Pollen Quantitative and Genetic Competitiveness of Rice (Oryza sativa L.) and Their Effects on Gene Flow
Abstract
1. Introduction
2. Results
2.1. Quantification of Pollen’s Quantitative and Genetic Competitiveness
2.2. Optimization of the Rice Gene Flow Model
2.3. Sensitivity Analysis of Rice Pollen Competitiveness
3. Discussion
3.1. Quantitative Pollen Competitiveness in Rice
3.2. Genetic Competitiveness of Rice Pollen
3.3. Self-Pollination and Cross-Pollination
4. Material and Methods
4.1. Data Sources
4.1.1. Dual-Donor Experiment
4.1.2. Gene Flow Experiments
4.1.3. Detection of Gene Flow Rate
4.2. Rice Gene Flow Model
4.2.1. Simulating Pollen Diffusion
4.2.2. Simulating Gene Flow
4.2.3. Model Validation
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. World Food and Agriculture-Statistical Yearbook; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Du, X.X.; Piao, Z.; Kim, K.M.; Lee, G.S. Gene flow from transgenic rice to conventional rice in China. Plant Breed. Biotech. 2021, 9, 259–271. [Google Scholar] [CrossRef]
- Kumar, K.; GGambhir ADass Tripathi, A.K.; Singh, A.; Jha, A.K.; Yadava, P.; Choudhary, M.; Rakshit, S. Genetically modified crops: Current status and future prospects. Planta 2020, 251, 91. [Google Scholar] [CrossRef]
- Lu, B.; Fu, Q.; Shen, Z. Commercialization of transgenic rice in China: Potential environmental biosafety issues. Biodivers. Sci. 2008, 16, 426. [Google Scholar]
- Lu, B.R. Challenges of transgenic crop commercialization in China. Nat. Plants 2016, 2, 16077. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, Y.; Hallerman, E.M.; Wu, K. Biosafety management and commercial use of genetically modified crops in China. Plant Cell Rep. 2014, 33, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Yuan, Q.; Pei, X.; Wang, F.; Hu, N.; Yao, K.; Wang, Z. Rice transgene flow: Its patterns, model and risk management. Plant Biotech. J. 2014, 12, 1259–1270. [Google Scholar] [CrossRef]
- Messeguer, J.; Marfà, V.; Català, M.M.; Guiderdoni, E.; Melé, E. A field study of pollen-mediated gene flow from Mediterranean GM rice to conventional rice and the red rice weed. Mol. Breed. 2004, 13, 103–112. [Google Scholar] [CrossRef]
- Rong, J.; Song, Z.; Su, J.; Xia, H.; Lu, B.R.; Wang, F. Low frequency of GM pollen from Bt/CpTI rice to its nontransgenic counterparts planted at close spacing. New Phytol. 2005, 168, 559–566. [Google Scholar] [CrossRef]
- Rong, J.; Lu, B.R.; Song, Z.; Su, J.; Snow, A.A.; Zhang, X.; Sun, S.; Chen, R.; Wang, F. Dramatic reduction of crop-to-crop gene flow within a short distance from transgenic rice fields. New Phytol. 2006, 173, 346–353. [Google Scholar] [CrossRef]
- Bae, H.K.; Oo, M.M.; Jeon, J.E.; Nguyen, T.D.; Oh, S.A.; Oh, S.D.; Kweon, S.J.; Eun, M.Y.; Park, S.K. Evaluation of gene flow from GM to Non-GM rice. Plant Breed. Biotech. 2013, 1, 162–170. [Google Scholar] [CrossRef]
- Han, S.M.; Lee, B.; Won, O.J.; Hwang, K.S.; Suh, S.J.; Kim, C.G.; Park, K.W. Gene flow from herbicide resistant genetically modified rice to conventional rice (Oryza sativa L.) cultivars. J. Ecol. Environ. 2015, 38, 397–403. [Google Scholar] [CrossRef][Green Version]
- Song, Z.P.; Lu, B.R.; Zhu, Y.G.; Chen, J.K. Gene flow from cultivated rice to the wild species Oryza rufipogonunder experimental field conditions. New Phytol. 2003, 157, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Lee, D.S.; Song, Z.P.; Suh, H.S.; Lu, B.R. Gene flow from cultivated rice (Oryza sativa) to its weedy and wild relatives. Ann. Bot. 2004, 93, 67–73. [Google Scholar] [CrossRef]
- Lu, B.R.; Yang, C. Gene flow from genetically modified rice to its wild relatives: Assessing potential ecological consequences. Biotechnol. Adv. 2009, 27, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Tovar, E.; Villafañe, C.; Bocanegra, J.L.; Moreno, R. Distribution, genetic diversity and potential spatiotemporal scale of alien gene flow in crop wild relatives of rice (Oryza spp.) in Colombia. Rice 2017, 10, 13. [Google Scholar] [CrossRef]
- Yao, K.; Hu, N.; Chen, W.; Li, R.; Yuan, Q.; Wang, F.; Qian, Q.; Jia, S. Establishment of a rice transgene flow model for predicting maximum distances of gene flow in southern China. New Phytol. 2008, 180, 217–228. [Google Scholar] [CrossRef]
- Beckie, H.J.; Hall, L.M. Simple to complex: Modelling crop pollen-mediated gene flow. Plant Sci. 2008, 175, 615–628. [Google Scholar] [CrossRef]
- Rong, J.; Song, Z.; de Jong, T.J.; Zhang, X.; Sun, S.; Xu, X.; Xia, H.; Liu, B.; Lu, B.R. Modelling pollen-mediated gene flow in rice: Risk assessment and management of transgene escape. Plant Biotech. J. 2010, 8, 452–464. [Google Scholar] [CrossRef]
- Wang, L.; Haccou, P.; Lu, B.R. High-resolution gene flow model for assessing environmental impacts of transgene escape based on biological parameters and wind speed. PLoS ONE 2016, 11, e0149563. [Google Scholar] [CrossRef]
- Song, Z.; Lu, B.; Zhu, Y.; Chen, J. Pollen competition between cultivated and wild rice species (Oryza sativa and O. rufipogon). New Phytol. 2002, 153, 289–296. [Google Scholar] [CrossRef]
- Hu, N.; Hu, J.C.; Jiang, X.D.; Xiao, W.; Yao, K.M.; Li, L.; Li, X.H.; Pei, X.W. Application of the maximum threshold distances to reduce gene flow frequency in the coexistence between genetically modified (GM) and non-GM maize. Evol. Appl. 2022, 15, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Shivrain, V.K.; Burgos, N.R.; Gealy, D.R.; Moldenhauer, K.A.K.; Baquireza, C.J. Maximum outcrossing rate and genetic compatibility between red rice (Oryza sativa) biotypes and Clearfield™ rice. Weed Sci. 2008, 56, 807–813. [Google Scholar] [CrossRef]
- Zhu, X.; Gou, Y.; Heng, Y.; Ding, W.; Li, Y.; Zhou, D.; Li, X.; Liang, C.; Wu, C.; Wang, H.; et al. Targeted manipulation of grain shape genes effectively improves outcrossing rate and hybrid seed production in rice. Plant Biotech. J. 2022, 21, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Shirk, A.J.; Wallin, D.O.; Cushman, S.A.; Rice, C.G.; Warheit, K.I. Inferring landscape effects on gene flow: A new model selection framework. Mol. Ecol. 2010, 19, 3603–3619. [Google Scholar] [CrossRef]
- Goggi, A.S.; Lopez-Sanchez, H.; Caragea, P.; Westgate, M.; Arritt, R.; Clark, C.A. Gene flow in maize fields with different local pollen densities. Int. J. Biometeorol. 2007, 51, 493–503. [Google Scholar] [CrossRef]
- Muraya, M.M.; Geiger, H.H.; de Villiers, S.; Sagnard, F.; Kanyenji, B.M.; Kiambi, D.; Parzies, H.K. Investigation of pollen competition between wild and cultivated sorghums (Sorghum bicolor (L.) Moench) using simple sequence repeats markers. Euphytica 2010, 178, 393–401. [Google Scholar] [CrossRef][Green Version]
- Shivrain, V.K.; Burgos, N.R.; Sales, M.A.; Mauromoustakos, A.; Gealy, D.R.; Smith, K.L.; Black, H.L.; Jia, M. Factors affecting the outcrossing rate between Clearfield™ rice and red rice (Oryza sativa). Weed Sci. 2017, 57, 394–403. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, Z.; Zhu, X.; Tang, Y.; Ye, L.; Yu, H.; Li, Y.; Zhang, N.; Liu, T.; Wang, T.; et al. A minimal genome design to maximally guarantee fertile inter-subspecific hybrid rice. Mol. Plant 2023, 16, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Xia, H.; Zhu, Y.; Wang, Y.; Lu, B.R. Asymmetric gene flow between traditional and hybrid rice varieties (Oryza sativa) indicated by nuclear simple sequence repeats and implications for germplasm conservation. New Phytol. 2004, 163, 439–445. [Google Scholar] [CrossRef]
- Zhao, J.F.; He, G.L.; He, M.D.; Ke, Z.; Zhai, N.X.; Fuya, X.N.; Pei, X.W.; Yuan, Q.H. The competitiveness of transgene flow between Indica and Japonica rice pollen donor. Biotech. Bull. 2018, 34, 96–101. (In Chinese) [Google Scholar]
- Pei, X.W.; Yuan, Q.H.; Hu, N.; Wang, F.; Yao, K.M.; Jia, S.R. Rice Transgenic Flow; China Science Publishing: Beijing, China, 2016. (In Chinese) [Google Scholar]
- Katul, G.G.; Porporato, A.; Nathan, R.; Siqueira, M.; Soons, M.B.; Poggi, D.; Horn, H.S.; Levin, S.A. Mechanistic analytical models for long-distance seed dispersal by wind. Am. Nat. 2005, 166, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.P.; Lu, B.R.; Chen, J.K. A study of pollen viability and longevity in Oryza rufipogon, O. sativa, and their hybrids. Int. Rice Res. Notes 2001, 26, 31–32. [Google Scholar]
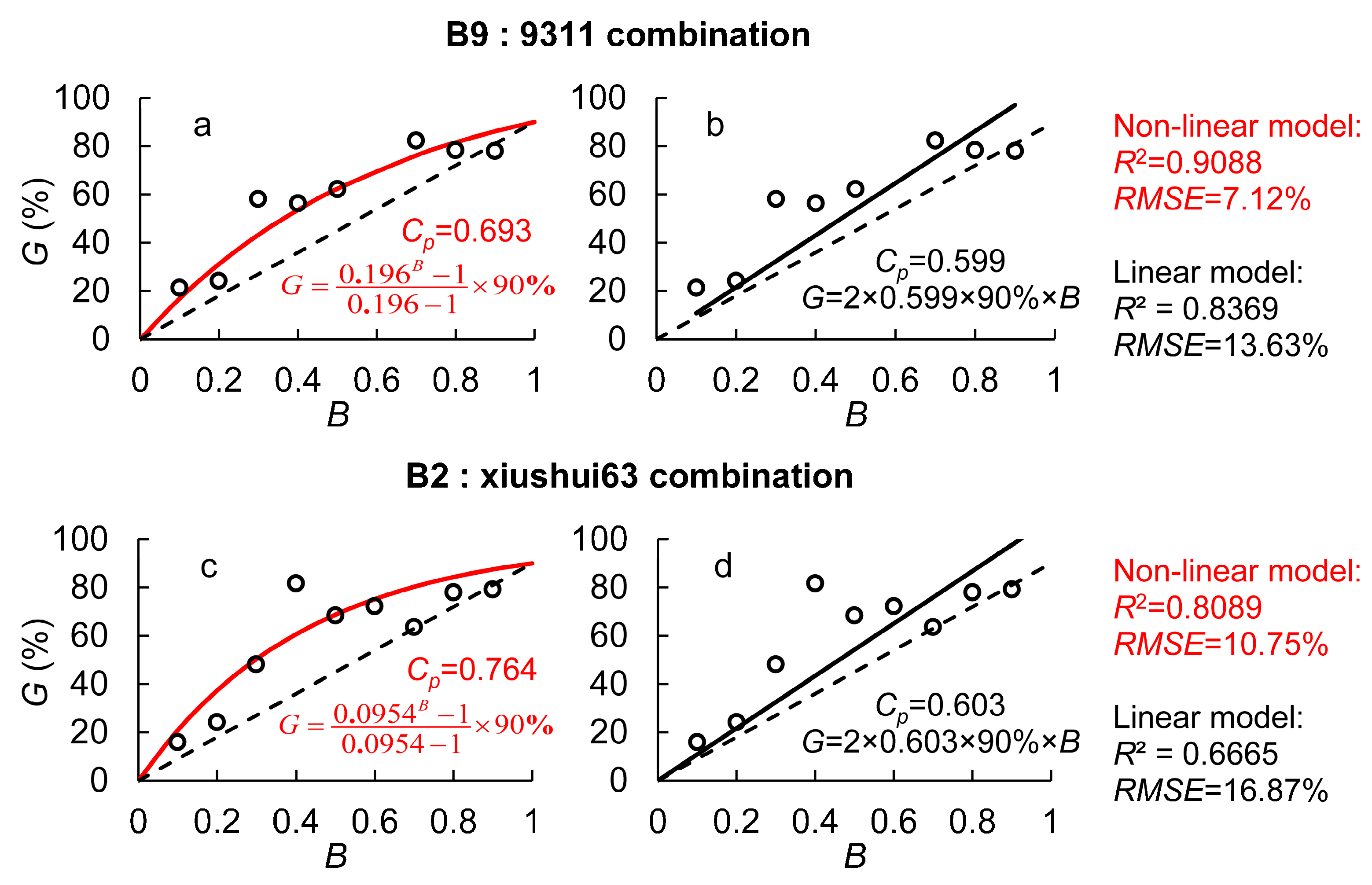




| B | G (%) | |
|---|---|---|
| B9: 9311 | B2: Xiushui63 | |
| 0.1 | 21.23 | 15.92 |
| 0.2 | 24.20 | 24.16 |
| 0.3 | 58.01 | 48.13 |
| 0.4 | 56.26 | 81.66 |
| 0.5 | 62.12 | 68.42 |
| 0.6 | - | 72.18 |
| 0.7 | 82.23 | 63.61 |
| 0.8 | 78.36 | 77.96 |
| 0.9 | 77.94 | 79.18 |
| Cp | 0.15 | 0.3 | 0.5 | 0.7 | 0.85 | |
|---|---|---|---|---|---|---|
| MTD1% | Cb = 90% | 46.8 | 70.5 | 89.3 | 99.6 | 121.3 |
| Cb = 10% | 16.3 | 28.7 | 43.6 | 59.3 | 69.5 | |
| MTD0.1% | Cb = 90% | 91.8 | 123.7 | 142.4 | 148.4 | 160.3 |
| Cb = 10% | 48.4 | 71.8 | 91.3 | 103.5 | 124.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, N.; Wang, D.; Yuan, Q.; Liu, Y.; Jiang, H.; Pei, X. Pollen Quantitative and Genetic Competitiveness of Rice (Oryza sativa L.) and Their Effects on Gene Flow. Plants 2025, 14, 1980. https://doi.org/10.3390/plants14131980
Hu N, Wang D, Yuan Q, Liu Y, Jiang H, Pei X. Pollen Quantitative and Genetic Competitiveness of Rice (Oryza sativa L.) and Their Effects on Gene Flow. Plants. 2025; 14(13):1980. https://doi.org/10.3390/plants14131980
Chicago/Turabian StyleHu, Ning, Dantong Wang, Qianhua Yuan, Yang Liu, Huizi Jiang, and Xinwu Pei. 2025. "Pollen Quantitative and Genetic Competitiveness of Rice (Oryza sativa L.) and Their Effects on Gene Flow" Plants 14, no. 13: 1980. https://doi.org/10.3390/plants14131980
APA StyleHu, N., Wang, D., Yuan, Q., Liu, Y., Jiang, H., & Pei, X. (2025). Pollen Quantitative and Genetic Competitiveness of Rice (Oryza sativa L.) and Their Effects on Gene Flow. Plants, 14(13), 1980. https://doi.org/10.3390/plants14131980






