Optimized Extraction, Comprehensive Chemical Profiling, and Antioxidant Evaluation of Volatile Oils from Wurfbainia villosa (Lour.) Škorničk. & A.D.Poulsen Leaves
Abstract
1. Introduction
2. Results and Discussion
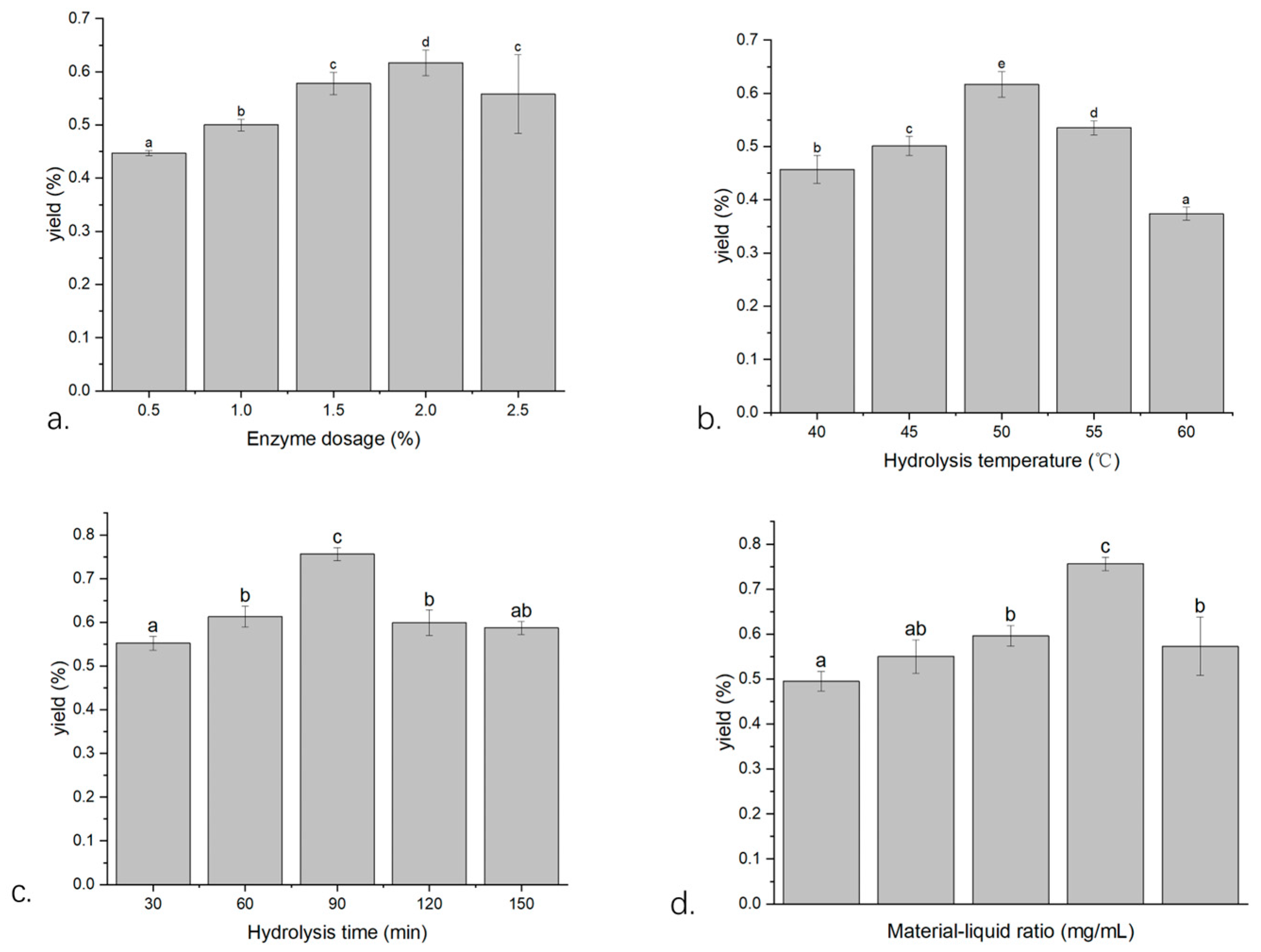
2.1. Single-Factor Screening
2.1.1. The Effect of the Enzyme Dosage on the Extraction Yield of Volatile Oil from W. villosa Leaves
2.1.2. The Effect of Enzymatic Hydrolysis Temperature on the Extraction Yield of Volatile Oil from W. villosa Leaves
2.1.3. The Effect of Enzymatic Hydrolysis Time on the Extraction Yield of Volatile Oil from W. villosa Leaves
2.1.4. The Effect of Material/Liquid Ratio on the Extraction Yield of Volatile Oil from W. villosa Leaves
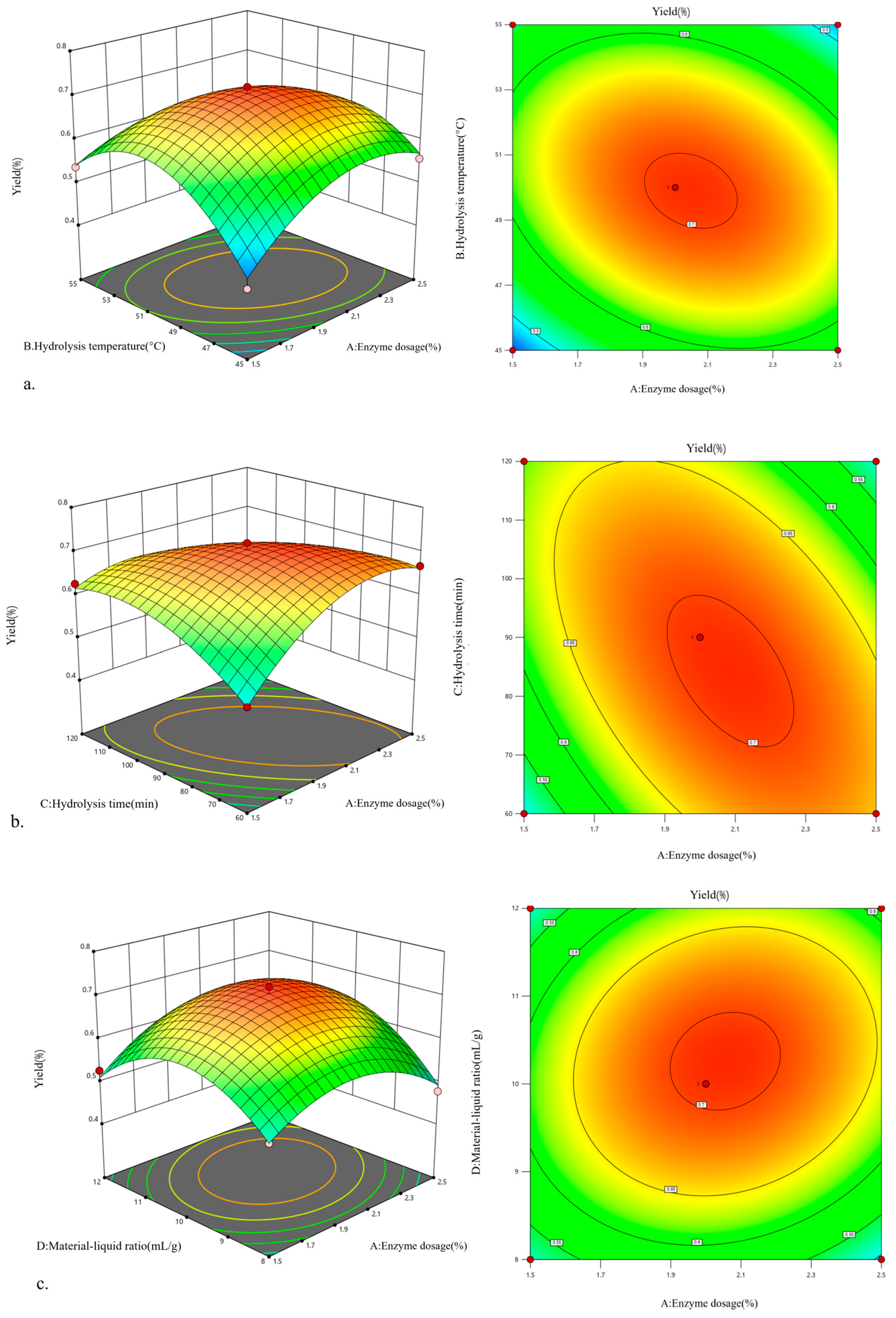
2.2. Optimization of Cellulase-HD Process for W. villosa Leaf Volatile Oil Extraction Using a Box–Behnken Design
2.3. Optimization and Validation of Hydrolysis Conditions
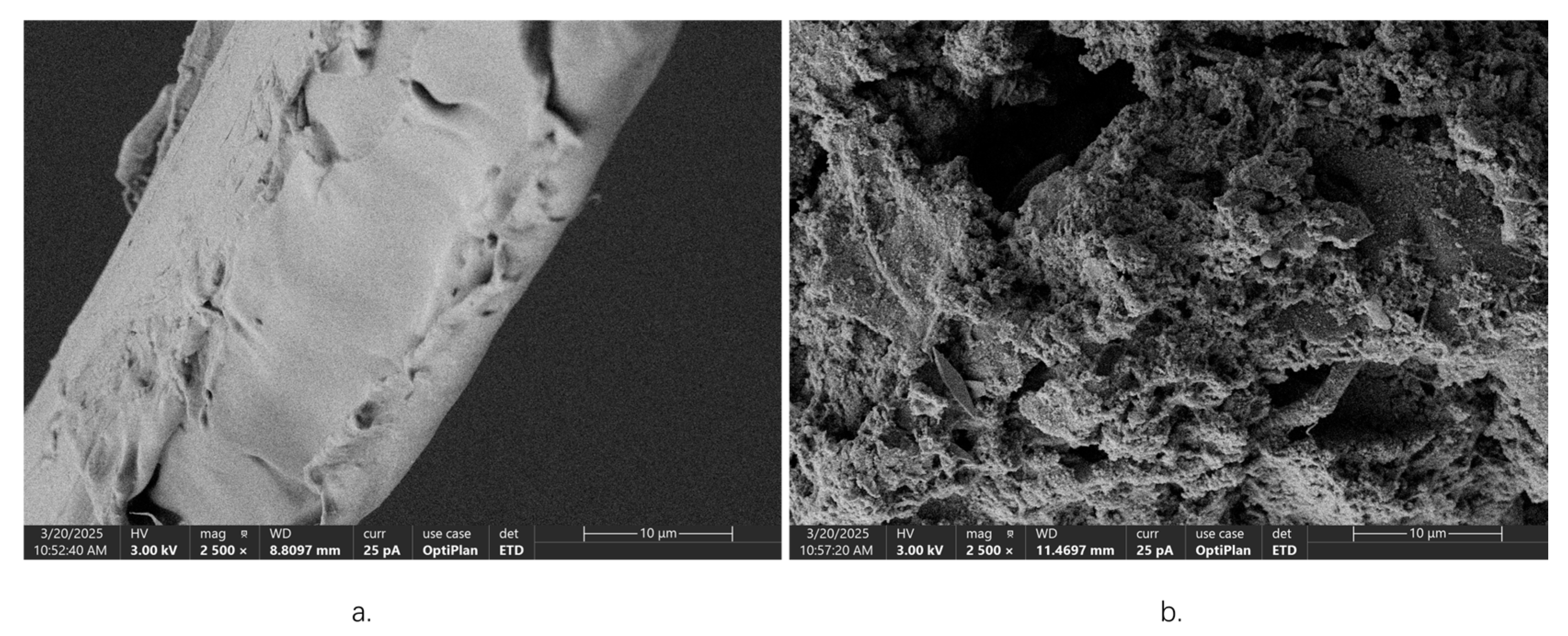
2.4. Microstructure Analysis of W. villosa Leaves
2.5. Chemical Composition Analysis of W. villosa Leaf Volatile Oil
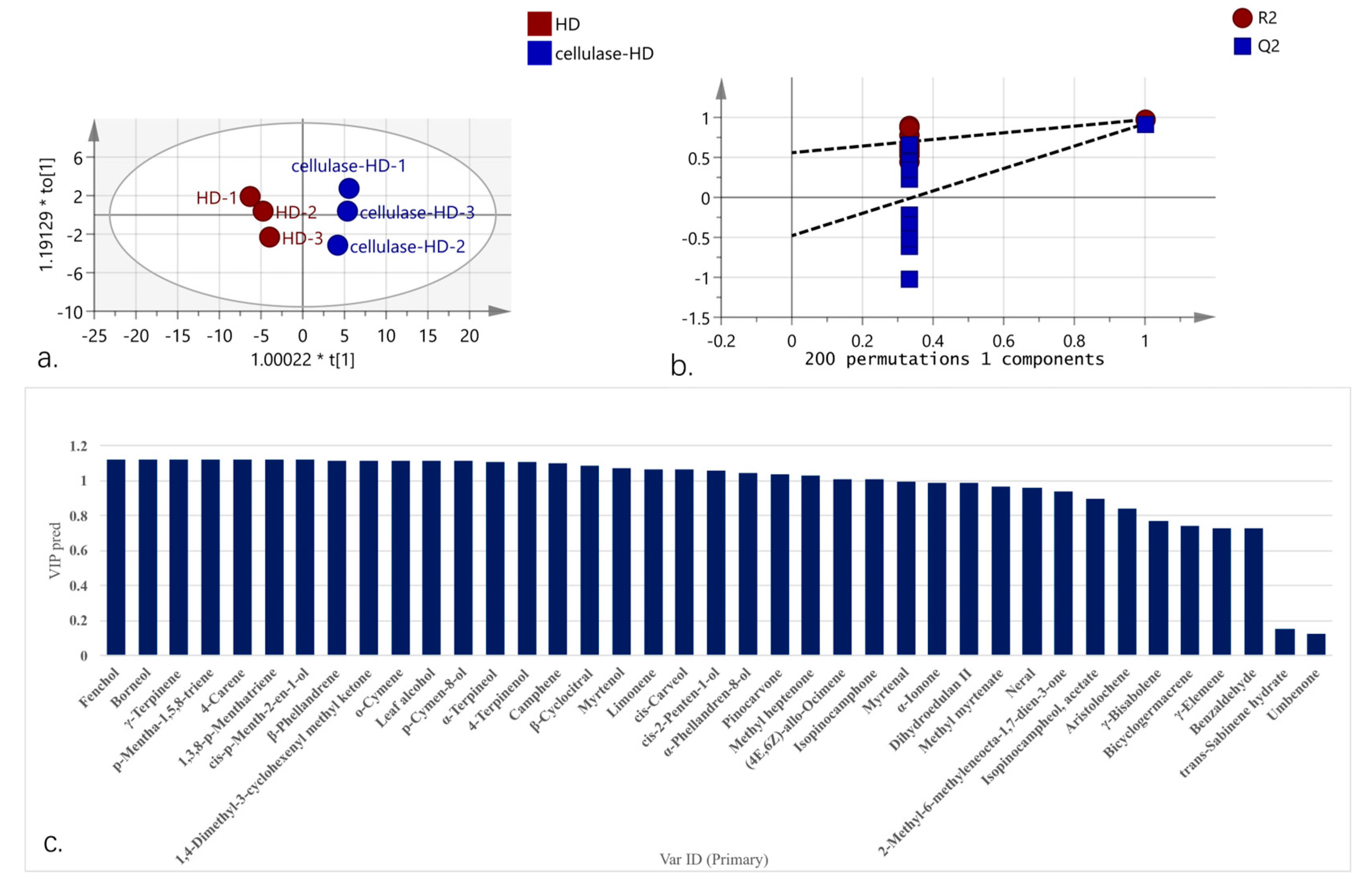
2.6. Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) Analysis of Volatile Oil Components from HD and Cellulase-HD Extracts
2.7. Antioxidant Activity of W. villosa Leaf Volatile Oil
2.7.1. DPPH Radical Scavenging Assay
2.7.2. ABTS Radical Scavenging Assay
2.7.3. Total Antioxidant Capacity (TAC)
2.8. Identification of Antioxidant Active Ingredients
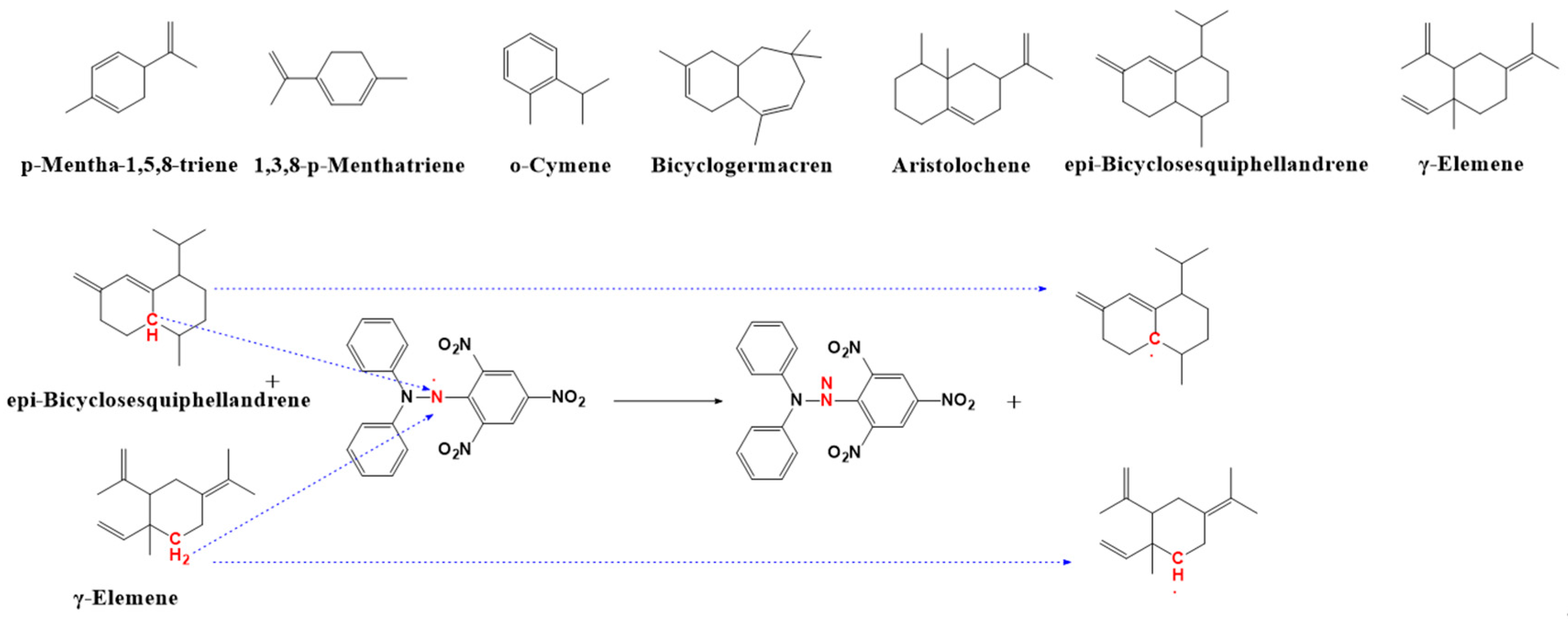
2.8.1. Antioxidant Components of DPPH Free Radicals
2.8.2. Antioxidant Components of ABTS Free Radicals
3. Materials and Methods
3.1. Materials and Ecological Characteristics
3.2. Chemicals and Reagents
3.3. Instruments and Equipment
3.4. Extraction of Volatile Oil from W. villosa Leaves
3.4.1. Volatile Oil Extraction from W. villosa leaves by Hydrodistillation (HD)
3.4.2. Cellulase-Assisted Hydrodistillation (Cellulase-HD)
Single-Factor Screening
Box–Behnken Experimental Design
3.5. Chemical Composition Analysis of Volatile Oil from W. villosa Leaves
3.6. Scanning Electron Microscope (SEM) Analysis
3.7. The Determination of the Antioxidant Capacity of Volatile Oil from W. villosa Leaves
3.7.1. DPPH Radical Scavenging Activity Assay
3.7.2. ABTS Radical Scavenging Activity Assay
3.7.3. Total Antioxidant Capacity Assay
3.8. Screening of Antioxidant Components
3.8.1. DPPH Radical Scavenging Activity Assessment
3.8.2. ABTS Radical Scavenging Activity Assessment
3.9. Data Processing and Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kara, M.; Haoudi, N.H.; Tahiri, H.E.N.; Mernissi, R.E.; Assouguem, A.; Slali, H.; Bahhou, J. Chemical Profiling, Antioxidant and Antimicrobial Activities, and In Silico Evaluation of Gardenia jasminoides Essential Oil. Plants 2025, 14, 1055. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.M.; Tian, K.; Hu, H.J.; Li, P.F.; Tian, X.J. Extraction process optimization of essential oil from Mellissa officinalis L. using a new ultrasound-microwave hybrid-assisted Clevenger hydrodistillation. Ind. Crops Prod. 2023, 203, 117165. [Google Scholar] [CrossRef]
- Liu, Y.C.; Qu, M.C.; Zhao, Y.L.; Wang, D.Y. Research progress on extraction methods, component analysis and bioactivities of Toona sinensis essential oil. China Food Addit. 2024, 35, 289–294. [Google Scholar] [CrossRef]
- Sena, J.D.S.; Rodrigues, S.A.; Sakumoto, K.; Inumaro, R.S.; Maldonado, P.G. Antioxidant Activity, Antiproliferative Activity, Antiviral Activity, NO Production Inhibition, and Chemical Composition of Essential Oils and Crude Extracts of Leaves, Flower Buds, and Stems of Tetradenia riparia. Pharmaceuticals 2024, 17, 888. [Google Scholar] [CrossRef]
- Bai, D.M.; Li, X.Y.; Wang, S.G.; Zhang, T.Y.; Wei, Y.M. Advances in extraction methods, chemical constituents, pharmacological activities, molecular targets and toxicology of volatile oil from Acorus calamus var. angustatus Besser. Front. Pharmacol. 2022, 13, 1004529. [Google Scholar] [CrossRef]
- Wang, Y.T.; Yan, M.X.; Qin, R.Q.; Gong, Y.L. Enzymolysis–Microwave-Assisted Hydrodistillation for Extraction of Volatile Oil from Atractylodes chinensis and Its Hypoglycemic Activity in vitro. J. AOAC Int. 2021, 104, 1196–1205. [Google Scholar] [CrossRef]
- Meng, Q.Y.; Xie, Y.; Fu, T.L.; Chen, N.; Ye, C.C. Optimization of the extraction process of volatile oil from Jasmine flowers assisted by enzymatic hydrolysis and steam distillation and analysis of its components. Chin. Tradit. Pat. Med. 2023, 45, 891–896. [Google Scholar]
- Jiang, D.M.; Mo, J.M.; Jiang, L.L.; Zang, Q.M.; Xu, L.C. Study on enzymatic extraction technology and antioxidant activity of volatile oil from Curcuma zedoary. Food Ind. 2023, 44, 1–4. [Google Scholar]
- Li, Y.Y.; Huang, L.; Liu, F.; Zhao, M.Q. Optimization of Enzymatic Hydrolysis-assisted Extraction of Essential Oil from Rosemary by Response Surface Methodology and Its GC-MS Component Analysis. Chin. Condit. 2025, 50, 209–214. [Google Scholar]
- Suo, S.Z.; Lai, Y.F.; Li, M.; Song, Q.R.; Cai, J. Phytochemicals, pharmacology, clinical application, patents, and products of Amomi fructus. Food Chem. Toxicol. 2018, 119, 31–36. [Google Scholar] [CrossRef]
- Feng, L.L.; Wang, Z.C.; Lei, Z.W.; Zhang, X.F.; Zhai, B.T. Amomum villosum Lour.: An insight into ethnopharmacological, phytochemical, and pharmacological overview. J. Ethnopharmacol. 2024, 335, 118615. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.N.; Li, J.X.; Su, J.; Ding, X. Unveiling the potential mechanisms of Amomi fructus against gastric ulcers via integrating network pharmacology and in vivo experiments. J. Ethnopharmacol. 2023, 319, 117179. [Google Scholar] [CrossRef]
- Rim, K.H.; Paulrayer, A.; Seul, K.Y.; Gwan, K.Y.; Gon, R.D. Amomum villosum Lour. fruit extract ameliorates high-fat diet-induced body mass gain and adipogenic pathways in C57BL/6 mice. King Saud Univ. Sci. 2021, 33, 101473. [Google Scholar] [CrossRef]
- Kim, H.R.; Paulrayer, A.; Kwon, Y.G.; Ryu, D.G.; Beak, D.G. Acute effects of Amomum villosum Lour. fruit extract on postprandial glycemia and insulin secretion: A single-blind, placebo-controlled, crossover study in healthy subjects. Saudi J. Biol. Sci. 2020, 27, 2968–2971. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, J.S.; Kim, H.G.; Lee, H.W.; Fang, Z.G. Ethyl Acetate Fraction of Amomum villosum var. xanthioides Attenuates Hepatic Endoplasmic Reticulum Stress-Induced Non-Alcoholic Steatohepatitis via Improvement of Antioxidant Capacities. Antioxidants 2021, 10, 998. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sun, D.J.; Xiong, L.L.; Zhang, Z.Q.; Li, Y.X. Phenolics and terpenoids with good anti-inflammatory activity from the fruits of Amomum villosum and the anti-inflammatory mechanism of active diterpene. Bioorg. Chem. 2024, 145, 107190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.Y.; Cao, F.F.; Zhao, Y.L.; Wang, H.B.; Chen, L.M. Antibacterial Ingredients and Modes of the Methanol-Phase Extract from the Fruit of Amomum villosum Lour. Plants 2024, 13, 834. [Google Scholar] [CrossRef]
- Yue, J.J.; Zhang, S.L.; Zheng, B.; Raza, F.; Luo, Z.H. Efficacy and Mechanism of Active Fractions in Fruit of Amomum villosum Lour. for Gastric Cancer. J. Cancer 2021, 12, 5991–5998. [Google Scholar] [CrossRef]
- Lu, S.H.; Zhang, T.; Gu, W.; Yang, X.X.; Lu, J.M. Volatile Oil of Amomum villosum Inhibits Nonalcoholic Fatty Liver Disease via the Gut-Liver Axis. BioMed Res. Int. 2018, 2018, 3589874. [Google Scholar] [CrossRef]
- Tu, X.H.; Liu, Y.J.; Yao, Y.L.; Li, W.X.; Luo, P. Effects of four drying methods on Amomum villosum Lour. ‘Guiyan1’ volatile organic compounds analyzed via headspace solid phase microextraction and gas chromatography-mass spectrometry coupled with OPLS-DA. RSC Adv. 2022, 12, 26485–26496. [Google Scholar] [CrossRef]
- Li, N.; Gao, M.J.; Wu, Y. GCMS was used to analyze the volatile components of Amomum villosum essential oil produced in Guizhou. Guangzhou Chem. Ind. 2024, 52, 63–66. [Google Scholar]
- Zhou, X.M.; Zhang, Y.; Luo, M.Q.; Lian, Y.; Cheng, Q.Y. Comparative analysis of the volatile constituents of Amomi fructus and their protective effects on GES-1 cells. J. Hainan Med. Univ. 2024, 30, 721–730. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Shi, L.J.; Zhang, H.H.; Jing, W.H.; Hong, Z. Study on Extraction Technology of Amomum villosum Leaf Oil and Analysis of Its Chemical Components. Food Drug 2021, 23, 97–101. [Google Scholar]
- Huang, F.T.; Wang, M.J.; Zhang, D.Y. Chemical constituents from Amomum villosum leaf oil and its activity of promoting wound healing. J. Guangdong Pharm. Univ. 2017, 33, 466–470. [Google Scholar] [CrossRef]
- Wang, M.J. Chemical Composition Analysis and Preliminary Pharmacological Study of Amomum villosum Leaf Oil. Master’s Thesis, Guangzhou University of Chinese Medicine, Guangzhou, China, 2017. [Google Scholar]
- Zhang, X.M.; Wang, C.; Qin, X.L.; Hou, S.N.; Zhen, L.P. Optimization of Extraction Technology of Total Flavonoids from Passiflora edulis peel by Ultrasonic Assisted with Complex Enzymeand Its Antioxidant Activity. Sci. Technol. Food Ind. 2022, 43, 215–222. [Google Scholar] [CrossRef]
- Duan, S.; Li, S.R.; Wu, X.T.; Zhang, X.Q. Optimization of cellulase assisted alkali extraction of Cyperus esculentus L. meal protein by response surface methodology. Cereals Oils 2022, 35, 110–114. [Google Scholar]
- Li, X.; Li, C.Y.; Zeng, X.X.; Wang, F. Optimization of Aqueous Enzymatic Extraction of Safflower Oil by Response Surface Methodology. Food Sci. 2017, 38, 231–238. [Google Scholar]
- Kang, H.D.; Zhao, X.H.; Zhang, X.X.; Zhang, Q.; Geng, Y.T.; Zhao, W. Study on the enzyme-assisstes extraction peocess of essential oil from Ledum palustre L. and its antioxidant activity. Nat. Prod. Res. Dev. 2022, 34, 1678–1689+1726. [Google Scholar] [CrossRef]
- Li, M.J.; Xu, B.C.; Zhao, D.S.; Liu, L.L.; Chen, S.X. Advance on key technologies of aqueous enzymatic extraction of oil. Food Ferment. Ind. 2024, 50, 381–387. [Google Scholar] [CrossRef]
- Wang, Y.H.; Xia, Y.L.; Zhang, X.; Yang, H.; Mei, G.F.; Yu, B.; Yi, J.M.; Xue, H.L. Optimization of Extraction Process of Essential Oil from Bergamot Peel and Analysis of Its Components and Antioxidant Activity. Sci. Technol. Food Ind. 2023, 44, 230–239. [Google Scholar] [CrossRef]
- Pan, L.J.; Zhao, D.D.; Xu, X.J.; Yang, S.Y.; Gao, X.Y.; Lu, Q.; Chen, C.X. Enzymatic pretreatment solvent-free microwave extraction of fresh Cirsium setosum essential oil and analysis of antioxidant activity. Food Ferment. Ind. 2025, 6, 1–21. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.Q.; Feng, J.; Li, C.Y.; Chuon, M.R.; Sun, S.; Taing, B. Optimization of the Cellulose-Assisted Extraction Technique of Sulforaphane from Broccoli by Response Surface Method. Sci. Technol. Food Ind. 2020, 45, 188–195. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Pan, X.D.; Guo, Y.; Gao, W.B. Enzyme-deep eutectic solvent pre-treatment for extraction of essential oil from Mentha haplocalyx Briq. leaves: Kinetic, chemical composition and inhibitory enzyme activity. Ind. Crops Prod. 2022, 177, 114429. [Google Scholar] [CrossRef]
- Guo, C.C.; Cui, Y.; Gao, S.; Su, B.Y.; Ma, A.J. Effects of Different Pre-drying Temperatures on Quality Characteristics and Flavor Components of Kernels from Dried Walnuts with Green Husks. Food Sci. 2025, 46, 274–282. [Google Scholar]
- Ma, X.L.; Wang, P.; Geng, Q.Z.; Tian, H.L.; Yan, H.Y. Effect of Heating Temperature on the Flavor Quality of Xinjiang Spicy Chicken Seasoning Sauce Investigated by Sensory Evaluation Combined with GC-MS and OPLS-DA. Food Sci. 2024, 45, 127–134. [Google Scholar]
- Kuang, G.L.; Li, S.; Ning, T.T.; Zhao, G.Z. Differential Volatile Metabolites between Sichuan Baoning Vinegar and Shanxi Aged Vinegar Determined by GC-MS Fingerprint and Multivariate Statistics. Food Sci. 2020, 41, 227–232. [Google Scholar]
- Zhang, H.; An, K.J.; Fu, M.Q.; Yu, Y.S.; Wu, J.J. Effects of Different Extraction and GC-MS Injection Methods on the Volatile Composition of Pomelo Peel Essential Oil. FSTA 2019, 35, 264–273+238. [Google Scholar] [CrossRef]
- Zhu, J.D.; Xu, Z.Y.; Xu, L. Chemical composition, antioxidant activities, and enzyme inhibitory effects of Lespedeza bicolour Turcz. essential oil. J. Enzyme Inhib. Med. Chem. 2025, 40, 2460053. [Google Scholar] [CrossRef]
- Zhou, Y.S.; Gao, C.; Song, L.; Dong, L.; Yu, L.H. Regulation of Terpinen-4-ol on Antioxidant Function of Lungs, Spleen and Pancreas of Weaned Piglets with Oxidative Stress. Chin. J. Anim. Nutr. 2025, 37, 226–235. [Google Scholar]
- Fathalizadeh, M.; Tabrizi, M.H.; Tehranipour, M. A novel alpha-terpineol-loaded niosome formulation coated with hyaluronic acid and evaluation of its anticancer properties in vitro. J. Mol. Liq. 2025, 424, 127139. [Google Scholar] [CrossRef]
- Wang, W.S.; Wang, Y.; Chen, Y.; Jia, H.E.; Guo, D.M. Study on borneol enhancing the antioxidant effect of edaravone in rats with the cerebral ischemia-reperfusion. Jilin J. Chin. Med. 2021, 41, 1497–1501. [Google Scholar] [CrossRef]
- Xu, R.R.; Duan, T.H.; Li, R.; Tian, W.; Niu, L.Y. Establishment of UPLC Fingerprint of Wild Qingqiao from Hebei Province and Study on Its Antioxidant Spectrum-Effect Relationship. Chin. J. Med. Pharm. 2025, 60, 579–588. [Google Scholar]
- Wojtunik, K.A.; Ciesla, L.M.; Waksmundzka-Hajnos, M. Model studies on the antioxidant activity of common terpenoid constituents of essential oils by means of the 2,2-diphenyl-1-picrylhydrazyl method. J. Agric. Food Chem. 2014, 62, 9088–9094. [Google Scholar] [CrossRef]
- Ahmed, Z.B.; Mohamed, Y.; Johan, V.; Dejaegher, B.; Demeyer, K. Defining a standardized methodology for the determination of the antioxidant capacity: Case study of Pistacia atlantica leaves. Analyst 2019, 145, 557–571. [Google Scholar] [CrossRef]
- Pei, L.; Li, F. Theoretical investigation on the antioxidative property of imine Resveratrol. Chem. Res. Appl. 2019, 31, 612–618. [Google Scholar]
- Ran, C.X.; Hu, J.; Deng, H.L. Analysis of volatile components and screening of free radical-scavenging active components in fresh Guhong tangerine peel essential oil. Food Mach. 2025, 41, 136–144. [Google Scholar] [CrossRef]
- Feng, L.; Huang, H.S. Key points of Planting and cultivation management techniques for Amomum villosum under the forest. World Trop. Agric. Inf. 2022, 6, 28–29. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020.
- Tan, D.; Deng, H.Q.; Zhu, S.Q. Optimization of cellulase assisted aqueous extraction of active components from Zingiber officinale Roscoe. Cereals Oils 2023, 36, 75–78. [Google Scholar]
- Sun, M.Z.; Yang, Y.Y.; Yu, Y.N.; Chen, H.; Zhao, Q.Y. Composition Analysis Based on Gas Chromatography-Orbitrap-Mass Spectrometry and Evaluation of the Antioxidant Activity of Four Plants Essential Oils. Sci. Technol. Food Ind. 2022, 43, 338–354. [Google Scholar] [CrossRef]
- Li, X.J.; Yang, L.H.; Chen, Y.M.; Cao, K.H.; He, J.M. Antioxidant and antibacterial activity of essential oil extracted from leaves of the Pistacia weinmannifolia by three methods. Food Ferment. Ind. 2020, 46, 93–98. [Google Scholar] [CrossRef]
- Gao, W.T.; Lei, W.P.; Chen, L.; Yang, X.Q.; Li, J.M. Identification of Characteristic Volatile Substances by the Combined Use of Different Analytical Methods and Antioxidant Properties of White Peppers from Different Geographical Origins. Food Sci. 2025, 46, 235–246. [Google Scholar]
- Wang, S.Y.; Lv, H.; Gao, M.; Chen, T.T.; Zhao, C.X. Rapid Screening of Antioxidants of Oil Extracted from Bangmaoluoxinfu (Trichonephila clavata) Based on GC-MS Coupled with DPPH Assay. Chin. Arch. Tradit. Chin. Med. 2025, 43, 108–111+272. [Google Scholar] [CrossRef]
- Wang, C.; Li, R.; Wu, Y.; Wang, Y.; Tang, S.H. Rapid Screening of Radical Scavengers in Essential Oil from Piper longum L. as Food Seasoning. Food Sci. 2021, 42, 226–231. [Google Scholar]




| No. | A: Enzyme Dosage (%) | B: Hydrolysis Temperature (°C) | C: Hydrolysis Time (min) | D: Material/Liquid Ratio (g/mL) | Yield (%) |
|---|---|---|---|---|---|
| 1 | 2 | 50 | 120 | 12 | 0.495 |
| 2 | 2 | 50 | 60 | 12 | 0.649 |
| 3 | 2.5 | 45 | 90 | 10 | 0.557 |
| 4 | 1.5 | 45 | 90 | 10 | 0.421 |
| 5 | 2 | 55 | 90 | 12 | 0.515 |
| 6 | 2.5 | 50 | 90 | 12 | 0.591 |
| 7 | 2 | 50 | 90 | 10 | 0.692 |
| 8 | 2 | 55 | 60 | 10 | 0.500 |
| 9 | 2 | 55 | 90 | 8 | 0.469 |
| 10 | 2 | 45 | 90 | 8 | 0.499 |
| 11 | 2.5 | 50 | 60 | 10 | 0.668 |
| 12 | 2 | 45 | 60 | 10 | 0.574 |
| 13 | 2.5 | 50 | 90 | 8 | 0.478 |
| 14 | 1.5 | 50 | 60 | 10 | 0.499 |
| 15 | 2.5 | 50 | 120 | 10 | 0.523 |
| 16 | 1.5 | 50 | 120 | 10 | 0.627 |
| 17 | 2 | 50 | 60 | 8 | 0.469 |
| 18 | 2 | 55 | 120 | 10 | 0.542 |
| 19 | 1.5 | 50 | 90 | 8 | 0.514 |
| 20 | 2.5 | 55 | 90 | 10 | 0.478 |
| 21 | 1.5 | 50 | 90 | 12 | 0.527 |
| 22 | 2 | 50 | 120 | 8 | 0.557 |
| 23 | 2 | 45 | 120 | 10 | 0.506 |
| 24 | 2 | 45 | 90 | 12 | 0.485 |
| 25 | 2 | 50 | 90 | 10 | 0.704 |
| 26 | 2 | 50 | 90 | 10 | 0.720 |
| 27 | 1.5 | 55 | 90 | 10 | 0.537 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 0.1617 | 14 | 0.0115 | 34.15 | <0.0001 ** | significant |
| A—Enzyme dosage | 0.0024 | 1 | 0.0024 | 7.12 | 0.0205 * | |
| B—Hydrolysis temperature | 8.333 × 10−8 | 1 | 8.333E-08 | 0.0002 | 0.9877 | |
| C—Hydrolysis time | 0.0010 | 1 | 0.0010 | 2.93 | 0.1128 | |
| D—Material/liquid ratio | 0.0063 | 1 | 0.0063 | 18.77 | 0.0010 ** | |
| AB | 0.0095 | 1 | 0.0095 | 28.11 | 0.0002 ** | |
| AC | 0.0186 | 1 | 0.0186 | 55.10 | <0.0001 ** | |
| AD | 0.0025 | 1 | 0.0025 | 7.39 | 0.0186 * | |
| BC | 0.0030 | 1 | 0.0030 | 8.95 | 0.0113 * | |
| BD | 0.0009 | 1 | 0.0009 | 2.66 | 0.1287 | |
| CD | 0.0146 | 1 | 0.0146 | 43.30 | <0.0001 ** | |
| A2 | 0.0329 | 1 | 0.0329 | 97.19 | <0.0001 ** | |
| B2 | 0.0776 | 1 | 0.0776 | 229.49 | <0.0001 ** | |
| C2 | 0.0161 | 1 | 0.0161 | 47.49 | <0.0001 ** | |
| D2 | 0.0533 | 1 | 0.0533 | 157.72 | <0.0001 ** | |
| Residual | 0.0041 | 12 | 0.0003 | |||
| Lack of Fit | 0.0037 | 10 | 0.0004 | 1.86 | 0.4005 | not significant |
| Pure Error | 0.0004 | 2 | 0.0002 | |||
| Cor Total | 0.1657 | 26 | ||||
| R2 = 0.9577 | Adjusted R2 = 0.9469 | |||||
| No. | RT | Name | Structural Formula | CAS | KI | KI* | Relative Amount (%) | |
|---|---|---|---|---|---|---|---|---|
| HD | Cellulase-HD | |||||||
| Olefins | ||||||||
| 1 | 18.99 | Norbornane | C10H16 | 497-32-5 | 980 | 940 | / | 0.648 ± 0.116% |
| 2 | 19.24 | Camphene | C10H16 | 79-92-5 | 1005 | 1043 | 1.235 ± 0.274 | 2.897 ± 0.114% |
| 3 | 22.12 | β-Phellandrene | C10H16 | 555-10-2 | 1070 | 1183 | 41.881 ± 6.262 | 10.634 ± 1.381% |
| 5 | 23.61 | β-Myrcene | C10H16 | 123-35-3 | 1114 | 1137 | 8.656 ± 2.591 | / |
| 7 | 23.76 | β-Pinene | C10H16 | 18172-67-3 | 1125 | 1118 | 4.743 ± 1.395 | / |
| 4 | 24.21 | α-Phellandrene | C10H16 | 99-83-2 | 1149 | 1164 | / | 0.967 ± 0.345% |
| 6 | 24.71 | α-Terpinene | C10H16 | 99-86-5 | 1172 | 1178 | 2.691 ± 0.780 | / |
| 8 | 25.73 | Limonene | C10H16 | 5989-27-5 | 1206 | / | 8.444 ± 1.907 | 13.352 ± 0.264% |
| 9 | 27.06 | α-Terpinene | C10H16 | 2867-05-2 | 1225 | 1210 | 0.519 ± 0.082 | / |
| 10 | 27.24 | trans-β-Ocimene | C10H16 | 3779-61-1 | 1228 | 1247 | / | 1.073 ± 0.210% |
| 11 | 27.95 | γ-Terpinene | C10H16 | 99-85-4 | 1237 | 1243 | 6.601 ± 1.324 | 14.981 ± 0.274% |
| 12 | 29.84 | 4-Carene | C10H16 | 29050-33-7 | 1264 | 1149 | 1.541 ± 0.220 | 5.572 ± 0.135% |
| 13 | 30.83 | trans-Isolimonene | C10H16 | 6876-12-6 | 1275 | / | 0.044 ± 0.037 | / |
| 14 | 34.01 | (4E,6Z)-allo-Ocimene | C10H16 | 7216-56-0 | 1325 | 1370 | 0.506 ± 0.117 | 0.829 ± 0.098% |
| 15 | 37.24 | Neo-allo-ocimene | C12H24 | 74630-41-4 | 1374 | 1392 | / | / |
| 16 | 37.38 | p-Mentha-1,5,8-triene | C10H14 | 21195-59-5 | 1376 | 1375 | 0.052 ± 0.012 | 0.157 ± 0.005% |
| 17 | 37.79 | 1,3,8-p-Menthatriene | C10H14 | 18368-95-1 | 1383 | / | 0.039 ± 0.006 | 0.165 ± 0.008% |
| 18 | 39.65 | γ-Elemene | C15H24 | 29873-99-2 | 1425 | 1434 | 0.215 ± 0.079 | 0.335 ± 0.097% |
| 19 | 44.25 | γ-Bisabolene | C15H24 | 242794-76-9 | 1561 | / | 0.811 ± 0.215 | 0.335 ± 0.097% |
| 20 | 46.34 | Humulene | C15H24 | 6753-98-6 | 1638 | 1665 | / | 0.411 ± 0.108% |
| 21 | 47.31 | Aristolochene | C15H24 | 26620-71-3 | 1707 | 1669 | 0.245 ± 0.089 | 0.383 ± 0.072% |
| 22 | 48.21 | Bicyclogermacrene | C15H24 | 24703-35-3 | 1756 | 1752 | 0.150 ± 0.063 | 0.220 ± 0.042% |
| 23 | 48.53 | epi-Bicyclosesquiphellandrene | C15H24 | 54274-73-6 | 1775 | 1760 | / | 0.213 ± 0.059% |
| alcohol | ||||||||
| 24 | 31.26 | cis-2-Penten-1-ol | C5H10O | 1576-95-0 | 1284 | 1296 | 0.134 ± 0.068 | 0.293 ± 0.009% |
| 25 | 32.88 | 1-Hexanol | C6H14O | 111-27-3 | 1307 | 1325 | / | 0.134 ± 0.016% |
| 26 | 34.51 | Leaf alcohol | C6H12O | 928-96-1 | 1332 | 1351 | 0.188 ± 0.034 | 0.428 ± 0.017% |
| 27 | 42.08 | trans-Sabinene hydrate | C10H18O | 17699-16-0 | 1505 | 1483 | 0.093 ± 0.013 | 0.088 ± 0.036% |
| 28 | 42.29 | trans-Pinene hydrate | C10H18O | 4948-29-2 | 1511 | 1432 | / | 0.054 ± 0.008% |
| 29 | 43.28 | Fenchol | C10H18O | 1632-73-1 | 1536 | 1543 | 0.394 ± 0.105 | 1.843 ± 0.118% |
| 30 | 44.02 | 4-Terpineol | C10H18O | 562-74-3 | 1555 | 1552 | 4.974 ± 0.656 | 10.145 ± 0.971% |
| 31 | 44.65 | cis-p-Menth-2-en-1-ol | C10H18O | 29803-82-5 | 1570 | 1563 | 0.130 ± 0.017 | 0.288 ± 0.016% |
| 32 | 45.59 | cis-Verbenol | C10H16O | 18881-04-4 | 1593 | 1645 | 0.286 ± 0.407 | / |
| 33 | 46.23 | trans-Verbenol | C10H16O | 1820-09-3 | 1630 | 1648 | 0.225 ± 0.046 | / |
| 34 | 46.63 | α-Terpineol | C10H18O | 10482-56-1 | 1659 | 1690 | 2.751 ± 0.603 | 8.085 ± 0.940% |
| 35 | 46.94 | Borneol | C10H18O | 507-70-0 | 1682 | 1698 | 0.675 ± 0.168 | 2.333 ± 0.154% |
| 36 | 47.35 | α-Phellandren-8-ol | C10H16O | 1686-20-0 | 1709 | 1714 | 0.264 ± 0.067 | 0.486 ± 0.066% |
| 37 | 47.55 | cis-Carveol | C10H16O | 1000374-16-8 | 1720 | 1774 | 0.035 ± 0.008 | 0.068 ± 0.010% |
| 38 | 49.56 | Myrtenol | C10H16O | 19894-97-4 | 1815 | 1807 | 1.855 ± 0.422 | 3.073 ± 0.236% |
| 39 | 50.64 | 4-Carenol | C10H16O | 6617-35-2 | 1843 | 1816 | / | 0.486 ± 0.066% |
| 40 | 50.77 | p-Cymen-8-ol | C10H14O | 1197-01-9 | 1847 | 1852 | 0.173 ± 0.041 | 0.392 ± 0.039% |
| ketone | ||||||||
| 41 | 31.95 | 2,2,6-Trimethylcyclohexanone | C9H16O | 2408-37-9 | 1293 | 1282 | / | 0.070 ± 0.006% |
| 42 | 32.26 | Methyl heptenone | C8H14O | 110-93-0 | 1297 | 1317 | 0.046 ± 0.019 | 0.126 ± 0.024% |
| 43 | 36.11 | Fenchone | C10H16O | 7787-20-4 | 1357 | 1383 | / | 0.114 ± 0.013% |
| 44 | 38.18 | Thujone | C10H16O | 471-15-8 | 1389 | / | / | 0.079 ± 0.020% |
| 45 | 41.43 | 1,4-Dimethyl-3-cyclohexenyl methyl ketone | C10H16O | 43219-68-7 | 1485 | 1491 | 0.156 ± 0.043 | 0.484 ± 0.031% |
| 46 | 41.95 | 2-Methyl-6-methyleneocta-1,7-dien-3-one | C10H14O | 41702-60-7 | 1502 | 1345 | 0.177 ± 0.017 | 0.257 ± 0.042% |
| 47 | 42.66 | Isopinocamphone | C10H16O | 15358-88-0 | 1520 | 1555 | 0.929 ± 0.125 | 1.417 ± 0.182% |
| 48 | 43.37 | Pinocarvone | C10H14O | 30460-92-5 | 1549 | 1566 | 1.030 ± 0.139 | 1.580 ± 0.167% |
| 49 | 45.43 | Umbenone | C10H14O | 24545-81-1 | 1590 | 1614 | 0.071 ± 0.015 | 0.068 ± 0.031% |
| 50 | 51.23 | α-Ionone | C13H20O | 127-41-3 | 1859 | 1863 | 0.047 ± 0.018 | 0.112 ± 0.023% |
| aldehydes | ||||||||
| 51 | 35.13 | Nonanal | C9H18O | 124-19-6 | 1342 | 1348 | / | 0.052 ± 0.013% |
| 52 | 41.49 | Benzaldehyde | C7H6O | 100-52-7 | 1487 | 1480 | 0.196 ± 0.055 | 0.752 ± 0.645% |
| 53 | 44.86 | β-Cyclocitral | C10H16O | 432-25-7 | 1576 | 1586 | 0.049 ± 0.006 | 0.094 ± 0.007% |
| 54 | 45.01 | α-Thujenal | C10H14O | 57129-54-1 | 1579 | / | / | 0.084 ± 0.022% |
| 55 | 45.23 | Myrtenal | C10H14O | 564-94-3 | 1585 | 1597 | 1.935 ± 0.316 | 2.740 ± 0.292% |
| 56 | 47.65 | Neral | C10H16O | 106-26-3 | 1726 | 1733 | 0.137 ± 0.024 | 0.227 ± 0.032% |
| esters | ||||||||
| 57 | 43.82 | Isopinocampheol, acetate | C12H20O2 | 1000462-98-1 | 1549 | / | 0.570 ± 0.096 | 0.842 ± 0.141% |
| 58 | 45.70 | β-Sabinyl acetate | C12H18O2 | 3536-54-7 | 1597 | 1615 | / | 1.173 ± 0.106% |
| 59 | 46.75 | Methyl myrtenate | C11H16O2 | 30649-97-9 | 1668 | 1670 | 1.966 ± 0.403 | 2.727 ± 0.169% |
| 60 | 49.43 | Methyl acetylsalicylate | C10H10O4 | 580-02-9 | 1812 | 1822 | 0.200 ± 0.048 | / |
| 61 | 49.67 | cis-Chrysanthenyl formate | C10H16O | 1000151-75-4 | 1818 | / | / | 0.541 ± 0.025% |
| other | ||||||||
| 62 | 29.18 | o-Cymene | C10H14 | 527-84-4 | 1254 | 1276 | 1.653 ± 0.378 | 4.038 ± 0.090% |
| 63 | 37.24 | 1-Methyl-1-ethylcyclopentane | C8H16 | 16747-50-5 | 1374 | / | 0.027 ± 0.008 | / |
| 64 | 37.62 | β,β-Dimethylstyrene | C10H12 | 768-49-0 | 1380 | / | 0.137 ± 0.025 | / |
| 65 | 40.49 | Dihydroedulan II | C13H22O | 41678-32-4 | 1453 | 1492 | 0.124 ± 0.019 | 0.207 ± 0.031% |
| Classification | Name | DPPH | ABTS |
|---|---|---|---|
| Olefins | Norbornane | 33.39 ± 0.61% | 6.46 ± 0.69% |
| Camphene | 34.47 ± 0.89% | 11.24 ± 0.01% | |
| β-Phellandrene | 30.71 ± 1.59% | 22.45 ± 0.33% | |
| α-Phellandrene | 43.47 ± 1.84% | 3.29 ± 0.64% | |
| Limonene | 37.04 ± 0.13% | 25.93 ± 0.03% | |
| trans-β-Ocimene | 39.46 ± 7.21% | 12.45 ± 1.74% | |
| γ-Terpinene | 40.35 ± 2.05% | 36.23 ± 0.15% | |
| 4-Carene | 45.02 ± 3.35% | 4.68 ± 1.58% | |
| (4E,6Z)-allo-Ocimene | 43.09 ± 2.99% | 20.12 ± 1.08% | |
| p-Mentha-1,5,8-triene | 54.42 ± 0.34% | 37.99 ± 3.63% | |
| 1,3,8-p-Menthatriene | 53.91 ± 1.69% | 23.65 ± 2.82% | |
| γ-Elemene | 78.91 ± 2.01% | 57.67 ± 1.14% | |
| γ-Bisabolene | 15.91 ± 1.74% | 57.19 ± 0.45% | |
| Humulene | 54.05 ± 0.86% | 36.67 ± 4.28% | |
| Aristolochene | 58.36 ± 0.61% | 29.18 ± 1.25% | |
| Bicyclogermacren | 55.72 ± 3.10% | / | |
| epi-Bicyclosesquiphellandrene | 71.51 ± 3.42% | / | |
| Alcohol | Fenchol | 25.73 ± 2.69% | 64.80 ± 1.97% |
| 4-Terpinenol | 18.38 ± 1.32% | 62.77 ± 5.54% | |
| cis-p-Menth-2-en-1-ol | 24.52 ± 2.26% | / | |
| α-Terpineol | 18.54 ± 0.30% | 66.95 ± 1.11% | |
| Borneol | 20.89 ± 1.81% | 65.04 ± 2.61% | |
| α-Phellandren-8-ol | 32.44 ± 3.15% | 55.96 ± 2.41% | |
| Myrtenol | 15.65 ± 0.33% | 39.56 ± 0.99% | |
| Ketone | Methyl heptenone | 30.49 ± 3.66% | / |
| 1,4-Dimethyl-3-cyclohexenyl methyl ketone | 43.55 ± 5.67% | 66.55 ± 1.09% | |
| Isopinocamphone | 30.34 ± 10.08% | 66.58 ± 2.57% | |
| Pinocarvone | 30.14 ± 2.82% | 64.85 ± 1.75% | |
| Aldhydes | β-Cyclocitral | 38.88 ± 2.69% | / |
| Myrtenal | 27.54 ± 1.70% | 65.57 ± 4.43% | |
| Efters | Isopinocampheol, acetate | 40.85 ± 5.05% | 62.94 ± 2.79% |
| Methyl myrtenate | 35.15 ± 1.21% | 65.03 ± 4.20% | |
| Other | o-Cymene | 55.03 ± 3.02% | 20.25 ± 1.00% |
| Dihydroedulan II | 44.90 ± 0.37% | 54.55 ± 2.66% |
| Factor | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A—Enzyme dosage (%) | 1.5 | 2.0 | 2.5 |
| B—Hydrolysis temperature (°C) | 45 | 50 | 55 |
| C—Hydrolysis time (min) | 60 | 90 | 120 |
| D—Material/liquid ratio (mL/g) | 8:1 | 10:1 | 12:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Lv, B.; Nian, X.; Xie, X.; Yang, X. Optimized Extraction, Comprehensive Chemical Profiling, and Antioxidant Evaluation of Volatile Oils from Wurfbainia villosa (Lour.) Škorničk. & A.D.Poulsen Leaves. Plants 2025, 14, 2041. https://doi.org/10.3390/plants14132041
Gu Y, Lv B, Nian X, Xie X, Yang X. Optimized Extraction, Comprehensive Chemical Profiling, and Antioxidant Evaluation of Volatile Oils from Wurfbainia villosa (Lour.) Škorničk. & A.D.Poulsen Leaves. Plants. 2025; 14(13):2041. https://doi.org/10.3390/plants14132041
Chicago/Turabian StyleGu, Yuancong, Bangyu Lv, Xingrui Nian, Xinrui Xie, and Xinhe Yang. 2025. "Optimized Extraction, Comprehensive Chemical Profiling, and Antioxidant Evaluation of Volatile Oils from Wurfbainia villosa (Lour.) Škorničk. & A.D.Poulsen Leaves" Plants 14, no. 13: 2041. https://doi.org/10.3390/plants14132041
APA StyleGu, Y., Lv, B., Nian, X., Xie, X., & Yang, X. (2025). Optimized Extraction, Comprehensive Chemical Profiling, and Antioxidant Evaluation of Volatile Oils from Wurfbainia villosa (Lour.) Škorničk. & A.D.Poulsen Leaves. Plants, 14(13), 2041. https://doi.org/10.3390/plants14132041






