.J.; Shrikhande, A.J. Characterization of Anthocyanins in Grape Juices by Ion Trap Liquid Chromatography−Mass Spectrometry. J. Agric. Food Chem. 2003, 51, 1839–1844. [Google Scholar] [CrossRef]
- Jansen, G.; Flamme, W. Coloured Potatoes (Solanum tuberosum L.)—Anthocyanin Content and Tuber Quality. Genet. Resour. Crop Evol. 2006, 53, 1321–1331. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, B.; Guo, Y.; Na, T.; Guo, Y. The Impact of Different Nitrogen Levels on the Tuber Yield and Anthocyanin Synthesis of Purple Potatoes. Agriculture 2024, 14, 125. [Google Scholar] [CrossRef]
- Kita, A.; Bąkowska-Barczak, A.; Hamouz, K.; Kułakowska, K.; Lisińska, G. The Effect of Frying on Anthocyanin Stability and Antioxidant Activity of Crisps from Red- and Purple-Fleshed Potatoes (Solanum tuberosum L.). J. Food Compos. Anal. 2013, 32, 169–175. [Google Scholar] [CrossRef]
- Lee, B.-S.; Lee, C.; Yang, S.; Park, E.K.; Ku, S.-K. Suppressive effects of pelargonidin on lipopolysaccharide-induced inflammatory responses. Chem.-Biol. Interact. 2019, 302, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gu, M.; Gong, P.; Wang, J.; Hu, Y. Glycosides changed the stability and antioxidant activity of pelargonidin. LWT 2021, 147, 111581. [Google Scholar] [CrossRef]
- Karthi, N.; Karthiga, A.; Kalaiyarasu, T.; Stalin, A.; Manju, V. Exploration of cell cycle regulation and modulation of the DNA methylation mechanism of pelargonidin: Insights from the molecular modeling approach. Comput. Biol. Chem. 2017, 70, 175–185. [Google Scholar] [CrossRef]
- Rajan, V.K.; Ragi, C.; Muraleedharan, K. A computational exploration into the structure, antioxidant capacity, toxicity and drug-like activity of the anthocyanidin “Petunidin”. Heliyon 2019, 5, e02115. [Google Scholar] [CrossRef]
- Fossen, T.; Andersen, Ø.M. Anthocyanins from tubers and shoots of the purple potato, Solanum tuberosum. J. Hortic. Sci. Biotechnol. 2000, 75, 360–363. [Google Scholar] [CrossRef]
- Hamouz, K.; Lachman, J.; Pazderů, K.; Tomášek, J.; Hejtmánková, K.; Pivec, V. Differences in Anthocyanin Content and Antioxidant Activity of Potato Tubers with Different Flesh Colour. Plant Solid. Environ. 2011, 57, 478–485. [Google Scholar] [CrossRef]
- Fogelman, E.; Oren-Shamir, M.; Hirschberg, J.; Mandolino, G.; Parisi, B.; Ovadia, R.; Tanami, Z.; Faigenboim, A.; Ginzberg, I. Nutritional Value of Potato (Solanum tuberosum) in Hot Climates: Anthocyanins, Carotenoids, and Steroidal Glycoalkaloids. Planta 2019, 249, 1143–1155. [Google Scholar] [CrossRef]
- Kim, M.-K.; Kim, H.; Koh, K.; Kim, H.-S.; Lee, Y.S.; Kim, Y.H. Identification and Quantification of Anthocyanin Pigments in Colored Rice. Nutr. Res. Pract. 2008, 2, 46. [Google Scholar] [CrossRef]
- Novikov, D.A.; Novochadov, V.V. Statistical Methods in Medical and Biological Experiments (Typical Cases); Izd-vo VGMU: Volgograd, Russia, 2005. [Google Scholar]


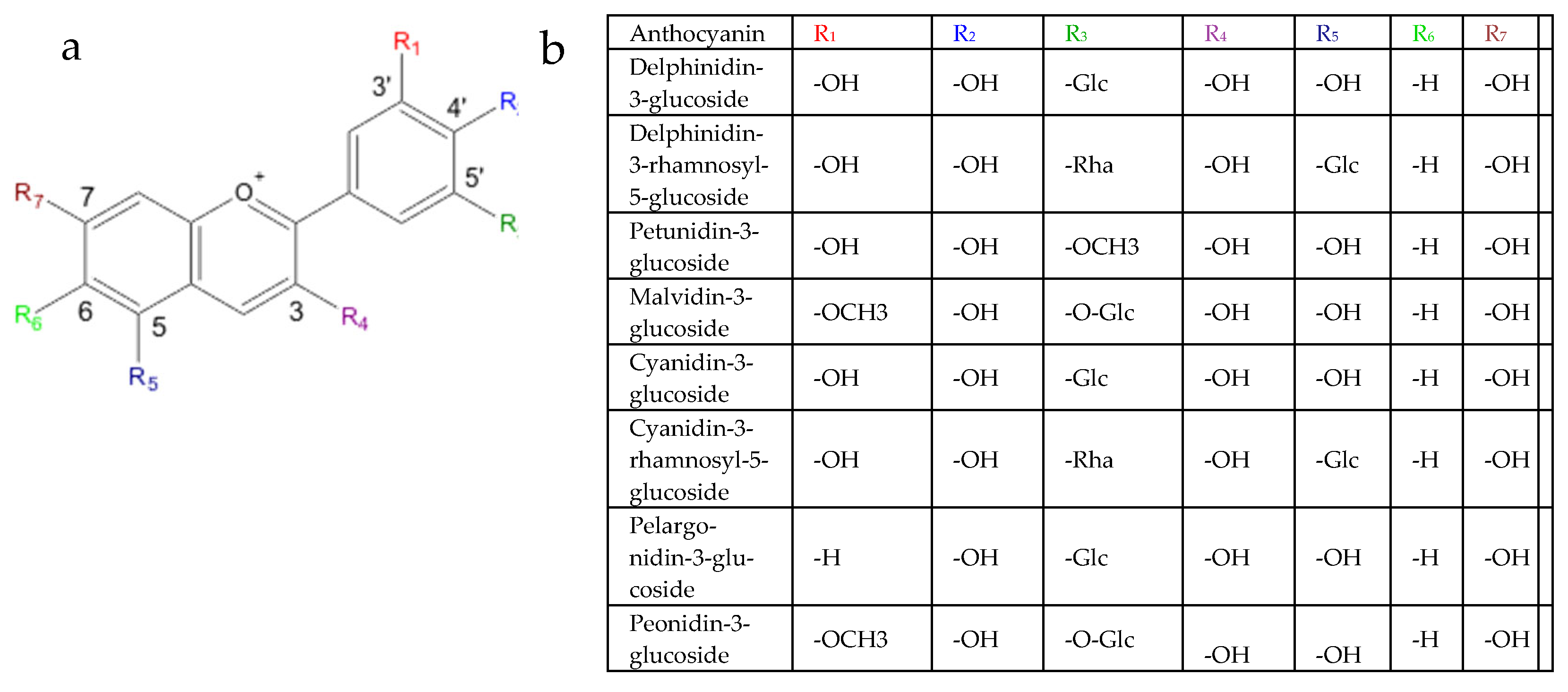



| Variety/Hybrid | Leaf | Tuber | Correlation Coefficient * (r) | |
|---|---|---|---|---|
| Anthocyanin Pigmentation of the Midrib on the Upper Side | Total Content of Anthocyanins, g/kg | Total Content of Anthocyanins, g/kg | ||
| Yellow and cream tuber skin | ||||
| Kazachok, st | None | <0.0005 | 0.0021 ± 0.0001 | 0.85 |
| Yantar’, st | None | 0.0086 ± 0.001 | 0.0018 ± 0.0001 | 0.78 |
| Dachnyi, st | None | 0.0103 ± 0.001 | 0.0010 ± 0.0001 | 0.75 |
| Sante, st | None | <0.0005 | 0.0010 ± 0.0001 | 0.85 |
| Vitesse | None | <0.0005 | 0.0009 ± 0.0001 | 0.80 |
| Meteor | None | <0.0005 | 0.0029 ± 0.0001 | 0.85 |
| Krepysh | None | <0.0005 | 0.0012 ± 0.0001 | 0.86 |
| Sarma | None | <0.0005 | 0.0022 ± 0.0001 | 0.90 |
| Azart | None | 0.0420 ± 0.001 | <0.0005 | −0.96 |
| Pamyati Rogacheva | None | <0.0005 | <0.0005 | 0.92 |
| Shchedrik | None | <0.0005 | <0.0005 | 0.95 |
| Tamyr | None | <0.0005 | 0.0020 ± 0.0001 | 0.85 |
| Nadezhda | None | 0.0955 ± 0.002 | <0.0005 | −0.85 |
| Dubrava | None | <0.0005 | <0.0005 | 0.98 |
| Zol’skii | None | 0.0977 ± 0.002 | <0.0005 | −0.70 |
| Krinitsa | None | 0.0013 ± 0.001 | 0.0021 ± 0.0001 | 0.86 |
| Ragneda | None | 0.0012 ± 0.001 | 0.0013 ± 0.0001 | 0.87 |
| Yellow tuber skin with pink spots, pink and red tuber skin | ||||
| Pamyati Kulakova | Light | 0.0839 ± 0.002 | 0.0032 ± 0.0001 | 0.32 |
| Ol’skii | Light | 0.0801 ± 0.002 | 0.0053 ± 0.0001 | 0.45 |
| Matushka | Light | 0.1256 ± 0.003 | 0,0400 ± 0.0002 | 0.95 |
| Bashkirskii | Light | 0.1140 ± 0.002 | 0.0311 ± 0.0002 | 0.74 |
| Ognivo | Light | 0.0913 ± 0.002 | 0.0032 ± 0.0001 | −0.56 |
| Yubilar | Light | 0.1311 ± 0.003 | 0.0146 ± 0.0001 | 0.50 |
| Kuznechanka | Moderate | 0.3203 ± 0.005 | 0.0922 ± 0.0003 | 0.98 |
| Sirenevyi tuman | Moderate | 0.3073 ± 0.005 | 0.0198 ± 0.0001 | 0.75 |
| Zhukovskii rannii | Light | 0.1035 ± 0.002 | 0.0302 ± 0.0001 | 0.52 |
| Povin’ | Light | 0.1642 ± 0.003 | 0.0504 ± 0.0002 | 0.84 |
| Zhuravinka | Light | 0.1203 ± 0.002 | 0.0368 ± 0.0001 | 0.45 |
| Pri-15-7-16 Irbitskii × Avrora | Light | 0.1756 ± 0.003 | 0.0874 ± 0.0003 | 0.78 |
| Pri-15-41-8 Russkaya krasavitsa × Irbitskii | Light | 0.1853 ± 0.003 | 0.0851 ± 0.0003 | 0.85 |
| Pri-12-35-4 Debryansk × Mustang | Light | 0.2201 ± 0.003 | 0.0620 ± 0.0002 | 0.74 |
| Manifest | Light | 0.0751 ± 0.002 | 0.1131 ± 0.0003 | 0.68 |
| Mayak | Light | 0.0829 ± 0.002 | 0.1087 ± 0.0003 | 0.95 |
| Romanze | Light | 0.1685 ± 0.003 | 0.0576 ± 0.0002 | 0.87 |
| Purple and blue-purple tuber skin | ||||
| Tsyganka Lora | Bright | 0.1863 ± 0.003 | 0.1154 ± 0.0003 | 0.95 |
| Chernyi prints | Bright | 0.3700 ± 0.005 | 0.1831 ± 0.0004 | 0.91 |
| Vasilek | Bright | 0.4340 ± 0.005 | 0.1950 ± 0.0004 | 0.95 |
| Pri-15-12-14 Purple potato × Manifest | Moderate | 0.3657 ± 0.005 | 0.1377 ± 0.0003 | 0.97 |
| Pri-14-52-2 Lomonosovskii × Purple potato | Moderate | 0.2565 ± 0.004 | 0.2233 ± 0.0004 | 0.93 |
| Fioletovyi | Bright | 0.3754 ± 0.004 | 0.2040 ± 0.0004 | 0.97 |
| Variety/Hybrid | Anthocyanin | Molecular Ion [M + H]+ | Emergence Time | Content of Anthocyanins, g/kg | |
|---|---|---|---|---|---|
| Skin | Flesh | ||||
| Yellow and cream tuber skin | |||||
| Kazachok, st | Petunidin-3-glucoside | 479.3; 317.2 | 35.0 | 0.0015 ± 0.0001 | <0.0005 |
| Yantar’, st | Petunidin-3-glucoside | 479.3; 317.2 | 35.0 | 0.0012 ± 0.0001 | <0.0005 |
| Sante, st | Malvidin-3-glucoside | 493.3; 331.3 | 44.0 | 0.0012 ± 0.0001 | <0.0005 |
| Dachnyi, st | Malvidin-3-glucoside | 493.3; 331.3 | 44.0 | 0.0019 ± 0.0001 | <0.0005 |
| Vitesse | Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0019 ± 0.0001 | <0.0005 |
| Meteor | Malvidin-3-glucoside | 493.3; 331.3 | 44.0 | 0.0009 ± 0.0001 | <0.0005 |
| Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0023 ± 0.0001 | <0.0005 | |
| Krepysh | Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0015 ± 0.0001 | <0.0005 |
| Sarma | Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0002 ± 0.0001 | <0.0005 |
| Azart | Malvidin-3-glucoside | 493.3; 331.3 | 44.0 | 0.0010 ± 0.0001 | <0.0005 |
| Pamyati Rogacheva | Malvidin-3-glucoside | 493.3; 331.3 | 44.0 | 0.0014 ± 0.0001 | <0.0005 |
| Shchedrik | Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0008 ± 0.0001 | <0.0005 |
| Tamyr | Petunidin-3-glucoside | 479.3; 317.2 | 35.0 | 0.0028 ± 0.0001 | <0.0005 |
| Nadezhda | Malvidin-3-glucoside | 493.3; 331.3 | 44.0 | 0.0013 ± 0.0001 | <0.0005 |
| Dubrava | Malvidin-3-glucoside | 493.3; 331.3 | 44.0 | 0.0012 ± 0.0001 | <0.0005 |
| Zol’skii | Petunidin-3-glucoside | 479.3; 317.2 | 35.0 | 0.0009 ± 0.0001 | <0.0005 |
| Krinitsa | Malvidin-3-glucoside | 493.3; 331.3 | 44.0 | 0.0015 ± 0.0001 | <0.0005 |
| Ragneda | Petunidin-3-glucoside | 479.3; 317.2 | 35.0 | 0.0021 ± 0.0001 | <0.0005 |
| Mean | 0.0015 | <0.0005 | |||
| V, % | 9.7 | 0.1 | |||
| Yellow tuber skin with pink spots, pink and red skin | |||||
| Pamyati Kulakova | Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0056 ± 0.0001 | <0.0005 |
| Ol’skii | Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0070 ± 0.0001 | <0.0005 |
| Matushka | Delphinidin-3-rhamnosyl-5-glucoside | 627.3; 465.3; 303.2 | 17.5 | 0.0026 ± 0.0001 | <0.0005 |
| Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0308 ± 0.0002 | <0.0005 | |
| Petunidin-3-glucoside | 479.3; 317.2 | 35.0 | 0.0007 ± 0.0001 | <0.0005 | |
| Bashkirskii | Delphinidin-3-glucoside | 465.3; 303.2 | 25.0 | 0.0010 ± 0.0001 | <0.0005 |
| Delphinidin-3-rhamnosyl-5-glucoside | 627.3; 465.3; 303.2 | 17.5 | 0.0035 ± 0.0001 | <0.0005 | |
| Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0302 ± 0.0002 | 0.0012 ± 0.0001 | |
| Petunidin-3-glucoside | 479.3; 317.2 | 35.0 | 0.0025 ± 0.0001 | <0.0005 | |
| Ognivo | Delphinidin-3-glucoside | 465.3; 303.2 | 25.0 | 0.0003 ± 0.0001 | <0.0005 |
| Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0030 ± 0.0001 | <0.0005 | |
| Yubilar | Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0124 ± 0.0001 | <0.0005 |
| Delphinidin-3-glucoside | 465.3; 303.2 | 25.0 | 0.0003 ± 0.0001 | <0.0005 | |
| Kuznechanka | Delphinidin-3-glucoside | 465.3; 303.2 | 25.0 | 0.0077 ± 0.0001 | <0.0005 |
| Delphinidin-3-rhamnosyl-5-glucoside | 627.3; 465.3; 303.2 | 17.5 | 0.0100 ± 0.0001 | <0.0005 | |
| Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0852 ± 0.0003 | 0.0032 ± 0.0001 | |
| Sirenevyi tuman | Delphinidin-3-glucoside | 465.3; 303.2 | 25.0 | 0.0030 ± 0.0001 | <0.0005 |
| Malvidin-3-glucoside | 493.3; 331.3 | 44.0 | 0.0120 ± 0.0001 | <0.0005 | |
| Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0060 ± 0.0001 | <0.0005 | |
| Zhukovskii rannii | Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0352 ± 0.0002 | <0.0005 |
| Povin’ | Delphinidin-3-glucoside | 465.3; 303.2 | 25.0 | 0.0050 ± 0.0001 | <0.0005 |
| Delphinidin-3-rhamnosyl-5-glucoside | 627.3; 465.3; 303.2 | 17.5 | 0.0075 ± 0.0001 | <0.0005 | |
| Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0302 ± 0.0002 | 0.0009 ± 0.0001 | |
| Petunidin-3-glucoside | 479.3; 317.2 | 35.0 | 0.0050 ± 0.0001 | <0.0005 | |
| Cyanidin-3-rhamnosyl-5-glucoside | 611.3; 499.3; 287.2 | 19.0 | 0.0110 ± 0.0001 | <0.0005 | |
| Zhuravinka | Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0403 ± 0.0002 | 0.0021 ± 0.0001 |
| Pri-15-7-16 Irbitskii × Avrora | Delphinidin-3-glucoside | 465.3; 303.2 | 25.0 | 0.0020 ± 0.0001 | <0.0005 |
| Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.2041 ± 0.0003 | <0.0005 | |
| Malvidin-3-glucoside | 493.3; 331.3 | 44.0 | 0.0108 ± 0.0001 | <0.0005 | |
| Petunidin-3-arabinoside | 479.3; 317.2 | 16.0 | 0.0401 ± 0.0002 | 0.0014 ± 0.0001 | |
| Cyanidin-3-glucoside | 449.2; 287.2 | 27.5 | 0.0991 ± 0.0002 | 0.0023 ± 0.0001 | |
| Pri-15-41-8 Russkaya krasavitsa × Irbitskii | Pelargonidin-3-glucoside | 433.3; 271.1 | 15.9 | 0.1301 ± 0.0004 | <0.0005 |
| Malvidin-3-glucoside | 493.3; 331.3 | 44.0 | 0.0052 ± 0.0001 | <0.0005 | |
| Delphinidin-3-glucoside | 465.3; 303.2 | 15.9 | 0.0150 ± 0.0001 | <0.0005 | |
| Petunidin-3-arabinoside | 479.3; 317.2 | 16.0 | 0.0167 ± 0.0001 | <0.0005 | |
| Cyanidin-3-glucoside | 449.2; 287.2 | 10.8 | 0.0045 ± 0.0001 | <0.0005 | |
| Pri-12-35-4 Debryansk × Mustang | Delphinidin-3-glucoside | 465.3; 303.2 | 25.0 | 0.0035 ± 0.0001 | 0.0012 ± 0.0001 |
| Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.1101 ± 0.0002 | <0.0005 | |
| Petunidin-3-arabinoside | 479.3; 317.2 | 16.0 | 0.0078 ± 0.0001 | <0.0005 | |
| Manifest | Delphinidin-3-glucoside | 465.3; 303.2 | 25.0 | 0.0050 ± 0.0001 | <0.0005 |
| Delphinidin-3-rhamnosyl-5-glucoside | 627.3; 465.3; 303.2 | 17.5 | 0.0050 ± 0.0001 | <0.0005 | |
| Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0795 ± 0.0002 | 0.0226 ± 0.0001 | |
| Petunidin-3-glucoside | 479.3; 317.2 | 35.0 | 0.0010 ± 0.0001 | <0.0005 | |
| Mayak | Delphinidin-3-glucoside | 465.3; 303.2 | 25.0 | 0.0040 ± 0.0001 | <0.0005 |
| Delphinidin-3-rhamnosyl-5-glucoside | 627.3; 465.3; 303.2 | 17.5 | 0.0025 ± 0.0001 | <0.0005 | |
| Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.1130 ± 0.0003 | 0.0029 ± 0.0001 | |
| Petunidin-3-glucoside | 479.3; 317.2 | 35.0 | 0.0120 ± 0.0001 | <0.0005 | |
| Romanze | Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0621 ± 0.0002 | 0.0014 ± 0.0001 |
| Delphinidin-3-rhamnosyl-5-glucoside | 627.3; 465.3; 303.2 | 17.5 | 0.0104 ± 0.0001 | <0.0005 | |
| Malvidin-3-glucoside | 493.3; 331.3 | 44.0 | 0.0031 ± 0.0001 | <0.0005 | |
| Mean | 0.0738 | 0.0015 | |||
| V, % | 38.2 | 15.7 | |||
| Purple and blue-purple tuber skin | |||||
| Tsyganka Lora | Petunidin-3-glucoside | 479.3; 317.2 | 35.0 | 0.1140 ± 0.0003 | 0.0126 ± 0.0002 |
| Cyanidin-3-glucoside | 449.2; 287.2 | 27.5 | 0.0301 ± 0.0002 | <0.0005 | |
| Chernyi prints | Cyanidin-3-glucoside | 449.2; 287.2 | 27.5 | 0.0708 ± 0.0002 | 0.0103 ± 0.0002 |
| Cyanidin-3-rhamnosyl-5-glucoside | 611.3; 499.3; 287.2 | 19.0 | 0.0304 ± 0.0002 | <0.0005 | |
| Petunidin-3-glucoside | 479.3; 317.2 | 35.0 | 0.1407 ± 0.0003 | 0.0241 ± 0.0003 | |
| Vasilek | Delphinidin-3-rhamnosyl-5-glucoside | 627.3; 465.3; 303.2 | 17.5 | 0.0090 ± 0.0001 | <0.0005 |
| Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0451 ± 0.0002 | <0.0005 | |
| Petunidin-3-glucoside | 479.3; 317.2 | 35.0 | 0.1801 ± 0.0004 | 0.0261 ± 0.0003 | |
| Cyanidin-3-rhamnosyl-5-glucoside | 611.3; 499.3; 287.2 | 19.0 | 0.0020 ± 0.0001 | <0.0005 | |
| Pri-15-12-14 Purple potato × Manifest | Petunidin-3-arabinoside | 479.3; 317.2 | 16.0 | 0.3500 ± 0.0005 | 0.0911 ± 0.0003 |
| Cyanidin-3-glucoside | 449.2; 287.2 | 27.5 | 0.1402 ± 0.0004 | 0.0398 ± 0.0002 | |
| Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0050 ± 0.0001 | <0.0005 | |
| Delphinidin-3-glucoside | 627.3; 465.3; 303.2 | 17.5 | 0.0631 ± 0.0001 | 0.0017 ± 0.0001 | |
| Malvidin-3-glucoside | 493.3; 331.3 | 22.5 | 0.0100 ± 0.0001 | 0.0010 ± 0.0001 | |
| Pri-14-52-2 Lomonosovskii × Purple potato | Petunidin-3-arabinoside | 479.3; 317.2 | 16.0 | 0.3579 ± 0.0005 | 0.1430 ± 0.0004 |
| Cyanidin-3-glucoside | 449.2; 287.2 | 27.5 | 0.0804 ± 0.0001 | 0.0812 ± 0.0003 | |
| Pelargonidin-3-glucoside | 433.3; 271.1 | 37.5 | 0.0841 ± 0.0002 | 0.0012 ± 0.0001 | |
| Delphinidin-3-glucoside | 627.3; 465.3; 303.2 | 17.5 | 0.0101 ± 0.0001 | 0.0015 ± 0.0001 | |
| Malvidin-3-glucoside | 493.3; 331.3 | 22.5 | 0.0021 ± 0.0001 | 0.0011 ± 0.0001 | |
| Fioletovyi | Delphinidin-3-glucoside | 465.3; 303.2 | 25.0 | 0.0351 ± 0.0002 | 0.0026 ± 0.0001 |
| Malvidin-3-glucoside | 493.3; 331.3 | 44.0 | 0.0603 ± 0.0002 | 0.0031 ± 0.0001 | |
| Petunidin-3-glucoside | 479.3; 317.2 | 35.0 | 0.0055 ± 0.0001 | 0.0021 ± 0.0001 | |
| Cyanidin-3-glucoside | 449.2; 287.2 | 27.5 | 0.1210 ± 0.0004 | 0.0352 ± 0.0002 | |
| Cyanidin-3-rhamnosyl-5-glucoside | 611.3; 499.3; 287.2 | 19.0 | 0.0089 ± 0.0001 | 0.0024 ± 0.0001 | |
| Mean | 0.3349 | 0.0803 | |||
| V, % | 52.1 | 47.3 | |||
| Variety/Hybrid | Origin | Color | |
|---|---|---|---|
| Corolla | Tuber Flesh | ||
| Yellow and cream tuber skin | |||
| Kazachok, st | Russia | White | Yellow |
| Yantar’, st | Russia | White | Bright yellow |
| Dachnyi, st | Russia | White | White |
| Sante, st | Netherlands | White | Yellow |
| Meteor | Russia | White | Yellow |
| Krepysh | Russia | Pale red-purple with white streaks | White |
| Sarma | Russia | White | Yellow |
| Azart | Russia | Pale blue-purple | White |
| Pamyati Rogacheva | Russia | Pale red-purple | Yellow |
| Nadezhda | Russia | Blue-purple with white streaks | Cream |
| Dubrava | Russia | Pale red-purple | Cream |
| Zol’skii | Russia | Pale red-purple with white streaks | Yellow |
| Krinitsa | Belarus | White | Yellow |
| Ragneda | Belarus | White | Yellow |
| Tamyr | Kazakhstan | White | Light yellow |
| Vitesse | Germany | White | Yellow |
| Shchedrik | Ukraine | White | White |
| Yellow tuber skin with pink spots, pink and red tuber skin | |||
| Pamyati Kulakova | Russia | White | White |
| Ol’skii | Russia | White | Cream |
| Matushka | Russia | White | Cream |
| Bashkirskii | Russia | Pale red-purple | White |
| Ognivo | Russia | Red-purple with white streaks | Cream |
| Yubilyar | Russia | Red-purple | Yellow |
| Kuznechanka | Russia | Pale red-purple | White |
| Sirenevyi tuman | Russia | Pale red-purple with white streaks | Light yellow |
| Zhukovskii rannii | Russia | Red-purple | White |
| Zhuravinka | Russia | Red-purple | Yellow |
| Pri-15-7-16 Irbitskii × Avrora * | Russia | Red-purple with white streaks | Yellow |
| Pri-15-41-8 Russkaya krasavitsa × Irbitskii * | Russia | Red-purple with white streaks | White |
| Pri-12-35-4 Derbyansk × Mustang * | Russia | Blue-purple | Yellow |
| Manifest | Russia | Red-purple with white streaks | Cream |
| Mayak | Russia | Pale red-purple | Cream |
| Povin’ | Ukraine | Red-purple | Yellow |
| Romanze | Germany | Red-purple | Yellow |
| Purple and blue-purple tuber skin | |||
| Tsyganka Lora | Russia (folk variety) | Red-purple | Cream |
| Chernyi prints | Russia (folk variety) | Red-purple with white streaks | Cream |
| Vasilek | Russia | Blue-purple with white streaks | Cream |
| Pri-15-12-14 Purple potato × Manifest * | Russia | Red-blue-purple | Yellow with purple pigmentation |
| Pri-14-52-2 Lomonosovskii × Purple potato * | Russia | Pale red-purple | Yellow with purple pigmentation |
| Fioletovyi * | Russia | Blue-purple with white streaks | Purple |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, I.V.; Nawaz, M.A.; Volkov, D.I.; Klykov, A.G.; Razgonova, M.P.; Golokhvast, K.S. Identification and Quantification of Anthocyanins in Various Organs of Potato Varieties (Solanum tuberosum L.) as Potential Visual Selection Markers During Breeding. Plants 2025, 14, 2042. https://doi.org/10.3390/plants14132042
Kim IV, Nawaz MA, Volkov DI, Klykov AG, Razgonova MP, Golokhvast KS. Identification and Quantification of Anthocyanins in Various Organs of Potato Varieties (Solanum tuberosum L.) as Potential Visual Selection Markers During Breeding. Plants. 2025; 14(13):2042. https://doi.org/10.3390/plants14132042
Chicago/Turabian StyleKim, Irina V., Muhammad A. Nawaz, Dmitry I. Volkov, Aleksey G. Klykov, Mayya P. Razgonova, and Kirill S. Golokhvast. 2025. "Identification and Quantification of Anthocyanins in Various Organs of Potato Varieties (Solanum tuberosum L.) as Potential Visual Selection Markers During Breeding" Plants 14, no. 13: 2042. https://doi.org/10.3390/plants14132042
APA StyleKim, I. V., Nawaz, M. A., Volkov, D. I., Klykov, A. G., Razgonova, M. P., & Golokhvast, K. S. (2025). Identification and Quantification of Anthocyanins in Various Organs of Potato Varieties (Solanum tuberosum L.) as Potential Visual Selection Markers During Breeding. Plants, 14(13), 2042. https://doi.org/10.3390/plants14132042









