Modeling Climate Refugia for Chengiodendron marginatum: Insights for Future Conservation Planning
Abstract
1. Introduction
2. Results
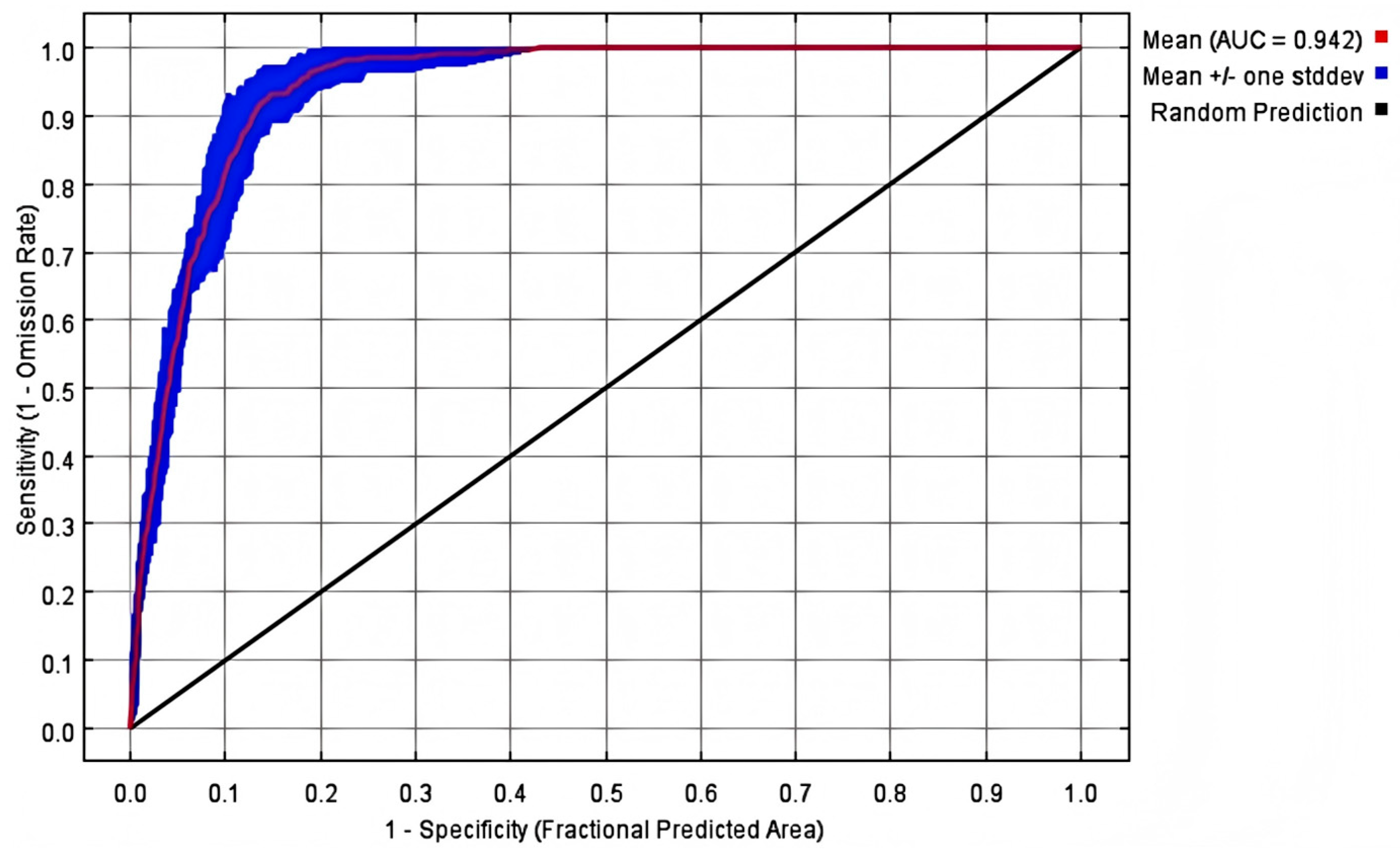
2.1. Accuracy of Maximum Entropy Model Detection
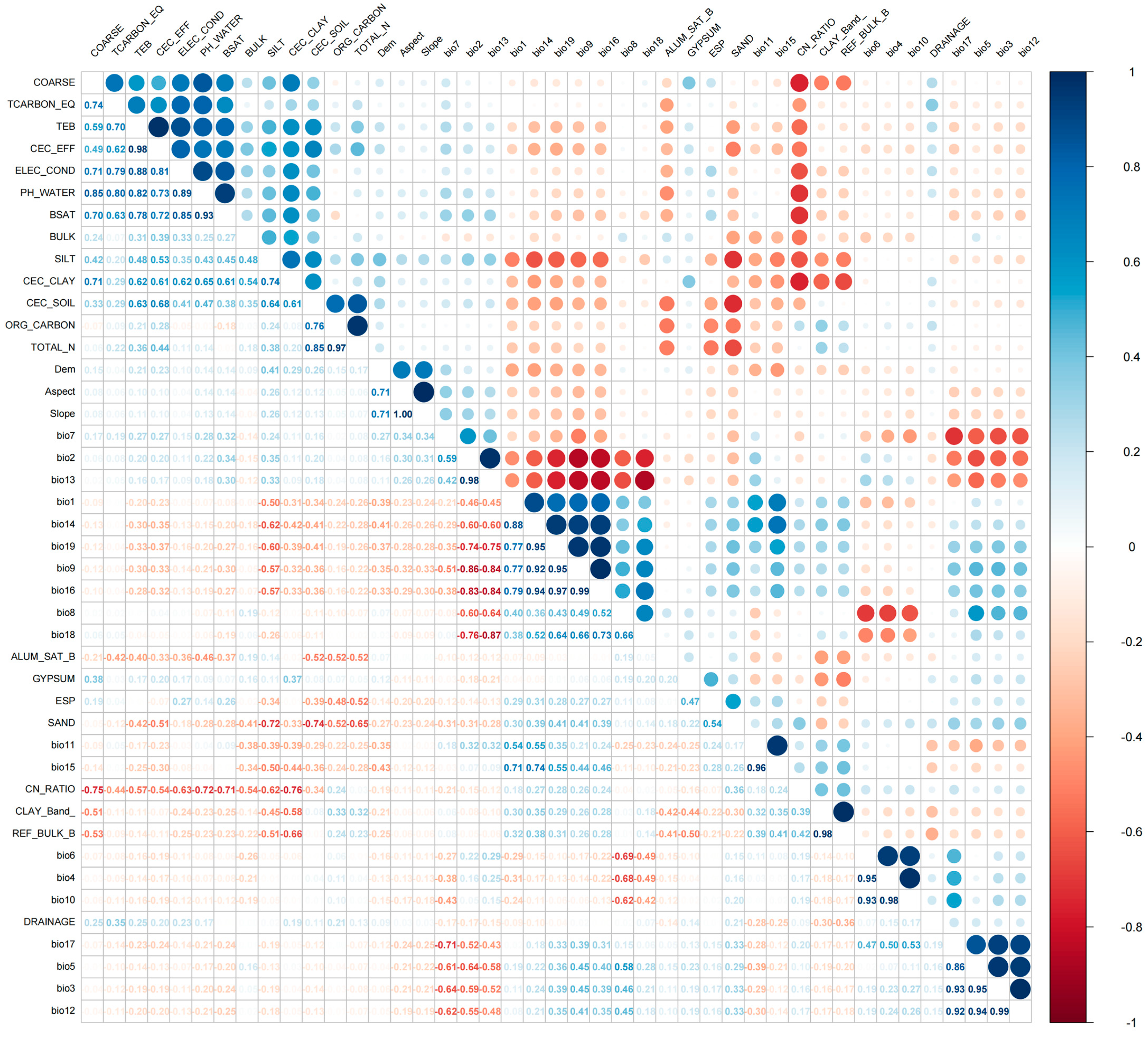
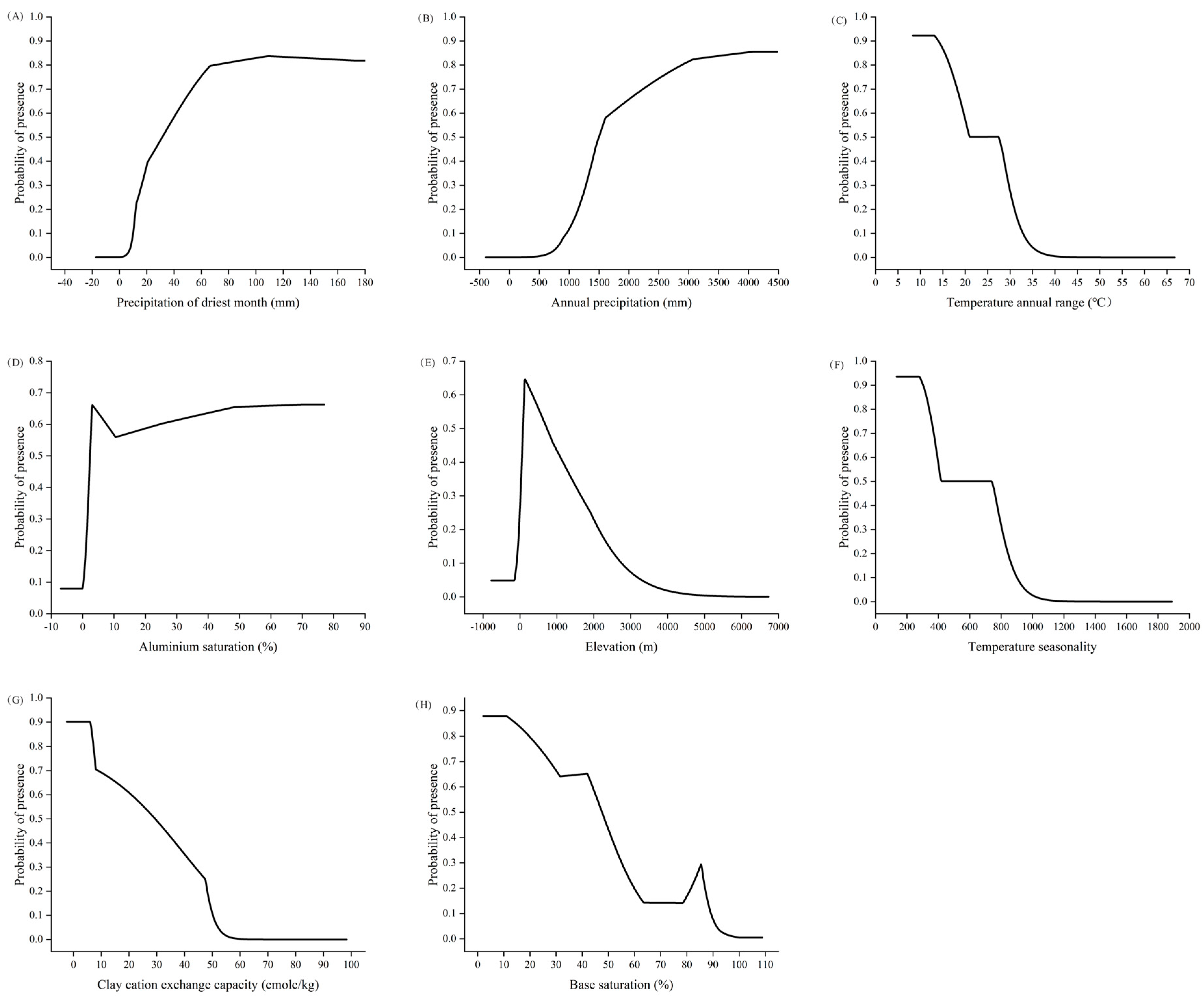
2.2. Analysis of Environmental Variables Influencing Distribution of C. marginatum
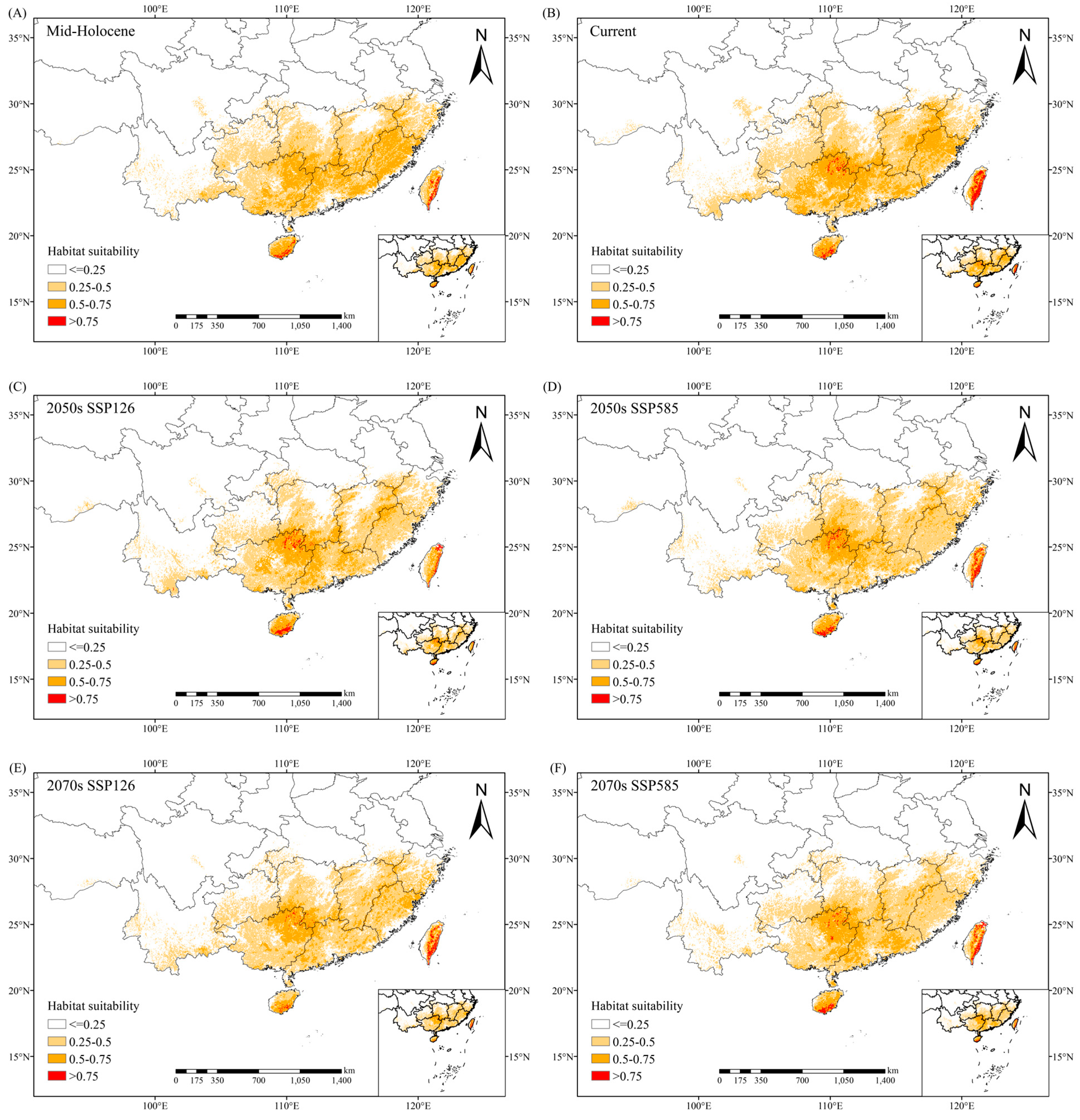
2.3. Distribution of Potential Suitable Habitats of C. marginatum
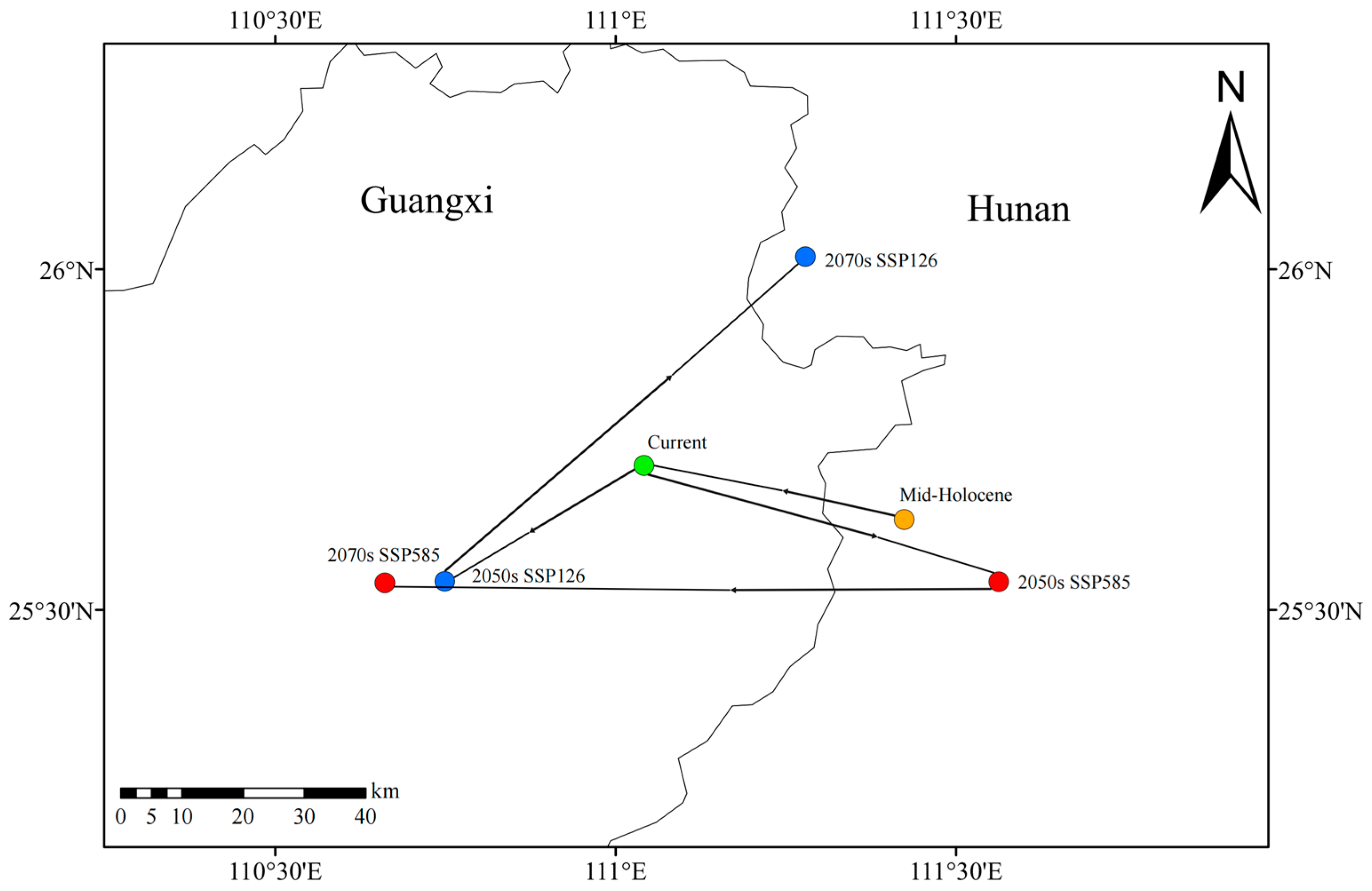
2.4. Migration of Suitable Habitat Center
3. Discussion
3.1. Model Evaluation and Limitations
3.2. Key Environmental Factors Influencing C. marginatum Distribution
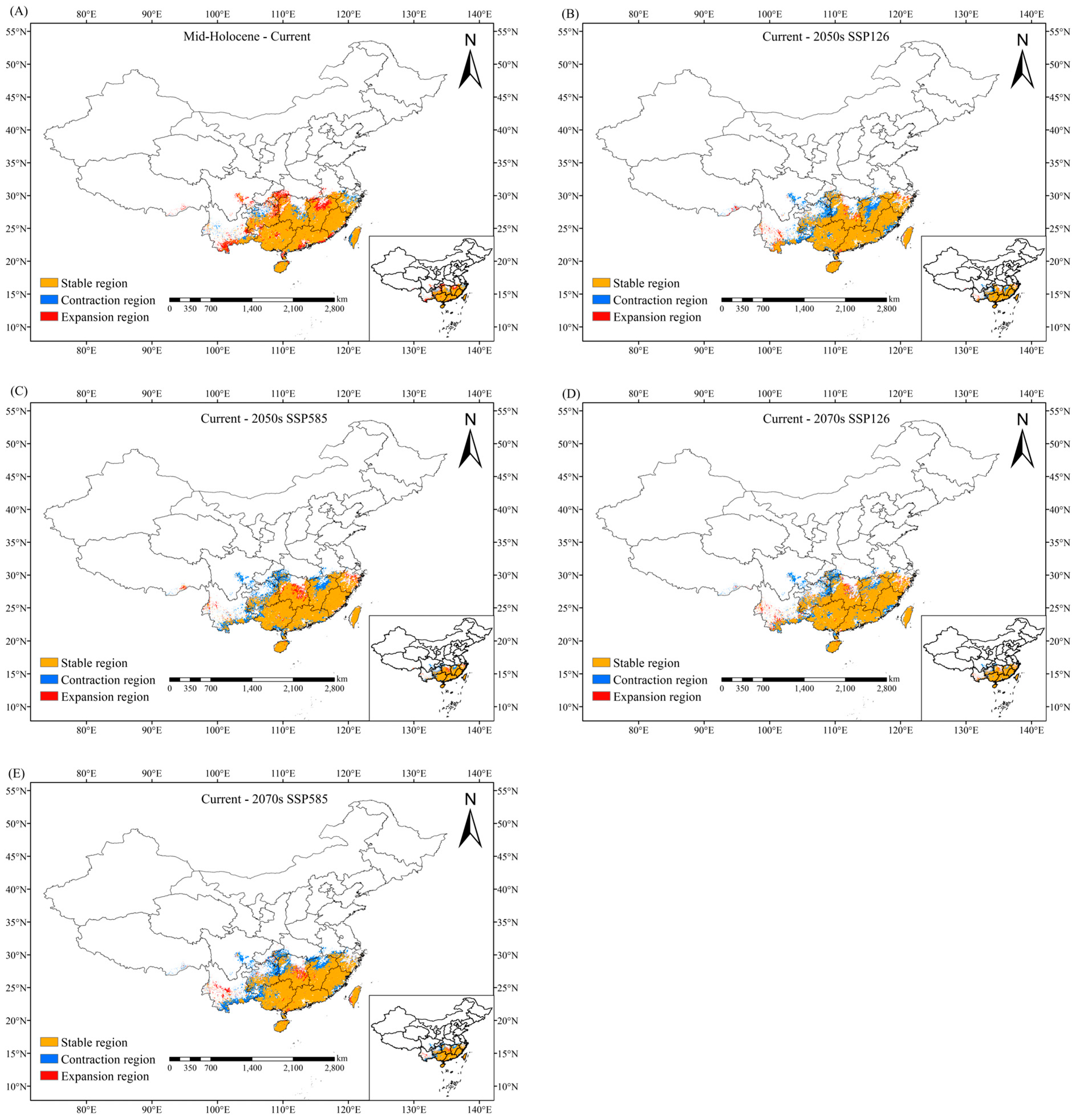
3.3. Changes in Suitable Habitat of C. marginatum Under Different Climate Scenarios
3.4. Conservation Strategy of C. marginatum
4. Materials and Methods
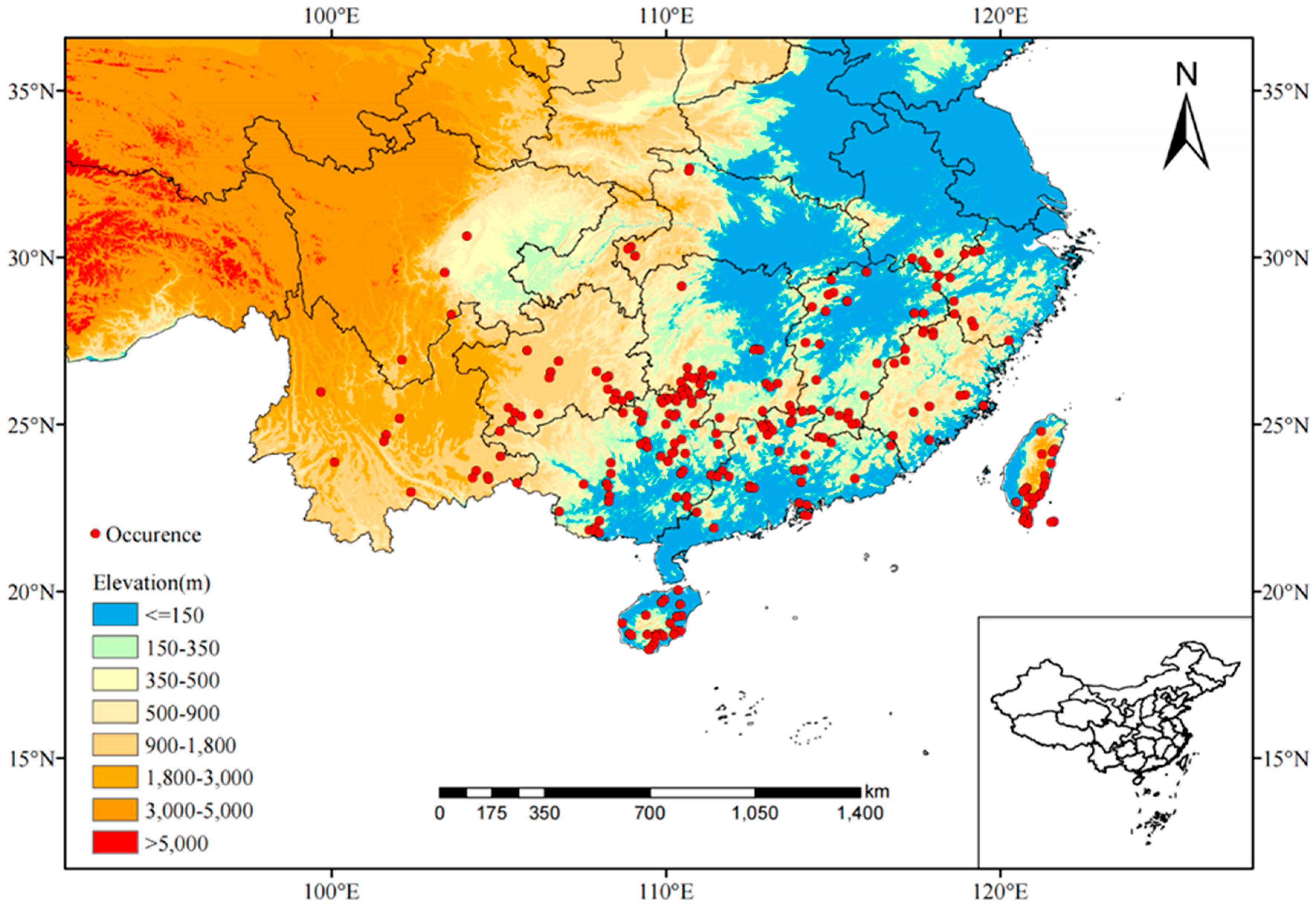
4.1. Species Data Collection
4.2. Environment Variable Data Collection
4.3. Model Construction and Evaluation
4.4. Centroid Migration Analysis of Suitable Habitats Across Time Periods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Data Type | Environmental Variables | Description | Unit |
|---|---|---|---|
| Bioclimatic variables | Bio1 | Annual Mean Temperature | °C |
| Bio2 | Mean Diurnal Range | °C | |
| Bio3 | Isothermality | - | |
| Bio4 | Temperature Seasonality | - | |
| Bio5 | Max Temperature of Warmest Month | °C | |
| Bio6 | Min Temperature of Coldest Month | °C | |
| Bio7 | Temperature Annual Range | °C | |
| Bio8 | Mean Temperature of Wettest Quarter | °C | |
| Bio9 | Mean Temperature of Driest Quarter | °C | |
| Bio10 | Mean Temperature of Warmest Quarter | °C | |
| Bio11 | Mean Temperature of Coldest Quarter | °C | |
| Bio12 | Annual Precipitation | mm | |
| Bio13 | Precipitation of Wettest Month | mm | |
| Bio14 | Precipitation of Driest Month | mm | |
| Bio15 | Precipitation Seasonality | - | |
| Bio16 | Precipitation of Wettest Quarter | mm | |
| Bio17 | Precipitation of Driest Quarter | mm | |
| Bio18 | Precipitation of Warmest Quarter | mm | |
| Bio19 | Precipitation of Coldest Quarter | mm | |
| Soil variables | ALUM_SAT | Aluminum Saturation | % ECEC |
| BSAT | Base Saturation | %CECsoil | |
| BULK | Bulk Density | g/cm3 | |
| CEC_CLAY | Clay Cation Exchange Capacity | cmolc/kg | |
| CEC_EFF | ECEC | cmolc/kg | |
| CEC_SOIL | CEC Soil | cmolc/kg | |
| CLAY | Clay | % weight | |
| CN_RATIO | Carbon/Nitrogen Ratio | (C/N) | |
| COARSE | Coarse Fragments | % volume | |
| DRAINAGE | Drainage | - | |
| ELEC_COND | Electric Conductivity | dS/m | |
| ESP | Exchangeable Sodium Percentage | % | |
| GYPSUM | Gypsum Content | % weight | |
| ORG_CARBON | Organic Carbon Content | % weight | |
| PH_WATER | pH in Water | −LOG(H+) | |
| REF_BULK | Reference Bulk Density | g/cm3 | |
| SAND | Sand | % weight | |
| SILT | Silt | % weight | |
| TCARBON_EQ | Calcium Carbonate | % weight | |
| TEB | TEB | cmolc/kg | |
| TOTAL_N | Total Nitrogen Content | g/kg | |
| Landform variables | DEM | Elevation | m |
| Slope | Degree of Slope | ° | |
| Aspect | Direction of Slope | - |
References
- Levin, D.A. Plant speciation in the age of climate change. Ann. Bot. 2019, 124, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Singh, H.; Maurya, S.; Kumar, C.; Kumar, A. Current scenario of climate change and its impact on plant diseases. Plant Sci. Today 2022, 10, 169–171. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Liu, T.; Zhang, C. The potential geographical distribution of Haloxylon across Central Asia under climate change in the 21st century. Agric. For. Meteorol. 2019, 275, 243–254. [Google Scholar] [CrossRef]
- Ostad-Ali-Askari, K.; Ghorbanizadeh, K.H.; Shayannejad, M.; Zareian, M.J. Effect of Climate Change on Precipitation Patterns in an Arid Region Using GCM Models: Case Study of Isfahan-Borkhar Plain. Nat. Hazards Rev. 2020, 21, 4020006. [Google Scholar] [CrossRef]
- Rong, S.; Luo, P.; Yi, H.; Yang, X.; Zhang, L.; Zeng, D.; Wang, L. Predicting Habitat Suitability and Adaptation Strategies of an Endangered Endemic Species, Camellia luteoflora Li ex Chang (Ericales: Theaceae) under Future Climate Change. Forests 2023, 14, 2177. [Google Scholar] [CrossRef]
- Ye, P.; Zhang, G.; Zhao, X.; Chen, H.; Si, Q.; Wu, J. Potential geographical distribution and environmental explanations of rare and endangered plant species through combined modeling: A case study of Northwest Yunnan, China. Ecol. Evol. 2021, 11, 13052–13067. [Google Scholar] [CrossRef]
- Robinson, N.M.; Nelson, W.A.; Costello, M.J.; Sutherland, J.E.; Lundquist, C.J. A Systematic Review of Marine-Based Species Distribution Models (SDMs) with Recommendations for Best Practice. Front. Mar. Sci. 2017, 4, 421. [Google Scholar] [CrossRef]
- Rosner-Katz, H.; Mccune, J.L.; Bennett, J.R. Using stacked SDMs with accuracy and rarity weighting to optimize surveys for rare plant species. Biodivers. Conserv. 2020, 29, 3209–3225. [Google Scholar] [CrossRef]
- Cianfrani, C.; Broennimann, O.; Loy, A.; Guisan, A. More than range exposure: Global otter vulnerability to climate change. Biol. Conserv. 2018, 221, 103–113. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.; Wan, J.; Han, S.; Wang, Q.; Nie, S. A model-based method to evaluate the ability of nature reserves to protect endangered tree species in the context of climate change. For. Ecol. Manag. 2014, 327, 48–54. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Li, L.; Liu, W.; Ai, J.; Cai, S.; Dong, J. Predicting Mangrove Distributions in the Beibu Gulf, Guangxi, China, Using the Maxent Model: Determining Tree Species Selection. Forests 2023, 14, 149. [Google Scholar] [CrossRef]
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Hutchinson, M.F. Bioclim: The first species distribution modelling package, its early applications and relevance to most current Maxent studies. Divers. Distrib. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Carpenter, G.; Gillison, A.N.; Winter, J. DOMAIN: A flexible modelling procedure for mapping potential distributions of plants and animals. Biodivers. Conserv. 1993, 2, 667–680. [Google Scholar] [CrossRef]
- Peterson, A.; Papes, M.; Eaton, M. Transferability and model evaluation in ecological niche modeling: A comparison of GARP and Maxent. Ecography 2007, 30, 550–560. [Google Scholar] [CrossRef]
- Elith, J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C. A statistical explanation of Maxent for ecologists. Divers. Distrib. 2010, 17, 43–57. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Rhoden, C.M.; Peterman, W.E.; Taylor, C.A.; Minasny, B. Maxent-directed field surveys identify new populations of narrowly endemic habitat specialists. PeerJ 2017, 5, e3632. [Google Scholar] [CrossRef]
- Yi, Y.; Cheng, X.; Yang, Z.; Wieprecht, S.; Zhang, S.; Wu, Y. Evaluating the ecological influence of hydraulic projects: A review of aquatic habitat suitability models. Renew. Sustain. Energy Rev. 2017, 68, 748–762. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Li, C.; Liu, Z. Optimized Maxent Model Predictions of Climate Change Impacts on the Suitable Distribution of Cunninghamia lanceolata in China. Forests 2020, 11, 302. [Google Scholar] [CrossRef]
- Xie, C.; Huang, B.; Jim, C.Y.; Han, W.; Liu, D. Predicting differential habitat suitability of Rhodomyrtus tomentosa under current and future climate scenarios in China. For. Ecol. Manag. 2021, 501, 119696. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, K.; Zhao, R.; Xie, L.; Li, Y.; Zhao, G.; Zhang, F. Prediction of potential suitable habitats in the 21st century and GAP analysis of priority conservation areas of Chionanthus retusus based on the MaxEnt and Marxan models. Front. Plant Sci. 2024, 15, 1304121. [Google Scholar] [CrossRef]
- Wang, G.; Xie, C.; Wei, L.; Gao, Z.; Yang, H.; Jim, C. Predicting Suitable Habitats for China’s Endangered Plant Handeliodendron bodinieri (H. Lév.) Rehder. Diversity 2023, 15, 1033. [Google Scholar] [CrossRef]
- Wei, L.; Wang, G.; Xie, C.; Gao, Z.; Huang, Q.; Jim, C.Y. Predicting suitable habitat for the endangered tree Ormosia microphylla in China. Sci. Rep. 2024, 14, 10330. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.; Wang, X.; Sylvester, S.P.; Xiang, Q.; Li, X.; Li, M.; Zhu, H.; Zhang, C.; Chen, L.; et al. Revisiting the phylogeny and taxonomy of Osmanthus (Oleaceae) including description of the new genus Chengiodendron. Phytotaxa 2020, 436, 283–292. [Google Scholar] [CrossRef]
- Chang, M.; Qiu, L.; Wei, Z.; Green, P.S. Osmanthus. In Flora of China; Wu, Z., Raven, P., Hong, D., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 1996; Volume 15, pp. 286–292. [Google Scholar]
- Chai, Z.; Zhang, M.; Li, L.; Zhang, C.; Duan, Y. Non-Targeted Metabolomics Analysis of the Petals of Osmanthus fragrans ‘Yanzhi Hong’ in Different Developmental Phases. J. Northwest For. Univ. 2022, 37, 107–113. [Google Scholar]
- Gu, H.; Yang, X.; Wang, L. Progress in Molecular Biological Studies of Osmanthus fragrans. J. Trop. Subtrop. Bot. 2025, 33, 220–228. [Google Scholar]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. Original Article: Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Kong, W.; Li, X.; Zou, H. Optimizing Maxent model in the prediction of species distribution. Chin. J. Appl. Ecol. 2019, 30, 2116–2128. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Molecular Regulation of Plant Responses to Environmental Temperatures. Mol. Plant. 2020, 13, 544–564. [Google Scholar] [CrossRef] [PubMed]
- Zeppel, M.J.B.; Wilks, J.V.; Lewis, J.D. Impacts of extreme precipitation and seasonal changes in precipitation on plants. Biogeosciences 2014, 11, 3083–3093. [Google Scholar] [CrossRef]
- Kong, F.; Tang, L.; He, H.; Yang, F.; Tao, J.; Wang, W. Assessing the impact of climate change on the distribution of Osmanthus fragrans using Maxent. Environ. Sci. Pollut. Res. 2021, 28, 34655–34663. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Zhang, L.; Zhang, J.; He, L.; Sun, R. Maxent Modeling for Predicting the Potential Geographical Distribution of Castanopsis carlesii under Various Climate Change Scenarios in China. Forests 2023, 14, 1397. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, Z.; Li, Z.; Liu, Y.; Fang, S. Predictive Modeling of Suitable Habitats for Cinnamomum camphora (L.) Presl Using Maxent Model under Climate Change in China. Int. J. Environ. Res. Public Health 2019, 16, 3185. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Fang, Y. Prediction of potential suitable distribution areas of Quercus fabri in China based on an optimized Maxent model. Scientia Silvae Sinicae 2018, 54, 154–164. [Google Scholar] [CrossRef]
- Mishra, A.K. Plant Adaptation to Global Climate Change. Atmosphere 2021, 12, 451. [Google Scholar] [CrossRef]
- Bertrand, R.; Lenoir, J.; Piedallu, C.; Riofrío-Dillon, G.; de Ruffray, P.; Vidal, C.; Pierrat, J.; Gégout, J. Changes in plant community composition lag behind climate warming in lowland forests. Nature 2011, 479, 517–520. [Google Scholar] [CrossRef]
- Cao, F.; Xu, Q.; Cao, S.; Long, J.; Qi, C. Advances of Global Warming Impact on Species Distribution. J. Cent. South Univ. For. Technol. 2008, 28, 86–89. [Google Scholar]
- Li, Y.; Zhang, X.; Fang, Y. Responses of the distribution pattern of Quercus chenii to climate change following the Last Glacial Maximum. Chin. J. Plant Ecol. 2016, 40, 1164–1178. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Z.; Chen, J.; Sun, W.; Shen, Z.; Jia, X. Holocene Precipitation Change in the Middle and Lower Reaches of the Yangtze River and Its Forcing Mechanisms. J. Earth Sci. 2025, 50, 699–717. [Google Scholar]
- Temunović, M.; Frascaria-Lacoste, N.; Franjić, J.; Satovic, Z.; Fernández-Manjarrés, J.F. Identifying refugia from climate change using coupled ecological and genetic data in a transitional Mediterranean-temperate tree species. Mol. Ecol. 2013, 22, 2128–2142. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, R.; Cameron, S.; Parra, J.; Jones, P.; Jarvis, A. Very high resolution interpolated climate surfaces of global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Wu, T.; Lu, Y.; Fang, Y.; Xin, X.; Li, L.; Li, W.; Jie, W.; Zhang, J.; Liu, Y.; Zhang, L.; et al. The Beijing Climate Center Climate System Model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Böhmelt, T. Employing the shared socioeconomic pathways to predict CO2 emissions. Environ. Sci. Policy 2017, 75, 56–64. [Google Scholar] [CrossRef]
- Sharma, S.; Arunachalam, K.; Bhavsar, D.; Kala, R. Modeling habitat suitability of Perilla frutescens with MaxEnt in Uttarakhand—A conservation approach. J. Appl. Res. Med. Aromat. Plants 2018, 10, 99–105. [Google Scholar] [CrossRef]
- FAO; IIASA. Harmonized World Soil Database Version 2.0; FAO: Rome, Italy; IIASA: Laxenburg, Austria, 2023. [Google Scholar]
- Li, X.; Wu, H.; Wang, L. Seasonal growing dynamics and vertical distribution of seedling root system in Osmanthus fragrans under the controlled root cultivation mode. J. For. Eng. 2013, 27, 59–62. [Google Scholar]
- Huang, J.; Li, G.; Li, J.; Zhang, X.; Yan, M.; Du, S. Projecting the Range Shifts in Climatically Suitable Habitat for Chinese Sea Buckthorn under Climate Change Scenarios. Forests 2018, 9, 9. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.; Zhang, Q.; Zhang, D.; Xie, C.; Liu, X. Prediction for the potential distribution area of Codonopsis pilosula at global scale based on Maxent model. Chin. J. Appl. Ecol. 2017, 28, 992–1000. [Google Scholar] [CrossRef]
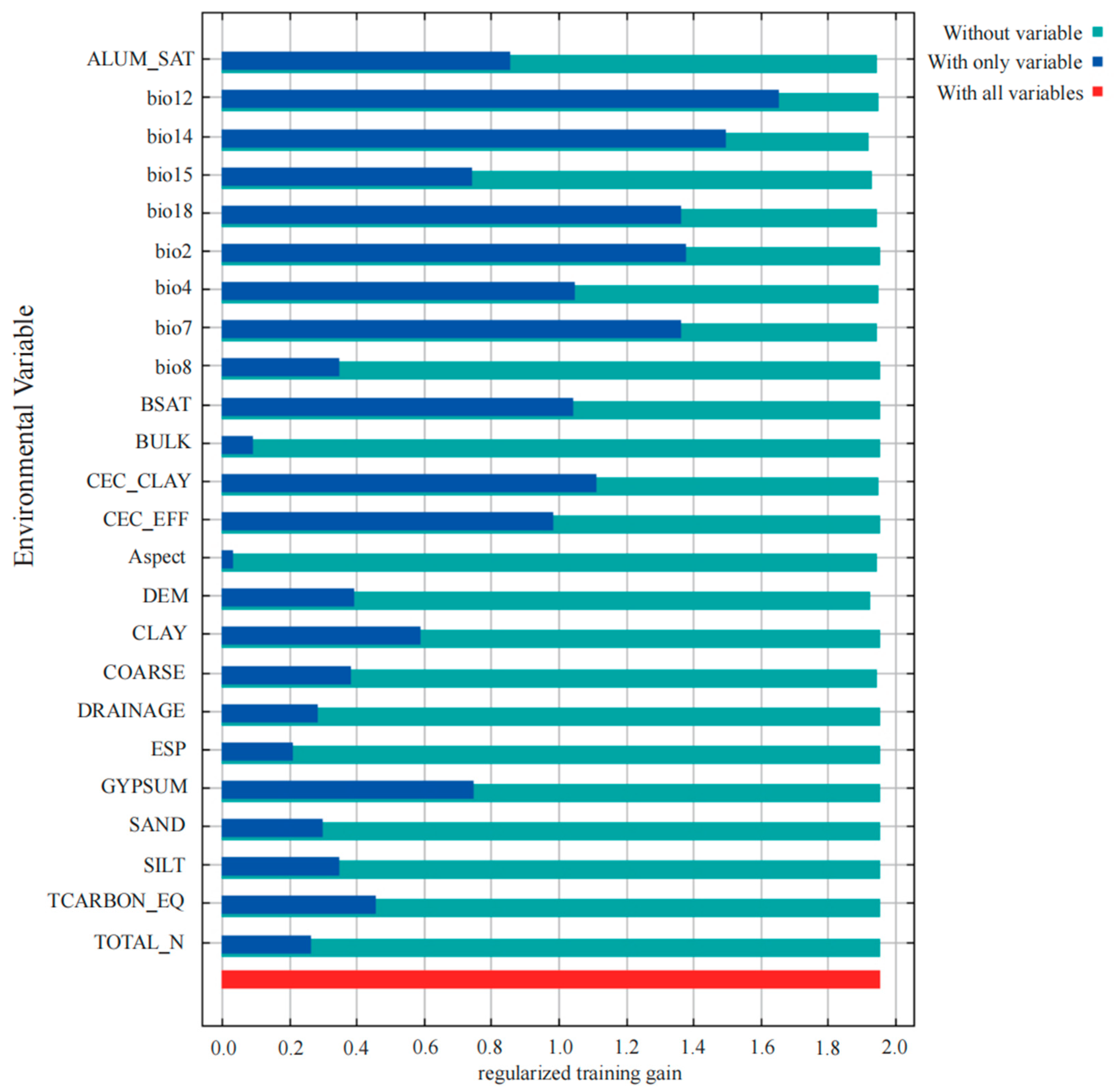
| Variables | Percent Contribution/% | Permutation Importance/% |
|---|---|---|
| bio14 | 58.3 | 26.9 |
| bio12 | 14.9 | 10.4 |
| bio7 | 5.5 | 21.4 |
| ALUM_SAT | 4.9 | 1.3 |
| DEM | 2.4 | 13.4 |
| bio4 | 2.3 | 1.3 |
| CEC_CLAY | 1.9 | 2.1 |
| BSAT | 1.7 | 1.8 |
| bio15 | 1.6 | 4.9 |
| bio2 | 1.1 | 1.9 |
| SAND | 0.8 | 0.4 |
| Aspect | 0.6 | 0.8 |
| bio18 | 0.6 | 2.9 |
| TCARBON_EQ | 0.4 | 0.5 |
| CLAY | 0.4 | 0 |
| CEC_EFF | 0.4 | 0.1 |
| GYPSUM | 0.4 | 2.3 |
| DRAINAGE | 0.4 | 0.3 |
| COARSE | 0.4 | 2.2 |
| BULK | 0.3 | 0.1 |
| SILT | 0.3 | 0.7 |
| bio8 | 0.2 | 0.1 |
| TOTAL_N | 0.2 | 0 |
| ESP | 0.1 | 0.1 |
| Climate Scenario | Non-Suitable Area | Ratio/% | Low Suitability Area | Ratio/% | Medium-Suitability Area | Ratio/% | High Suitability Area | Ratio/% | Total Suitable Area | Ratio/% | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Current | 822.27 | 65.72 | 31.04 | 1.62 | 98.38 | ||||||
| Mid-Holocene | 831.22 | 1.09 | 62.05 | −5.58 | 26.65 | −14.14 | 0.67 | −58.64 | 89.37 | −9.16 | |
| SSP126 | 2050s | 836.37 | 1.71 | 63.52 | −3.35 | 19.71 | −36.50 | 1.05 | −35.19 | 84.28 | −14.33 |
| 2070s | 834.28 | 1.46 | 67.65 | 2.94 | 17.82 | −42.59 | 0.90 | −44.44 | 86.37 | −12.21 | |
| SSP585 | 2050s | 834.63 | 1.50 | 66.68 | 1.46 | 18.03 | −41.91 | 1.30 | −19.75 | 86.01 | −12.57 |
| 2070s | 838.47 | 1.97 | 62.94 | −4.23 | 18.11 | −41.66 | 1.13 | −30.25 | 82.18 | −16.47 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Yan, Q.; Li, Y.; Yan, Z.; Fu, C.; Jiang, B.; Chen, L. Modeling Climate Refugia for Chengiodendron marginatum: Insights for Future Conservation Planning. Plants 2025, 14, 1961. https://doi.org/10.3390/plants14131961
Yu Z, Yan Q, Li Y, Yan Z, Fu C, Jiang B, Chen L. Modeling Climate Refugia for Chengiodendron marginatum: Insights for Future Conservation Planning. Plants. 2025; 14(13):1961. https://doi.org/10.3390/plants14131961
Chicago/Turabian StyleYu, Zhirun, Quanhong Yan, Yilin Li, Zheng Yan, Chenlong Fu, Bo Jiang, and Lin Chen. 2025. "Modeling Climate Refugia for Chengiodendron marginatum: Insights for Future Conservation Planning" Plants 14, no. 13: 1961. https://doi.org/10.3390/plants14131961
APA StyleYu, Z., Yan, Q., Li, Y., Yan, Z., Fu, C., Jiang, B., & Chen, L. (2025). Modeling Climate Refugia for Chengiodendron marginatum: Insights for Future Conservation Planning. Plants, 14(13), 1961. https://doi.org/10.3390/plants14131961






