Impact of Drought and Biostimulant in Greenhouse Tomato: Agronomic and Metabolomic Insights
Abstract
1. Introduction
2. Results
2.1. Growth Traits and Fruit Yield
2.2. Leaf Non-Destructive and Destructive Pigment Measurements
2.3. Fruit Quality
2.4. Fruit Nutrient Composition
2.5. Fruit Antioxidants
2.6. Fruit Carbohydrates
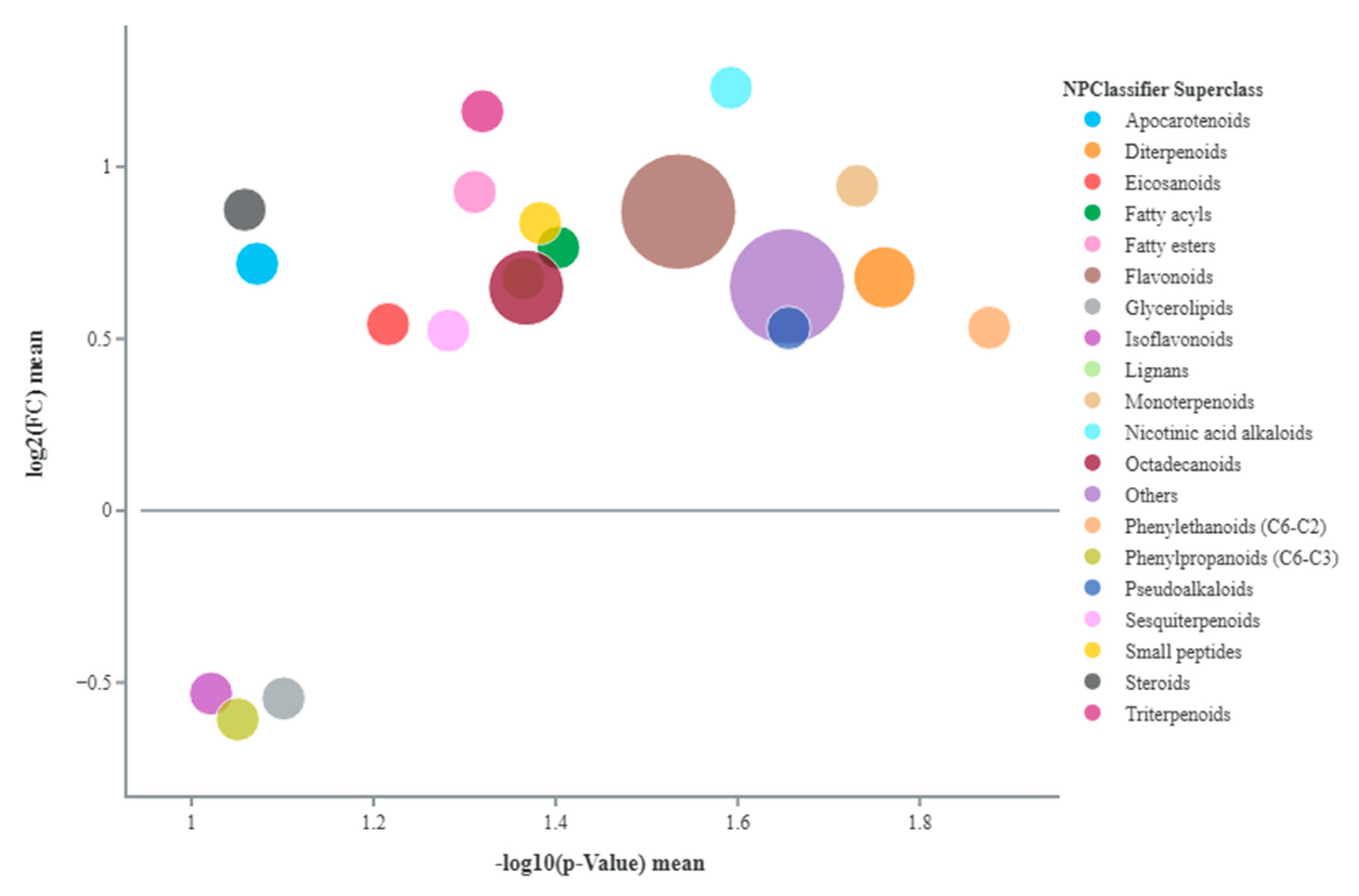
2.7. Fruit Metabolomics
3. Discussion
4. Materials and Methods
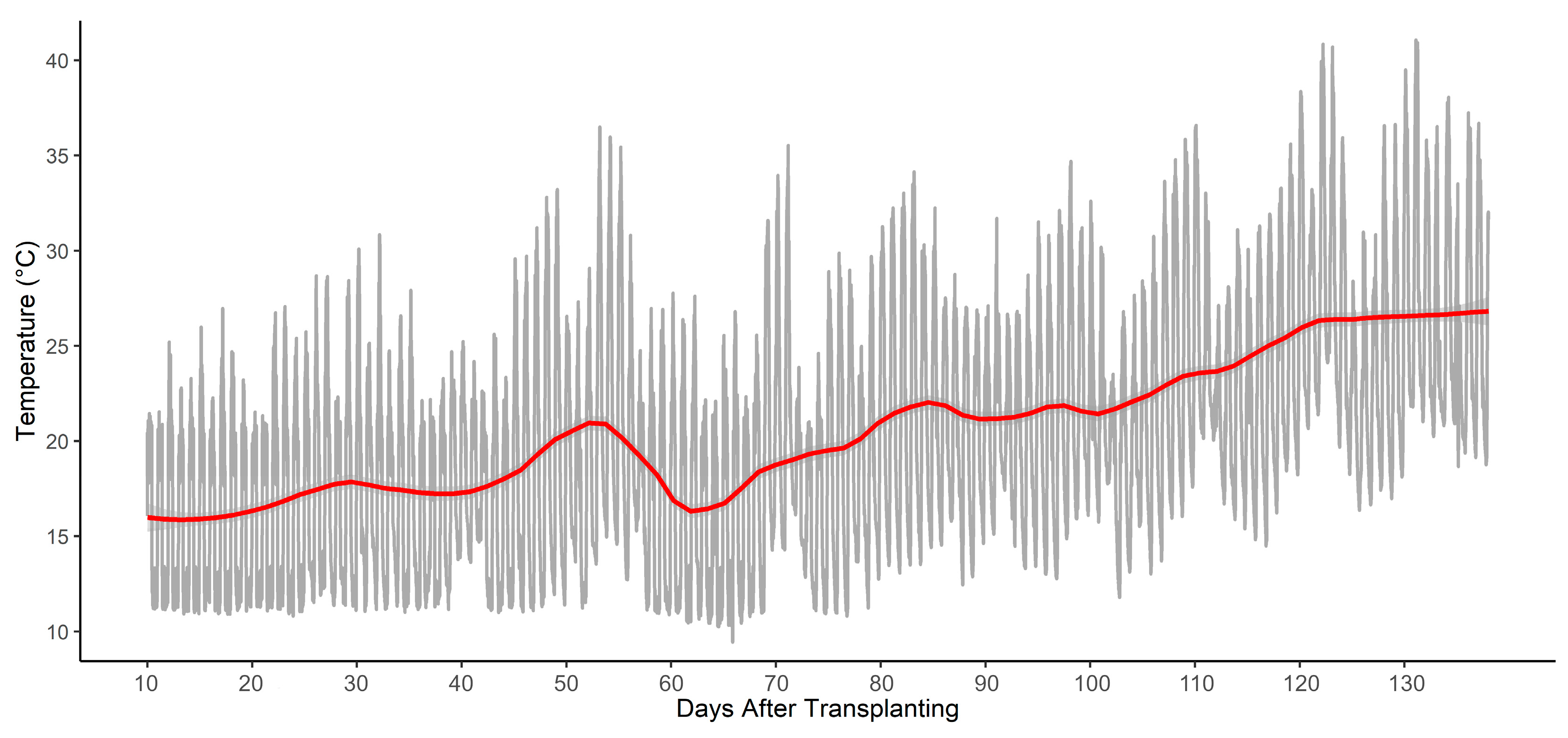
4.1. Plant Materials and Growth Conditions
4.2. Biostimulant Application
4.3. Fruit Yields and Yield Component
4.4. Leaf Non-Destructive and Destructive Pigments Measurements
4.5. Fruit Quality Assessment
4.6. Fruit Nutrient Composition
4.7. Fruit Antioxidants Assesment
4.8. Fruit Carbohydrates Composition
4.9. Fruit Metabolomics
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Furtak, K.; Wolińska, A. The Impact of Extreme Weather Events as a Consequence of Climate Change on the Soil Moisture and on the Quality of the Soil Environment and Agriculture—A Review. Catena 2023, 231, 107378. [Google Scholar] [CrossRef]
- Nikolaou, G.; Neocleous, D.; Christou, A.; Kitta, E.; Katsoulas, N. Implementing Sustainable Irrigation in Water-Scarce Regions under the Impact of Climate Change. Agronomy 2020, 10, 1120. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of Drought Stress on Sugar Metabolism in Leaves and Roots of Soybean Seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Alleviate the Adverse Effects of Drought Stress: A Comprehensive Review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef]
- Razi, K.; Muneer, S. Drought Stress-Induced Physiological Mechanisms, Signaling Pathways and Molecular Response of Chloroplasts in Common Vegetable Crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Triantaphylidès, C.; Havaux, M. Singlet Oxygen in Plants: Production, Detoxification and Signaling. Trends Plant Sci. 2009, 14, 219–228. [Google Scholar] [CrossRef]
- Beveridge, T.; Loubert, E.; Harrison, J.E. Simple Measurement of Phenolic Esters in Plant Cell Walls. Food Res. Int. 2000, 33, 775–783. [Google Scholar] [CrossRef]
- Solimene, S.; Coluccia, D.; Bernardo, A. Environmental Impact of Different Business Models: An LCA Study of Fresh Tomato Production in Italy. Sustainability 2023, 15, 10365. [Google Scholar] [CrossRef]
- Kalra, A.; Goel, S.; Elias, A.A. Understanding Role of Roots in Plant Response to Drought: Way Forward to Climate-Resilient Crops. Plant Genome 2024, 17, e20395. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, W.; Du, T.; Kang, S.; Davies, W.J. Responses of Water Accumulation and Solute Metabolism in Tomato Fruit to Water Scarcity and Implications for Main Fruit Quality Variables. J. Exp. Bot. 2020, 71, 1249–1264. [Google Scholar] [CrossRef]
- Barbagallo, R.N.; Di Silvestro, I.; Patanè, C. Yield, Physicochemical Traits, Antioxidant Pattern, Polyphenol Oxidase Activity and Total Visual Quality of Field-Grown Processing Tomato Cv. Brigade as Affected by Water Stress in Mediterranean Climate. J. Sci. Food Agric. 2013, 93, 1449–1457. [Google Scholar] [CrossRef]
- Jin, N.; Jin, L.; Wang, S.; Meng, X.; Ma, X.; He, X.; Zhang, G.; Luo, S.; Lyu, J.; Yu, J. A Comprehensive Evaluation of Effects on Water-Level Deficits on Tomato Polyphenol Composition, Nutritional Quality and Antioxidant Capacity. Antioxidants 2022, 11, 1585. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant Action of a Plant-Derived Protein Hydrolysate Produced through Enzymatic Hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef] [PubMed]
- Francesca, S.; Cirillo, V.; Raimondi, G.; Maggio, A.; Barone, A.; Rigano, M.M. A Novel Protein Hydrolysate-Based Biostimulant Improves Tomato Performances under Drought Stress. Plants 2021, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Agliassa, C.; Mannino, G.; Molino, D.; Cavalletto, S.; Contartese, V.; Margherita, C.; Secchi, F. A New Protein Hydrolysate-Based Biostimulant Applied by Fertigation Promotes Relief from Drought Stress in Capsicum Annuum L. Plant Physiol. Biochem. 2021, 166, 1076–1086. [Google Scholar] [CrossRef]
- Leporino, M.; Rouphael, Y.; Bonini, P.; Colla, G.; Cardarelli, M. Protein Hydrolysates Enhance Recovery from Drought Stress in Tomato Plants: Phenomic and Metabolomic Insights. Front. Plant Sci. 2024, 15, 1357316. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Miras Moreno, M.B.; Reynaud, H.; Canaguier, R.; Trtílek, M.; et al. A Combined Phenotypic and Metabolomic Approach for Elucidating the Biostimulant Action of a Plant-Derived Protein Hydrolysate on Tomato Grown under Limited Water Availability. Front. Plant Sci. 2019, 10, 493. [Google Scholar] [CrossRef]
- Patanè, C.; Tringali, S.; Sortino, O. Effects of Deficit Irrigation on Biomass, Yield, Water Productivity and Fruit Quality of Processing Tomato under Semi-Arid Mediterranean Climate Conditions. Sci. Hortic. 2011, 129, 590–596. [Google Scholar] [CrossRef]
- Ahmed, M.; Ullah, H.; Piromsri, K.; Tisarum, R.; Cha-um, S.; Datta, A. Effects of an Ascophyllum nodosum Seaweed Extract Application Dose and Method on Growth, Fruit Yield, Quality, and Water Productivity of Tomato under Water-Deficit Stress. S. Afr. J. Bot. 2022, 151, 95–107. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, L.; Shen, Q.; Yang, J.; Han, X.; Tian, F.; Wu, J. Effects of Water Stress on Photosynthesis, Yield, and Water Use Efficiency in Winter Wheat. Water 2020, 12, 2127. [Google Scholar] [CrossRef]
- Rosa, A.P.; Barão, L.; Chambel, L.; Cruz, C.; Santana, M.M. Early Identification of Plant Drought Stress Responses: Changes in Leaf Reflectance and Plant Growth Promoting Rhizobacteria Selection-The Case Study of Tomato Plants. Agronomy 2023, 13, 183. [Google Scholar] [CrossRef]
- Makela, P.; Karkkainen, J.; Somersalo, S. Effect of Glycinebetaine on Chloroplast Ultrastructure, Chlorophyll and Protein Content, and RuBPCO Activities in Tomato Grown under Drought or Salinity. Biol. Plant. 2000, 43, 471–475. [Google Scholar] [CrossRef]
- Rodriguez, J.; Rios, D.; Rodriguez, E.; Diaz, C. Physico-Chemical Changes During Ripening of Conventionally, Ecologically and Hydroponically Cultivated Tyrlain (TY 10016) Tomatoes. Int. J. Agric. Res. 2006, 1, 452–461. [Google Scholar]
- Ripoll, J.; Urban, L.; Brunel, B.; Bertin, N. Water Deficit Effects on Tomato Quality Depend on Fruit Developmental Stage and Genotype. J. Plant Physiol. 2016, 190, 26–35. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Shennan, C.; Grattan, S.R.; May, D.M. Tomato Fruit Yields and Quality under Water Deficit and Salinity. J. Am. Soc. Hortic. Sci. 2019, 116, 215–221. [Google Scholar] [CrossRef]
- Levander, O.A. Fruit and Vegetable Contributions to Dietary Mineral Intake in Human Health and Disease. HortScience 1990, 25, 1486–1488. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Shennan, C.; Grattan, S.R. Developmental Changes in Tomato Fruit Composition in Response to Water Deficit and Salinity. Physiol. Plant. 1991, 83, 177–185. [Google Scholar] [CrossRef]
- Ahmed, N.; Zhang, B.; Chachar, Z.; Li, J.; Xiao, G.; Wang, Q.; Hayat, F.; Deng, L.; Narejo, M.N.; Bozdar, B.; et al. Micronutrients and Their Effects on Horticultural Crop Quality, Productivity and Sustainability. Sci. Hortic. 2024, 323, 112512. [Google Scholar] [CrossRef]
- Rai, S.; Singh, P.K.; Mankotia, S.; Swain, J.; Satbhai, S.B. Iron Homeostasis in Plants and Its Crosstalk with Copper, Zinc, and Manganese. Plant Stress. 2021, 1, 100008. [Google Scholar] [CrossRef]
- Lu, S.W.; Li, T.L.; Jiang, J. Tomato Key Sucrose Metabolizing Enzyme Activities and Gene Expression Under NaCl and PEG Iso-Osmotic Stresses. Agric. Sci. China 2009, 8, 1046–1052. [Google Scholar] [CrossRef]
- Luo, A.; Zhou, C.; Chen, J. The Associated With Carbon Conversion Rate and Source–Sink Enzyme Activity in Tomato Fruit Subjected to Water Stress and Potassium Application. Front. Plant Sci. 2021, 12, 681145. [Google Scholar] [CrossRef]
- Singh, P.; Choudhary, K.K.; Chaudhary, N.; Gupta, S.; Sahu, M.; Tejaswini, B.; Sarkar, S. Salt Stress Resilience in Plants Mediated through Osmolyte Accumulation and Its Crosstalk Mechanism with Phytohormones. Front. Plant Sci. 2022, 13, 1006617. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.K. Role of Amino Acids in Plant Responses to Stress. Biol. Plant. 2002, 45, 481–487. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Hima Kumari, P.; Sunita, M.S.L.; Sreenivasulu, N. Role of Proline in Cell Wall Synthesis and Plant Development and Its Implications in Plant Ontogeny. Front. Plant Sci. 2015, 6, 544. [Google Scholar] [CrossRef] [PubMed]
- Galili, G.; Tang, G.; Zhu, X.; Gakiere, B. Lysine Catabolism: A Stress and Development Super-Regulated Metabolic Pathway. Curr. Opin. Plant Biol. 2001, 4, 261–266. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, N.; Zhen, F.; Ren, P.; Li, F. Metabolism of Endogenous Arginine in Tomato Fruit Harvested at Different Ripening Stages. Sci. Hortic. 2014, 179, 349–355. [Google Scholar] [CrossRef]
- Gao, F.; Mei, X.; Li, Y.; Guo, J.; Shen, Y. Update on the Roles of Polyamines in Fleshy Fruit Ripening, Senescence, and Quality. Front. Plant Sci. 2021, 12, 610313. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Isayenkov, S.V. The Role of Anthocyanins in Plant Tolerance to Drought and Salt Stresses. Plants 2023, 12, 2558. [Google Scholar] [CrossRef]
- Hou, X.; Rivers, J.; León, P.; McQuinn, R.P.; Pogson, B.J. Synthesis and Function of Apocarotenoid Signals in Plants. Trends Plant Sci. 2016, 21, 792–803. [Google Scholar] [CrossRef]
- Simkin, A.J. Carotenoids and Apocarotenoids in Planta: Their Role in Plant Development, Contribution to the Flavour and Aroma of Fruits and Flowers, and Their Nutraceutical Benefits. Plants 2021, 10, 2321. [Google Scholar] [CrossRef] [PubMed]
- Gosch, C.; Halbwirth, H.; Stich, K. Phloridzin: Biosynthesis, Distribution and Physiological Relevance in Plants. Phytochemistry 2010, 71, 838–843. [Google Scholar] [CrossRef]
- Schijlen, E.; Ric De Vos, C.H.; Jonker, H.; Van Den Broeck, H.; Molthoff, J.; Van Tunen, A.; Martens, S.; Bovy, A. Pathway Engineering for Healthy Phytochemicals Leading to the Production of Novel Flavonoids in Tomato Fruit. Plant Biotechnol. J. 2006, 4, 433–444. [Google Scholar] [CrossRef]
- Tohge, T.; Scossa, F.; Wendenburg, R.; Frasse, P.; Balbo, I.; Watanabe, M.; Alseekh, S.; Jadhav, S.S.; Delfin, J.C.; Lohse, M.; et al. Exploiting Natural Variation in Tomato to Define Pathway Structure and Metabolic Regulation of Fruit Polyphenolics in the Lycopersicum Complex. Mol. Plant 2020, 13, 1027–1046. [Google Scholar] [CrossRef]
- Dere, S.; Kusvuran, S.; Dasgan, H.Y. Does Drought Increase the Antioxidant Nutrient Capacity of Tomatoes? Int. J. Food Sci. Technol. 2022, 57, 6633–6645. [Google Scholar] [CrossRef]
- Hano, S.; Shibuya, T.; Imoto, N.; Ito, A.; Imanishi, S.; Aso, H.; Kanayama, Y. Scientia Horticulturae Serotonin Content in Fresh and Processed Tomatoes and Its Accumulation during Fruit Development. Sci. Hortic. 2017, 214, 107–113. [Google Scholar] [CrossRef]
- Conti, V.; Romi, M.; Guarnieri, M.; Cantini, C.; Cai, G. Italian Tomato Cultivars under Drought Stress Show Different Content of Bioactives in Pulp and Peel of Fruits. Foods 2022, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Tieman, D.; Taylor, M.; Schauer, N.; Fernie, A.R.; Hanson, A.D.; Klee, H.J. Tomato Aromatic Amino Acid Decarboxylases Participate in Synthesis of the Flavor Volatiles 2-Phenylethanol and 2-Phenylacetaldehyde. Proc. Natl. Acad. Sci. USA 2006, 103, 8287–8292. [Google Scholar] [CrossRef]
- Shohat, H.; Eliaz, N.I.; Weiss, D. Gibberellin in Tomato: Metabolism, Signaling and Role in Drought Responses. Mol. Hortic. 2021, 1, 15. [Google Scholar] [CrossRef]
- Park, M.; Malka, S.K. Gibberellin Delays Metabolic Shift during Tomato Ripening by Inducing Auxin Signaling. Front. Plant Sci. 2022, 13, 1045761. [Google Scholar] [CrossRef]
- Nir, I.; Moshelion, M.; Weiss, D.; Transferase, M. The Arabidopsis GIBBERELLIN METHYL TRANSFERASE 1 Suppresses Gibberellin Activity, Reduces Whole-Plant Transpiration and Promotes Drought Tolerance in Transgenic Tomato. Plant Cell Environ. 2014, 37, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Huang, H.; Jingjing, W.; Xiaoyun, W.; Bingqin, X.; Xuehui, Y.; Lulu, S.; Rui, Y.; Jianli, W.; Aidong, S.; et al. Jasmonic Acid Enhances Osmotic Stress Responses by MYC2- Mediated Inhibition of Protein Phosphatase 2C1 and Response Regulators 26 Transcription Factor in Tomato. Plant J. 2023, 113, 546–561. [Google Scholar] [CrossRef] [PubMed]
- El-Nakhel, C.; Cristofano, F.; Colla, G.; Pii, Y.; Secomandi, E.; De Gregorio, M.; Buffagni, V.; Garcia-Perez, P.; Lucini, L.; Rouphael, Y. Vegetal-Derived Biostimulants Distinctively Command the Physiological and Metabolomic Signatures of Lettuce Grown in Depleted Nitrogen Conditions. Sci. Hortic. 2023, 317, 112057. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Stadtman, F.H.; Buhlert, J.E.; Marsh, G.L. Titratable Acidity of Tomato Juice As Affected By Break Procedure. J. Food Sci. 1977, 42, 379–382. [Google Scholar] [CrossRef]
- Bremner, J.M. Total Nitrogen. Am. Soc. Agron. 1965, 10, 1049–1178. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Verh. Dtsch. Ges. Inn. Med. 1967, 73, 366–369. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Ierna, A.; Mauromicale, G. Variation of Polyphenols in a Germplasm Collection of Globe Artichoke. Food Res. Int. 2012, 46, 544–551. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Adsule, P.; Dan, A. Simplified Extraction Procedure in the Rapid Spectrophotometric Method for Lycopene Estimation in Tomato. J. Food Sci. Technol. 1979, 16, 216–217. [Google Scholar]
- Bonini, P.; Kind, T.; Tsugawa, H.; Barupal, D.K.; Fiehn, O. Retip: Retention Time Prediction for Compound Annotation in Untargeted Metabolomics. Anal. Chem. 2020, 92, 7515–7522. [Google Scholar] [CrossRef] [PubMed]
| Source of Variation | Yield (g/Plant) | Marketable Fruits | |||
|---|---|---|---|---|---|
| Marketable | Not Marketable | Total | Number (n./Plant) | Mean Weight (g/Fruit) | |
| Irrigation | |||||
| W | 2484.32 ± 40.49 | 528.60 ± 22.26 | 3012.35 ± 51.37 | 152.98 ± 2.65 | 16.41 ± 0.16 |
| S | 1471.79 ± 23.49 | 368.83 ± 11.18 | 1798.64 ± 23.32 | 103.98 ± 1.49 | 14.39 ± 0.13 |
| Biostimulant | |||||
| U | 2078.02 ± 121.57 | 434.48 ± 19.60 | 2454.01 ± 124.56 | 131.83 ± 6.00 | 15.41 ± 0.24 |
| PH-F | 1971.25 ± 94.03 | 462.10 ± 27.88 | 2345.24 ± 106.5 | 129.41 ± 4.57 | 15.25 ± 0.23 |
| PH-R | 1915.33 ± 97.02 | 447.02 ± 29.24 | 2436.41 ± 133.11 | 125.06 ± 4.95 | 15.55 ± 0.30 |
| Irrigation × Biostimulant | |||||
| W × U | 2614.52 ± 66.40 | 471.52 ± 29.99 | 3031.71 ± 86.46 | 157.94 ± 5.40 | 16.49 ± 0.20 |
| W × PH-F | 2420.29 ± 71.90 | 567.67 ± 38.49 | 2932.55 ± 49.57 | 150.75 ± 4.44 | 16.09 ± 0.28 |
| W × PH-R | 2427.31 ± 62.47 | 547.81 ± 44.61 | 3062.80 ± 113.39 | 150.07 ± 3.66 | 16.64 ± 0.33 |
| S × U | 1452.11 ± 23.46 | 397.44 ± 22.47 | 1793.79 ± 37.98 | 102.00 ± 2.34 | 14.40 ± 0.20 |
| S × PH-F | 1492.28 ± 38.69 | 356.53 ± 15.70 | 1831.35 ± 39.04 | 108.06 ± 2.39 | 14.30 ± 0.11 |
| S × PH-R | 1467.34 ± 49.93 | 352.54 ± 18.48 | 1768.27 ± 44.58 | 101.63 ± 2.76 | 14.45 ± 0.33 |
| Significance | |||||
| Irrigation | *** | *** | *** | *** | *** |
| Biostimulant | ns | ns | ns | ns | ns |
| Irrigation × Biostimulant | ns | ns | ns | ns | ns |
| Source of Variation | Pigment Concentration (mg/g FW) | |||
|---|---|---|---|---|
| Clorophyll a | Clorophyll b | Total Chlorophyll | Carotenoids | |
| Irrigation | ||||
| W | 1.188 ± 0.013 | 0.450 ± 0.004 | 1.637 ± 0.017 | 0.333 ± 0.004 |
| S | 1.359 ± 0.021 | 0.500 ± 0.005 | 1.858 ± 0.027 | 0.365 ± 0.005 |
| Biostimulant | ||||
| U | 1.285 ± 0.022 ab | 0.479 ± 0.006 ab | 1.764 ± 0.027 ab | 0.351 ± 0.005 |
| PH-F | 1.227 ± 0.017 b | 0.462 ± 0.005 b | 1.688 ± 0.022 b | 0.341 ± 0.005 |
| PH-R | 1.305 ± 0.030 a | 0.483 ± 0.008 a | 1.788 ± 0.039 a | 0.355 ± 0.007 |
| Irrigation × Biostimulant | ||||
| W × U | 1.232 ± 0.023 bc | 0.463 ± 0.007 | 1.695 ± 0.030 bc | 0.342 ± 0.006 |
| W × PH-F | 1.151 ± 0.019 c | 0.438 ± 0.005 | 1.589 ± 0.023 c | 0.325 ± 0.006 |
| W × PH-R | 1.181 ± 0.023 c | 0.447 ± 0.007 | 1.628 ± 0.030 c | 0.332 ± 0.006 |
| S × U | 1.337 ± 0.034 ab | 0.494 ± 0.009 | 1.831 ± 0.043 ab | 0.359 ± 0.007 |
| S × PH-F | 1.306 ± 0.022 b | 0.486 ± 0.006 | 1.790 ± 0.028 b | 0.357 ± 0.006 |
| S × PH-R | 1.430 ± 0.046 a | 0.518 ± 0.013 | 1.948 ± 0.059 a | 0.378 ± 0.010 |
| Significance | ||||
| Irrigation | *** | *** | *** | *** |
| Biostimulant | * | * | * | ns |
| Irrigation × Biostimulant | * | ns | * | ns |
| Source of Variation | pH | TSS (°Brix) | TA (g Citric Acid/100 mL Juice) | TSS/TA | DM (%) | Firmness (N) |
|---|---|---|---|---|---|---|
| Irrigation | ||||||
| W | 4.32 ± 0.01 | 8.55 ± 0.05 | 0.46 ± 0.01 | 18.81 ± 0.24 | 9.30 ± 0.08 | 0.95 ± 0.02 |
| S | 4.29 ± 0.01 | 8.83 ± 0.04 | 0.48 ± 0.01 | 18.65 ± 0.29 | 9.72 ± 0.07 | 1.09 ± 0.02 |
| Biostimulant | ||||||
| U | 4.31 ± 0.01 | 8.65 ± 0.06 | 0.46 ± 0.01 | 18.99 ± 0.35 | 9.49 ± 0.08 | 1.02 ± 0.02 |
| PH-F | 4.30 ± 0.01 | 8.76 ± 0.06 | 0.48 ± 0.01 | 18.44 ± 0.25 | 9.54 ± 0.10 | 1.00 ± 0.03 |
| PH-R | 4.32 ± 0.01 | 8.66 ± 0.07 | 0.47 ± 0.01 | 18.74 ± 0.35 | 9.38 ± 0.12 | 1.02 ± 0.03 |
| Irrigation × Biostimulant | ||||||
| W × U | 4.34 ± 0.02 | 8.49 ± 0.11 | 0.46 ± 0.01 | 18.90 ± 0.51 | 9.37 ± 0.10 | 0.99 ± 0.04 |
| W × PH-F | 4.31 ± 0.02 | 8.58 ± 0.07 | 0.45 ± 0.01 | 19.11 ± 0.26 | 9.39 ± 0.14 | 0.91 ± 0.04 |
| W × PH-R | 4.33 ± 0.01 | 8.58 ± 0.09 | 0.47 ± 0.01 | 18.47 ± 0.43 | 9.14 ± 0.16 | 0.95 ± 0.02 |
| S × U | 4.27 ± 0.02 | 8.79 ± 0.05 | 0.47 ± 0.01 | 19.08 ± 0.48 | 9.64 ± 0.13 | 1.05 ± 0.02 |
| S × PH-F | 4.29 ± 0.02 | 8.94 ± 0.08 | 0.50 ± 0.01 | 17.82 ± 0.37 | 9.78 ± 0.07 | 1.12 ± 0.02 |
| S × PH-R | 4.30 ± 0.02 | 8.77 ± 0.09 | 0.47 ± 0.01 | 19.06 ± 0.58 | 9.76 ± 0.13 | 1.09 ± 0.04 |
| Significance | ||||||
| Irrigation | * | *** | * | ns | *** | *** |
| Biostimulant | ns | ns | ns | ns | ns | ns |
| Irrigation × Biostimulant | ns | ns | ns | ns | ns | ns |
| Source of Variation | Macronutrient Concentration (g/kg DW) | Micronutrient Concentration (mg/kg DW) | |||||
|---|---|---|---|---|---|---|---|
| N | P | K | Fe | Mn | Zn | Cu | |
| Irrigation | |||||||
| W | 21.85 ± 0.32 | 7.86 ± 0.43 | 50.64 ± 0.86 | 304.53 ± 10.53 | 62.74 ± 2.61 | 25.44 ± 0.83 | 5.63 ± 0.15 |
| S | 21.62 ± 0.30 | 6.85 ± 0.63 | 40.75 ± 1.26 | 280.66 ± 18.75 | 42.81 ± 1.45 | 18.17 ± 0.51 | 5.07 ± 0.16 |
| Biostimulant | |||||||
| U | 21.49 ± 0.36 | 8.03 ± 0.64 | 43.93 ± 1.90 | 250.01 ± 14.36 b | 52.77 ± 3.84 | 21.93 ± 1.61 | 5.52 ± 0.24 |
| PH-F | 21.86 ± 0.44 | 7.21 ± 0.76 | 47.59 ± 2.17 | 325.85 ± 13.38 a | 57.71 ± 4.82 | 22.21 ± 1.49 | 5.23 ± 0.27 |
| PH-R | 21.85 ± 0.34 | 6.68 ± 0.65 | 44.98 ± 1.97 | 303.05 ± 19.52 ab | 51.57 ± 3.84 | 21.63 ± 1.17 | 5.25 ± 0.11 |
| Irrigation × Biostimulant | |||||||
| W × U | 21.82 ± 0.59 | 8.15 ± 0.76 | 49.55 ± 1.38 | 266.78 ± 14.98 | 61.95 ± 4.38 | 25.15 ± 2.21 | 6.02 ± 0.13 |
| W × PH-F | 21.48 ± 0.63 | 7.83 ± 0.88 | 52.90 ± 0.48 | 314.57 ± 16.91 | 64.59 ± 5.20 | 25.99 ± 1.02 | 5.52 ± 0.36 |
| W × PH-R | 22.14 ± 0.56 | 7.48 ± 0.49 | 49.67 ± 1.81 | 325.95 ± 14.82 | 61.69 ± 4.73 | 25.19 ± 1.03 | 5.46 ± 0.09 |
| S × U | 21.15 ± 0.41 | 7.90 ± 1.09 | 39.25 ± 1.51 | 236.03 ± 22.81 | 43.60 ± 3.49 | 18.07 ± 0.26 | 5.19 ± 0.33 |
| S × PH-F | 22.15 ± 0.67 | 6.47 ± 1.32 | 42.28 ± 2.61 | 342.77 ± 21.66 | 43.95 ± 1.21 | 17.67 ± 1.09 | 4.90 ± 0.38 |
| S × PH-R | 21.57 ± 0.42 | 6.22 ± 0.96 | 40.95 ± 2.48 | 283.41 ± 33.44 | 41.46 ± 1.23 | 18.59 ± 1.00 | 5.10 ± 0.17 |
| Significance | |||||||
| Irrigation | ns | ns | *** | ns | *** | *** | * |
| Biostimulant | ns | ns | ns | * | ns | ns | ns |
| Irrigation × Biostimulant | ns | ns | ns | ns | ns | ns | ns |
| Source of Variation | mg Trolox eq./g FW | mg GAE eq./g FW | mg QE eq./g FW | |
|---|---|---|---|---|
| FRAP | DPPH | Polyphenols | Flavonoids | |
| Irrigation | ||||
| W | 22.106 ± 0.593 | 11.742 ± 0.077 | 31.576 ± 0.646 | 0.252 ± 0.016 |
| S | 25.935 ± 0.437 | 11.519 ± 0.109 | 33.817 ± 0.657 | 0.304 ± 0.016 |
| Biostimulant | ||||
| U | 22.293 ± 0.582 b | 11.657 ± 0.128 | 32.141 ± 0.921 | 0.254 ± 0.021 |
| PH-F | 26.034 ± 0.891 a | 11.699 ± 0.136 | 32.844 ± 0.785 | 0.278 ± 0.025 |
| PH-R | 23.706 ± 0.496 b | 11.516 ± 0.072 | 33.104 ± 0.897 | 0.311 ± 0.011 |
| Irrigation × Biostimulant | ||||
| W × U | 19.622 ± 0.414 | 11.797 ± 0.116 | 30.864 ± 1.184 | 0.247 ± 0.039 |
| W × PH-F | 23.779 ± 1.304 | 11.753 ± 0.173 | 31.701 ± 1.014 | 0.243 ± 0.026 |
| W × PH-R | 22.581 ± 0.690 | 11.662 ± 0.106 | 32.162 ± 1.294 | 0.271 ± 0.008 |
| S × U | 24.519 ± 0.406 | 11.518 ± 0.228 | 33.418 ± 1.297 | 0.261 ± 0.020 |
| S × PH-F | 28.740 ± 0.618 | 11.645 ± 0.216 | 33.987 ± 1.078 | 0.313 ± 0.040 |
| S × PH-R | 24.831 ± 0.574 | 11.371 ± 0.082 | 34.047 ± 1.229 | 0.338 ± 0.006 |
| Significance | ||||
| Irrigation | *** | ns | * | * |
| Biostimulant | *** | ns | ns | ns |
| Irrigation × Biostimulant | ns | ns | ns | ns |
| Source of Variation | Soluble Carbohydrates (mg/g DW) | Insoluble Carbohydrate (mg/g DW) | |||
|---|---|---|---|---|---|
| Glucose | Sucrose | Fructose | Total | Starch | |
| Irrigation | |||||
| W | 233.62 ± 12.82 | 27.22 ± 2.30 | 296.91 ± 22.13 | 557.76 ± 34.13 | 6.03 ± 1.40 |
| S | 271.47 ± 7.44 | 20.20 ± 2.50 | 325.04 ± 16.22 | 616.71 ± 23.37 | 4.88 ± 0.58 |
| Biostimulant | |||||
| U | 266.25 ± 10.88 | 18.48 ± 3.68 | 312.66 ± 24.18 | 597.34 ± 35.04 | 4.18 ± 0.72 |
| PH-F | 233.52 ± 12.63 | 25.50 ± 2.20 | 298.60 ± 17.56 | 557.62 ± 26.98 | 6.26 ± 2.10 |
| PH-R | 257.87 ± 18.68 | 27.16 ± 2.86 | 321.67 ± 31.28 | 606.69 ± 49.00 | 5.92 ± 0.50 |
| Irrigation × Biostimulant | |||||
| W × U | 272.94 ± 20.66 ab | 23.74 ± 3.52 | 331.04 ± 50.30 | 627.71 ± 71.98 | 3.42 ± 0.07 |
| W × PH-F | 208.40 ± 12.81 c | 27.98 ± 3.58 | 291.28 ± 34.69 | 527.65 ± 49.70 | 8.67 ± 3.99 |
| W × PH-R | 219.53 ± 13.80 bc | 29.97 ± 5.32 | 268.41 ± 33.74 | 517.91 ± 51.34 | 5.10 ± 0.79 |
| S × U | 259.57 ± 10.95 abc | 13.22 ± 5.27 | 294.29 ± 7.54 | 567.08 ± 6.31 | 4.95 ± 1.42 |
| S × PH-F | 258.65 ± 1.48 abc | 23.03 ± 2.29 | 305.92 ± 16.87 | 587.59 ± 16.49 | 3.85 ± 0.61 |
| S × PH-R | 296.21 ± 9.14 a | 24.34 ± 2.19 | 374.92 ± 30.28 | 695.47 ± 38.54 | 5.83 ± 0.77 |
| Significance | |||||
| Irrigation | ** | * | ns | ns | ns |
| Biostimulant | ns | ns | ns | ns | ns |
| Irrigation × Biostimulant | * | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leporino, M.; Cardarelli, M.; Bonini, P.; Proietti, S.; Moscatello, S.; Colla, G. Impact of Drought and Biostimulant in Greenhouse Tomato: Agronomic and Metabolomic Insights. Plants 2025, 14, 2000. https://doi.org/10.3390/plants14132000
Leporino M, Cardarelli M, Bonini P, Proietti S, Moscatello S, Colla G. Impact of Drought and Biostimulant in Greenhouse Tomato: Agronomic and Metabolomic Insights. Plants. 2025; 14(13):2000. https://doi.org/10.3390/plants14132000
Chicago/Turabian StyleLeporino, Marzia, Mariateresa Cardarelli, Paolo Bonini, Simona Proietti, Stefano Moscatello, and Giuseppe Colla. 2025. "Impact of Drought and Biostimulant in Greenhouse Tomato: Agronomic and Metabolomic Insights" Plants 14, no. 13: 2000. https://doi.org/10.3390/plants14132000
APA StyleLeporino, M., Cardarelli, M., Bonini, P., Proietti, S., Moscatello, S., & Colla, G. (2025). Impact of Drought and Biostimulant in Greenhouse Tomato: Agronomic and Metabolomic Insights. Plants, 14(13), 2000. https://doi.org/10.3390/plants14132000


.png)





