Soil-Driven Coupling of Plant Community Functional Traits and Diversity in Desert–Oasis Transition Zone
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sample Collection
2.3. Measurement of Plant Functional Traits and Soil Indicators
2.3.1. Measurement of Leaf Functional Traits
2.3.2. Measurement of Fine Root Functional Traits
2.4. Measurement of Soil Indicators
2.5. Species Diversity Calculations
2.6. Calculation of Community Mean Trait Values
2.7. Functional Diversity Calculations
2.8. Statistical Analyses
3. Results
3.1. Vegetation Characteristics and Plant Diversity at Different Stages of Fixation in Sand Dunes
3.2. Characterizing Variations in Functional Traits of Plant Communities at Different Stages of Fixation in Sand Dunes
3.2.1. Leaf Functional Traits
3.2.2. Fine Root Functional Traits
3.3. Correlations Between Plant Community Functional Traits and Plant Diversity
3.4. Response of Plant Functional Traits and Community Composition to Environmental Changes
3.5. Analysis of Factors Influencing Plant Community Functional Diversity and Their Mechanisms
4. Discussion
4.1. Habitat Differences-Induced Community Recombination Led to Significant Alterations in the Functional Traits of Plant Communities
4.2. Functional Traits and Diversity Synergize to Reflect Adaptive Strategies for Plant Community Survival
4.3. Soil Factors Regulate Fine Root Functional Traits and Diversity in Desert Communities by Influencing Species Diversity, Which in Turn Drives Community Succession
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, M.D. An ecological perspective on extreme climatic events: A synthetic definition and framework to guide future research. J. Ecol. 2011, 99, 656–663. [Google Scholar] [CrossRef]
- Jing, Z.B.; Cheng, J.M.; Su, J.S.; Bai, Y.; Jin, J.W. Changes in plant community composition and soil properties under 3-decade grazing exclusion in semiarid grassland. Ecol. Eng. 2014, 64, 171–178. [Google Scholar] [CrossRef]
- Xie, J.B.; Wang, Z.Y.; Li, Y. Stomatal opening ratio mediates trait coordinating network adaptation to environmental gradients. New Phytol. 2022, 235, 907–922. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Isbell, F.; Gonzalez, A.; Loreau, M.; Cowles, J.; Díaz, S.; Hector, A.; Mace, G.M.; Wardle, D.A.; O’Connor, M.I.; Duffy, J.E.; et al. Linking the Influence and Dependence of People on Biodiversity across Scales. Nature 2017, 546, 65–72. [Google Scholar] [CrossRef]
- Branquinho, C.; Serrano, H.C.; Nunes, A.; Pinho, P.; Matos, P. Essential biodiversity change indicators for evaluating the effects of Anthropocene in ecosystems at a global scale. In From Assessing to Conserving Biodiversity: Conceptual and Practical Challenges; Springer: Cham, Swizterland, 2019; pp. 137–163. [Google Scholar] [CrossRef]
- Roswell, M.; Dushoff, J.; Winfree, R. A conceptual guide to measuring species diversity. Oikos 2021, 130, 321–338. [Google Scholar] [CrossRef]
- Onoda, Y.; Westoby, M.; Adler, P.B.; Choong, A.M.F.; Clissold, F.J.; Cornelissen, J.H.C.; Díaz, S.; Dominy, N.J.; Elgart, A.; Enrico, L.; et al. Global patterns of leaf mechanical properties. Ecol. Lett. 2011, 14, 301–312. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast–slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Jardine, E.C.; Thomas, G.H.; Forrestel, E.J.; Lehmann, C.E.R.; Osborne, C.P. The global distribution of grass functional traits within grassy biomes. J. Biogeogr. 2020, 47, 553–565. [Google Scholar] [CrossRef]
- Speed, J.D.M.; Chimal-Ballesteros, J.A.; Martin, M.D.; Barrio, I.C.; Vuorinen, K.E.M.; Soininen, E.M. Will borealization of Arctic tundra herbivore communities be driven by climate warming or vegetation change? Glob. Change Biol. 2021, 27, 6568–6577. [Google Scholar] [CrossRef]
- Grime, J.P. Benefits of plant diversity to ecosystems: Immediate, filter and founder effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Ricotta, C.; Moretti, M. CWM and Rao’s quadratic diversity: A unified framework for functional ecology. Oecologia 2011, 167, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Tilman, D. Distinguishing between the effects of species diversity and species composition. Oikos 1997, 80, 185. [Google Scholar] [CrossRef]
- Laliberté, E.; Zemunik, G.; Turner, B.L. Environmental filtering explains variation in plant diversity along resource gradients. Science 2014, 345, 1602–1605. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Wright, I.J.; Ackerly, D.D.; Bongers, F.; Harms, K.E.; Ibarra-Manriquez, G.; Martinez-Ramos, M.; Mazer, S.J.; Muller-Landau, H.C.; Paz, H.; Pitman, N.C.A.; et al. Relationships among ecologically important dimensions of plant trait variation in seven Neotropical forests. Ann. Bot. 2007, 99, 1003–1015. [Google Scholar] [CrossRef]
- Kembel, S.W.; Cahill, J.F., Jr. Independent evolution of leaf and root traits within and among temperate grassland plant communities. PLoS ONE 2011, 6, e19992. [Google Scholar] [CrossRef]
- Fan, B.; Zhao, C.; Zhang, X.; Sun, K. Impacts of sand burial and wind erosion on regeneration and growth of a desert clonal shrub. Front. Plant Sci. 2018, 9, 1696. [Google Scholar] [CrossRef]
- Yang, P.; Huang, L.; He, S.; Zeng, X.; Chen, Y.; Wang, H. Adaptive strategies employed by Clonal Plants in heterogeneous patches. Forests 2023, 14, 1648. [Google Scholar] [CrossRef]
- Ding, X.; Su, P.; Zhou, Z.; Shi, R.; Yang, J. Responses of plant bud bank characteristics to the enclosure in different desertified Grasslands on the Tibetan Plateau. Plants 2021, 10, 141. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; DeForest, J.L.; Burton, A.J.; Allen, M.F.; Ruess, R.W.; Hendrick, R.L. Fine root architecture of nine North American trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- Jackson, R.B.; Mooney, H.A.; Schulze, E.D. A global budget for fine root biomass, surface area, and nutrient contents. Proc. Natl. Acad. Sci. USA 1997, 94, 7362–7366. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Wang, L.; Jin, D.; Liu, H.; He, J. Alpine climate alters the relationships between leaf and root morphological traits but not chemical traits. Oecologia 2014, 175, 445–455. [Google Scholar] [CrossRef]
- Zhao, G.S.; Liu, M.; Shi, P.L.; Zong, N.; Zhang, X.; Zhang, X.Z. Variation of leaf and root traits and ecological adaptive strategies along a precipitation gradient on Changtang Plateau. Acta Ecol. Sin. 2020, 40, 295–309. (In Chinese) [Google Scholar] [CrossRef]
- Luo, Y.K.; Hu, H.F.; Zhao, M.Y.; Li, H.; Liu, S.S.; Fang, J.Y. Latitudinal pattern and the driving factors of leaf functional traits in 185 shrub species across Eastern China. J. Plant Ecol. 2019, 12, 67–77. [Google Scholar] [CrossRef]
- Zhou, W.; Li, H.B.; Zeng, H. Variations of root traits in three Xizang grassland communities along a precipitation gradient. Chin. J. Plant Ecol. 2018, 42, 1094–1102. (In Chinese) [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Roumet, C.; Birouste, M.; Picon-Cochard, C.; Ghestem, M.; Osman, N.; Vrignon-Brenas, S.; Cao, K.; Stokes, A. Root structure–function relationships in 74 species: Evidence of a root economics spectrum related to carbon economy. New Phytol. 2016, 210, 815–826. [Google Scholar] [CrossRef]
- Sharma, P.; Rathee, S.; Ahmad, M.; Siddiqui, M.H.; Alamri, S.; Kaur, S.; Kohli, R.K.; Singh, H.P.; Batish, D.R. Leaf functional traits and resource use strategies facilitate the spread of invasive plant Parthenium hysterophorus across an elevational gradient in western Himalayas. BMC Plant Biol. 2024, 24, 234. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramos, I.M.; Roumet, C.; Cruz, P.; Blanchard, A.; Autran, P.; Garnier, E. Evidence for a ‘plant community economics spectrum’driven by nutrient and water limitations in a Mediterranean rangeland of southern France. J. Ecol. 2012, 100, 1315–1327. [Google Scholar] [CrossRef]
- Chapin, F.S. The mineral nutrition of wild plants. Annu. Rev. Ecol. Syst. 1980, 11, 233–260. [Google Scholar] [CrossRef]
- Baraloto, C.; Timothy Paine, C.E.; Poorter, L.; Beauchene, J.; Bonal, D.; Domenach, A.; Hérault, B.; Patiño, S.; Roggy, J.; Chave, J. Decoupled leaf and stem economics in rain forest trees. Ecol. Lett. 2010, 13, 1338–1347. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Q.; Zhao, N.; Yu, G.; He, N. Complex trait relationships between leaves and absorptive roots: Coordination in tissue N concentration but divergence in morphology. Ecol. Evol. 2017, 7, 2697–2705. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Oleksyn, J.; Eissenstat, D.M.; Reich, P.B. Fine root decomposition rates do not mirror those of leaf litter among temperate tree species. Oecologia 2010, 162, 505–513. [Google Scholar] [CrossRef]
- Liu, G.; Freschet, G.T.; Pan, X.; Cornelissen, J.H.C.; Li, Y.; Dong, M. Coordinated variation in leaf and root traits across multiple spatial scales in Chinese semi-arid and arid ecosystems. New Phytol. 2010, 188, 543–553. [Google Scholar] [CrossRef]
- Li, F.L.; Bao, W.K. New insights into leaf and fine-root trait relationships: Implications of resource acquisition among 23 xerophytic woody species. Ecol. Evol. 2015, 5, 5344–5351. [Google Scholar] [CrossRef] [PubMed]
- Valverde-Barrantes, O.J.; Smemo, K.A.; Blackwood, C.B. Fine root morphology is phylogenetically structured, but nitrogen is related to the plant economics spectrum in temperate trees. Funct. Ecol. 2015, 29, 796–807. [Google Scholar] [CrossRef]
- Xie, Y.; Bie, Q.; Lu, H.; He, L. Spatio-Temporal Changes of Oases in the Hexi Corridor over the Past 30 Years. Sustainability 2018, 10, 4489. [Google Scholar] [CrossRef]
- Shao, W.; Wang, Q.; Guan, Q.; Zhang, J.; Yang, X.; Liu, Z. Environmental sensitivity assessment of land desertification in the Hexi Corridor, China. Catena 2023, 220, 106728. [Google Scholar] [CrossRef]
- Feng, X.; Qu, J.; Fan, Q.; Tan, L.; An, Z. Characteristics of desertification and short-term effectiveness of differing treatments on shifting sand dune stabilization in an Alpine Rangeland. Int. J. Environ. Res. Public Health 2019, 16, 4968. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, K.; An, Z.; Zhang, Y. Near-surface wind field characteristics of the desert-oasis transition zone in Dunhuang, China. J. Arid. Land 2024, 16, 654–667. [Google Scholar] [CrossRef]
- Sun, F.; Wang, Y.; Chen, Y.; Li, Y.; Zhang, Q.; Qin, J.; Kayumba, P.M. Historic and simulated desert-oasis ecotone changes in the arid Tarim River Basin, China. Remote Sens. 2021, 13, 647. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, J.; Li, X.; Zhu, S.; Liu, H.; Zhan, K. Long-term changes of Tamarix-vegetation in the oasis-desert ecotone and its driving factors: Implication for dryland management. Environ. Earth Sci. 2009, 59, 765–774. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, J.; Wang, H.; Xie, T.; Li, Q.; Shan, L. Fine Root Traits across Different Root Orders and Their Associations with Leaf Traits in 15 Co-Occurring Plant Species from the Desert–Oasis Transition Zone in the Hexi Corridor, Gansu Province, China. Plants 2024, 13, 2472. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cai, Y.; Yang, Q.; Gong, Y.; Lv, G. Factors that alter the relative importance of abiotic and biotic drivers on the fertile island in a desert-oasis ecotone. Sci. Total Environ. 2019, 697, 134096. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tariq, A.; Zeng, F.; Sardans, J.; Peñuelas, J.; Zhang, Z.; Islam, W.; Xu, M. “Fertile islands” beneath three desert vegetation on soil phosphorus fractions, enzymatic activities, and microbial biomass in the desert-oasis transition zone. Catena 2022, 212, 106090. [Google Scholar] [CrossRef]
- Yan, F.; Cong, R.C. Study on classification progress and cataloging system of sandy land in China. Geogr. Res. 2015, 34, 455–465. [Google Scholar] [CrossRef]
- Cheng, J.; Chu, P.; Chen, D.; Bai, Y. Functional correlations between specific leaf area and specific root length along a regional environmental gradient in Inner Mongolia grasslands. Funct. Ecol. 2016, 30, 985–997. [Google Scholar] [CrossRef]
- Margalef, R. Information theory in ecology. Gen. Syst. 1958, 3, 36–71. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. Species-diversity and pattern-diversity in the study of ecological succession. J. Theor. Biol. 1966, 10, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Lepš, J.; Bello, F.; Lavorel, S.; Berman, S. Quantifying and interpreting functional diversity of natural communities: Practical considerations matter. Preslia 2006, 78, 481–501. [Google Scholar]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, C.; Zhang, W. Vegetation coverage, species richness, and dune stability in the southern part of Gurbantünggüt Desert. Ecol. Res. 2011, 26, 79–86. [Google Scholar] [CrossRef]
- Li, X.R.; Zhang, Z.S.; Tan, H.J.; Gao, Y.H.; Liu, L.C.; Wang, X.P. Ecological restoration and recovery in the wind-blown sand hazard areas of northern China: Relationship between soil water and carrying capacity for vegetation in the Tengger Desert. Sci. China Life Sci. 2014, 57, 539–548. [Google Scholar] [CrossRef]
- Liu, M.Z.; Jiang, G.M.; Yu, S.L.; Li, Y.K.; Gao, L.M.; Niu, S.L.; Jiang, C.D.; Peng, Y. Dynamics of plant community traits during an 18-year natural restoration in the degraded sandy grassland of Hunshandak Sandland. Acta Ecol. Sin. 2004, 24, 1734–1740. (In Chinese) [Google Scholar]
- Fan, D.; Zhang, Y.; Qin, S.; Wu, B. Relationships between Artemisia ordosica communities and environmental factors following sand-dune stabilization in the Mu Us desert, northwest China. J. For. Res. 2017, 28, 115–124. [Google Scholar] [CrossRef]
- Xie, X.; Hu, Y.; Pan, X.; Liu, F.; Song, Y.; Dong, M. Biomass allocation of stoloniferous and rhizomatous plant in response to resource availability: A phylogenetic meta-analysis. Front. Plant Sci. 2016, 7, 603. [Google Scholar] [CrossRef] [PubMed]
- Violle, C.; Navas, M.L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Sun, D.; Lei, Z.; Carriquí, M.; Zhang, Y.; Liu, T.; Wang, S.; Song, K.; Zhu, L.; Zhang, W.; Zhang, Y. Reductions in mesophyll conductance under drought stress are influenced by increases in cell wall chelator-soluble pectin content and denser microfibril alignment in cotton. J. Exp. Bot. 2025, 76, 1116–1130. [Google Scholar] [CrossRef]
- Beyer, F.; Hertel, D.; Leuschner, C. Fine root morphological and functional traits in Fagus sylvatica and Fraxinus excelsior saplings as dependent on species, root order and competition. Plant Soil 2013, 373, 143–156. [Google Scholar] [CrossRef]
- Kubisch, P.; Hertel, D.; Leuschner, C. Do ectomycorrhizal and arbuscular mycorrhizal temperate tree species systematically differ in root order-related fine root morphology and biomass? Front. Plant Sci. 2015, 6, 64. [Google Scholar] [CrossRef]
- Yu, W.; Wang, C.; Huang, Z.; Wang, D.; Liu, G. Variations in the traits of fine roots of different orders and their associations with leaf traits in 12 co-occuring plant species in a semiarid inland dune. Plant Soil 2022, 472, 193–206. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.M.; Guo, D.L.; Helmisaari, H.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Fajardo, A.; Siefert, A. Intraspecific trait variation and the leaf economics spectrum across resource gradients and levels of organization. Ecology 2018, 99, 1024–1030. [Google Scholar] [CrossRef]
- Wen, Z.; Zheng, H.; Smith, J.R.; Ouyang, Z. Plant functional diversity mediates indirect effects of land-use intensity on soil water conservation in the dry season of tropical areas. For. Ecol. Manag. 2021, 480, 118646. [Google Scholar] [CrossRef]
- Díaz, S.; Cabido, M. Vive la différence: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Standish, R.J.; Gove, A.D.; Grigg, A.H.; Daws, M.I. Beyond species richness and community composition: Using plant functional diversity to measure restoration success in jarrah forest. Appl. Veg. Sci. 2021, 24, e12607. [Google Scholar] [CrossRef]
- Fukami, T.; Martijn Bezemer, T.; Mortimer, S.R.; van der Putten, W.H. Species divergence and trait convergence in experimental plant community assembly. Ecol. Lett. 2005, 8, 1283–1290. [Google Scholar] [CrossRef]
- Rahman, I.U.; Hart, R.E.; Ijaz, F.; Afzal, A.; Iqbal, Z.; Calixto, E.S.; Abd_Allah, E.F.; Alqarawi, A.A.; Hashem, A.; Al-Arjani, A.F.; et al. Environmental variables drive plant species composition and distribution in the moist temperate forests of Northwestern Himalaya, Pakistan. PLoS ONE 2022, 17, e0260687. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, M.M.; Bonser, S.P.; Morgan, J.W.; McNamara, A.S.; Vesk, P.A. What does species richness tell us about functional trait diversity? Predictions and evidence for responses of species and functional trait diversity to land-use change. Glob. Ecol. Biogeogr. 2010, 19, 423–431. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Peng, Z.; Tian, J.; Song, F.; Wei, G.; Wang, H. Distinct abundance patterns of nitrogen functional microbes in desert soil profiles regulate soil nitrogen storage potential along a desertification development gradient. Catena 2020, 194, 104716. [Google Scholar] [CrossRef]
- Tang, Z.S.; An, H.; Shangguan, Z.P. The impact of desertification on carbon and nitrogen storage in the desert steppe ecosystem. Ecol. Eng. 2015, 84, 92–99. [Google Scholar] [CrossRef]
- Zemunik, G.; Turner, B.L.; Lambers, H.; Laliberté, E. Increasing plant species diversity and extreme species turnover accompany declining soil fertility along a long-term chronosequence in a biodiversity hotspot. J. Ecol. 2016, 104, 792–805. [Google Scholar] [CrossRef]
- Zhou, X.; Feng, W.; He, B.; Qin, S.; Zhang, Y. Isolation and screening of soil phosphate-solubilizing bacteria and their phosphate solubilization mechanisms in the Mu Us Desert. Trans. Chin. Soc. Agric. Eng. 2024, 40, 109–118. (In Chinese) [Google Scholar] [CrossRef]
- Voortman, B.R.; Fujita, Y.; Bartholomeus, R.P.; Aggenbach, C.J.S.; Witte, J.P.M. How the evaporation of dry dune grasslands evolves during the concerted succession of soil and vegetation. Ecohydrology 2017, 10, e1848. [Google Scholar] [CrossRef]
- Wu, X.; Shen, C.; Ma, X.; Hu, L.; He, Y.; Shang, H.; Fu, D. Soil Moisture and Available Phosphorus as the Factors Driving Variation in Functional Characteristics across Different Restoration Communities in a Subtropical Mountain Ecosystem. Biology 2023, 12, 427. [Google Scholar] [CrossRef] [PubMed]
- Schönbeck, L.; Lohbeck, M.; Bongers, F.; Ramos, M.M.; Sterck, F. How do light and water acquisition strategies affect species selection during secondary succession in moist tropical forests? Forests 2015, 6, 2047–2065. [Google Scholar] [CrossRef]
- Conradi, T.; Kollmann, J. Species pools and environmental sorting control different aspects of plant diversity and functional trait composition in recovering grasslands. J. Ecol. 2016, 104, 1314–1325. [Google Scholar] [CrossRef]
- Cooksley, H.; Dreyling, L.; Esler, K.J.; Griebenow, S.; Neumann, G.; Valentine, A.; Schleuning, M.; Schurr, M.F. Functional traits shape plant–plant interactions and recruitment in a hotspot of woody plant diversity. New Phytol. 2024, 241, 1100–1114. [Google Scholar] [CrossRef]
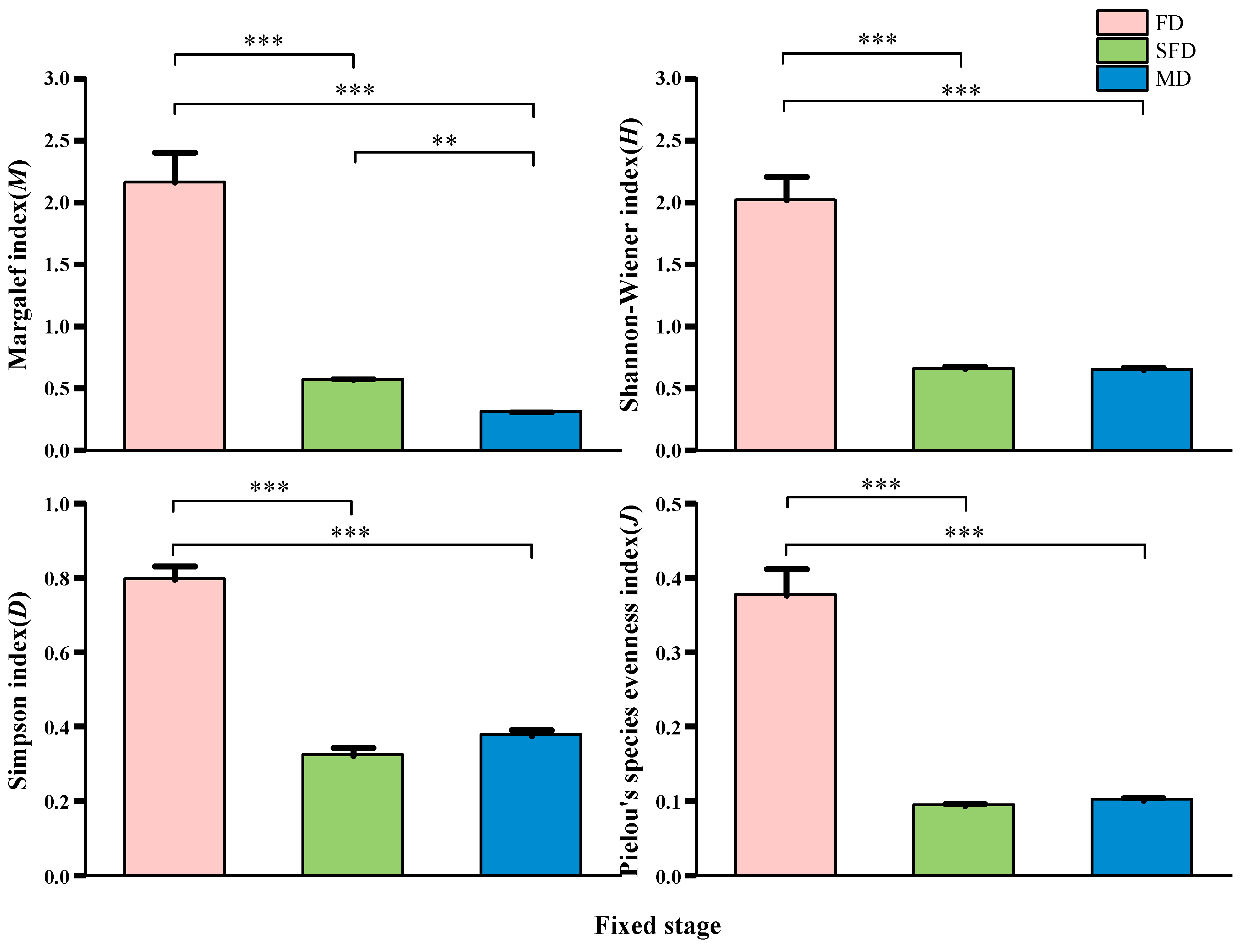
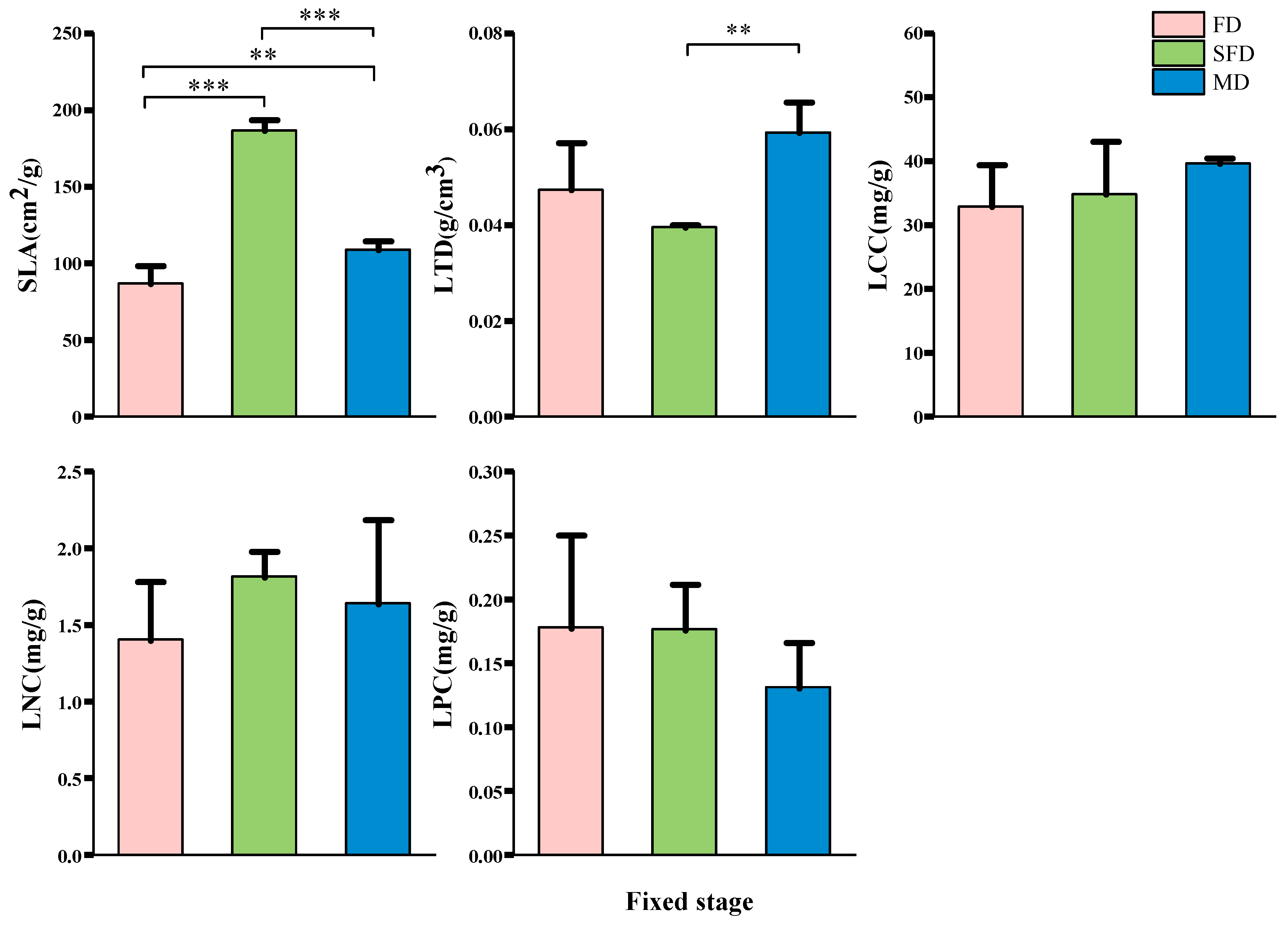
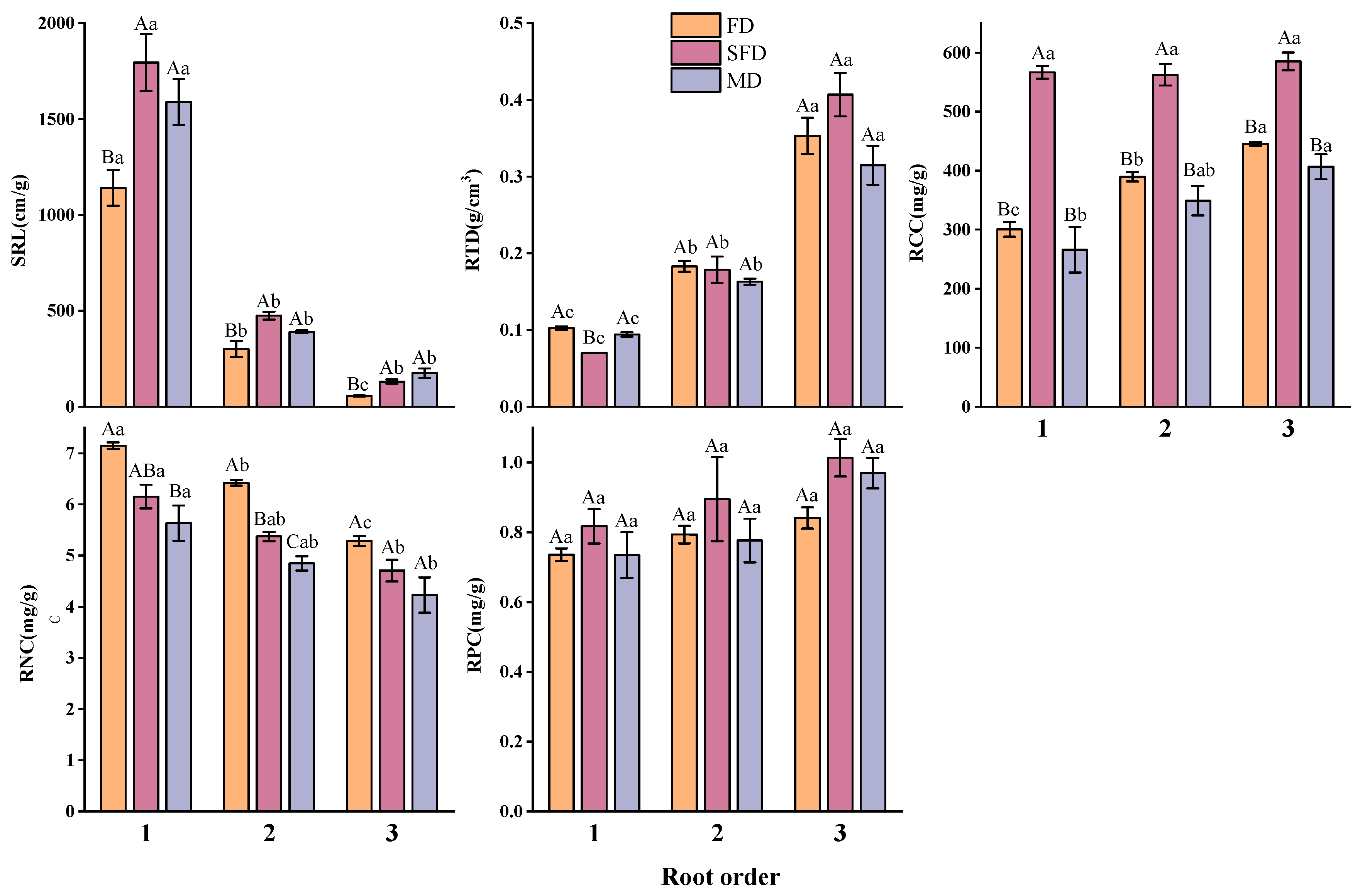
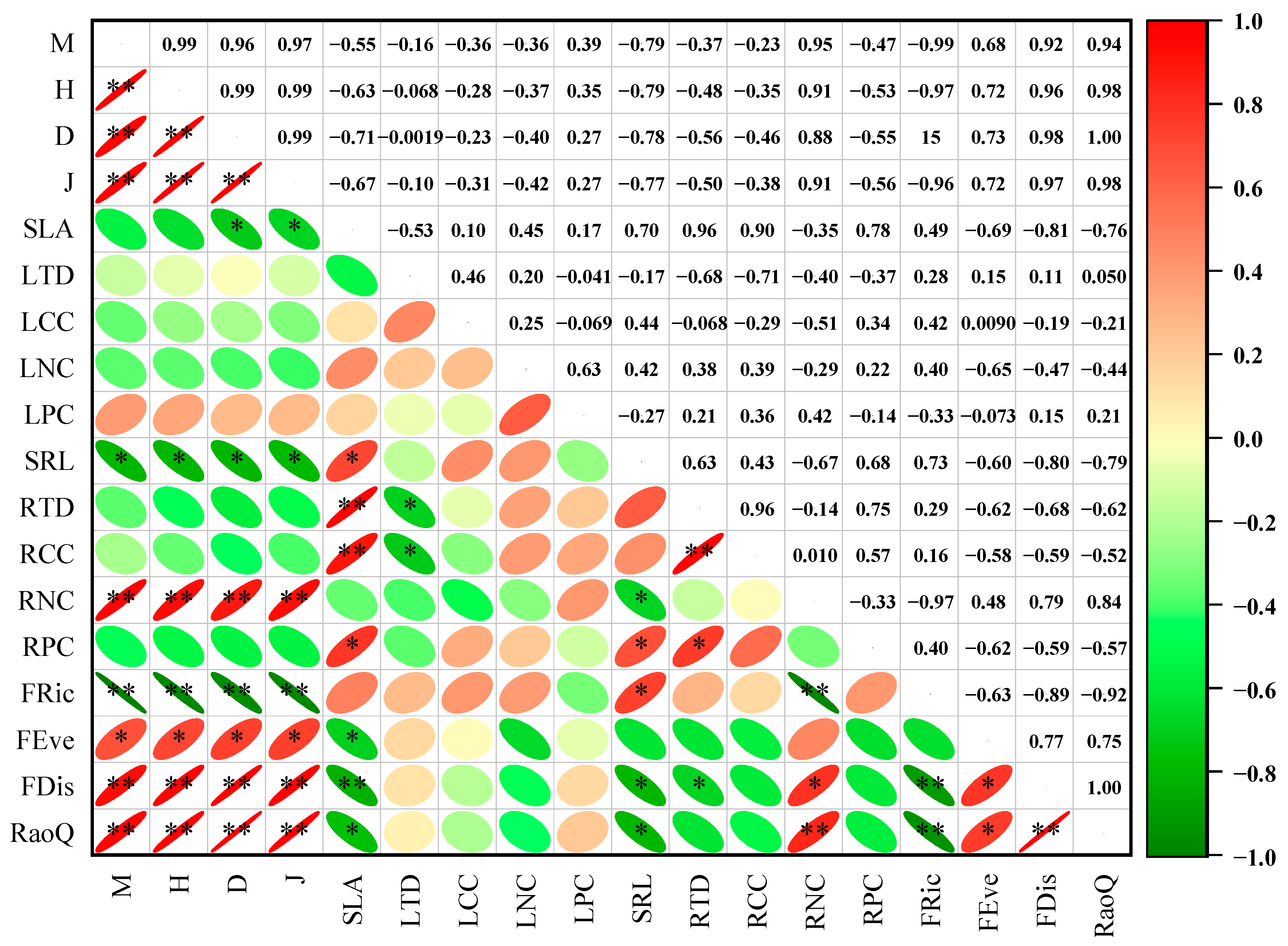
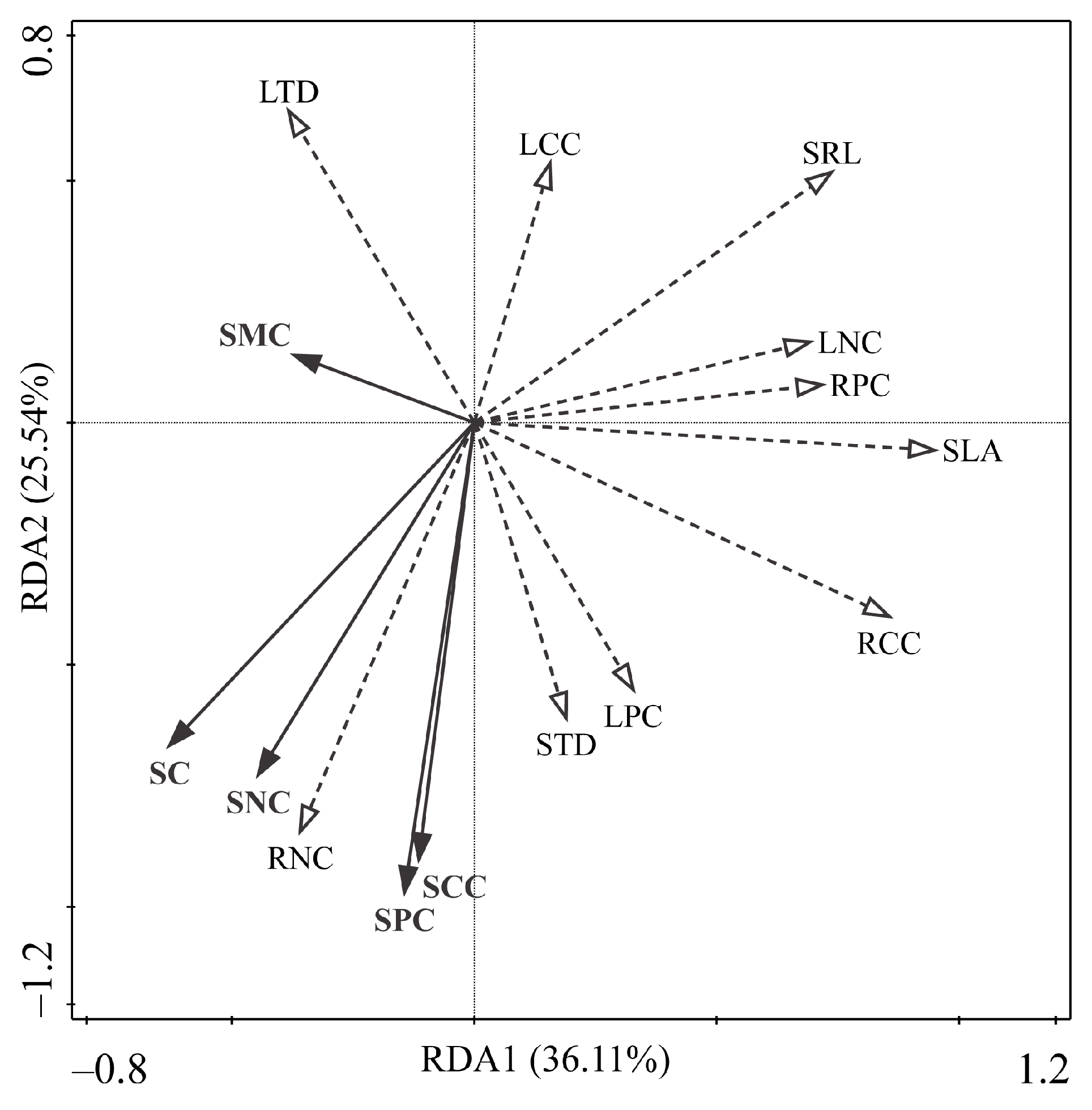

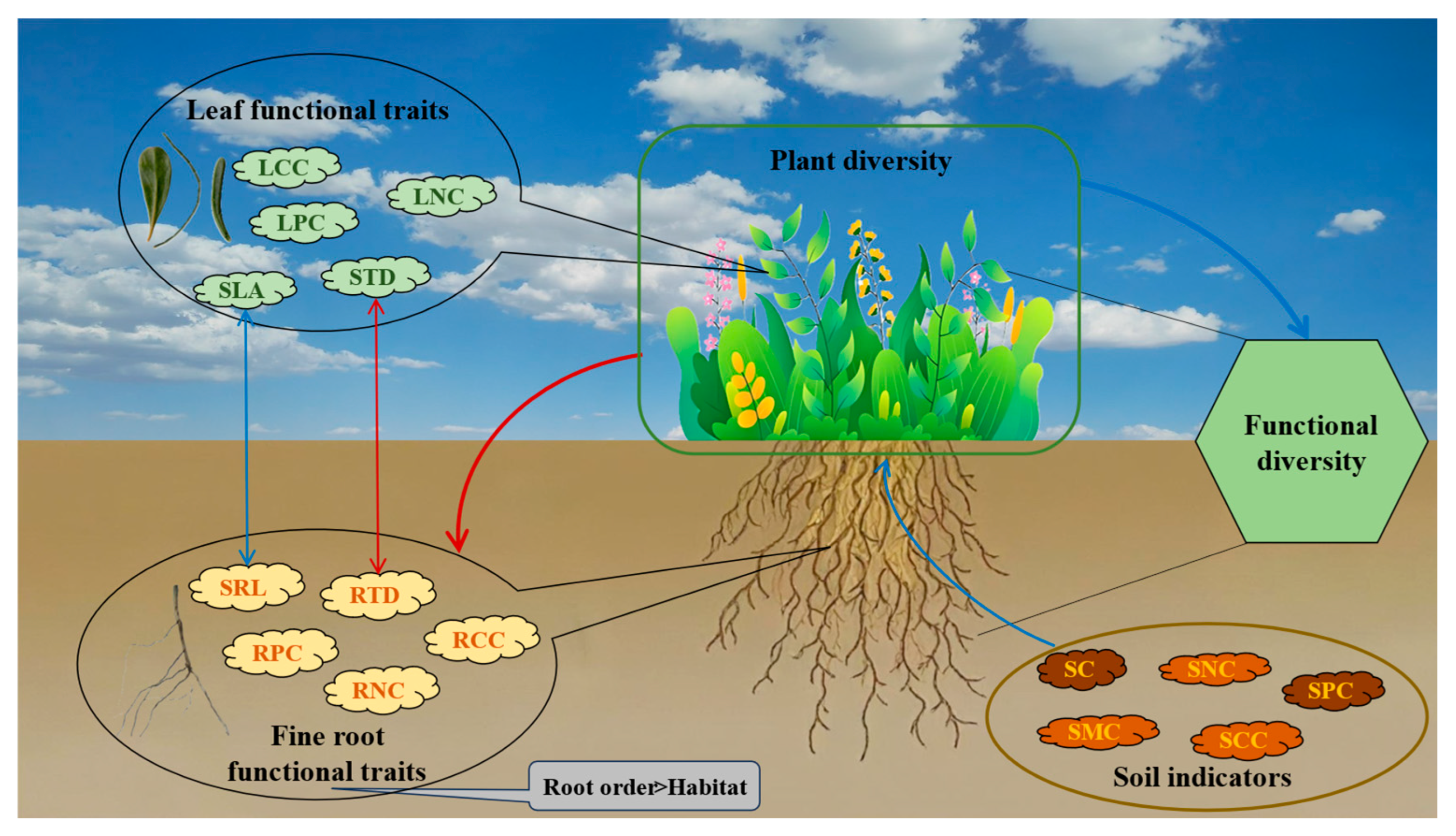
| Soil Indicators | Fixed Dune | Semi-Fixed Dune | Mobile Dune |
|---|---|---|---|
| SC (Pa) | 35.555 ± 1.647 | 17.720 ± 0.903 | 15.952 ± 2.582 |
| SMC (%) | 1.375 ± 0.460 | 2.292 ± 0.591 | 4.012 ± 0.930 |
| SCC (mg/g) | 1.151 ± 0.015 | 0.993 ± 0.017 | 0.757 ± 0.018 |
| SNC (mg/g) | 0.091 ± 0.007 | 0.058 ± 0.003 | 0.046 ± 0.008 |
| SPC (mg/g) | 0.095 ± 0.002 | 0.074 ± 0.004 | 0.043 ± 0.004 |
| Number | Species | Family | Genus | Life Forms |
|---|---|---|---|---|
| 1 | Calligonum arborescens | Polygonaceae | Calligonum | Shrub |
| 2 | Haloxylon ammodendron | Chenopodiaceae | Haloxylon | Shrub |
| 3 | Nitraria sphaerocarpa | Zygophyllaceae | Nitraria | Shrub |
| 4 | Caragana korshinskii | Leguminosae | Caragana | Shrub |
| 5 | Reaumuria songarica | Tamaricaceae | Reaumuria | Shrub |
| 6 | Karelinia caspia | Compositae | Karelinia | Perennial herb |
| 7 | Cynanchum chinense | Asclepiadaceae | Cynanchum | Perennial herb |
| 8 | Salsola passerina | Chenopodiaceae | Salsola | Perennial herb |
| 9 | Inula salsoloides | Compositae | Inula | Perennial herb |
| 10 | Artemisia desertorum | Compositae | Artemisia | Perennial herb |
| 11 | Echinops gmelini | Compositae | Echinops | Annual herb |
| 12 | Suaeda glauca | Chenopodiaceae | Suaeda | Annual herb |
| 13 | Bassia dasyphylla | Chenopodiaceae | Bassia | Annual herb |
| 14 | Halogeton arachnoideus | Chenopodiaceae | Halogeton | Annual herb |
| 15 | Agriophyllum squarrosum | Chenopodiaceae | Agriophyllum | Annual herb |
| Fixed Stage | Dominant Species | Common Species |
|---|---|---|
| Fixed dune | Karelinia caspia (26.83%) Reaumuria songarica (14.48%) Salsola passerina (7.56%) Echinops gmelini (7.05%) Bassia dasyphylla (7.05%) Nitraria sphaerocarpa (6.30%) Artemisia desertorum (5.79%) | Cynanchum chinense (4.79%) Inula salsoloides (4.03%) Halogeton arachnoideus (3.90%) Suaeda glauca (3.65%) Haloxylon ammodendron (3.15%) Caragana korshinskii (2.77%) Calligonum arborescens (2.64%) |
| Semi-fixed dune | Bassia dasyphylla (81.08%) Agriophyllum squarrosum (13.07%) | Nitraria sphaerocarpa (2.89%) Calligonum arborescens (1.51%) Haloxylon ammodendron (1.45%) |
| Mobile dune | Agriophyllum squarrosum (76.00%) Artemisia desertorum (20.09%) | Nitraria sphaerocarpa (3.91%) |
| Functional Diversity | Fixed Dune | Semi-Fixed Dune | MOBILE DUNE |
|---|---|---|---|
| FRic | 0.010 ± 0.008 c | 6.621 ± 0.192 b | 8.514 ± 0.045 a |
| FEve | 0.811 ± 0.024 a | 0.414 ± 0.029 b | 0.579 ± 0.149 ab |
| FDis | 2.887 ± 0.044 a | 1.148 ± 0.082 c | 1.601 ± 0.033 b |
| RaoQ | 8.718 ± 0.294 a | 2.620 ± 0.264 c | 3.743 ± 0.120 b |
| Stepwise Regression Equation | R2 | F | Significant |
|---|---|---|---|
| M = −8.359 + 1.496 RNC + 22.471 LTD | 0.939 | 62.706 | p < 0.001 |
| H = −3.605 + 1.031 RNC − 0.002 RCC | 0.955 | 85.697 | p < 0.001 |
| D = −1.216 + 0.349 RNC − 0.001 RCC + 0.05 LCC | 0.990 | 255.519 | p < 0.001 |
| J = −0.750 + 0.210 RNC − 0.001 RCC | 0.976 | 163.659 | p < 0.001 |
| FRic = 36.474 − 6.145 RNC + 0.006 RCC | 0.968 | 120.947 | p < 0.001 |
| FEve = 1.018 − 0.003 SLA | 0.400 | 6.327 | p < 0.05 |
| FDis = −3.932 − 0.010 SLA + 0.902 RNC + 0.031 LCC | 0.987 | 206.769 | p < 0.001 |
| RaoQ = −10.542 + 3.888 RNC − 0.014 RCC | 0.976 | 160.516 | p < 0.001 |
| Parameters | Explains/% | Contribution/% | F | p |
|---|---|---|---|---|
| SC | 25.8 | 36.4 | 2.4 | 0.034 |
| SMC | 4.0 | 5.7 | 0.4 | 0.724 |
| SCC | 6.0 | 8.5 | 0.8 | 0.616 |
| SNC | 6.9 | 9.6 | 0.8 | 0.54 |
| SPC | 28.3 | 39.8 | 3.7 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Z.; Xie, T.; Shan, L.; Wang, H.; Ma, J.; Yue, Y.; Yuan, M.; Li, Q.; He, C.; Zhao, Y. Soil-Driven Coupling of Plant Community Functional Traits and Diversity in Desert–Oasis Transition Zone. Plants 2025, 14, 1997. https://doi.org/10.3390/plants14131997
Fan Z, Xie T, Shan L, Wang H, Ma J, Yue Y, Yuan M, Li Q, He C, Zhao Y. Soil-Driven Coupling of Plant Community Functional Traits and Diversity in Desert–Oasis Transition Zone. Plants. 2025; 14(13):1997. https://doi.org/10.3390/plants14131997
Chicago/Turabian StyleFan, Zhuopeng, Tingting Xie, Lishan Shan, Hongyong Wang, Jing Ma, Yuanzhi Yue, Meng Yuan, Quangang Li, Cai He, and Yonghua Zhao. 2025. "Soil-Driven Coupling of Plant Community Functional Traits and Diversity in Desert–Oasis Transition Zone" Plants 14, no. 13: 1997. https://doi.org/10.3390/plants14131997
APA StyleFan, Z., Xie, T., Shan, L., Wang, H., Ma, J., Yue, Y., Yuan, M., Li, Q., He, C., & Zhao, Y. (2025). Soil-Driven Coupling of Plant Community Functional Traits and Diversity in Desert–Oasis Transition Zone. Plants, 14(13), 1997. https://doi.org/10.3390/plants14131997





