Antioxidant and Anti-Inflammatory Effects of Traditional Medicinal Plants for Urolithiasis: A Scoping Review
Abstract
1. Introduction
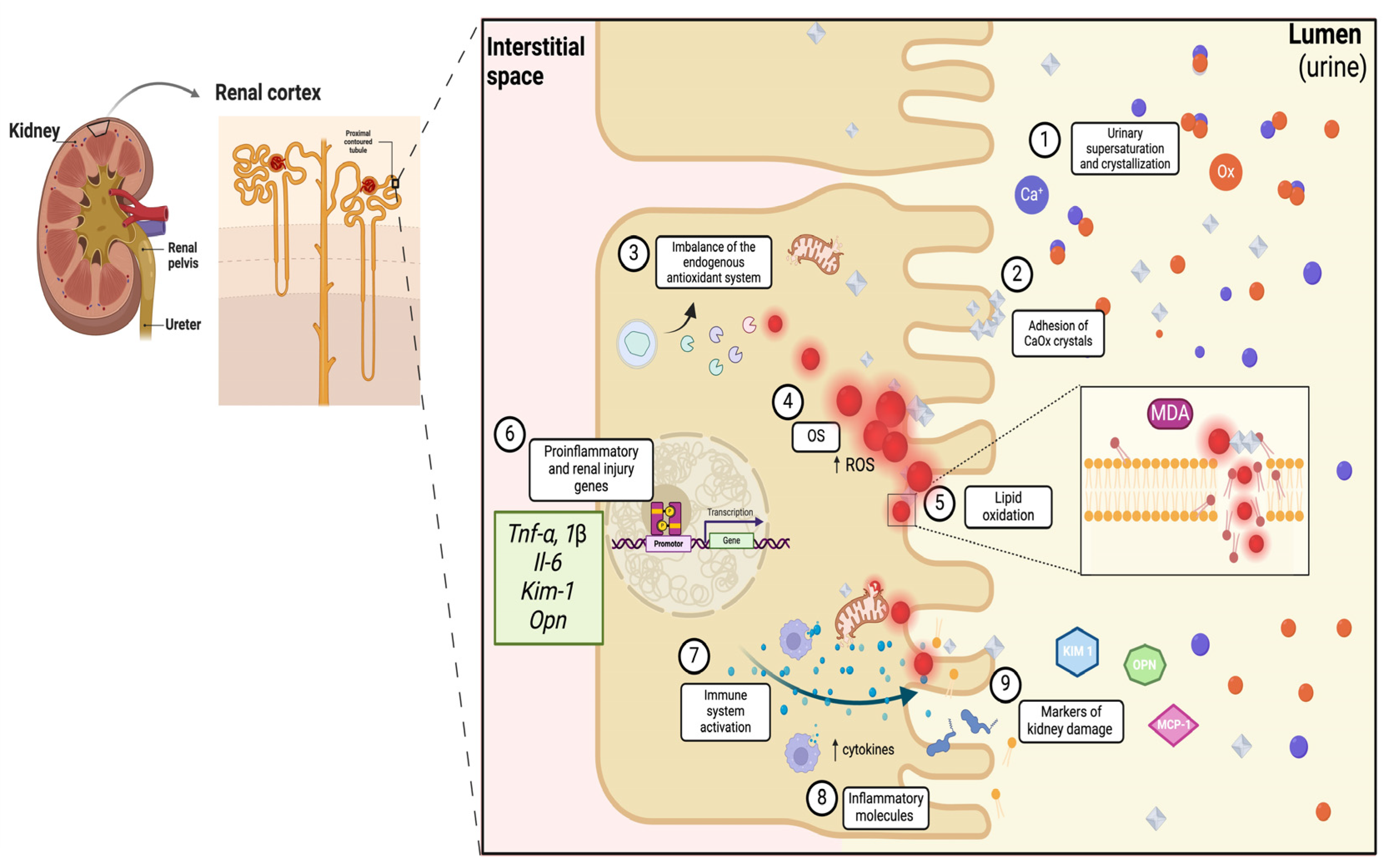
1.1. Oxidative Stress and Inflammation: Processes That Promote Urolithiasis
1.2. Traditional Medicinal Plants: Alternatives for Urolithiasis Management
2. Results and Discussion
2.1. Antioxidant and Anti-Inflammatory Effects of Traditional Medicinal Plants in Urolithiasis
| Scientific Name | Common Name | Region | Study Design | Antioxidant Biomarkers | Inflammatory Biomarkers | Reference |
|---|---|---|---|---|---|---|
| in vitro | ||||||
| Glechoma longituba | Naki | Beijing, China | Cell lines HK-2 were incubated with CaOx crystals (67 μg/cm2) and the effect of the Aq extract of the dried aerial part of Glechoma longituba (AExGl) was evaluated for 24 h. C (medium) CaOx (crystals: 67 μg/cm2) CaOx + Potassium citrate at different amounts: CaOx + AExGl (0.5 mg/mL) CaOx + AExGl (1 mg/mL) CaOx + AExGl (2 mg/mL) CaOx + AExGl (4 mg/mL) | CaOx vs. CaOx + 1 ↓ MDA ↑ SOD CaOx + 2 and CaOx + 4 ↓ MDA ↑ SOD, CAT Potassium citrate vs. CaOx + 1 ↓ MDA ↑ SOD CaOx + 2 and CaOx + 4 ↓ MDA ↑ SOD, CAT | CaOx vs. CaOx + 0.5, CaOx + 1, CaOx + 2 and CaOx + 4 ↓ KIM-1 ↓ OPN Potassium citrate vs. CaOx + 1, CaOx + 2, CaOx + 3 and CaOx + 4 ↓ KIM-1 ↓ OPN | [68] |
| Bergenia ligulata | Bergenia ligulata (Saxifragaceae) | Chandni Chowk, New Delhi, India | Cell lines HK-2 were incubated with Na2Ox (2 mM) and evaluated the effect of the EtOH extract of plant and rhizome Bergenia ligulata (EexBl) for 24 h. C (DMEM medium) Ox (2mM) Ox (2mM) + Cystone (200 μg/mL) Ox (2mM)+ EexBl (200 µg/mL) | Ox vs. EexBl + Ox ↓↓↓ H2O2 | Ox vs. EexBl + Ox ↓↓↓ Opn, (Mapk14-p38 MAPK), Nfkb | [69] |
| In vivo | ||||||
| Lygodium japonicum | Lygodium japonicum | Ulsan, Corea | Wistar rats were induced to UL with EG (75%) ad libitum for 28 days. The animals were divided into the following experimental groups (n = 6 per group). Treatment with EtOH extract of Lygodi Spora (EexLS) was administered orally for 28 days. C (water) EG EG + distilled water (1 mL) EG + EexLS (400 mg/kg BW) | In kidney EG vs. EG + EexLS ↑↑ SOD ↓↓ MDA EG + distilled water vs. EG + EexLS ↑↑ SOD ↓↓ MDA | In kidney EG vs. EG + EexLS ↓↓ % Chronic inflammation | [70] |
| Citrus limon | Lemon peel | China | Wistar rats were induced to UL with EG (75%) ad libitum for 30 days. The animals were divided into the following two experimental groups (n = 6 per group). Treatment with aqueous-MeOH extract of lemon peel (MExLP) daily for 20 days. C (water) EG EG + MExLP (100 mg/kg BW) | In kidney EG vs. EG + MExLP ↓↓↓ MDA | In kidney EG + 100 vs. EG ↓↓↓ OPN ↓↓↓ Nfkb, Tnf-α, Il-6 | [71] |
| Xanthium strumarium | Common Cocklebur, Donkeybur | India | Wistar rats were induced to UL with EG (75%) and NH4Cl (1%) ad libitum for 14 days and EG alone for the next 14 days. The animals were divided into the following experimental groups (n = 6 per group). Treatment with EtOH-Aq extract of the Xanthium strumarium fruit (AExXs) was administered orally from 14 days. C (water) EG + NH4Cl EG + NH4Cl + Water (vehicle control) EG + cystone (100 mg/kg BW) EG + AExXs (500 mg/kg BW) | In kidney EG + NH4Cl vs. EG + AExXs ↓↓ MDA ↑↑ CAT, SOD EG + Potassium citrate ↓↓ MDA ↑↑ CAT, SOD | In kidney EG + NH4Cl vs. EG + 500 mg/kg ↓↓ % OPN EG + Potassium citrate ↓↓ %OPN | [72] |
| Tribulus terrestris | Tribulus terrestris | Bangalore, India | Wistar rats were induced to UL with EG (0.4%) with NH4Cl (1%) for 15 days and EG (4%) from the 16th to 28th day. The animals were divided into the following experimental groups (n = 6 per group). Treatment with Aq extract of fruit of Tribulus terrestris (AExTt) administered orally from the 16th to 28th day. C (water) EG + NH4Cl EG + NH4Cl + Vehicle EG + cystone (750 mg/kg BW) EG + NH4Cl+ AExTt (75 mg/kg BW) EG + NH4Cl+ AExTt (225 mg/kg BW) EG + NH4Cl+ AExTt (750 mg/kg BW) | In kidney EG + NH4Cl vs. EG + NH4Cl +75, EG + NH4Cl + 225, EG + NH4Cl 750 ↓↓↓ MDA, CAT ↑↑↑ GSH | In kidney EG + NH4Cl vs. EG + NH4Cl + 750 ↓↓↓ Mapk14 and p38MAPK | [73] |
2.1.1. Antioxidant Effect of Traditional Medicinal Plants on Urolithiasis
2.1.2. Anti-Inflammatory Activity of Traditional Medicinal Plants in Urolithiasis
3. Molecular Effects of Bioactive Compounds in Traditional Medicinal Plants on Urolithiasis
Antioxidant and Anti-Inflammatory Effects of Bioactive Compounds from Plant Extracts
| Scientific Name | Common Name | Plant Part | Qualitative Compounds | Compounds of Interest or Major Proportion | Method of Identification | Reference |
|---|---|---|---|---|---|---|
| Glechoma longituba | Nakai | Aerial part | Terpenoids Steroids Flavonoids Polyphenols Alkaloids | (2E)-3-3,4-Dihydroxyphenyl)-2-propenoyl|oxy} malonic acid | HPLC-HR MS | [97,98] |
| Trans-caffeic acid * | ||||||
| Rosmarinic acid * | ||||||
| Luteolin-7-O-di-glucuronide | ||||||
| Apigenin-7-O-di-glucuronide | ||||||
| Luteolin-7-O-glucuronide | ||||||
| Apigenin-7-O-glucuronide * | ||||||
| Bergenia ligulata | Paashanbheda, bheda, Ayurveda | Plant and rhizome | Alkaloids Glycosides Saponins Carbohydrates Phenols Flavonoids Diterpenes | Phenol, 2,4-bis(1,1-dimethylethyl) | GC-MS LC-MS | [99,100,101] |
| Squalene * | ||||||
| Bergenin * | ||||||
| Lygodium japonicum. | Lygodium japonicum | Phenols Glucosides Flavonoids | Methyl Protocatechuate Caffeic acid * Chlorogenic acid * Linarin Apigenin * Narimgerina Kaempferol * Quercetin * | Miniature mass spectrometry | [102] | |
| Citrus limon | Lemon | Lemon peel | Phenols Flavonoids | Caffeic Ferulic * Hesperidin * Eriocitrin * Diosmin * Rutin * Cynarosides | HPLC UPLC-PDA | [103,104,105] |
| Xanthium strumarium | Common Cocklebur, Donkeybur | Fruit | Alkaloids Flavonoids Triterpenoids Terpenoids Tannins Saponins Quinones Coumarins Carbohydrates Glysides Phenolics | Chlorogenic acid * |
HPLC GC–MS | [106,107,108] |
| 3-O-caffeoylquinic acid | ||||||
| 1-O-caffeoylquinic acid | ||||||
| 4-O-caffeoylquinic acid | ||||||
| 1,3-O-dicaffeoylquinic acid | ||||||
| 1,4-O-dicaffeoylquinic acid | ||||||
| 1,5-O-dicaffeoylquinic acid | ||||||
| 4,5-O-dicaffeoylquinic acid | ||||||
| 1,3,5-O-tricaffeoylquinic acid | ||||||
| 3,4,5-O-tricaffeoylquinic acid | ||||||
| Tribulus terrestris | Gokharu | Fruit | Carbohydrates Amino acids and peptides Glycosides Tannins Terpenoids Phenols Saponins Alkaloids Flavonoids | Terrestrosin 1 | UHPLC/Q-TOF MSE | [109,110,111] |
| Polianthoside D | ||||||
| Parvispinoside B | ||||||
| Purpureagitosid | ||||||
| Desglucolanatigonin Il | ||||||
| F-gitonin | ||||||
| 25R-tribulosin | ||||||
| Ginsenoside Rb | ||||||
| Tigogenin-3-O-b-D-xylopyranosyl-(1 fi 2)-[bD-xylopyranosyl-(1 fi 4)]-[a-L-rhamnopyranosyl-(1 fi 2)]-b-D-galactopyranoside | ||||||
| Phytol * |
4. Challenges and Future Directions
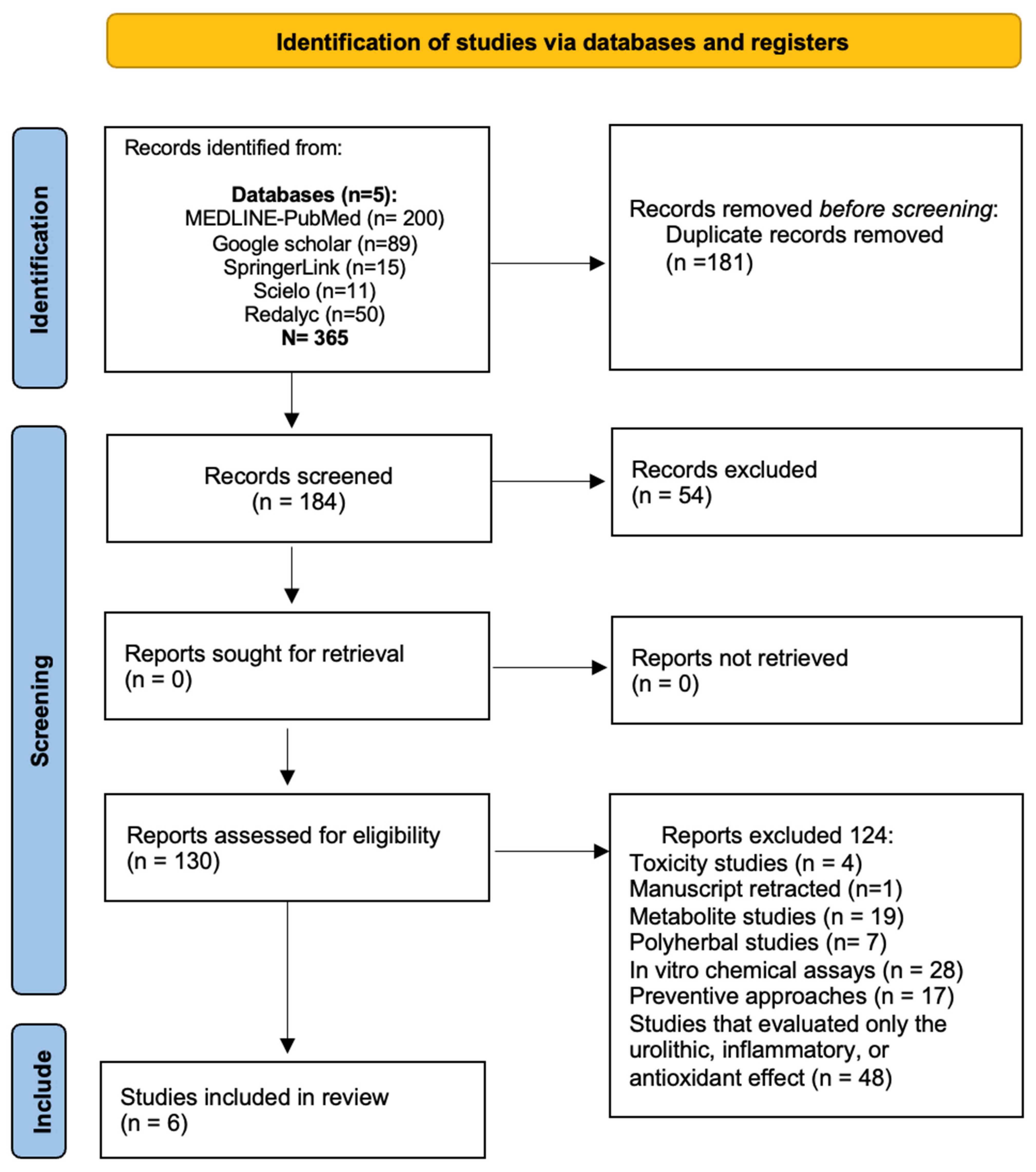
5. Search and Inclusion Methods
Search Strategy
6. Conclusions and Recommendations
7. Limitations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| UL | urolithiasis |
| OS | oxidative stress |
| OPN | osteopontin |
| CaOx | calcium oxalate |
| CaPO4 | calcium phosphate |
| ROS | reactive oxygen species |
| Nrf2 | nuclear factor 2 |
| SOD | superoxide dismutase |
| CAT | catalase |
| GPx | glutathione peroxidase |
| GSH | glutathione |
| MDA | malondialdehyde |
| KIM-1 | kidney injury molecule-1 |
| NF-κB | kappa-light chain enhancer of activated B cells |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| TNF-α | tumor necrosis factor-alpha |
| MCP-1 | monocyte chemoattractant protein |
| WHO | The World Health Organization |
| EG | ethylene glycol |
| NH4Cl | ammonium chloride |
| HK-2 | kidney epithelial cell line |
| BW | body weight |
| OH | reactive hydroxyl |
| COX-2 | cyclooxygenase-2 |
| PGE2 | prostaglandin E2 |
References
- Corbo, J.; Wang, J. Kidney and Ureteral Stones. Emerg. Med. Clin. N. Am. 2019, 37, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Medina-Escobedo, M.; Sánchez-Pozos, K.; Gutiérrez-Solis, A.L.; Avila-Nava, A.; González-Rocha, L.; Lugo, R. Recurrence of Nephrolithiasis and Surgical Events Are Associated with Chronic Kidney Disease in Adult Patients. Medicina 2022, 58, 420. [Google Scholar] [CrossRef]
- Alelign, T.; Petros, B. Kidney Stone Disease: An Update on Current Concepts. Adv. Urol. 2018, 2018, 3068365. [Google Scholar] [CrossRef]
- Aggarwal, K.P.; Narula, S.; Kakkar, M.; Tandon, C. Nephrolithiasis: Molecular Mechanism of Renal Stone Formation and the Critical Role Played by Modulators. Biomed. Res. Int. 2013, 2013, 292953. [Google Scholar] [CrossRef]
- Shastri, S.; Patel, J.; Sambandam, K.K.; Lederer, E.D. Kidney Stone Pathophysiology, Evaluation and Management: Core Curriculum 2023. Am. J. Kidney Dis. 2023, 82, 617–634. [Google Scholar] [CrossRef] [PubMed]
- Tamborino, F.; Cicchetti, R.; Mascitti, M.; Litterio, G.; Orsini, A.; Ferretti, S.; Basconi, M.; De Palma, A.; Ferro, M.; Marchioni, M.; et al. Pathophysiology and Main Molecular Mechanisms of Urinary Stone Formation and Recurrence. Int. J. Mol. Sci. 2024, 25, 3075. [Google Scholar] [CrossRef]
- Sheng, X.; Ward, M.D.; Wesson, J.A. Adhesion between Molecules and Calcium Oxalate Crystals: Critical Interactions in Kidney Stone Formation. J. Am. Chem. Soc. 2003, 125, 2854–2855. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Plata, I.T.; Medina-Escobedo, M.; Basulto-Martínez, M.; Avila-Nava, A.; Gutiérrez-Solis, A.L.; Méndez-Domínguez, N.; Lugo, R. Implementation of a Technique Based on Hounsfield Units and Hounsfield Density to Determine Kidney Stone Composition. Tomography 2021, 7, 606–613. [Google Scholar] [CrossRef]
- Khan, S.R. Reactive Oxygen Species as the Molecular Modulators of Calcium Oxalate Kidney Stone Formation: Evidence from Clinical and Experimental Investigations. J. Urol. 2013, 189, 803–811. [Google Scholar] [CrossRef]
- Banik, S.D.; Avila-Nava, A.; Lugo, R.; Chim Aké, R.; Gutiérrez Solis, A.L. Association Between Low-Grade Inflammation and Hyperuricemia in Adults With Metabolic Syndrome in Yucatán, México. Can. J. Diabetes 2022, 46, 369–374. [Google Scholar] [CrossRef]
- Setthawong, V.; Srisubat, A.; Potisat, S.; Lojanapiwat, B.; Pattanittum, P. Extracorporeal Shock Wave Lithotripsy (ESWL) versus Percutaneous Nephrolithotomy (PCNL) or Retrograde Intrarenal Surgery (RIRS) for Kidney Stones. Cochrane Database Syst. Rev. 2023, 2023, CD007044. [Google Scholar] [CrossRef]
- Pearle, M.S.; Goldfarb, D.S.; Assimos, D.G.; Curhan, G.; Denu-Ciocca, C.J.; Matlaga, B.R.; Monga, M.; Penniston, K.L.; Preminger, G.M.; Turk, T.M.T.; et al. Medical Management of Kidney Stones: AUA Guideline. J. Urol. 2014, 192, 316–324. [Google Scholar] [CrossRef]
- Skolarikos, A.; Straub, M.; Knoll, T.; Sarica, K.; Seitz, C.; Petřík, A.; Türk, C. Metabolic Evaluation and Recurrence Prevention for Urinary Stone Patients: EAU Guidelines. Eur. Urol. 2015, 67, 750–763. [Google Scholar] [CrossRef]
- Dion, M.; Ankawi, G.; Chew, B.; Paterson, R.; Sultan, N.; Hoddinott, P.; Razvi, H. CUA Guideline on the Evaluation and Medical Management of the Kidney Stone Patient—2016 Update. Can. Urol. Assoc. J. 2016, 10, E347–E358. [Google Scholar] [CrossRef]
- Alok, S.; Jain, S.K.; Verma, A.; Kumar, M.; Sabharwal, M. Pathophysiology of Kidney, Gallbladder and Urinary Stones Treatment with Herbal and Allopathic Medicine: A Review. Asian Pac. J. Trop. Dis. 2013, 3, 496–504. [Google Scholar] [CrossRef]
- Abdelmola, A.; Bahri, A.; Abuallut, I.; Refaei, B.; Hakami, W.; Abutaleb, A.; Mahzari, S.; Mashragi, M.; Es′haq, S.; Aldarbi, K. Prevalence, Knowledge, and Perception about the Use of Herbal Medicines Jazan—Saudi Arabia. J. Family Med. Prim. Care 2021, 10, 2386. [Google Scholar] [CrossRef]
- Noor, F.; Qamar, M.T.U.; Ashfaq, U.A.; Albutti, A.; Alwashmi, A.S.S.; Aljasir, M.A. Network Pharmacology Approach for Medicinal Plants: Review and Assessment. Pharmaceuticals 2022, 15, 572. [Google Scholar] [CrossRef]
- World Health Organization. WHO Traditional Medicine Strategy: 2014–2023; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Canaway, R.; Leach, M.; Hunter, J. Setting an Agenda for Strengthening the Evidence-Base for Traditional and Complementary Medicines: Perspectives from an Expert Forum in Australia. Adv. Integr. Med. 2018, 5, 103–111. [Google Scholar] [CrossRef]
- Khan, A.; Bashir, S.; Khan, S.R. Antiurolithic Effects of Medicinal Plants: Results of in Vivo Studies in Rat Models of Calcium Oxalate Nephrolithiasis—A Systematic Review. Urolithiasis 2021, 49, 95–122. [Google Scholar] [CrossRef]
- Arya, P. Kidney Stones Formation and Use of Medicinal Plants as Antiurolithiatic Agents. Univers. J. Pharm. Res. 2017, 2, 42–48. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, L.; Wang, Y.; Xu, R.; Yang, H.; Peng, J. Therapeutic Effects of Chinese Herbal Medicines for Treatment of Urolithiasis: A Review. Chin. Herb. Med. 2023, 15, 526–532. [Google Scholar] [CrossRef]
- Sansores-España, D.; Pech-Aguilar, A.G.; Cua-Pech, K.G.; Medina-Vera, I.; Guevara-Cruz, M.; Gutiérrez-Solis, A.L.; Reyes-García, J.G.; Avila-Nava, A. Plants Used in Mexican Traditional Medicine for the Management of Urolithiasis: A Review of Preclinical Evidence, Bioactive Compounds, and Molecular Mechanisms. Molecules 2022, 27, 2008. [Google Scholar] [CrossRef]
- Allam, E.A.H. Urolithiasis Unveiled: Pathophysiology, Stone Dynamics, Types, and Inhibitory Mechanisms: A Review. Afr. J. Urol. 2024, 30, 34. [Google Scholar] [CrossRef]
- Akram, M.; Idrees, M. Progress and Prospects in the Management of Kidney Stones and Developments in Phyto-Therapeutic Modalities. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419848220. [Google Scholar] [CrossRef]
- Kasote, D.M.; Jagtap, S.D.; Thapa, D.; Khyade, M.S.; Russell, W.R. Herbal Remedies for Urinary Stones Used in India and China: A Review. J. Ethnopharmacol. 2017, 203, 55–68. [Google Scholar] [CrossRef]
- Allam, E.A.H.; Sabra, M.S. Plant-Based Therapies for Urolithiasis: A Systematic Review of Clinical and Preclinical Studies. Int. Urol. Nephrol. 2024, 56, 3687–3718. [Google Scholar] [CrossRef]
- Bahmani, M.; Baharvand-Ahmadi, B.; Tajeddini, P.; Rafieian-Kopaei, M.; Naghdi, N. Identification of Medicinal Plants for the Treatment of Kidney and Urinary Stones. J. Ren. Inj. Prev. 2016, 5, 129–133. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, K.; Di, L.; Wang, P.; Liu, Z.; Zhang, J.; Yue, P.; Song, W.; Zhang, J.; Chen, T.; et al. Traditional Herbal Medicine and Nanomedicine: Converging Disciplines to Improve Therapeutic Efficacy and Human Health. Adv. Drug Deliv. Rev. 2021, 178, 113964. [Google Scholar] [CrossRef]
- Liu, P.; Liu, S.; Chen, G.; Wang, P. Understanding Channel Tropism in Traditional Chinese Medicine in the Context of Systems Biology. Front. Med. 2013, 7, 277–279. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Mnayer, D.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Bezerra, C.F.; Coutinho, H.D.M.; Salehi, B.; Martorell, M.; del Mar Contreras, M.; Soltani-Nejad, A.; et al. Echinacea Plants as Antioxidant and Antibacterial Agents: From Traditional Medicine to Biotechnological Applications. Phytother. Res. 2018, 32, 1653–1663. [Google Scholar] [CrossRef]
- Saenz-Medina, J.; Muñoz, M.; Rodriguez, C.; Sanchez, A.; Contreras, C.; Carballido-Rodríguez, J.; Prieto, D. Endothelial Dysfunction: An Intermediate Clinical Feature between Urolithiasis and Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 912. [Google Scholar] [CrossRef]
- Chaiyarit, S.; Thongboonkerd, V. Mitochondrial Dysfunction and Kidney Stone Disease. Front. Physiol. 2020, 11, 1289. [Google Scholar] [CrossRef]
- Wigner, P.; Grębowski, R.; Bijak, M.; Szemraj, J.; Saluk-Bijak, J. The Molecular Aspect of Nephrolithiasis Development. Cells 2021, 10, 1926. [Google Scholar] [CrossRef]
- Liu, T.; Sun, L.; Zhang, Y.; Wang, Y.; Zheng, J. Imbalanced GSH/ROS and Sequential Cell Death. J. Biochem. Mol. Toxicol. 2022, 36, e22942. [Google Scholar] [CrossRef]
- Napolitano, G.; Fasciolo, G.; Venditti, P. Mitochondrial Management of Reactive Oxygen Species. Antioxidants 2021, 10, 1824. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Venditti, P.; Di Meo, S. The Role of Reactive Oxygen Species in the Life Cycle of the Mitochondrion. Int. J. Mol. Sci. 2020, 21, 2173. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Biological Effect of Protein Modifications by Lipid Peroxidation Products. Chem. Phys. Lipids 2019, 221, 46–52. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Yang, L. Kidney Injury Molecule-1. Curr. Opin. Crit. Care 2010, 16, 556–561. [Google Scholar] [CrossRef]
- Hinojosa-Gonzalez, D.E.; Eisner, B.H. Biomarkers in Urolithiasis. Urol. Clin. N. Am. 2023, 50, 19–29. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Feng, D.; Fu, Y.; Wu, H.; Lu, J.; Bao, J. Calcium-Sensing Receptor Promotes Calcium Oxalate Crystal Adhesion and Renal Injury in Wistar Rats by Promoting ROS Production and Subsequent Regulation of PS Ectropion, OPN, KIM-1, and ERK Expression. Ren. Fail. 2021, 43, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Wang, N. Kidney Injury Molecule-1 in Kidney Disease. Ren. Fail. 2016, 38, 1567–1573. [Google Scholar] [CrossRef]
- Humphreys, B.D.; Xu, F.; Sabbisetti, V.; Grgic, I.; Naini, S.M.; Wang, N.; Chen, G.; Xiao, S.; Patel, D.; Henderson, J.M.; et al. Chronic Epithelial Kidney Injury Molecule-1 Expression Causes Murine Kidney Fibrosis. J. Clin. Investig. 2013, 123, 4023–4035. [Google Scholar] [CrossRef]
- Huang, M.Y.; Chaturvedi, L.S.; Koul, S.; Koul, H.K. Oxalate Stimulates IL-6 Production in HK-2 Cells, a Line of Human Renal Proximal Tubular Epithelial Cells. Kidney Int. 2005, 68, 497–503. [Google Scholar] [CrossRef]
- Wang, X.; Xie, L.; Liu, C. CCR2 Antagonist Attenuates Calcium Oxalate-Induced Kidney Oxidative Stress and Inflammation by Regulating Macrophage Activation. Exp. Anim. 2024, 73, 23–0113. [Google Scholar] [CrossRef] [PubMed]
- Washino, S.; Hosohata, K.; Miyagawa, T. Roles Played by Biomarkers of Kidney Injury in Patients with Upper Urinary Tract Obstruction. Int. J. Mol. Sci. 2020, 21, 5490. [Google Scholar] [CrossRef]
- Jonassen, J.A.; Cao, L.-C.; Honeyman, T.; Scheid, C.R. Mechanisms Mediating Oxalate-Induced Alterations in Renal Cell Functions. Crit. Rev. Eukaryot. Gene Expr. 2003, 13, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative Stress, Free Radicals and Antioxidants: Potential Crosstalk in the Pathophysiology of Human Diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Chaiyarit, S.; Thongboonkerd, V. Mitochondria-Derived Vesicles and Their Potential Roles in Kidney Stone Disease. J. Transl. Med. 2023, 21, 294. [Google Scholar] [CrossRef]
- Mulay, S.R.; Evan, A.; Anders, H.-J. Molecular Mechanisms of Crystal-Related Kidney Inflammation and Injury. Implications for Cholesterol Embolism, Crystalline Nephropathies and Kidney Stone Disease. Nephrol. Dial. Transplant. 2014, 29, 507–514. [Google Scholar] [CrossRef]
- Ming, S.; Tian, J.; Ma, K.; Pei, C.; Li, L.; Wang, Z.; Fang, Z.; Liu, M.; Dong, H.; Li, W.; et al. Oxalate-Induced Apoptosis through ERS-ROS–NF-ΚB Signalling Pathway in Renal Tubular Epithelial Cell. Mol. Med. 2022, 28, 88. [Google Scholar] [CrossRef]
- World Health Organization Traditional Medicine. Available online: https://www.who.int/news-room/questions-and-answers/item/traditional-medicine (accessed on 15 May 2025).
- World Health Organization. Traditional, Complementary and Integrative Medicine. Available online: https://www.who.int/health-topics/traditional-complementary-and-integrative-medicine#tab=tab_1 (accessed on 15 May 2025).
- Smith-Hall, C.; Larsen, H.O.; Pouliot, M. People, Plants and Health: A Conceptual Framework for Assessing Changes in Medicinal Plant Consumption. J. Ethnobiol. Ethnomed. 2012, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Devine, S.N.O.; Kolog, E.A.; Atinga, R. Toward a Knowledge-Based System for African Traditional Herbal Medicine: A Design Science Research Approach. Front. Artif. Intell. 2022, 5, 856705. [Google Scholar] [CrossRef]
- Lucía, C.-P.A.; Jacqueline, B.-R.; Alberto, B.-R.L.; David, B.-A.; Beatriz, R.-A. Actualized Inventory of Medicinal Plants Used in Traditional Medicine in Oaxaca, Mexico. J. Ethnobiol. Ethnomed. 2021, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Uprety, Y.; Asselin, H.; Dhakal, A.; Julien, N. Traditional Use of Medicinal Plants in the Boreal Forest of Canada: Review and Perspectives. J. Ethnobiol. Ethnomed. 2012, 8, 7. [Google Scholar] [CrossRef]
- Tamene, S.; Negash, M.; Makonda, F.B.; Chiwona-Karltun, L. Influence of Socio-Demographic Factors on Medicinal Plant Knowledge among Three Selected Ethnic Groups in South-Central Ethiopia. J. Ethnobiol. Ethnomed. 2024, 20, 29. [Google Scholar] [CrossRef]
- Li, H.; Huang, C.; Li, Y.; Wang, P.; Sun, J.; Bi, Z.; Xia, S.; Xiong, Y.; Bai, X.; Huang, X. Ethnobotanical Study of Medicinal Plants Used by the Yi People in Mile, Yunnan, China. J. Ethnobiol. Ethnomed. 2024, 20, 22. [Google Scholar] [CrossRef]
- Balkrishna, A.; Sharma, I.P.; Arya, V. Ethno-Medicinal Study of Traditional Medicinal Plants Used by Tribal Communities of Uttarakhand, India. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2024, 94, 277–299. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Bibak, H.; Ghara, A.R.; Sahebkar, A.; Shakeri, A. Ethnobotany of the Medicinal Plants Used by the Ethnic Communities of Kerman Province, Southeast Iran. J. Ethnobiol. Ethnomed. 2021, 17, 31. [Google Scholar] [CrossRef]
- Jiang, Q.; Dong, C.; He, Z.; Wang, Y.; Jiang, R.; Liao, W.; Yang, S. Research Landscape and Pharmacological Mechanisms of Traditional Chinese Medicines in Treating and Preventing Urolithiasis: Unearthing an Anti-Urolithic Treasure Trove. J. Ethnopharmacol. 2024, 334, 118502. [Google Scholar] [CrossRef]
- Gorain, S.; Barik, S.; Mandal, M.; Patra, M.; Rajwar, A.K.; Gope, D.; Biswas, S.J. Traditional Herbal Remedies Used in the Management of Urolithiasis by the Tribals of Purulia District, West Bengal, India. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2024, 95, 325–338. [Google Scholar] [CrossRef]
- Pammi, S.S.S.; Suresh, B.; Giri, A. Antioxidant Potential of Medicinal Plants. J. Crop Sci. Biotechnol. 2023, 26, 13–26. [Google Scholar] [CrossRef]
- Ahmed, S.; Hasan, M.M.; Khan, H.; Mahmood, Z.A.; Patel, S. The Mechanistic Insight of Polyphenols in Calcium Oxalate Urolithiasis Mitigation. Biomed. Pharmacother. 2018, 106, 1292–1299. [Google Scholar] [CrossRef]
- Guevara-Cruz, M.; Medina-Vera, I.; Cu-Cañetas, T.E.; Cordero-Chan, Y.; Torres, N.; Tovar, A.R.; Márquez-Mota, C.; Talamantes-Gómez, J.M.; Pérez-Monter, C.; Lugo, R.; et al. Chaya Leaf Decreased Triglycerides and Improved Oxidative Stress in Subjects With Dyslipidemia. Front. Nutr. 2021, 8, 666243. [Google Scholar] [CrossRef]
- Liang, Q.; Li, X.; Zhou, W.; Su, Y.; He, S.; Cheng, S.; Lu, J.; Cao, W.; Yan, Y.; Pei, X.; et al. An Explanation of the Underlying Mechanisms for the In Vitro and In Vivo Antiurolithic Activity of Glechoma Longituba. Oxid. Med. Cell. Longev. 2016, 2016, 3134919. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Tandon, S.; Kumar, D.; Kaur, T.; Kesari, K.K.; Tandon, C. Insights into the Cytoprotective Potential of Bergenia ligulata against Oxalate-Induced Oxidative Stress and Epithelial–Mesenchymal Transition (EMT) via TGFβ1/P38MAPK Pathway in Human Renal Epithelial Cells. Urolithiasis 2022, 50, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Bae, W.J.; Kim, S.J.; Hong, S.H.; Lee, J.Y.; Hwang, T.K.; Choi, Y.J.; Hwang, S.Y.; Kim, S.W. The Inhibitory Effect of an Ethanol Extract of the Spores of Lygodium japonicum on Ethylene Glycol-Induced Kidney Calculi in Rats. Urolithiasis 2014, 42, 309–315. [Google Scholar] [CrossRef]
- Sridharan, B.; Mehra, Y.; Ganesh, R.N.; Viswanathan, P. Regulation of Urinary Crystal Inhibiting Proteins and Inflammatory Genes by Lemon Peel Extract and Formulated Citrus Bioflavonoids on Ethylene Glycol Induced Urolithic Rats. Food Chem. Toxicol. 2016, 94, 75–84. [Google Scholar] [CrossRef]
- Panigrahi, P.N.; Dey, S.; Sahoo, M.; Choudhary, S.S.; Mahajan, S. Alteration in Oxidative/Nitrosative Imbalance, Histochemical Expression of Osteopontin and Antiurolithiatic Efficacy of Xanthium strumarium (L.) in Ethylene Glycol Induced Urolithiasis. Biomed. Pharmacother. 2016, 84, 1524–1532. [Google Scholar] [CrossRef]
- Kaushik, J.; Tandon, S.; Bhardwaj, R.; Kaur, T.; Singla, S.K.; Kumar, J.; Tandon, C. Delving into the Antiurolithiatic Potential of Tribulus Terrestris Extract Through –In Vivo Efficacy and Preclinical Safety Investigations in Wistar Rats. Sci. Rep. 2019, 9, 15969. [Google Scholar] [CrossRef]
- Heinrich, M. Ethnobotany and Its Role in Drug Development. Phytother. Res. 2000, 14, 479–488. [Google Scholar] [CrossRef]
- Abubakar, A.; Haque, M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Rodrigues, E.; Barnes, J. Pharmacovigilance of Herbal Medicines. Drug Saf. 2013, 36, 1–12. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on Tannins: Extraction Processes, Applications and Possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Plaskova, A.; Mlcek, J. New Insights of the Application of Water or Ethanol-Water Plant Extract Rich in Active Compounds in Food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Lü, J.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and Molecular Mechanisms of Antioxidants: Experimental Approaches and Model Systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Zeng, X.; Xi, Y.; Jiang, W. Protective Roles of Flavonoids and Flavonoid-Rich Plant Extracts against Urolithiasis: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2125–2135. [Google Scholar] [CrossRef]
- Hefer, M.; Huskic, I.M.; Petrovic, A.; Raguz-Lucic, N.; Kizivat, T.; Gjoni, D.; Horvatic, E.; Udiljak, Z.; Smolic, R.; Vcev, A.; et al. A Mechanistic Insight into Beneficial Effects of Polyphenols in the Prevention and Treatment of Nephrolithiasis: Evidence from Recent In Vitro Studies. Crystals 2023, 13, 1070. [Google Scholar] [CrossRef]
- Melo, L.F.M.d.; Aquino-Martins, V.G.d.Q.; Silva, A.P.d.; Oliveira Rocha, H.A.; Scortecci, K.C. Biological and Pharmacological Aspects of Tannins and Potential Biotechnological Applications. Food Chem. 2023, 414, 135645. [Google Scholar] [CrossRef]
- Sahakyan, G.; Vejux, A.; Sahakyan, N. The Role of Oxidative Stress-Mediated Inflammation in the Development of T2DM-Induced Diabetic Nephropathy: Possible Preventive Action of Tannins and Other Oligomeric Polyphenols. Molecules 2022, 27, 9035. [Google Scholar] [CrossRef]
- Molino, S.; Pilar Francino, M.; Ángel Rufián Henares, J. Why Is It Important to Understand the Nature and Chemistry of Tannins to Exploit Their Potential as Nutraceuticals? Food Res. Int. 2023, 173, 113329. [Google Scholar] [CrossRef]
- Swanson, B.G. Tannins and Polyphenols. In Encyclopedia of Food Sciences and Nutrition; Elsevier Academic Press: Cambridge, MA, USA, 2003; pp. 5729–5733. [Google Scholar]
- El-Saadony, M.T.; Yang, T.; Saad, A.M.; Alkafaas, S.S.; Elkafas, S.S.; Eldeeb, G.S.; Mohammed, D.M.; Salem, H.M.; Korma, S.A.; Loutfy, S.A.; et al. Polyphenols: Chemistry, Bioavailability, Bioactivity, Nutritional Aspects and Human Health Benefits: A Review. Int. J. Biol. Macromol. 2024, 277, 134223. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Chaudhuri, P.K. Structural Characteristics, Bioavailability and Cardioprotective Potential of Saponins. Integr. Med. Res. 2018, 7, 33–43. [Google Scholar] [CrossRef]
- Vigne, S.; Pot, C. Implication of Oxysterols and Phytosterols in Aging and Human Diseases. Adv. Exp. Med. Biol. 2024, 1440, 231–260. [Google Scholar] [CrossRef]
- Sordon, S.; Popłoński, J.; Milczarek, M.; Stachowicz, M.; Tronina, T.; Kucharska, A.Z.; Wietrzyk, J.; Huszcza, E. Structure–Antioxidant–Antiproliferative Activity Relationships of Natural C7 and C7–C8 Hydroxylated Flavones and Flavanones. Antioxidants 2019, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-H.; Baek, S.J. Molecular Targets of Dietary Polyphenols with Anti-Inflammatory Properties. Yonsei Med. J. 2005, 46, 585. [Google Scholar] [CrossRef] [PubMed]
- Kamran, S.; Sinniah, A.; Abdulghani, M.A.M.; Alshawsh, M.A. Therapeutic Potential of Certain Terpenoids as Anticancer Agents: A Scoping Review. Cancers 2022, 14, 1100. [Google Scholar] [CrossRef]
- Gutiérrez-del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef]
- Avunduk, S. Antiinflammatory Saponins. Stud. Nat. Prod. Chem. 2024, 81, 265–314. [Google Scholar]
- Rashid, S.; Sameti, M.; Alqarni, M.H.; Abdel Bar, F.M. In Vivo Investigation of the Inhibitory Effect of Peganum Harmala L. and Its Major Alkaloids on Ethylene Glycol-Induced Urolithiasis in Rats. J. Ethnopharmacol. 2023, 300, 115752. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Choi, Y.-J.; Ahn, Y.H. Identification of Polyphenol Glucuronide Conjugates in Glechoma hederacea Var. longituba Hot Water Extracts by High-Performance Liquid Chromatography-Tandem Mass Spectrometry (HPLC-MS/MS). Molecules 2020, 25, 4713. [Google Scholar] [CrossRef]
- Hahm, Y.H.; Cho, K.; Ahn, Y.H. Compositional Characteristics of Glucuronide Conjugates in Regional Glechoma hederacea Var. longituba Herbal Extracts Using a Set of Polyphenolic Marker Compounds. Plants 2021, 10, 2353. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, S. Evaluation of in Vitro and in Vivo Antileishmanial Potential of Bergenin Rich Bergenia ligulata (Wall.) Engl. Root Extract against Visceral Leishmaniasis in Inbred BALB/c Mice through Immunomodulation. J. Tradit. Complement. Med. 2018, 8, 251–260. [Google Scholar] [CrossRef]
- Singh, A.; Tandon, S.; Nandi, S.P.; Kaur, T.; Tandon, C. Downregulation of Inflammatory Mediators by Ethanolic Extract of Bergenia ligulata (Wall.) in Oxalate Injured Renal Epithelial Cells. J. Ethnopharmacol. 2021, 275, 114104. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, P.; Banerjee, J.; Dahal, P.; Khanal, H.; Gupta, A.K.; Kumar Dey, B.; Kumar Gupta, A. Phytochemical Screening and Biological Evaluation of Different Parts of Plant Bergenia ciliata. J. Pharmacogn. Phytochem. 2014, 3, 220–224. [Google Scholar]
- Gu, X.; Jia, S.; Hu, W.; Cui, M.; Hou, J.; Wang, R.; Zhang, M. Rapid Quality Evaluation of Chinese Herbal Medicines Using a Miniature Mass Spectrometer: Lygodium japonicum (Thunb.) Sw. as an Example. Anal. Methods 2023, 15, 430–435. [Google Scholar] [CrossRef]
- Sharif Nasirian, V.; Shahidi, S.; Tahermansouri, H.; Chekin, F. Application of Graphene Oxide in the Adsorption and Extraction of Bioactive Compounds from Lemon Peel. Food Sci. Nutr. 2021, 9, 3852–3862. [Google Scholar] [CrossRef]
- Durmus, N.; Gulsunoglu-Konuskan, Z.; Kilic-Akyilmaz, M. Recovery, Bioactivity, and Utilization of Bioactive Phenolic Compounds in Citrus Peel. Food Sci. Nutr. 2024, 12, 9974–9997. [Google Scholar] [CrossRef]
- Dong, X.; Hu, Y.; Li, Y.; Zhou, Z. The Maturity Degree, Phenolic Compounds and Antioxidant Activity of Eureka Lemon [Citrus limon (L.) Burm. f.]: A Negative Correlation between Total Phenolic Content, Antioxidant Capacity and Soluble Solid Content. Sci. Hortic. 2019, 243, 281–289. [Google Scholar] [CrossRef]
- Peng, W.; Han, P.; Yu, L.; Chen, Y.; Ye, B.; Qin, L.; Xin, H.; Han, T. Anti-Allergic Rhinitis Effects of Caffeoylquinic Acids from the Fruits of Xanthium strumarium in Rodent Animals via Alleviating Allergic and Inflammatory Reactions. Rev. Bras. Farmacogn. 2019, 29, 46–53. [Google Scholar] [CrossRef]
- Chandel, S.; Bagai, U.; Vashishat, N. Antiplasmodial Activity of Xanthium strumarium against Plasmodium Berghei-Infected BALB/c Mice. Parasitol. Res. 2012, 110, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Ingawale, A.S.; Sadiq, M.B.; Nguyen, L.T.; Ngan, T.B. Optimization of Extraction Conditions and Assessment of Antioxidant, α-Glucosidase Inhibitory and Antimicrobial Activities of Xanthium strumarium L. Fruits. Biocatal. Agric. Biotechnol. 2018, 14, 40–47. [Google Scholar] [CrossRef]
- Rajendrabhai, V. Detection of Phytochemical and Pharmacological Properties of Crude Extracts of Tribulus Terrestris Collected from Tribal Regions of Baglan (M.S.), India. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 508–511. [Google Scholar] [CrossRef]
- Kostova, I.; Dinchev, D. Saponins in Tribulus Terrestris—Chemistry and Bioactivity. Phytochem. Rev. 2005, 4, 111–137. [Google Scholar] [CrossRef]
- Meng, X.; Xing, J.; Liu, S.; Liu, Z.; Song, F. Comprehensive Chemical Profiling and Potential Chemical Marker’s Evaluation of Tribulus Terrestris by UPLC-QTOF-MS in Combination with Ion Mobility Spectrometry. J. Pharm. Biomed. Anal. 2022, 217, 114839. [Google Scholar] [CrossRef]
- GULCIN, I. Antioxidant Activity of Caffeic Acid (3,4-Dihydroxycinnamic Acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Aldoghachi, F.E.H.; Noor Al-Mousawi, U.M.; Shari, F.H. Antioxidant Activity of Rosmarinic Acid Extracted and Purified from Mentha Piperita. Arch. Razi Inst. 2021, 76, 1279–1287. [Google Scholar] [CrossRef]
- Kamalakararao, K.; Gopalakrishnan, V.K.; Hagos, Z.; Satyaprasad, Y.; Karri, K.C. In Vitro Antioxidant Activities of Isolated Bioactive Flavonoid Apigenin-7-O-β-D-Glucuronide Methyl Ester from Ethyl Acetate Leaf Extract of Manilkara zapota. Drug Invent. Today 2018, 10, 1142–1145. [Google Scholar]
- Cheng, L.; Ji, T.; Zhang, M.; Fang, B. Recent Advances in Squalene: Biological Activities, Sources, Extraction, and Delivery Systems. Trends Food Sci. Technol. 2024, 146, 104392. [Google Scholar] [CrossRef]
- Khan, H.; Amin, H.; Ullah, A.; Saba, S.; Rafique, J.; Khan, K.; Ahmad, N.; Badshah, S.L. Antioxidant and Antiplasmodial Activities of Bergenin and 11-O-Galloylbergenin Isolated from Mallotus philippensis. Oxid. Med. Cell. Longev. 2016, 2016, 1051925. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In Vitro and in Vivo Antioxidant Properties of Chlorogenic Acid and Caffeic Acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Han, H.; Dye, L.; Mackie, A. The Impact of Processing on the Release and Antioxidant Capacity of Ferulic Acid from Wheat: A Systematic Review. Food Res. Int. 2023, 164, 112371. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, M.; He, L.; Song, X.; Cao, T. Chlorogenic Acid: A Review on Its Mechanisms of Anti-Inflammation, Disease Treatment, and Related Delivery Systems. Front. Pharmacol. 2023, 14, 1218015. [Google Scholar] [CrossRef] [PubMed]
- Cavia-Saiz, M.; Busto, M.D.; Pilar-Izquierdo, M.C.; Ortega, N.; Perez-Mateos, M.; Muñiz, P. Antioxidant Properties, Radical Scavenging Activity and Biomolecule Protection Capacity of Flavonoid Naringenin and Its Glycoside Naringin: A Comparative Study. J. Sci. Food Agric. 2010, 90, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z.; Xiao, W. Antitumor, Antioxidant and Anti-Inflammatory Activities of Kaempferol and Its Corresponding Glycosides and the Enzymatic Preparation of Kaempferol. PLoS ONE 2018, 13, e0197563. [Google Scholar] [CrossRef]
- Qi, W.; Qi, W.; Xiong, D.; Long, M. Quercetin: Its Antioxidant Mechanism, Antibacterial Properties and Potential Application in Prevention and Control of Toxipathy. Molecules 2022, 27, 6545. [Google Scholar] [CrossRef]
- Kandemir, Ö.; Akaras, N.; Şimşek, H.; Gür, C.; İleritürk, M.; Küçükler, S.; Caglayan, C.; Kandemir, F.M. Nephroprotective Effects of Hesperidin on Ifosfamide-Induced Acute Nephrotoxicity in Rats: Role of NF-ΚB/TNF-α/IL-1β, P53/Caspase-3/Bax/Bcl-2, and ATF6/IRE1/PERK/GRP78 Signaling Pathways. Arch. Biochem. Biophys. 2025, 770, 110465. [Google Scholar] [CrossRef]
- Yao, L.; Liu, W.; Bashir, M.; Nisar, M.F.; Wan, C. (Craig) Eriocitrin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2022, 154, 113563. [Google Scholar] [CrossRef]
- Huwait, E.; Mobashir, M. Potential and Therapeutic Roles of Diosmin in Human Diseases. Biomedicines 2022, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-S.; Park, H.-R.; Lee, K.-A. A Comparative Study of Rutin and Rutin Glycoside: Antioxidant Activity, Anti-Inflammatory Effect, Effect on Platelet Aggregation and Blood Coagulation. Antioxidants 2021, 10, 1696. [Google Scholar] [CrossRef]
- Costa, J.; Islam, T.; Santos, P.; Ferreira, P.; Oliveira, G.; Alencar, M.; Paz, M.; Ferreira, É.; Feitosa, C.; Citó, A.; et al. Evaluation of Antioxidant Activity of Phytol Using Non- and Pre-Clinical Models. Curr. Pharm. Biotechnol. 2016, 17, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Islam, M.S.; Rahman, M.K.; Uddin, M.N.; Akanda, M.R. The Pharmacological and Biological Roles of Eriodictyol. Arch. Pharm. Res. 2020, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoglu, Z. Astragalin: A Bioactive Phytochemical with Potential Therapeutic Activities. Adv. Pharmacol. Sci. 2018, 2018, 9794625. [Google Scholar] [CrossRef]
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide Research Trends on Medicinal Plants. Int. J. Environ. Res. Public. Health 2020, 17, 3376. [Google Scholar] [CrossRef]
- Organización Mundial de la Salud. Medicina Tradicional; World Health Organization (WHO): Geneva, Switzerland, 2023. [Google Scholar]
- Begley, C.G.; Ioannidis, J.P.A. Reproducibility in Science. Circ. Res. 2015, 116, 116–126. [Google Scholar] [CrossRef]
- Butterweck, V.; Khan, S. Herbal Medicines in the Management of Urolithiasis: Alternative or Complementary? Planta Med. 2009, 75, 1095–1103. [Google Scholar] [CrossRef]
- Mutomba, W.F.; Symeonidis, E.N.; Mykoniatis, I.; Tzelves, L.; Tsaturyan, A.; Juliebo-Jones, P.; Tokas, T.; Sountoulides, P. Phytotherapy in Urolithiasis: An Updated Overview of Current Knowledge. J. Clin. Med. 2025, 14, 2885. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.R. Clinical Studies of Medicinal Plants for Their Antiurolithic Effects: A Systematic Review. Longhua Chin. Med. 2022, 5, 16. [Google Scholar] [CrossRef]
- World Health Organization. Programme on Traditional Medicine. In General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Bose, S.; Datta, R.; Kirlin, W.G. Toxicity Studies Related to Medicinal Plants. In Evidence Based Validation of Traditional Medicines; Springer Nature: Singapore, 2021; pp. 621–647. [Google Scholar]
- Mugale, M.N.; Dev, K.; More, B.S.; Mishra, V.S.; Washimkar, K.R.; Singh, K.; Maurya, R.; Rath, S.K.; Chattopadhyay, D.; Chattopadhyay, N. A Comprehensive Review on Preclinical Safety and Toxicity of Medicinal Plants. Clin. Complement. Med. Pharmacol. 2024, 4, 100129. [Google Scholar] [CrossRef]
- Heinrich, M.; Jalil, B.; Abdel-Tawab, M.; Echeverria, J.; Kulić, Ž.; McGaw, L.J.; Pezzuto, J.M.; Potterat, O.; Wang, J.-B. Best Practice in the Chemical Characterisation of Extracts Used in Pharmacological and Toxicological Research—The ConPhyMP—Guidelines12. Front. Pharmacol. 2022, 13, 953205. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Guidelines for the Testing of Chemicals; OECD: Paris, France, 2025. [Google Scholar]
- Patle, A.; Hatware, K.V.; Patil, K.; Sharma, S.; Gupta, G. Role of Herbal Medicine in the Management of Urolithiasis—A Review for Future Perspectives. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 97–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent Advances in the Extraction of Bioactive Compounds with Subcritical Water: A Review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Ai, S.; Li, Y.; Zheng, H.; Zhang, M.; Tao, J.; Liu, W.; Peng, L.; Wang, Z.; Wang, Y. Collision of Herbal Medicine and Nanotechnology: A Bibliometric Analysis of Herbal Nanoparticles from 2004 to 2023. J. Nanobiotechnol. 2024, 22, 140. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Aromataris, E.; Munn, Z. (Eds.) JBI Manual for Evidence Synthesis. Chapter 10.2.7: Data Extraction. Available online: https://jbi-global-wiki.refined.site/space/MANUAL/355862769/10.2.7+Data+extraction (accessed on 18 May 2025).
| Criterion | Description | |
|---|---|---|
| P | Population | Models of UL |
| I | Intervention | Use of traditional medicinal plant (roots, leaves, seeds, bark, or other constituent parts) |
| C | Comparator | Any comparator positive or negative or control group |
| O | Outcomes | Lithogenic, antioxidant, and inflammatory biomarkers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco-Hernández, B.; Ayora-Talavera, T.; Cano-Sosa, J.; Noriega, L.G.; Pacheco-López, N.A.; Vargas-Morales, J.M.; Medina-Vera, I.; Guevara-Cruz, M.; Chim-Aké, R.; Gutiérrez-Solis, A.L.; et al. Antioxidant and Anti-Inflammatory Effects of Traditional Medicinal Plants for Urolithiasis: A Scoping Review. Plants 2025, 14, 2032. https://doi.org/10.3390/plants14132032
Pacheco-Hernández B, Ayora-Talavera T, Cano-Sosa J, Noriega LG, Pacheco-López NA, Vargas-Morales JM, Medina-Vera I, Guevara-Cruz M, Chim-Aké R, Gutiérrez-Solis AL, et al. Antioxidant and Anti-Inflammatory Effects of Traditional Medicinal Plants for Urolithiasis: A Scoping Review. Plants. 2025; 14(13):2032. https://doi.org/10.3390/plants14132032
Chicago/Turabian StylePacheco-Hernández, Brenda, Teresa Ayora-Talavera, Julia Cano-Sosa, Lilia G. Noriega, Neith Aracely Pacheco-López, Juan M. Vargas-Morales, Isabel Medina-Vera, Martha Guevara-Cruz, Rodolfo Chim-Aké, Ana Ligia Gutiérrez-Solis, and et al. 2025. "Antioxidant and Anti-Inflammatory Effects of Traditional Medicinal Plants for Urolithiasis: A Scoping Review" Plants 14, no. 13: 2032. https://doi.org/10.3390/plants14132032
APA StylePacheco-Hernández, B., Ayora-Talavera, T., Cano-Sosa, J., Noriega, L. G., Pacheco-López, N. A., Vargas-Morales, J. M., Medina-Vera, I., Guevara-Cruz, M., Chim-Aké, R., Gutiérrez-Solis, A. L., Lugo, R., & Avila-Nava, A. (2025). Antioxidant and Anti-Inflammatory Effects of Traditional Medicinal Plants for Urolithiasis: A Scoping Review. Plants, 14(13), 2032. https://doi.org/10.3390/plants14132032











