Investigating the Mechanisms Underlying Citral-Induced Oxidative Stress and Its Contribution to Antifungal Efficacy on Magnaporthe oryzae Through a Multi-Omics Approach
Abstract
1. Introduction
2. Results
2.1. Citral Affected the Activities of Antioxidant Enzymes in M. oryzae
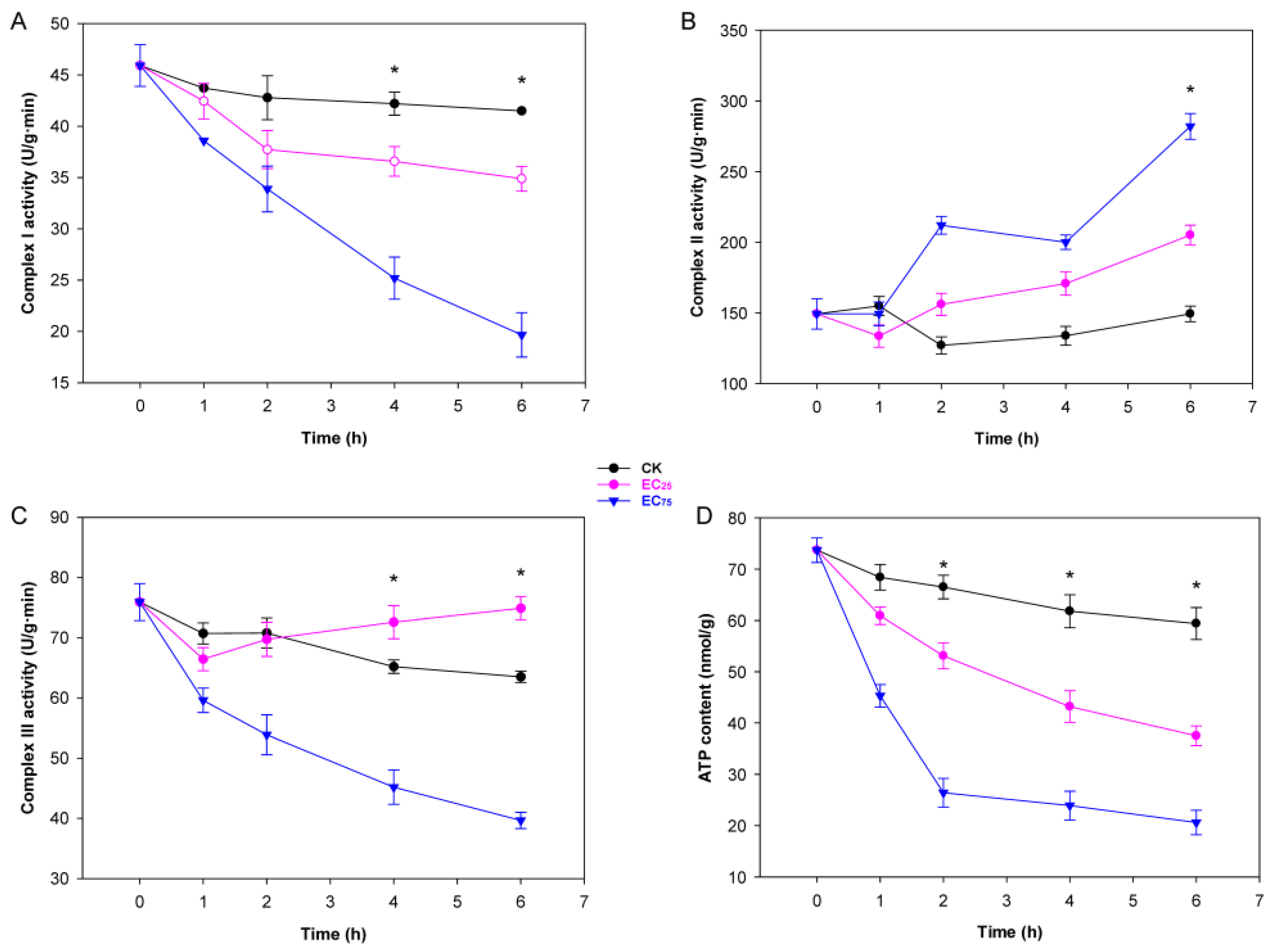
2.2. Citral Affected Mitochondrial Function and ATP Synthesis in M. oryzae
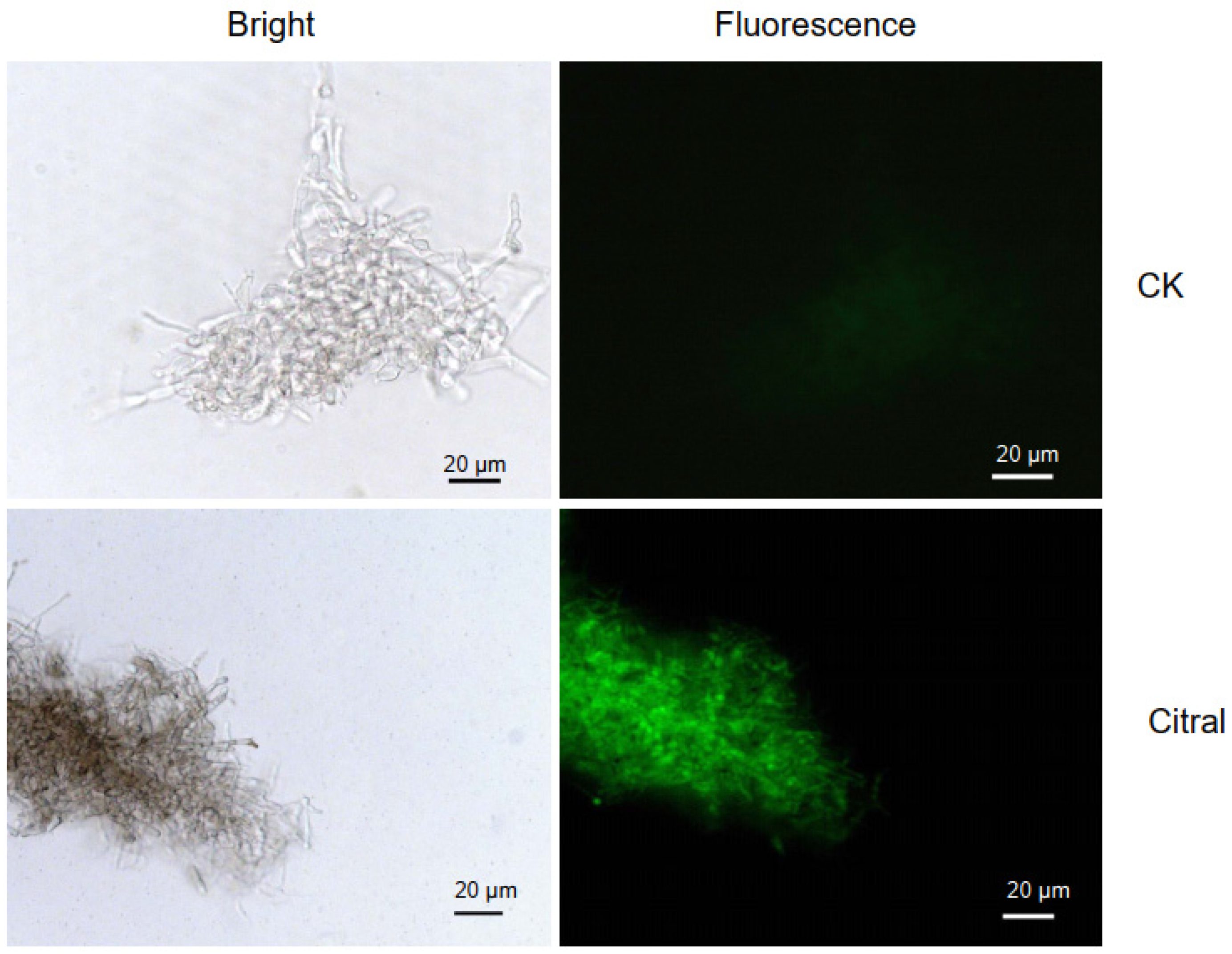
2.3. Citral Affected ROS Accumulation in M. oryzae
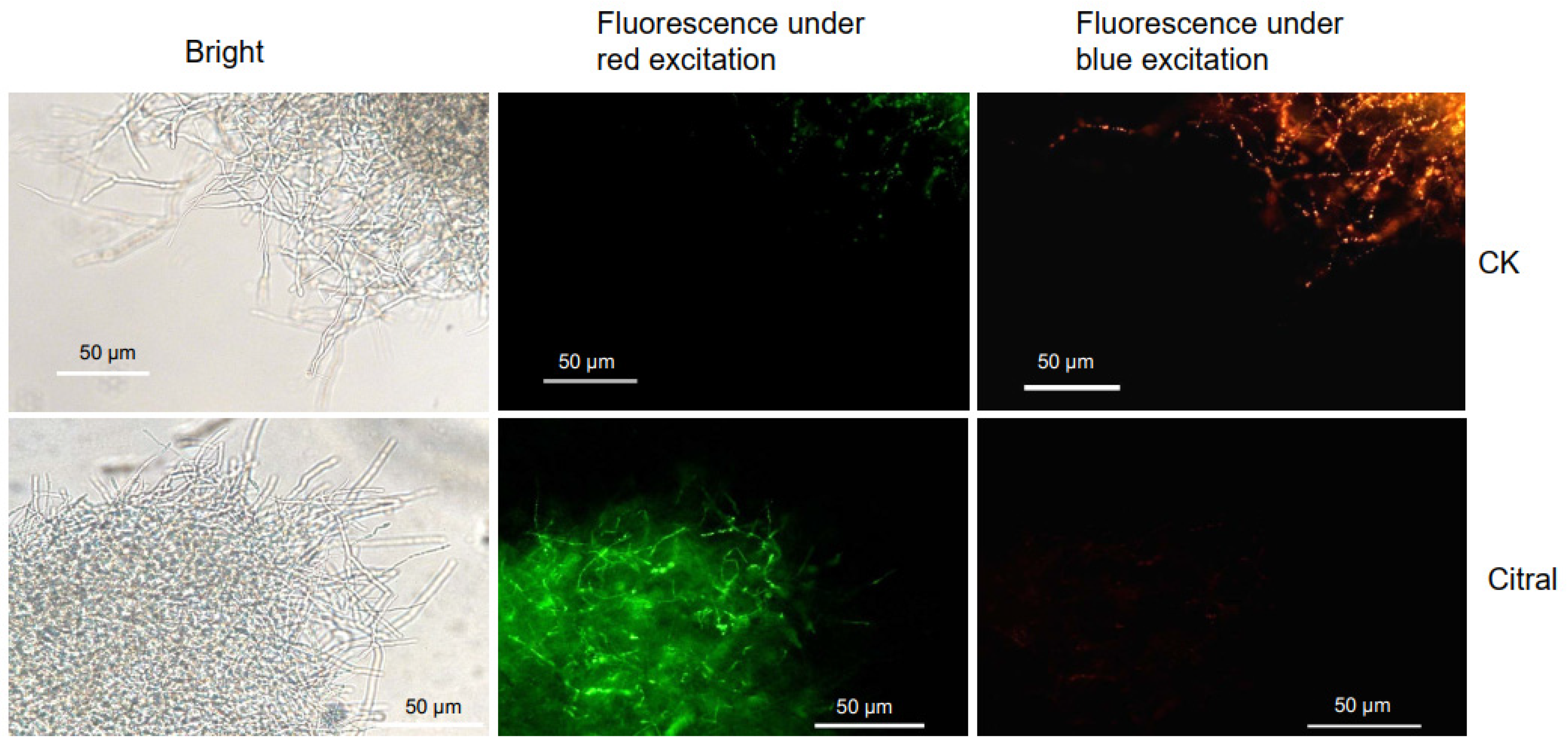
2.4. Citral Affected the MMP of M. oryzae
2.5. The Response of Transcriptome in M. oryzae to Citral Exposure
2.5.1. Illumina Sequencing and Sequence Assembly
2.5.2. Analysis of Differentially Expressed Genes
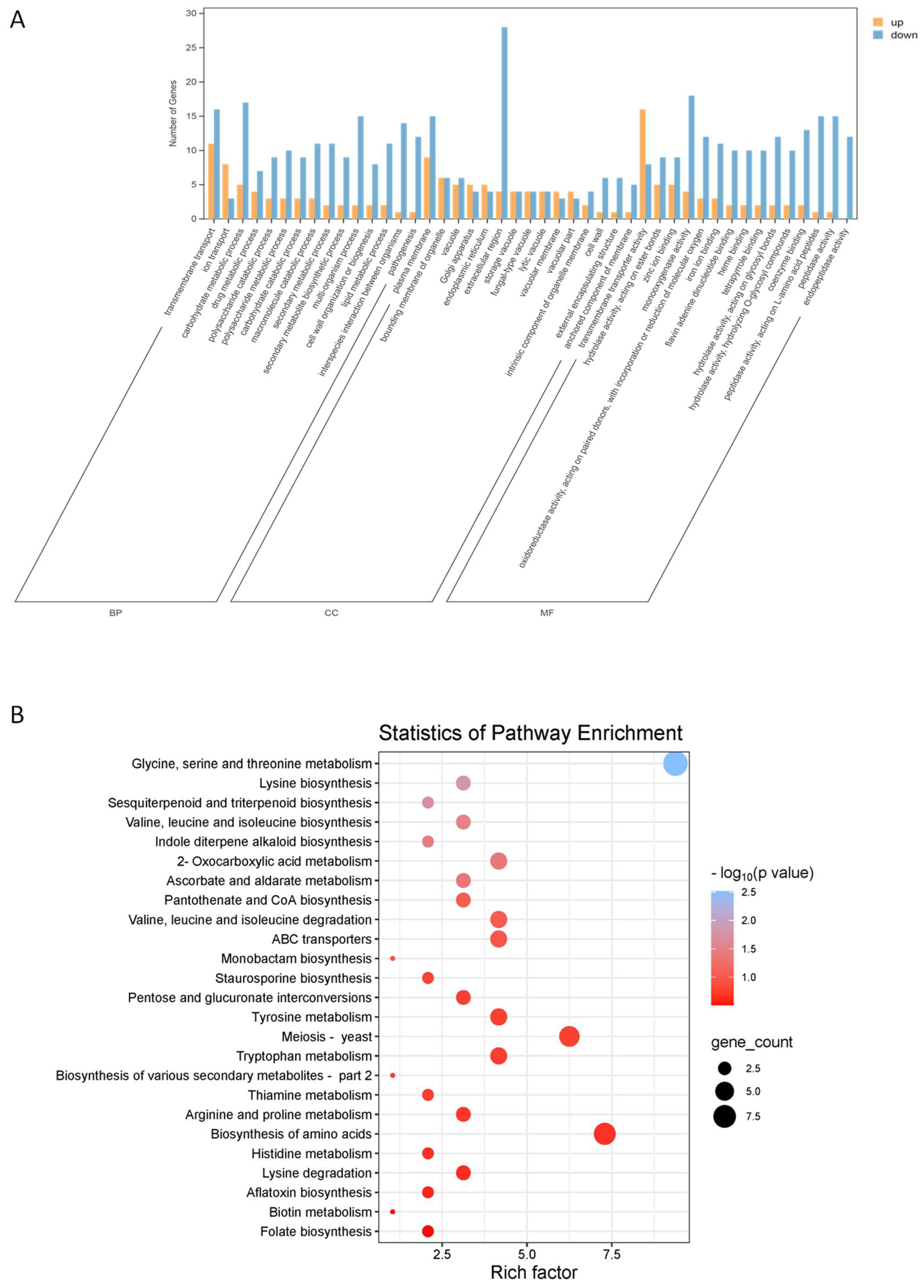
2.5.3. GO Functional Analysis and KEGG Enrichment Analysis
2.6. The Response of Metabolome in M. oryzae to Citral Exposure
2.6.1. Metabolite Detection and Clustering Analysis of Differentially Accumulated Metabolites (DAMs)
2.6.2. KEGG Enrichment Analysis of DAMs
2.7. Association Analysis of Transcriptome and WT Metabolome
3. Discussion
4. Materials and Methods
4.1. Chemicals, Strain, Culture Media and Reagents
4.2. Effect of Citral on Antioxidant Enzyme Activity
4.3. Determination of Mitochondrial Respiration Complex I, II and III Activity
4.4. Determination of ATP Content in Energy Metabolism
4.5. Imaging of Reactive Oxygen Species (ROS)
4.6. Assay of Mitochondrial Membrane Potential (MMP)
4.7. Transcriptome Analysis
4.8. Widely-Targeted Metabolite Profiling
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deng, Y.; Zhai, K.; Zhen, X.; Yang, D.; Zhu, X.; Liu, J.; He, Z. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science 2017, 355, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Talbot, N.J. On the trail of a cereal killer: Investigating the biology of Magnaporthe grisea. Annu. Rev. Microbiol. 2003, 57, 177–202. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kashyap, P.L.; Mahapatra, S.; Jasrotia, P.; Singh, G.P. New and emerging technologies for detecting Magnaporthe oryzae causing blast disease in crop plants. Crop Prot. 2021, 143, 105473. [Google Scholar] [CrossRef]
- Galhano, R.; Talbot, N.J. The biology of blast: Understanding how Magnaporthe oryzae invades rice plants. Fungal Biol. Rev. 2011, 25, 61–67. [Google Scholar] [CrossRef]
- Mohiddin, F.A.; Bhat, N.A.; Wani, S.H.; Bhat, A.H.; Ahanger, M.A.; Shikari, A.B.; Sofi, N.R.; Parveen, S.; Khan, G.H.; Bashir, Z.; et al. Combination of strobilurin and triazole chemicals for the management of blast disease in mushk budji-aromatic rice. J. Fungi 2021, 7, 1060. [Google Scholar] [CrossRef]
- Ghazanfar, M.U.; Wakil, W.; Sahi, S.T.; Kaku, K.S. Influence of various fungicides on the management of rice blast disease. Mycopath. 2009, 7, 29–34. [Google Scholar]
- Chen, Y.; Zhang, Y.; Yao, J.; Li, Y.; Yang, X.; Wang, W.; Zhang, A.; Gao, T. Frequency distribution of sensitivity of ustilaginoidea virens to four ebi fungicides, prochloraz, difenoconazole, propiconazole and tebuconazole, and their efficacy in controlling rice false smut in anhui province of china. Phytoparasitica 2013, 41, 277–284. [Google Scholar] [CrossRef]
- Xin, W.; Mao, Y.; Lu, F.; Li, T.; Wang, J.; Duan, Y.B.; Zhou, M. In vitro fungicidal activity and in planta control efficacy of coumoxy -strobin against Magnaporthe oryzae. Pestic. Biochem. Physiol. 2020, 162, 78–85. [Google Scholar] [CrossRef]
- Adukwu, E.C.; Bowles, M.; Edwards-Jones, V.; Bone, H. Antimicrobial activity, cytotoxicity and chemical analysis of lemongrass essential oil (Cymbopogon flexuosus) and pure citral. Appl. Microbiol. Biotechnol. 2016, 100, 9619–9627. [Google Scholar] [CrossRef]
- Saeed, K.; Pasha, I.; Jahangir Chughtai, M.F.; Ali, Z.; Bukhari, H.; Zuhair, M. Application of essential oils in food industry: Challenges and innovation. J. Essent. Oil Res. 2022, 34, 97–110. [Google Scholar] [CrossRef]
- Kim, I.H.; Oh, Y.A.; Lee, H.; Song, K.B.; Min, S.C. Grape berry coatings of lemongrass oil-incorporating nanoemulsion. Lwt Food Sci. Technol. 2014, 58, 1–10. [Google Scholar] [CrossRef]
- Wei, L.; Chen, C.; Wan, C.; Chen, M.; Chen, J. Citral delays postharvest senescence of kiwifruit by enhancing antioxidant capacity under cold storage. J. Food Qual. 2021, 2021, 6684172. [Google Scholar] [CrossRef]
- Gao, X.; Hu, X.; Mo, F.; Ding, Y.; Li, M.; Li, R. Repellency mechanism of natural guar gum-based film incorporated with citral against brown planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). Int. J. Mol. Sci. 2022, 23, 758. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kurmi, A.; Singh, V.; Singh, M.K.; Mishra, S.; Shankar, U.; Savita, A.; Gupta, H.; Yadav, N.P.; Sakia, D.; et al. Cymbopogon distans: A source of essential oil with potential antibacterial, antifungal, and mosquito-repelling properties. Food Biosci. 2024, 61, 104931. [Google Scholar] [CrossRef]
- Eraslan, E.C.; Çırçırlı, B.; Özkan, A.; Akgül, H. Anticancer mechanisms of action of macrofungus extracts. Eurasian J. Med. Biol. Sci. 2021, 1, 58–69. [Google Scholar]
- Mohammed, F.S.; Sevindik, E.; Uysal, I.; Sevindik, M. Miracle plant Moringa oleifera Lam.: Nutritional, mineral, essential oil contents and biological activities. Vegetos 2024, 38, 867–874. [Google Scholar] [CrossRef]
- Li, R.; Wu, X.; Yin, X.; Liang, J.; Li, M. The natural product citral can cause significant damage to the hyphal cell walls of Magnaporthe grisea. Molecules 2014, 19, 10279–10290. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Xing, Z.; Liu, X.; Bai, X.; Yang, Y.; Guo, D.; Xia, X.; Zhang, C.; Shi, C. Antibacterial effect of citral on yersinia enterocolitica and its mechanism. Food Control 2022, 135, 108775. [Google Scholar] [CrossRef]
- Dai, J.; Bai, M.; Li, C.; San Cheang, W.; Cui, H.; Lin, L. Antibacterial properties of citral against Staphylococcus aureus: From membrane damage to metabolic inhibition. Food Biosci. 2023, 53, 102770. [Google Scholar] [CrossRef]
- Richter, S.; Gatto, B.; Fabris, D.; Takao, K.I.; Kobayashi, S.; Palumbo, M. Clerocidin alkylates DNA through its epoxide function: Evidence for a fine tuned mechanism of action. Nucleic Acids Res. 2003, 31, 5149–5156. [Google Scholar] [CrossRef]
- Lima, I.O.; de Medeiros Nóbrega, F.; de Oliveira, W.A.; de Oliveira Lima, E.; Albuquerque Menezes, E.; Afrânio Cunha, F.; de Fátima Formiga Melo Diniz, M. Anti-Candida albicans effectiveness of citral and investigation of mode of action. Pharm. Biol. 2012, 50, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wu, X.; Yin, X.; Long, Y.; Li, M. Naturally produced citral can significantly inhibit normal physiology and induce cytotoxicity on Magnaporthe grisea. Pestic. Biochem. Phys. 2015, 118, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Zhang, R.; Yao, W. Synergistic inhibition effect of citral and eugenol against Aspergillus niger and their application in bread preservation. Food Chem. 2020, 310, 125974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, J.; Chen, H.; Song, Z.; Guo, H.; Yuan, Y.; Yue, T. Antibacterial activity of essential oils against Stenotrophomonas maltophilia and the effect of citral on cell membrane. Lwt 2020, 117, 108667. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Jing, C.; Mou, G.; Zhang, W.; Jin, Y.; Qin, L.; An, J.; Zhang, S.; Liu, Y. Antifungal Effects and Postharvest Diseases Control Potential of E, E-2, 4-Nonadienal against Rhizopus stolonifer. J. Agric. Food Chem. 2024, 72, 25509–25521. [Google Scholar] [CrossRef]
- Zheng, S.; Jing, G.; Wang, X.; Ouyang, Q.; Jia, L.; Tao, N. Citral exerts its antifungal activity against Penicillium digitatum by affecting the mitochondrial morphology and function. Food Chem. 2015, 178, 76–81. [Google Scholar] [CrossRef]
- OuYang, Q.; Tao, N.; Zhang, M. A damaged oxidative phosphorylation mechanism is involved in the antifungal activity of citral against Penicillium digitatum. Front. Microbiol. 2018, 9, 239. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, K.; Yang, H.; Zhang, Z.; Yuan, Y.; Yue, T. Effect of cinnamaldehyde and citral combination on transcriptional profile, growth, oxidative damage and patulin biosynthesis of Penicillium expansum. Front. Microbiol. 2018, 9, 597. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, N.; Wang, D.; Wang, M. Effects of essential oil citral on the growth, mycotoxin biosynthesis and transcriptomic profile of Alternaria alternata. Toxins 2019, 11, 553. [Google Scholar] [CrossRef]
- Wani, M.Y.; Ahmad, A.; Aqlan, F.M.; Al-Bogami, A.S. Citral derivative activates cell cycle arrest and apoptosis signaling pathways in Candida albicans by generating oxidative stress. Bioorg. Chem. 2021, 115, 105260. [Google Scholar] [CrossRef]
- Semenza, G.L. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem. J. 2007, 405, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pandyra, A.; Mullen, P.J.; Kalkat, M.; Yu, R.; Pong, J.T.; Li, Z.; Trudel, S.; Lang, K.S.; Minden, M.D.; Schimmer, A.D.; et al. Immediate Utility of Two Approved Agents to Target Both the Metabolic Mevalonate Pathway and Its Restorative Feedback Loop. Cancer Res. 2014, 74, 4772–4782. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.A.; Pöch, G. Evaluation of consistency for multiple experiments of a single combination in the time- dependence mixture toxicity assay. Toxicol. Mech. Methods 2017, 27, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Luiz, R.C.; Cecchini, A.L. Mitochondria as a Target for Monoterpenes. In Mitochondrial Physiology and Vegetal Molecules; Academic Press: Cambridge, MA, USA, 2021; pp. 357–375. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Hwang, J.H.; Hwang, I.S.; Liu, Q.H.; Woo, E.R.; Lee, D.G. (+)-Medioresinol leads to intracellular ROS accumulation and mitochondria-mediated apoptotic cell death in Candida albicans. Biochimie 2012, 94, 1784–1793. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Phys. C 2011, 153, 175–190. [Google Scholar] [CrossRef]
- de Arruda Grossklaus, D.; Bailão, A.M.; Rezende, T.C.V.; Borges, C.L.; de Oliveira, M.A.P.; Parente, J.A.; de Almeida Soares, C.M. Response to oxidative stress in Paracoccidioides yeast cells as determined by proteomic analysis. Microbes Infect. 2013, 15, 347–364. [Google Scholar] [CrossRef]
- Mo, F.; Hu, X.; Ding, Y.; Li, R.; Long, Y.; Wu, X.; Li, M. Naturally produced magnolol can significantly damage the plasma membrane of Rhizoctonia solani. Pestic. Biochem. Phys. 2021, 178, 104942. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Zeng, H.; Novoa, I.; Lu, P.D.; Calfon, M.; Sadri, N.; Yun, C.; Popko, B.; Paules, R.; et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 2003, 11, 619–633. [Google Scholar] [CrossRef]
- Daddam, J.R.; Sura, M.; Vocelle, D.; Laguna, J.G.; Gallagher, K.; Zhou, Z. The supply of branched-chain amino acids and branched-chain keto acids alter lipid metabolism, oxidative stress, and apoptosis in primary bovine hepatocytes. J. Nutr. Biochem. 2025, 137, 109839. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, D.; Gao, P.; Wu, Q.; Li, Z.; Li, S.; Zhu, L. Oxidative stress and metabolic process responses of Chlorella pyrenoidosa to nanoplastic exposure: Insights from integrated analysis of transcriptomics and metabolomics. Environ. Pollut. 2024, 357, 124466. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; An, B.; Hu, Y.; Tao, Y. 2,4-Bisphenol S triggers physiological changes, oxidative stress and lipidome alterations in Gram-positive Enterococcus faecalis at environmental concentrations. Environ. Pollut. 2025, 366, 125475. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, T.; Shahbaz, M.; Maqsood, M.F.; Zulfiqar, U.; Saman, R.U.; Lili, N.; Irshad, M.; Maqsood, S.; Haider, A.; Shahzad, B.; et al. Phenylalanine supply alleviates the drought stress in mustard (Brassica campestris) by modulating plant growth, photosynthesis, and antioxidant defense system. Plant Physiol. Biochem. 2023, 201, 107828. [Google Scholar] [CrossRef]
- Dong, Q.; Li, D.; Wu, Y.; Zhou, C.; Lin, Y.; Miao, P.; Li, J.; Pan, C. Exogenous nanoselenium alleviates imidacloprid-induced oxidative stress toxicity by improving phenylpropanoid metabolism and antioxidant defense system in Perilla frutescens (L.) Britt. J. Plant Physiol. 2023, 289, 154095. [Google Scholar] [CrossRef]
- Mishra, V.; Tripathi, D.K.; Rai, P.; Sharma, S.; Singh, V.P. Regulation of arsenate stress by nitric oxide and hydrogen sulfide in Oryza sativa seedlings: Implication of sulfur assimilation, glutathione biosynthesis, and the ascorbate-glutathione cycle and its genes. Plant Physiol. Biochem. 2024, 215, 109001. [Google Scholar] [CrossRef]
- Husain, T.; Prasad, S.M.; Singh, V.P. Ethylene and hydrogen sulfide regulate hexavalent chromium toxicity in two pulse crops: Implication on growth, photosynthetic activity, oxidative stress and ascorbate glutathione cycle. Plant Physiol. Biochem. 2024, 216, 109170. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Gao, B.; Sillanpää, M. Sterilization mechanism of CuCeOx on fungus: Oxidative damage and energy metabolism disequilibrium. J. Environ. Chem. Eng. 2024, 12, 114564. [Google Scholar] [CrossRef]
- Asadi, E.; Maresca, V.; Sorbo, S.; Keramat, B.; Basile, A. Effects of triacontanol on ascorbate-glutathione cycle in Brassica napus L. exposed to cadmium-induced oxidative stress. Ecotox. Environ. Safe 2017, 144, 268–274. [Google Scholar] [CrossRef]
- Singh, V.P.; Srivastava, P.K.; Prasad, S.M. Differential effect of UV-B radiation on growth, oxidative stress and ascorbate glutathione cycle in two cyanobacteria under copper toxicity. Plant Physiol. Biochem. 2012, 61, 61–70. [Google Scholar] [CrossRef]
- Khan, M.N. Melatonin regulates mitochondrial enzymes and ascorbate-glutathione system during plant responses to drought stress through involving endogenous calcium. S. Afr. J. Bot. 2023, 162, 622–632. [Google Scholar] [CrossRef]
- Wang, G.; Chen, X.; Zhang, C.; Li, M.; Sun, C.; Zhan, N.; Huang, X.; Li, T.; Deng, W. Biosynthetic pathway and the potential role of melatonin at different abiotic stressors and developmental stages in Tolypocladium guangdongense. Front. Microbiol. 2021, 12, 746141. [Google Scholar] [CrossRef]
- Morcillo-Parra, M.Á.; Beltran, G.; Mas, A.; Torija, M.J. Effect of several nutrients and environmental conditions on intracellular melatonin synthesis in Saccharomyces cerevisiae. Microorganisms 2020, 8, 853. [Google Scholar] [CrossRef] [PubMed]
- Asdullah, H.U.; Chen, F.; Hassan, M.A.; Abbas, A.; Sajad, S.; Rafiq, M.; Chen, Y. Recent advances and role of melatonin in post-harvest quality preservation of shiitake (Lentinula edodes). Front. Nutr. 2024, 11, 1348235. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Y.; Qian, J.; Si, W.; Tan, Q.; Xu, J.; Zhao, Y. Melatonin enhances the cadmium tolerance of mushrooms through antioxidant-related metabolites and enzymes. Food Chem. 2020, 330, 127263. [Google Scholar] [CrossRef]
- Fanjul-Moles, M.L.; López-Riquelme, G.O. Relationship between oxidative stress, circadian rhythms, and AMD. Oxid. Med. Cell Longev. 2016, 1, 7420637. [Google Scholar] [CrossRef]
- Jiménez, A.; Sevilla, F.; Martí, M.C. Reactive oxygen species homeostasis and circadian rhythms in plants. J. Exp. Bot 2021, 72, 5825–5840. [Google Scholar] [CrossRef]
- Song, X.; Zhao, Q.; Zhou, A.; Wen, X.; Li, M.; Li, R.; Liao, X.; Xu, T. The antifungal effects of citral on Magnaporthe oryzae occur via modulation of chitin content as revealed by RNA-Seq analysis. J. Fungi 2021, 7, 1023. [Google Scholar] [CrossRef]
- Zhao, Q.; Ding, Y.; Song, X.; Liu, S.; Li, M.; Li, R.; Ruan, H. Proteomic analysis reveals that naturally produced citral can significantly disturb physiological and metabolic processes in the rice blast fungus Magnaporthe oryzae. Pestic. Biochem. Phys. 2021, 175, 104835. [Google Scholar] [CrossRef]
- Ding, Y.; Yuan, J.; Wu, S.; Hu, K.; Ma, Y.; Gao, Y.; Li, M.; Li, R. pH/chitinase dual stimuli-responsive essential oil-delivery system based on mesoporous silica nanoparticles for control of rice blast. Pest. Manag. Sci. 2024, 80, 3215–3226. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Tang, J.; Yuan, Q.; Liu, H.; Huang, J.; Hsiang, T.; Bao, C.; Zheng, L. Ornithine decarboxylase of the fungal pathogen Colletotrichum higginsianum plays an important role in regulating global metabolic pathways and virulence. Environ. Microbiol. 2022, 24, 1093–1116. [Google Scholar] [CrossRef]

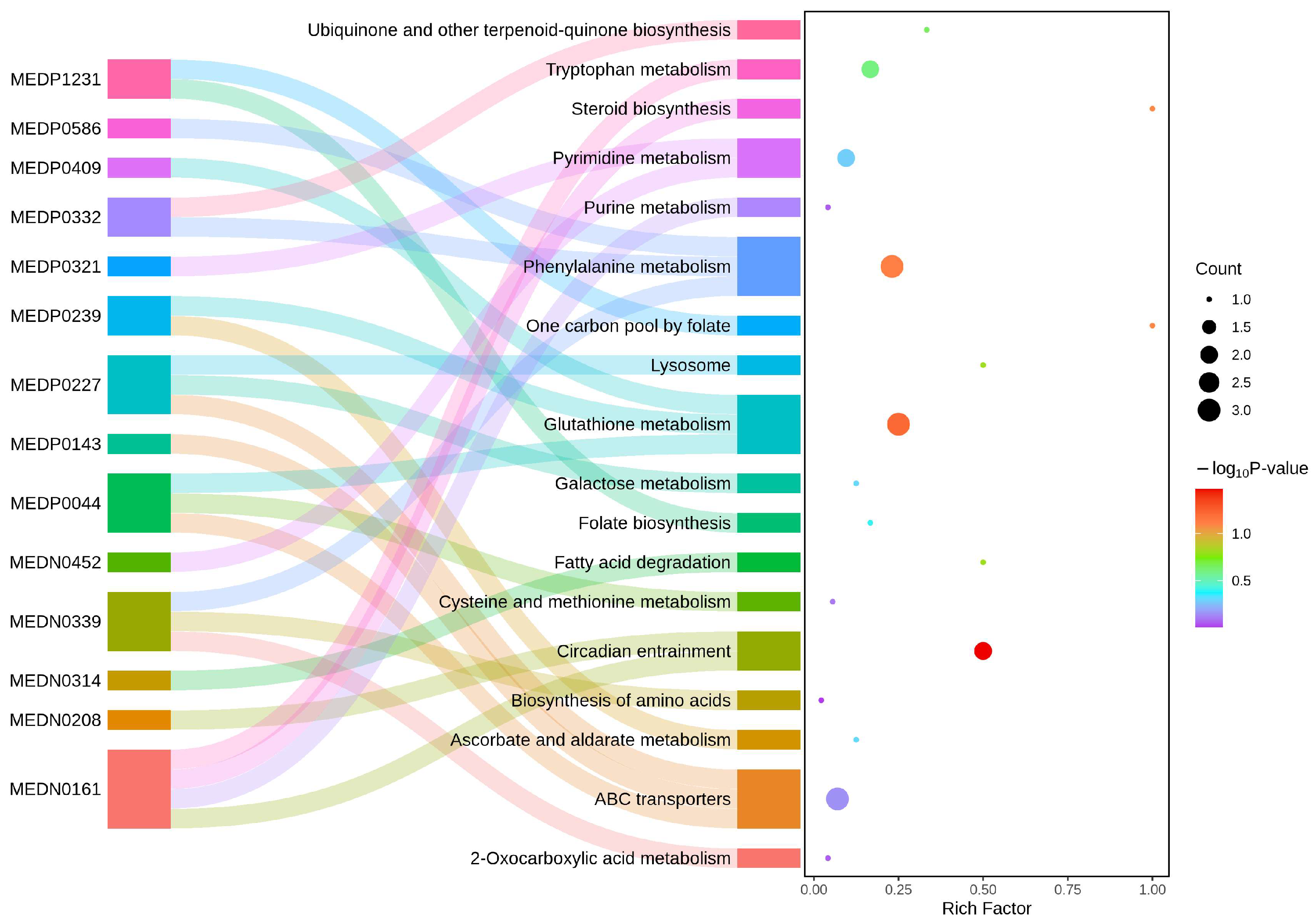
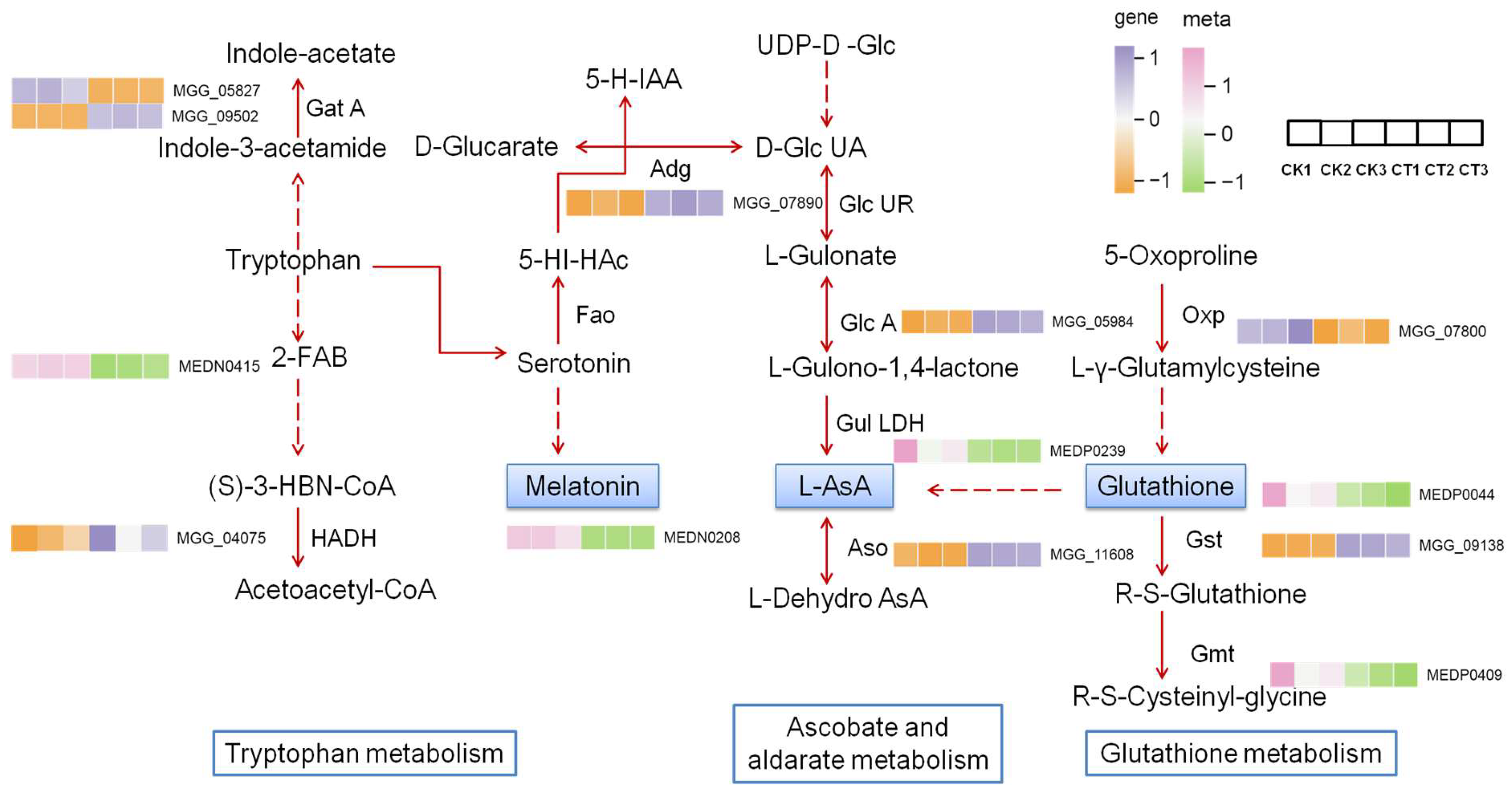
| Compounds | Index | VIP | Log2FC |
|---|---|---|---|
| Organic acids | |||
| 2-Methylsuccinic Acid | MEDN0285 | 1.389470915 | 1.457618 |
| Glutaric Acid | MEDN0314 | 1.389470915 | 1.457618 |
| Phenylpyruvic Acid | MEDN0339 | 1.391018692 | 1.420597 |
| Ethylmalonate | MEDN0413 | 1.389470915 | 1.457618 |
| 2-Furanoic Acid | MEDP0288 | 1.388682424 | −1.13016 |
| L-Dihydroorotic Acid | MEDP0321 | 1.337825865 | −1.11059 |
| Cinnamic Acid | MEDP0332 | 1.386860535 | −1.23325 |
| Folic acid | MEDP1231 | 1.389345578 | −1.35392 |
| Benzenes | |||
| 2-(Formylamino)Benzoic Acid | MEDN0415 | 1.384302381 | −1.03285 |
| (3,4-Dimethoxyphenyl) Acetic Acid | MEDP0109 | 1.337700645 | −1.72077 |
| 2-Methoxybenzoic Acid | MEDP0110 | 1.391767699 | 5.030013 |
| Anisic acid | MEDP0647 | 1.37730052 | 2.059254 |
| 4-Methylbenzoic acid | MEDP0846 | 1.393335542 | 12.7864 |
| Amino acids | |||
| Glutathione Reduced form | MEDP0044 | 1.241257863 | −1.07454 |
| N-Acetylthreonine | MEDP0386 | 1.383168672 | 1.174758 |
| Cys-Gly | MEDP0409 | 1.226508329 | −1.12269 |
| N-Acetylphenylalanine | MEDP0586 | 1.391699227 | 1.769527 |
| Nucleotides | |||
| Guanosine 3′,5′-Cyclic Monophosphate | MEDN0161 | 1.349898801 | −1.68473 |
| 2′-Deoxycytidine-5′-Monophosphate | MEDN0452 | 1.201755767 | −1.07781 |
| 7-Methylguanine | MEDP0381 | 1.356336443 | −1.00657 |
| CoEnzyme and vitamins | |||
| Vitamin D3 | MEDN0241 | 1.311694478 | 1.54347 |
| Biotin | MEDP0143 | 1.313201607 | −1.37375 |
| L-Ascorbate | MEDP0239 | 1.291994893 | −1.19252 |
| FA | |||
| Dodecanedioic Aicd | MEDP0308 | 1.392355211 | −1.97864 |
| Punicic Acid | MEDP0429 | 1.221991813 | −1.0298 |
| Hormones | |||
| Melatonin | MEDN0208 | 1.392900256 | −11.2892 |
| 3,3’,5-Triiodo-L-Thyronine | MEDP0184 | 1.390251632 | −1.15749 |
| Heterocyclic compounds | |||
| Isoxanthopterin | MEDP0384 | 1.38384504 | −1.03506 |
| Imidazoleacetic acid | MEDP0425 | 1.391592134 | 4.480622 |
| GL | |||
| Glycine linoleate | MEDN1288 | 1.365872365 | −1.27265 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Wang, R.; Tan, Y.; Liu, Y.; Ren, X.; Guo, C.; Li, R.; Li, M. Investigating the Mechanisms Underlying Citral-Induced Oxidative Stress and Its Contribution to Antifungal Efficacy on Magnaporthe oryzae Through a Multi-Omics Approach. Plants 2025, 14, 2001. https://doi.org/10.3390/plants14132001
Huang Y, Wang R, Tan Y, Liu Y, Ren X, Guo C, Li R, Li M. Investigating the Mechanisms Underlying Citral-Induced Oxidative Stress and Its Contribution to Antifungal Efficacy on Magnaporthe oryzae Through a Multi-Omics Approach. Plants. 2025; 14(13):2001. https://doi.org/10.3390/plants14132001
Chicago/Turabian StyleHuang, Yonghui, Ruoruo Wang, Yumei Tan, Yongxiang Liu, Xiyi Ren, Congtao Guo, Rongyu Li, and Ming Li. 2025. "Investigating the Mechanisms Underlying Citral-Induced Oxidative Stress and Its Contribution to Antifungal Efficacy on Magnaporthe oryzae Through a Multi-Omics Approach" Plants 14, no. 13: 2001. https://doi.org/10.3390/plants14132001
APA StyleHuang, Y., Wang, R., Tan, Y., Liu, Y., Ren, X., Guo, C., Li, R., & Li, M. (2025). Investigating the Mechanisms Underlying Citral-Induced Oxidative Stress and Its Contribution to Antifungal Efficacy on Magnaporthe oryzae Through a Multi-Omics Approach. Plants, 14(13), 2001. https://doi.org/10.3390/plants14132001







