Widely Targeted Metabolomics Reveals Metabolic Divergence in Abutilon theophrasti Populations Under Glufosinate Ammonium Treatment
Abstract
1. Introduction
2. Results
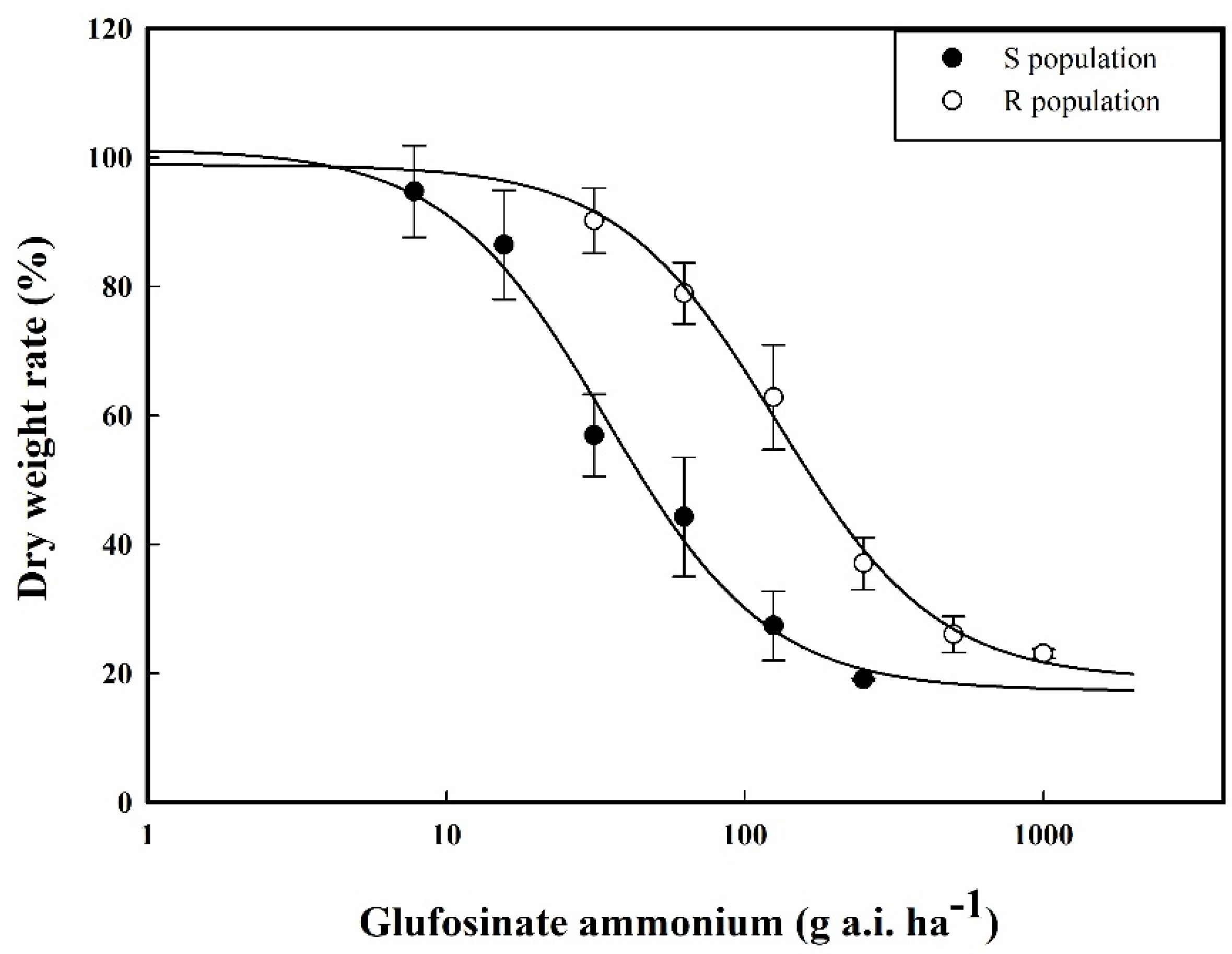
2.1. Results of the Whole-Plant Bioassay
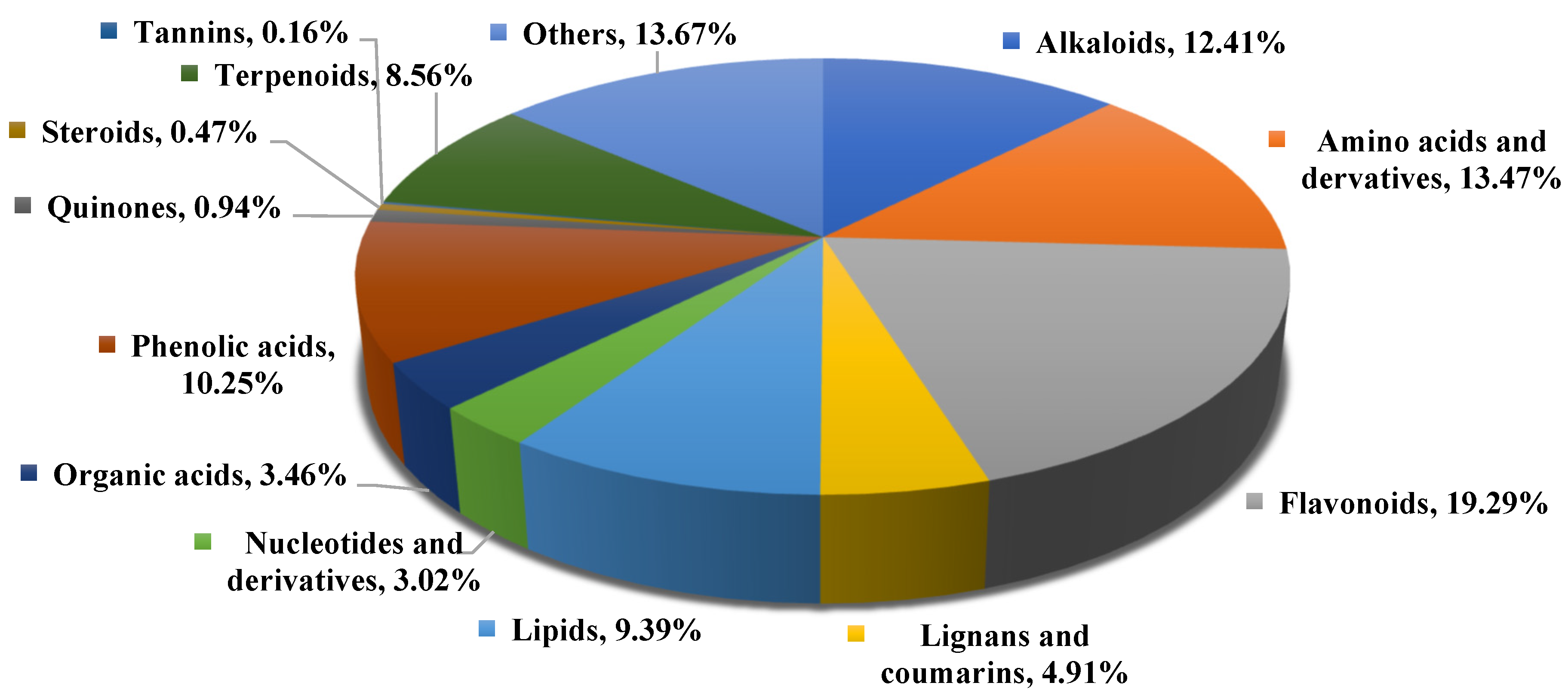
2.2. Overview of the Nonvolatile Metabolites of Abutilon theophrasti
2.3. Metabolite Qualitative–Quantitative Analysis and Sample Quality Control
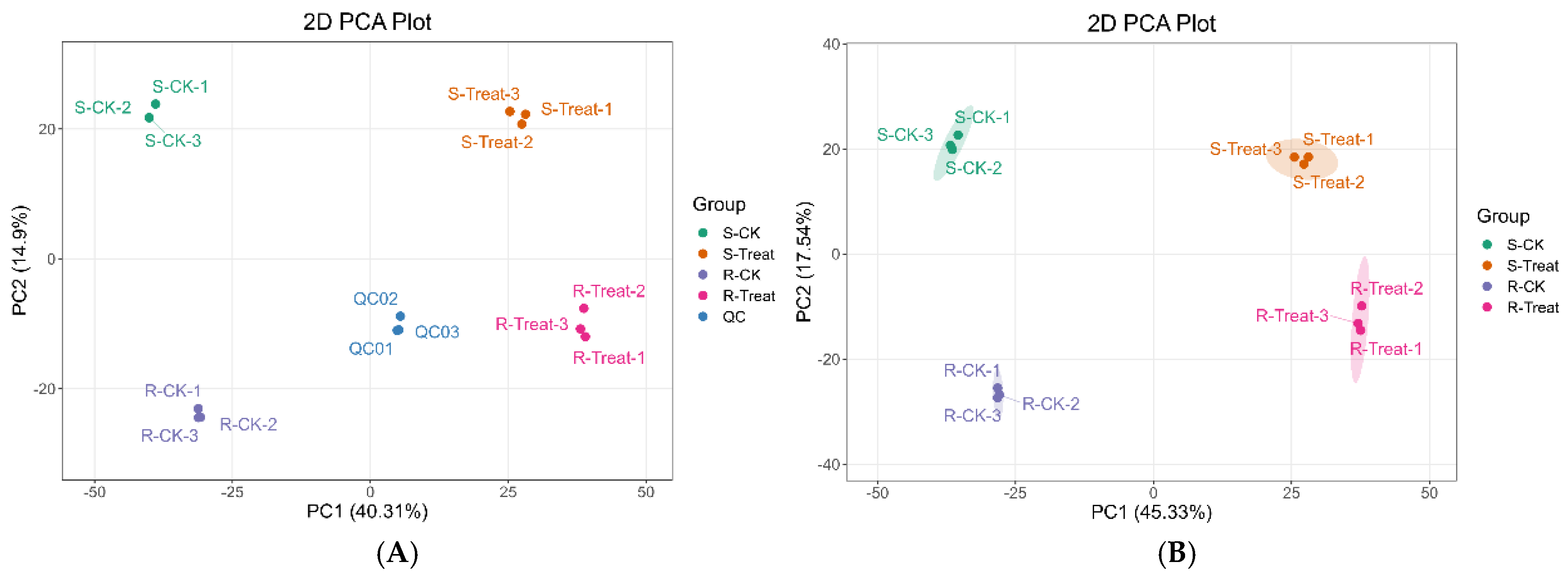
2.4. Principal Component Analysis (PCA) and Grouped Principal Component Analysis
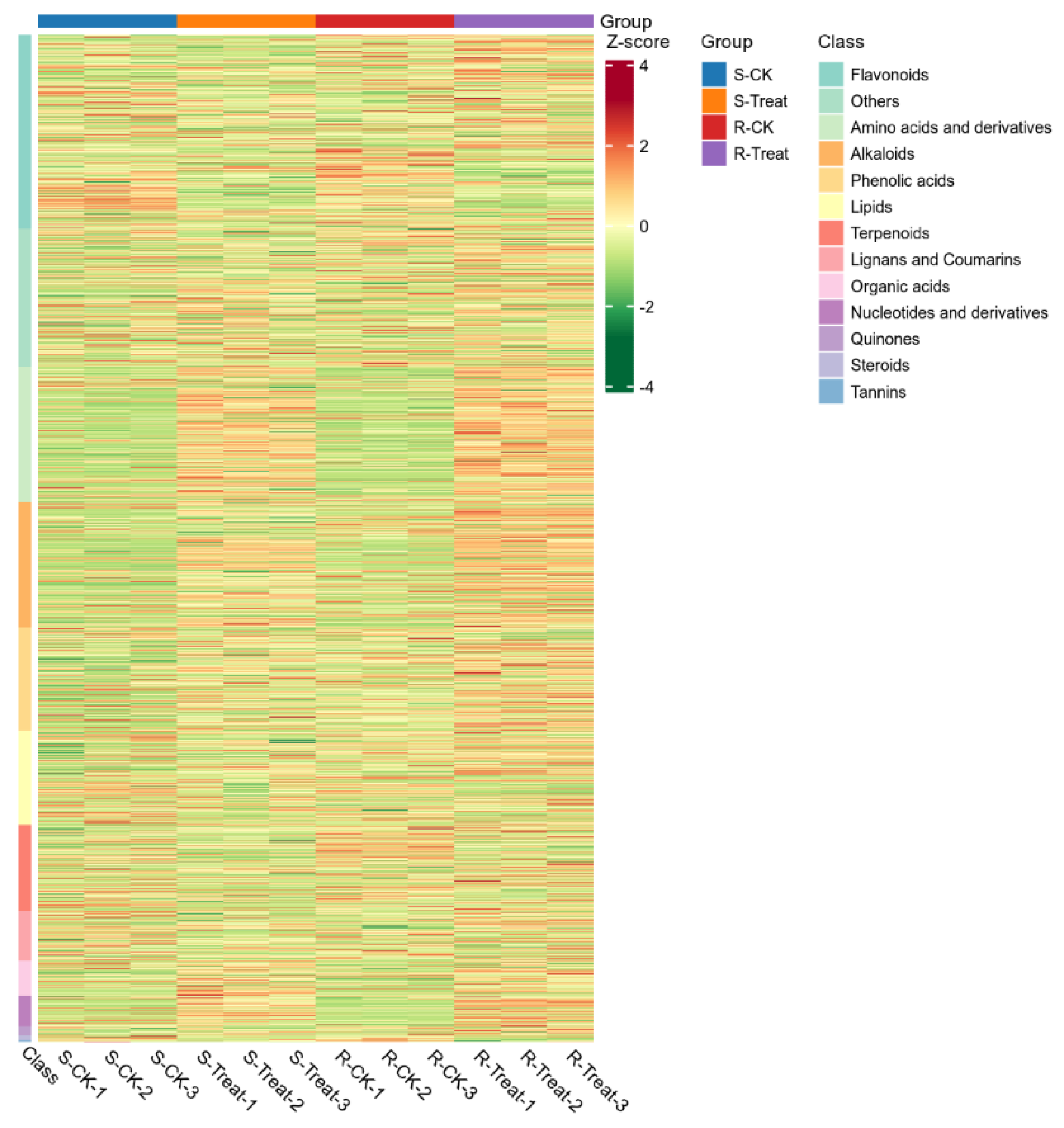
2.5. Cluster Analysis
2.6. Orthogonal Partial Least Squares-Discriminant Analysis (OPLS-DA) and Model Validation
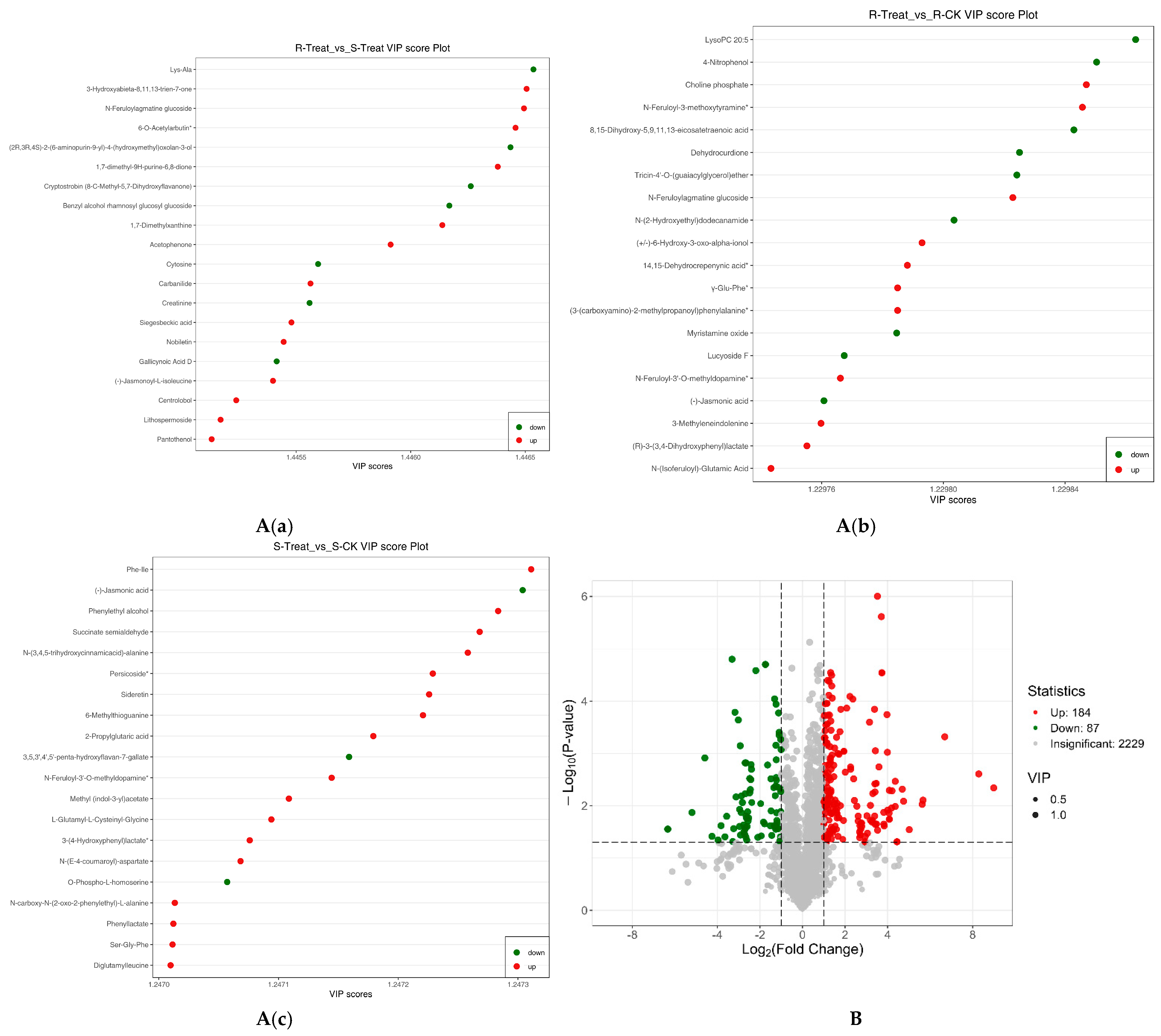
2.7. VIP Plot, and Volcano Plot of Differential Metabolites
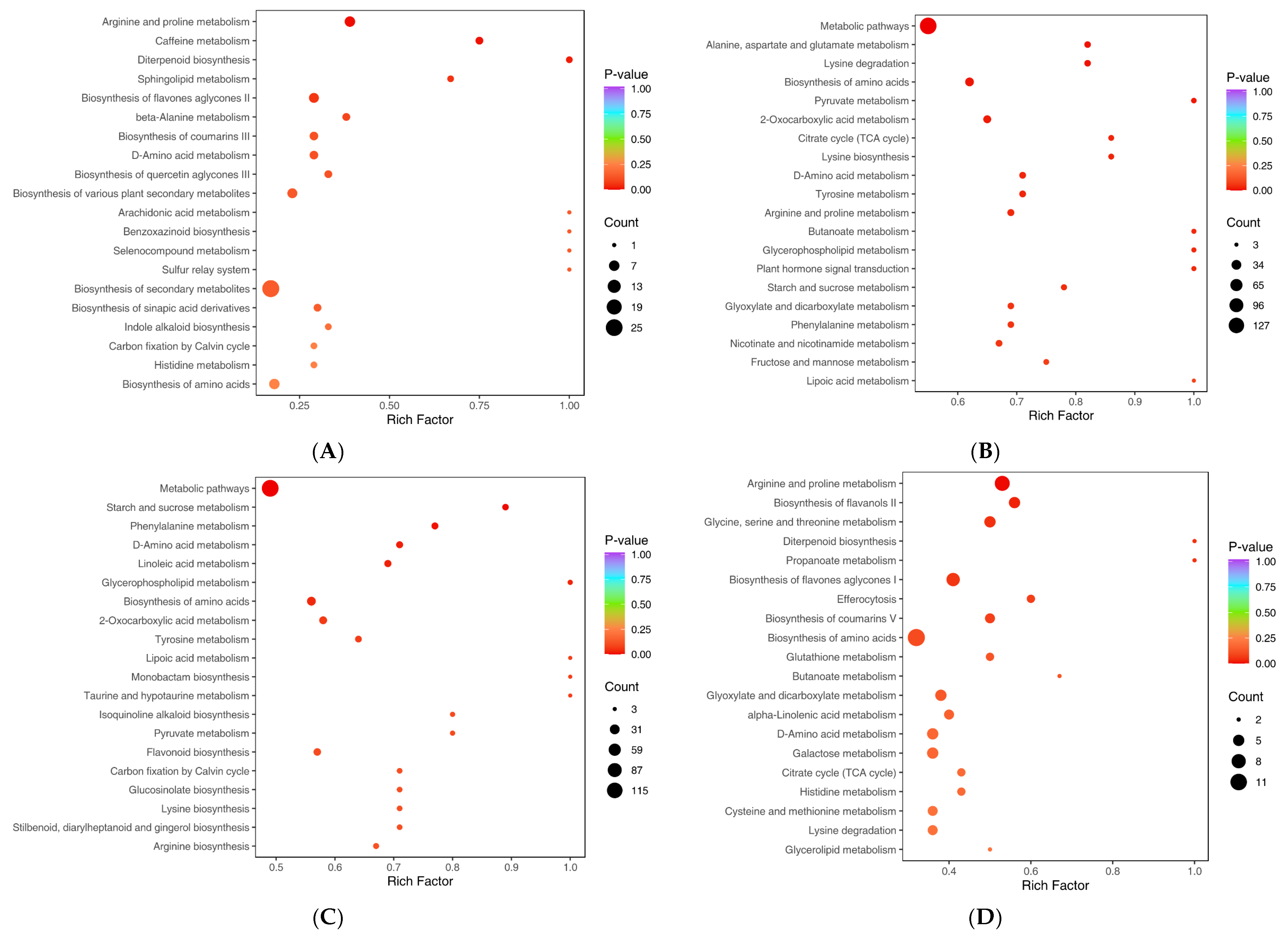
2.8. Metabolite Annotation and Metabolic Pathway Enrichment Analysis Result
3. Discussion
4. Materials and Methods
4.1. Metabolite Annotation and Metabolic Pathway Enrichment Analysis
Plant Materials and Herbicides
4.2. The Whole-Plant Bioassay
4.2.1. Plant Cultivation and Treatment
4.2.2. Data Analysis
4.2.3. Sample Collection
4.3. Sample Preparation and Extraction
4.3.1. Dry Sample Extraction
4.3.2. UPLC Conditions
4.3.3. ESI-Q TRAP-MS/MS
4.4. Data Analysis
4.4.1. Principal Component Analysis
4.4.2. Hierarchical Cluster Analysis and Pearson’s Correlation Coefficients
4.4.3. Differential Metabolites Selected
4.4.4. KEGG Annotation and Enrichment Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mcdonald, A.J.; Riha, S.J.; Mohler, C.L. Mining the record: Historical evidence for climatic influences on maize–Abutilon theophrasti competition. Weed Res. 2010, 44, 439–445. [Google Scholar] [CrossRef]
- Ziska, L.; Clements, D. Observed changes in soyabean growth and seed yield from Abutilon theophrasti competition as a function of carbon dioxide concentration. Weed Res. 2013, 53, 140–145. [Google Scholar] [CrossRef]
- Andersen, L.R.N. Longevity of velvetleaf (Abutilon theophrasti) seeds in soil under agricultural practices. Weed Sci. 1980, 28, 341–346. [Google Scholar] [CrossRef]
- Egea-Cobrero, V.; Bradley, K.; Calha, I.M.; Davis, A.S.; Dorado, J.; Forcella, F.; Lindquist, J.L.; Sprague, C.L.; Gonzalez-Andujar, J.L. Validation of predictive empirical weed emergence models of Abutilon theophrasti Medik based on intercontinental data. Weed Res. 2020, 60, 297–302. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: www.weedscience.org (accessed on 23 June 2025).
- Vencill, W.K.; Nichols, R.L.; Webster, T.M.; Soteres, J.K.; Mallory-Smith, C.; Burgos, N.R.; Johnson, W.G.; Mcclelland, M.R. Herbicide Resistance: Toward an understanding of resistance development and the impact of herbicide-resistant Crops. Weed Sci. 2012, 60, 2–30. [Google Scholar] [CrossRef]
- Avilaneto, R.; Ulguim, A.R.; Schneider, T.; Fortuna, C.; Holkem, A.S.; Somavilla, I.P. Interaction between saflufenacil and ammonium glufosinate to control ryegrass. Braz. J. Biol. 2025, 84, e286456. [Google Scholar] [CrossRef]
- Takano, H.K.; Dayan, F.E. Glufosinate-ammonium: A review of the current state of knowledge. Pest Manag. Sci. 2020, 76, 3911–3925. [Google Scholar] [CrossRef]
- Leason, M.; Cunliffe, D.; Parkin, D.; Lea, P.J.; Miflin, B.J. Inhibition of pea leaf glutamine synthetase by methionine sulphoximine, phosphinothricin and other glutamate analogues. Phytochemistry 2012, 21, 855–857. [Google Scholar] [CrossRef]
- Takano, H.K.; Beffa, R.; Preston, C.; Westra, P.; Dayan, F.E. Reactive oxygen species trigger the fast action of glufosinate. Planta 2019, 249, 1837–1849. [Google Scholar] [CrossRef]
- Davis, B.; Scott, R.C.; Norsworthy, J.K.; Gbur, E. Response of wheat (Tritium aestivum) to low rates of glyphosate and glufosinate. Crop Prot. 2013, 54, 181–184. [Google Scholar] [CrossRef]
- Shin, J.S.; Kim, K.M.; Lee, D.J.; Lee, S.B.; Burgos, N.R.; Kuk, Y.I. Resistance levels and fitness of glufosinate-resistant transgenic sweet potato in field experiments. Field Crops Res. 2011, 121, 324–332. [Google Scholar] [CrossRef]
- Meyer, C.J.; Peter, F.; Norsworthy, J.K.; Beffa, R. Uptake, translocation, and metabolism of glyphosate, glufosinate, and dicamba mixtures in Echinochloa crus-galli and Amaranthus palmeri. Pest Manag. Sci. 2020, 76, 3078–3087. [Google Scholar] [CrossRef] [PubMed]
- Brunharo, C.A.; Takano, H.K.; Mallory-Smith, C.A.; Dayan, F.E.; Hanson, B.D. Role of glutamine synthetase isogenes and herbicide metabolism in the mechanism of resistance to glufosinate in Lolium perenne L. spp. multiflorum biotypes from oregon. J. Agric. Food Chem. 2019, 67, 8431–8440. [Google Scholar] [CrossRef] [PubMed]
- Jugulam, S. Non-target-site resistance to herbicides: Recent developments. Plants 2019, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Annu. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef]
- Yu, Q.; Han, H.; Vila-Aiub, M.M.; Powles, S.B. AHAS herbicide resistance endowing mutations: Effect on AHAS functionality and plant growth. J. Exp. Bot. 2010, 61, 3925–3934. [Google Scholar] [CrossRef]
- Lee, L.J.; Ngim, J. A first report of glyphosate-resistant goosegrass (Eleusine indica (L) Gaertn) in Malaysia. Pest Manag. Sci. 2015, 56, 336–339. [Google Scholar] [CrossRef]
- Moretti, M.L.; Hanson, B.D. Reduced translocation is involved in resistance to glyphosate and paraquat in Conyza bonariensis and Conyza canadensis from California. Weed Res. 2017, 57, 25–34. [Google Scholar] [CrossRef]
- Serim, A.T.; Kaymak, S. The impact of glyphosate isopropylamine salt and glufosinate-ammonium on ssome soil pathogens causing disease in wheat. Proc. Bulg. Acad. Sci. 2023, 76, 1781–1788. [Google Scholar] [CrossRef]
- Lajmanovich, R.C.; Cabagna-Zenklusen, M.C.; Attademo, A.M.; Junges, C.M.; Peltzer, P.M.; Bassó, A.; Lorenzatti, E. Induction of micronuclei and nuclear abnormalities in tadpoles of the common toad (Rhinella arenarum) treated with the herbicides Liberty and glufosinate-ammonium. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2014, 769, 7–12. [Google Scholar] [CrossRef]
- Tan, B.; Tan, X.; Liu, C.; Zeng, Y.; Li, Y. Effects of lead stress on rice (Oryza sativa L.) growth and metabolism in the rhizosphere microenvironment: The role of eicosanoid compounds. Plant Growth Regul. 2022, 96, 483–495. [Google Scholar] [CrossRef]
- Fiehn, O. Combining Genomics, Metabolome analysis, and biochemical modelling to understand metabolic networks. Comp. Funct. Genom. 2001, 2, 155–168. [Google Scholar] [CrossRef]
- Jang, C.; Chen, L.; Rabinowitz, J.D. Metabolomics and isotope tracing. Cell 2018, 173, 822–837. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafez, O.H.; Othman, E.M.; Fahim, J.R.; Desoukey, S.Y.; Abdelmohsen, U.R. Metabolomics analysis and biological investigation of three Malvaceae plants. Phytochem. Anal. 2019, 31, 204–214. [Google Scholar] [CrossRef]
- André, K.; Aino, K. Plant secondary metabolite diversity and species interactions. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 115–138. [Google Scholar] [CrossRef]
- Fang, C.; Fernie, A.R.; Luo, J. Exploring the diversity of plant metabolism. Trends Plant Sci. 2019, 24, 16. [Google Scholar] [CrossRef]
- Luo, P.; Yin, P.; Hua, R.; Tan, Y.; Li, Z.; Qiu, G.; Yin, Z.; Xie, X.; Wang, X.; Chen, W. A Large-scale, multicenter serum metabolite biomarker identification study for the early detection of hepatocellular carcinoma. Hepatology 2018, 67, 662–675. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, R.; Song, Y.; He, J.; Sun, J.; Bai, J.; An, Z.; Dong, L.; Zhan, Q.; Abliz, Z. RRLC-MS/MS-based metabonomics combined with in-depth analysis of metabolic correlation network: Finding potential biomarkers for breast cancer. Analyst 2009, 134, 2003–2011. [Google Scholar] [CrossRef]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Kolukisaoglu, N. D-amino Acids in Plants: Sources, Metabolism, and Functions. Int. J. Mol. Sci. 2020, 21, 5421. [Google Scholar] [CrossRef] [PubMed]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.-P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef]
- Oihane, I.; Josy, T.B.; Laura, A.; André, M.; Remy, C.; Athanasios, T.; Savitski, M.M.; Victor, S.; Ronnie, P.-A.B.; Felipe, C. D-amino acids signal a stress-dependent run-away response in Vibrio cholerae. Nat. Microbiol. 2023, 8, 1549–1560. [Google Scholar] [CrossRef]
- Lam, H.; Oh, D.-C.; Cava, F.; Takacs, C.N.; Clardy, J.; de Pedro, M.A.; Waldor, M.K. D-Amino Acids Govern Stationary Phase Cell Wall Remodeling in Bacteria. Science 2009, 325, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Suárez Villada, J.S. D-Amino Acid Transport, Metabolism, and Its Link to Ethylene Regulation in Arabidopsis thaliana; Universität Tübingen: Tübingen, Germany, 2020. [Google Scholar]
- Yu, Q.; Powles, S.B. Resistance to AHAS inhibitor herbicides: Current understanding. Pest Manag. Sci. 2014, 70, 1340–1350. [Google Scholar] [CrossRef]
- Takano, H.K.; Beffa, R.; Preston, C.; Westra, P.; Dayan, F.E. A novel insight into the mode of action of glufosinate: How reactive oxygen species are formed. Photosynth. Res. 2020, 144, 361–372. [Google Scholar] [CrossRef]
- Lea, P.J.; Miflin, B.J. Glutamate synthase and the synthesis of glutamate in plants. Plant Physiol. Biochem. 2003, 41, 555–564. [Google Scholar] [CrossRef]
- Luo, Q.; Fu, H.; Hu, F.; Li, S.; Chen, Q.; Peng, S.; Yang, C.; Liu, Y.; Chen, Y. Effects of Biological Nitrogen Metabolism on Glufosinate-Susceptible and-Resistant Goosegrass (Eleusine indica L.). Int. J. Mol. Sci. 2023, 24, 13791. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Mallick, S. Chapter 35-Ameliorative Mechanisms of Polyamines Against Abiotic Stress in the Rice Plants. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J.K., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 725–735. [Google Scholar]
- Délye, C. Unravelling the genetic bases of non-target-site-based resistance (NTSR) to herbicides: A major challenge for weed science in the forthcoming decade. Pest Manag. Sci. 2013, 69, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Guo, Y.; Wang, Y.; Luo, C.; Cong, K. The effects of long-term application of fomesafen on weed seedbank and resistance levels of Amaranthus retroflexus L. Front. Plant Sci. 2024, 15, 14. [Google Scholar] [CrossRef]
| Populations | Glufosinate Ammonium (g a.i. ha−1) |
|---|---|
| S | 0, 23.4375, 46.875, 93.75, 187.5, 375, 750 * |
| SR | 0, 93.75, 187.5, 375, 750 *, 1500, 3000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Wang, Y.; Guo, Y.; Luo, C.; Cong, K. Widely Targeted Metabolomics Reveals Metabolic Divergence in Abutilon theophrasti Populations Under Glufosinate Ammonium Treatment. Plants 2025, 14, 1994. https://doi.org/10.3390/plants14131994
Guo X, Wang Y, Guo Y, Luo C, Cong K. Widely Targeted Metabolomics Reveals Metabolic Divergence in Abutilon theophrasti Populations Under Glufosinate Ammonium Treatment. Plants. 2025; 14(13):1994. https://doi.org/10.3390/plants14131994
Chicago/Turabian StyleGuo, Xiaotong, Yu Wang, Yulian Guo, Chan Luo, and Keqiang Cong. 2025. "Widely Targeted Metabolomics Reveals Metabolic Divergence in Abutilon theophrasti Populations Under Glufosinate Ammonium Treatment" Plants 14, no. 13: 1994. https://doi.org/10.3390/plants14131994
APA StyleGuo, X., Wang, Y., Guo, Y., Luo, C., & Cong, K. (2025). Widely Targeted Metabolomics Reveals Metabolic Divergence in Abutilon theophrasti Populations Under Glufosinate Ammonium Treatment. Plants, 14(13), 1994. https://doi.org/10.3390/plants14131994




