Residual Chlorine Interaction with Microelements in Plants Applied for Phytoremediation in Rain Gardens
Abstract
1. Introduction
2. Results and Discussion
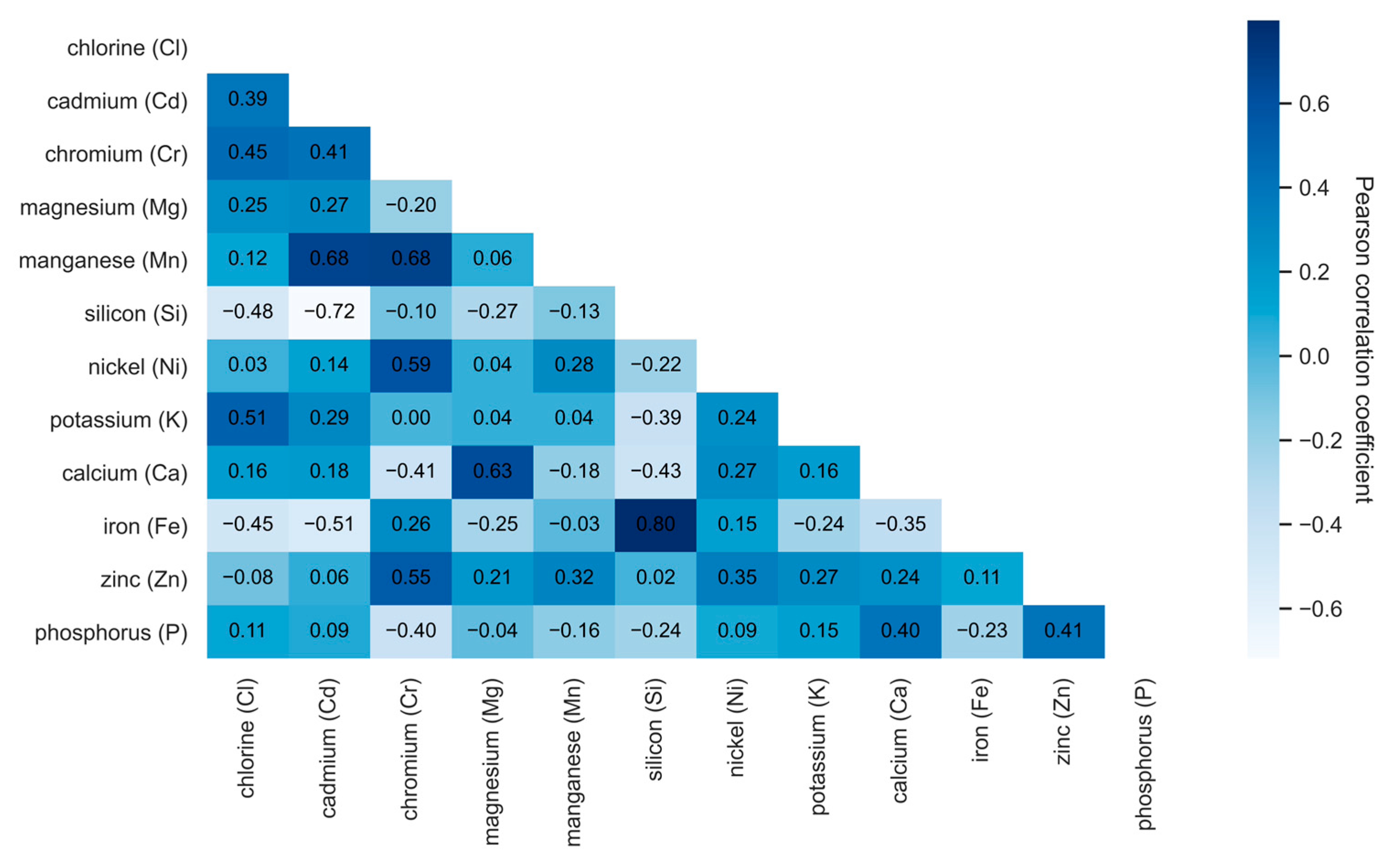
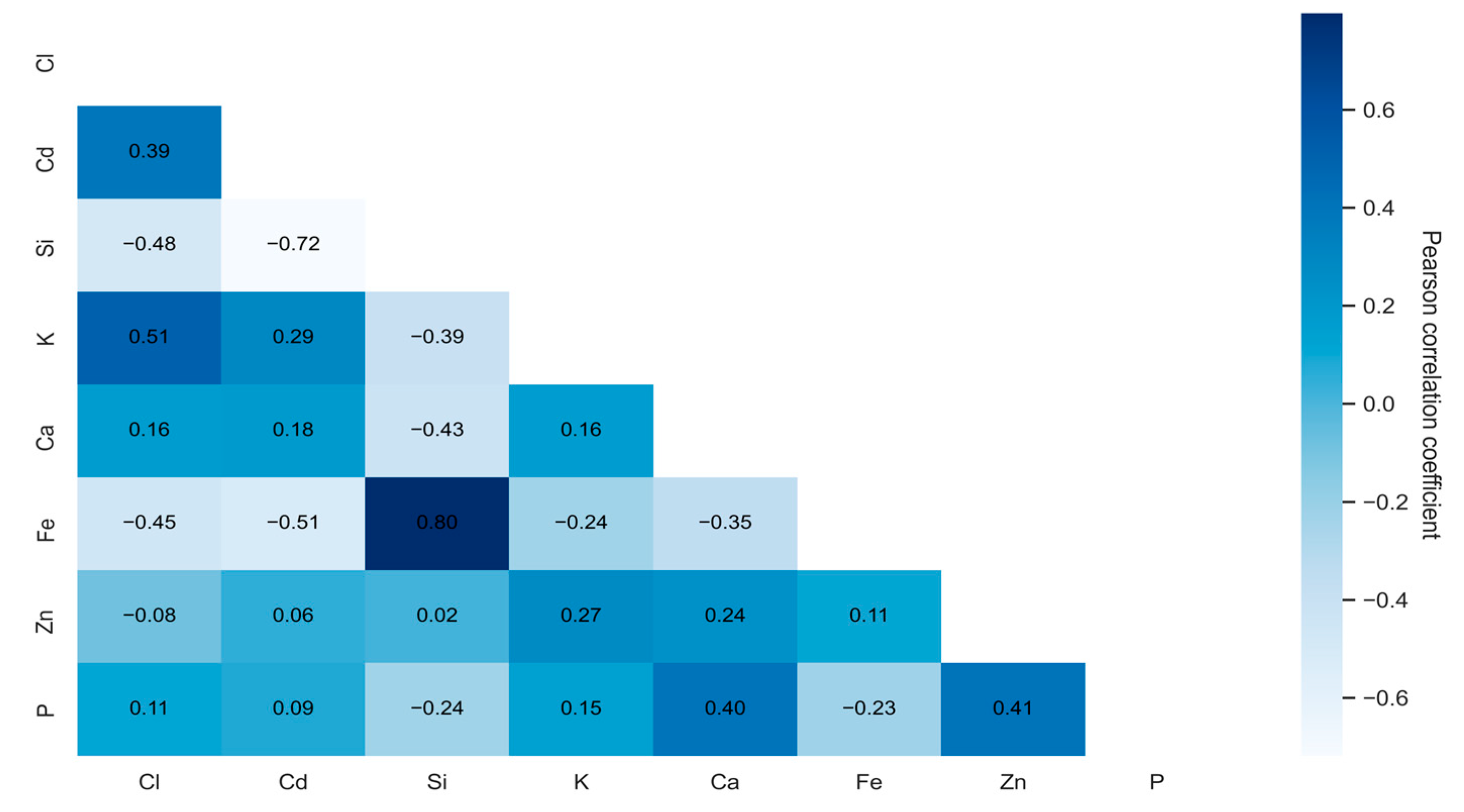
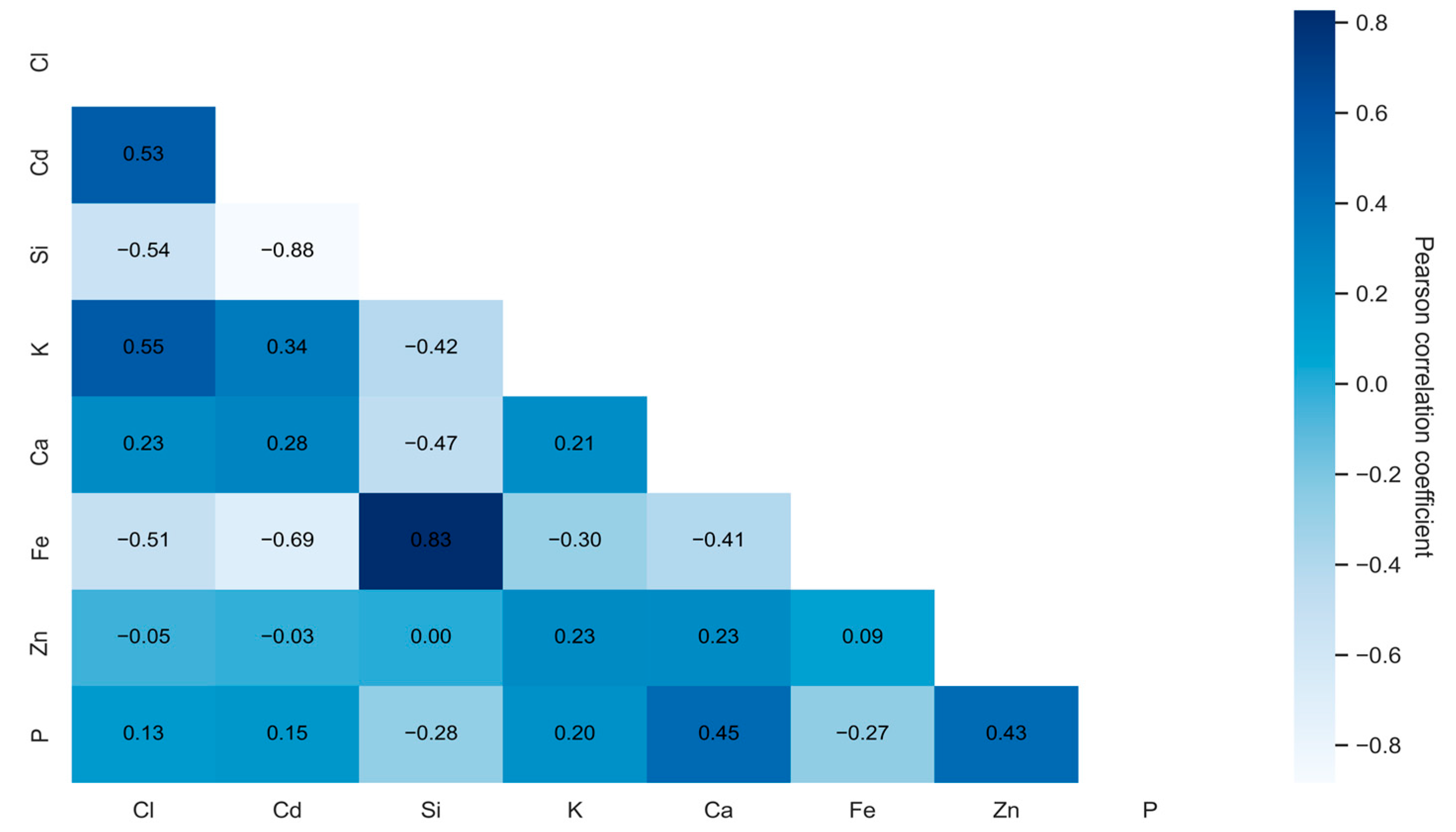
2.1. Correlation Analysis
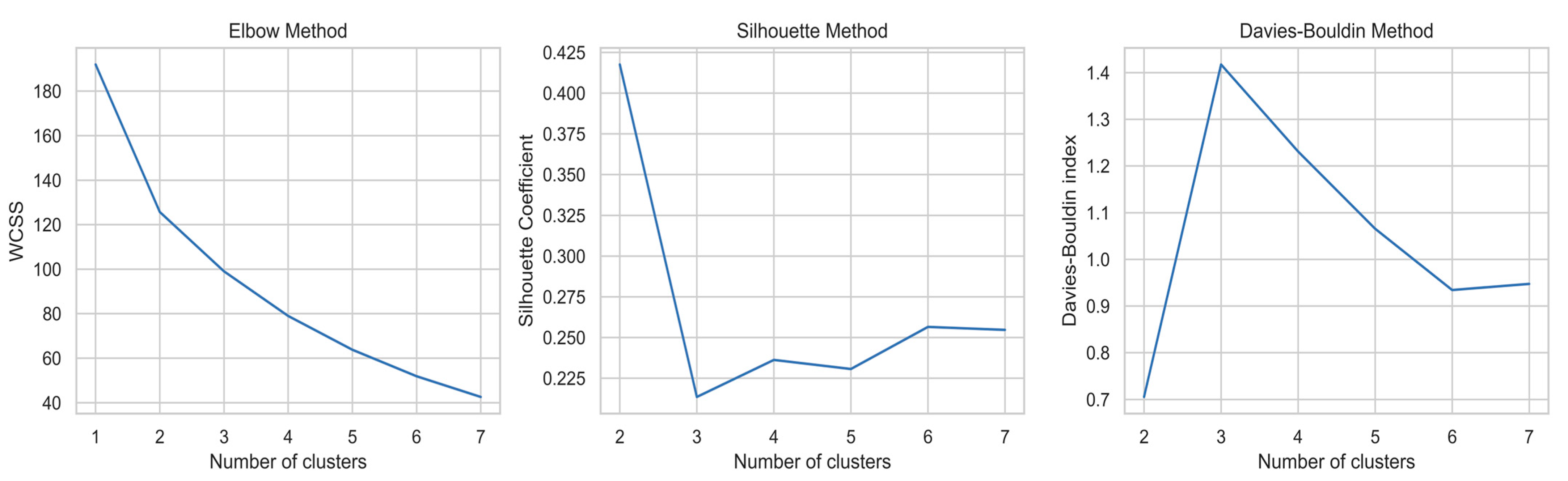
2.2. Cluster Analysis
3. Materials and Methods
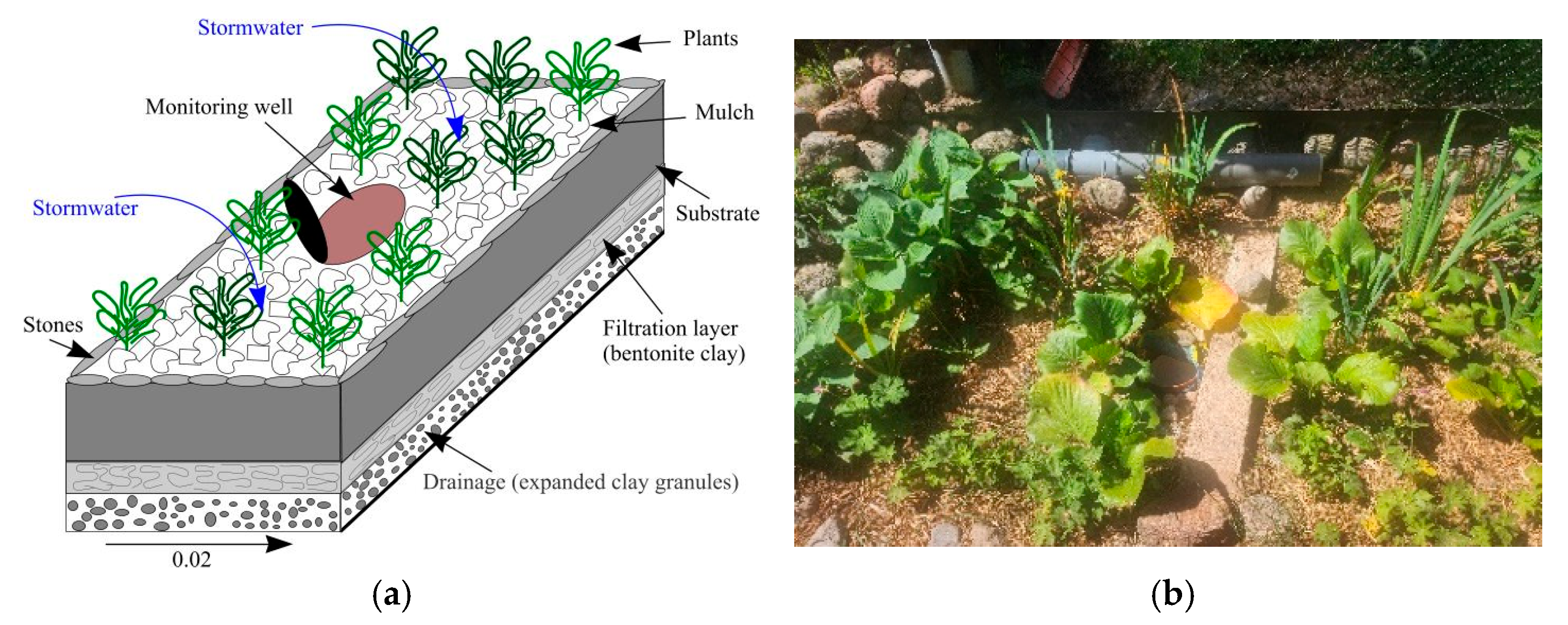
3.1. Field Experiment
3.1.1. Location Area and Experimental Model Installation
3.1.2. Plant Selection and Sampling
3.2. Data Analysis
- -
- Values close to 1 indicate a strong positive correlation;
- -
- Values near −1 suggest a strong negative correlation;
- -
- Values around 0 imply no linear relationship.
- -
- The Elbow method (evaluating the Within-Cluster Sum of Squares);
- -
- The Silhouette Score;
- -
- And the Davies–Bouldin index.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Wang, C.; Yu, Y.; Chen, Y.; Du, L.; Qu, X.; Peng, W.; Zhang, M.; Gui, C. Effect of Urban Stormwater Road Runoff of Different Land Use Types on an Urban River in Shenzhen, China. Water 2019, 11, 2545. [Google Scholar] [CrossRef]
- Müller, A.; Österlund, H.; Marsalek, J.; Viklander, M. The pollution conveyed by urban runoff: A review of sources. Sci. Total Environ. 2020, 709, 136125. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, J.; Zhou, K.; Feng, P.; Dong, L. The effects of surface pollution on urban river water quality under rainfall events in Wuqing district, Tianjin, China. J. Clean. Prod. 2021, 293, 126136. [Google Scholar] [CrossRef]
- Levin, P.S.; Howe, E.R.; Robertson, J.C. Impacts of stormwater on coastal ecosystems: The need to match the scales of management objectives and solutions. Philos. Trans. B 2020, 375, 20190460. [Google Scholar] [CrossRef]
- Mishra, J.; Botlaguduru, V. Impact of Stormwater Runoff on the Water Quality of Lakes in a Metropolitan Region in India. In Proceedings of the 9th World Congress on Civil, Structural, and Environmental Engineering (CSEE 2024), London, UK, 14–16 April 2024; p. No. ICEPTP 167. [Google Scholar] [CrossRef]
- Anh, N.T.; Le Duy Can, L.D.; Nhan, N.T.; Schmalz, B.; Luu, T.L. Influences of key factors on river water quality in urban and rural areas: A review. Case Stud. Chem. Environ. Eng. 2023, 8, 100424. [Google Scholar] [CrossRef]
- Wang, J.; Hu, C.; Ma, B.; Mu, X. Rapid Urbanization Impact on the Hydrological Processes in Zhengzhou, China. Water 2020, 12, 1870. [Google Scholar] [CrossRef]
- Liyanage, C.P.; Yamada, K. Impact of Population Growth on the Water Quality of Natural Water Bodies. Sustainability 2017, 9, 1405. [Google Scholar] [CrossRef]
- European Environment Agency Economic Losses from Weather- and Climate-Related Extremes in Europe, Published 14 Oct 2024. Available online: https://www.eea.europa.eu/en/analysis/indicators/economic-losses-from-climate-related (accessed on 27 April 2025).
- Garcia-Ayllon, S.; Radke, J. Geostatistical Analysis of the Spatial Correlation between Territorial Anthropization and Flooding Vulnerability: Application to the DANA Phenomenon in a Mediterranean Watershed. Appl. Sci. 2021, 11, 809. [Google Scholar] [CrossRef]
- UN General Assembly, Transforming our world: The 2030 Agenda for Sustainable Development, A/RES/70/1, 21 October 2015. Available online: https://docs.un.org/en/A/RES/70/1 (accessed on 3 January 2025).
- Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions the European Green Deal COM/2019/640 Final. European Commission. The European Green Deal. COM/2019/640 Final. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2019%3A640%3AFIN (accessed on 3 January 2025).
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committe and the Committee of the Regions a New Circular Economy Action Plant for a Cleaner and More Competetive Europe COM/2020/98 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1583933814386&uri=COM:2020:98:FIN (accessed on 3 January 2025).
- European Union, 2013 European Union: European Commission, Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions Green Infrastructure (GI)—Enhancing Europe’s Natural Capital, COM/2013/0249 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A52013DC0249 (accessed on 3 January 2025).
- Khan, A.H.A.; Kiyani, A.; Mirza, C.R.; Butt, T.A.; Barros, R.; Ali, B.; Iqbal, M.; Yousaf, S. Ornamental plants for the phytoremediation of heavy metals: Present knowledge and future perspectives. Environ. Res. 2021, 195, 110780. [Google Scholar] [CrossRef]
- Taguchi, V.J.; Weiss, P.T.; Gulliver, J.S.; Klein, M.R.; Hozalski, R.M.; Baker, L.A.; Finlay, J.C.; Keeler, B.L.; Nieber, J.L. It Is Not Easy Being Green: Recognizing Unintended Consequences of Green Stormwater Infrastructure. Water 2020, 12, 522. [Google Scholar] [CrossRef]
- Reu Junqueira, J.; Serrao-Neumann, S.; White, I. Developing and testing a cost-effectiveness analysis to prioritize green infrastructure alternatives for climate change adaptation. Water Environ. J. 2022, 37, 242–255. [Google Scholar] [CrossRef]
- Eckart, K.; McPhee, Z.; Bolisetti, T. Performance and implementation of low impact development—A review. Sci. Total Environ. 2017, 607–608, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.A.; Kiyani, A.; Arroyo-Velasco, B.; Rad, C.; Khan, M.A.; Curiel-Alegre, S.; Mazhar, I.; Rocio, B. Sustainable Decentralized Urban Water and Wastewater Treatment in Off-grid Areas of Developing Countries Using NbS and Integrated Green Technologies. In Nature-Based Solutions for Circular Management of Urban Water. Circular Economy and Sustainability; Stefanakis, A., Oral, H.V., Calheiros, C., Carvalho, P., Eds.; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Subpiramaniyam, S. Outdoor disinfectant sprays for the prevention of COVID-19: Are they safe for the environment? Sci. Total Environ. 2021, 759, 144289. [Google Scholar] [CrossRef]
- Wang, J.; Shen, J.; Ye, D. Disinfection technology of hospital wastes and wastewater: Suggestions for disinfection strategy during coronavirus Disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020, 262, 114665. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Healthcare Infection Control Practice Advisory Committee (HICPAC) Guideline for Disinfection and Sterilization in Healthcare Facilities 2019. Available online: https://stacks.cdc.gov/view/cdc/134910 (accessed on 12 June 2025).
- Li, T.; Wang, Z.; Wang, C.; Huang, J.; Zhou, M. Chlorination in the pandemic times: The current state of the art for monitoring chlorine residual in water and chlorine exposure in air. Sci. Total Environ. 2022, 838, 156193. [Google Scholar] [CrossRef]
- Valentukeviciene, M.; Andriulaityte, I.; Karczmarczyk, A.; Zurauskiene, R. Removal of Residual Chlorine from Stormwater Using Low-Cost Adsorbents and Phytoremediation. Environments 2024, 11, 101. [Google Scholar] [CrossRef]
- Homyer, K.M.; Mehendale, F.V. Time to rethink medical disinfection from a planetary health perspective. J. Glob. Health Rep. 2023, 7, e2023063. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 23665760, Sodium Hypochlorite. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-Hypochlorite (accessed on 18 January 2025).
- World Health Organization. Cleaning and Disinfection of Environmental Surfaces in the Context of COVID-19: Interim Guidance; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/handle/10665/332096 (accessed on 28 April 2025).
- Giné-Garriga, R.; Delepiere, A.; Ward, R.; Alvarez-Sala, J.; Alvarez-Murillo, I.; Mariezcurrena, V.; Sandberg, H.G.; Saikia, P.; Avello, P.; Thakar, K.; et al. COVID-19 water, sanitation, and hygiene response: Review of measures and initiatives adopted by governments, regulators, utilities, and other stakeholders in 84 countries. Sci. Total Environ. 2021, 795, 148789. [Google Scholar] [CrossRef]
- Hu, L.; Mao, J.; Zhon, R.; Zhao, H. Assessment of heavy metals mobilization in road deposited sediments induced by COVID-19 disinfection. Water Res. 2023, 243, 120393. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Lu, T.; Zhang, J.; Sun, L.; Hu, B.; Hu, J.; Peñuelas, J.; Zhu, L.; Qian, H. Residual chlorine disrupts the microbialcommunities and spreads antibiotic resistance in freshwater. J. Hazard. Mater. 2022, 423, 127152. [Google Scholar] [CrossRef]
- Vasilachi, I.C.; Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. Occurrence and Fate of Emerging Pollutants in Water Environment and Options for Their Removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- Silva, F.; Antão-Geraldes, A.M.; Zavattieri, C.; Afonso, M.J.; Freire, F.; Albuquerque, A. Improving Water Efficiency in a Municipal Indoor Swimming-Pool Complex: A Case Study. Appl. Sci. 2021, 11, 10530. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Tan, H.W.; Yean Pang, L.; Lim, S.; Chong, W.C. A state-of-the-art of phytoremediation approach for sustainable management of heavy metals recovery. Environ. Technol. Innov. 2023, 30, 103043. [Google Scholar] [CrossRef]
- Mocek-Płóciniak, A.; Mencel, J.; Zakrzewski, W.; Roszkowski, S. Phytoremediation as an Effective Remedy for Removing Trace Elements from Ecosystems. Plants 2023, 12, 1653. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Budzyńska, S.; Zine, H.; Vázquez-Núñez, E.; Talpur, S.A.; Hassan, M.; Barros, R. Dendroremediation: A sustainable nature-based solution for management of abandoned mining sites and brownfields. J. Clean. Prod. 2025, 501, 145342. [Google Scholar] [CrossRef]
- Yuliasni, R.; Kurniawan, S.B.; Marlena, B.; Hidayat, M.R.; Kadier, A.; Ma, P.C.; Imron, M.F. Recent Progress of Phytoremediation-Based Technologies for Industrial Wastewater Treatment. J. Ecol. Eng. 2023, 24, 208–220. [Google Scholar] [CrossRef]
- Hachoumi, I.; Pucher, B.; De Vito-Francesco, E.; Prenner, F.; Ertl, T.; Langergraber, G.; Fürhacker, M.; Allabashi, R. Impact of Green Roofs and Vertical Greenery Systems on Surface Runoff Quality. Water 2021, 13, 2609. [Google Scholar] [CrossRef]
- Schwammberger, P.; Walker, C.; Lucke, T. Using floating wetland treatments systems to reduce stormwater pollution from urban developments. GEOMATE J. 2017, 12, 45–50. [Google Scholar] [CrossRef]
- Wadzuk, B.; Delvecchio, T.; Sample-Lord, K.; Ahmed, M.; Welker, A. Nutrient removal in rain garden lysimeters with different soil types. J. Sustain. Water Built Environ. 2021, 7, 04020018. [Google Scholar] [CrossRef]
- Aryal, M. Phytoremediation strategies for mitigating environmental toxicants. Heliyon 2024, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Morash, J.; Wright, A.; LeBleu, C.; Meder, A.; Kessler, R.; Brantley, E.; Howe, J. Increasing Sustainability of Residential Areas Using Rain Gardens to Improve Pollutant Capture, Biodiversity and Ecosystem Resilience. Sustainability 2019, 11, 3269. [Google Scholar] [CrossRef]
- Berthon, K.; Thomas, F.; Bekessy, S. The role of ‘nativeness’ in urban greening to support animal biodiversity. Landsc. Urban Plan. 2021, 205, 103959. [Google Scholar] [CrossRef]
- Tartaglia, E.S.; Aronson, M.F. Plant native: Comparing biodiversity benefits, ecosystem services provisioning, and plant performance of native and non-native plants in urban horticulture. Urban Ecosyst. 2024, 27, 2587–2611. [Google Scholar] [CrossRef]
- Azizi, M.; Faz, A.; Zornoza, R.; Martinez-Martinez, S.; Acosta, J.A. Phytoremediation Potential of Native Plant Species in Mine Soils Polluted by Metal(loid)s and Rare Earth Elements. Plants 2023, 12, 1219. [Google Scholar] [CrossRef]
- Andriulaityte, I.; Valentukeviciene, M.; Zurauskiene, R. Research on the Reusability of Bentonite Waste Materials for Residual Chlorine Removal. Materials 2024, 17, 5647. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Masood, A.; Khan, M.; Syeed, S.; Khan, N. Cadmium Toxicity in Plants and Role of Mineral Nutrients in Its Alleviation. Am. J. Plant Sci. 2012, 3, 1476–1489. [Google Scholar] [CrossRef]
- Xu, X.; Du, X.; Wang, F.; Sha, J.; Chen, Q.; Tian, G.; Zhu, Z.; Ge, S.; Jiang, Y. Effects of Potassium Levels on Plant Growth, Accumulation and Distribution of Carbon, and Nitrate Metabolism in Apple Dwarf Rootstock Seedlings. Front. Plant Sci. 2020, 11, 904. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Alsafran, M.; Usman, K.; Ahmed, B.; Rizwan, M.; Saleem, M.H.; Al Jabri, H. Understanding the Phytoremediation Mechanisms of Potentially Toxic Elements: A Proteomic Overview of Recent Advances. Front. Plant Sci. 2022, 13, 881242. [Google Scholar] [CrossRef]
- Wu, B.; Peng, H.; Sheng, M.; Luo, H.; Wang, X.; Zhang, R.; Xu, F.; Xu, H. Evaluation of phytoremediation potential of native dominant plants and spatial distribution of heavy metals in abandoned mining area in Southwest China. Ecotoxicol. Environ. Saf. 2021, 220, 112368. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Kirihata, Y. Elemental composition of plants from the serpentine soil of Sugashima Island, Japan. Aust. J. Bot. 2015, 63, 252–260. [Google Scholar] [CrossRef]
- Jung, J.Y.; Shin, R.; Schachtman, D.P. Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis. Plant Cell 2009, 21, 607–621. [Google Scholar] [CrossRef]
- Dubos, B.; Alarcón, W.H.; López, J.E.; Ollivier, J. Potassium uptake and storage in oil palm organs: The role of chlorine and the influence of soil characteristics in the Magdalena valley, Colombia. Nutr. Cycl. Agroecosyst. 2011, 89, 219–227. [Google Scholar] [CrossRef]
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of Silicon with Essential and Beneficial Elements in Plants. Front. Plant Sci. 2021, 12, 697592. [Google Scholar] [CrossRef]
- Denarié, M.-E.; Nielsen, U.N.; Hartley, S.E.; Johnson, S.N. Silicon-Mediated Interactions Between Plant Antagonists. Plants 2025, 14, 1204. [Google Scholar] [CrossRef]
- Valentukeviciene, M.; Andriulaityte, I.; Chadysas, V. Assessment of Residual Chlorine Interaction with Different Microelements in Stormwater Sediments. Molecules 2023, 28, 5358. [Google Scholar] [CrossRef]
- Farid, I.M.; El-Hussieny, O.H.; El-Shinawy, R.S.; Abbas, H.H.; Mohamed Abbas, M.H.H.; Bassouny, M.A. Phosphorus and Micronutrient Interactions in soil and their Impacts on Maize Growth. Egypt. J. Soil Sci. 2023, 63, 405–416. [Google Scholar] [CrossRef]
- Pitelli, R.A.; Simes, R.P.; Pitelli, R.L.; Rocha, R.J.d.S.; Merenda, A.M.P.; da Cruz, F.P.; Lameiro, A.M.M.d.S.; Oliveira Junior, A.J.d.; Gomes, R.H.M. Exploratory Analysis on the Chemical Composition of Aquatic Macrophytes in a Water Reservoir—Rio de Janeiro, Brazil. Water 2025, 17, 582. [Google Scholar] [CrossRef]
- Lee, N.; Yoo, H.; Yang, H. Cluster Analysis of Medicinal Plantsand Targets Based on Multipartite Network. Biomolecules 2021, 11, 546. [Google Scholar] [CrossRef]
- Rai, S.; Singh, P.K.; Mankotia, S.; Swain, J.; Satbhai, S.B. Iron homeostasis in plants and its crosstalk with copper, zinc, and manganese. Plant Stress 2021, 1, 100008. [Google Scholar] [CrossRef]
- Liang, G. Iron uptake, signaling, and sensing in plants. Plant Commun. 2022, 3, 100349. [Google Scholar] [CrossRef]
- Biswal, B.; Singh, S.K.; Patra, A.; Mohapatra, K.K. Evaluation of phytoremediation capability of French marigold (Tagetes patula) and African marigold (Tagetes erecta) under heavy metals contaminated soils. Int. J. Phytoremediation 2022, 24, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Sivaram, A.K.; Logeshwaran, P.; Lockington, R.; Naidu, R.; Megharaj, M. Phytoremediation efficacy assessment of polycyclic aromatic hydrocarbons contaminated soils using garden pea (Pisum sativum) and earthworms (Eisenia fetida). Chemosphere 2019, 229, 227–235. [Google Scholar] [CrossRef]
- McKinney, W. Python for Data Analysis; OReilly Media. Inc.: Sebastopol, CA, USA, 2012. [Google Scholar]
- Waskom, M.L. Seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Pearson, K. VII. Mathematical contributions to the theory of evolution.—III. Regression, heredity, and panmixia. Philos. Trans. R. Soc. Lond. Ser. A Contain. Pap. A Math. Phys. Character 1896, 187, 253–318. [Google Scholar]
- Wooldridge, J.M. Introductory Econometrics: A Modern Approach, 6th ed.; Cengage Learning: Boston, MA, USA, 2016. [Google Scholar]
- Murtagh, F. A survey of recent advances in hierarchical clustering algorithms. Comput. J. 1983, 26, 354–359. [Google Scholar] [CrossRef]
- Ketchen, D.J.; Shook, C.L. The application of cluster analysis in strategic management research: An analysis and critique. Strateg. Manag. J. 1996, 17, 441–458. [Google Scholar] [CrossRef]
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Davies, D.L.; Bouldin, D.W. A cluster separation measure. IEEE Trans. Pattern Anal. Mach. Intell. 1979, 2, 224–227. [Google Scholar] [CrossRef]

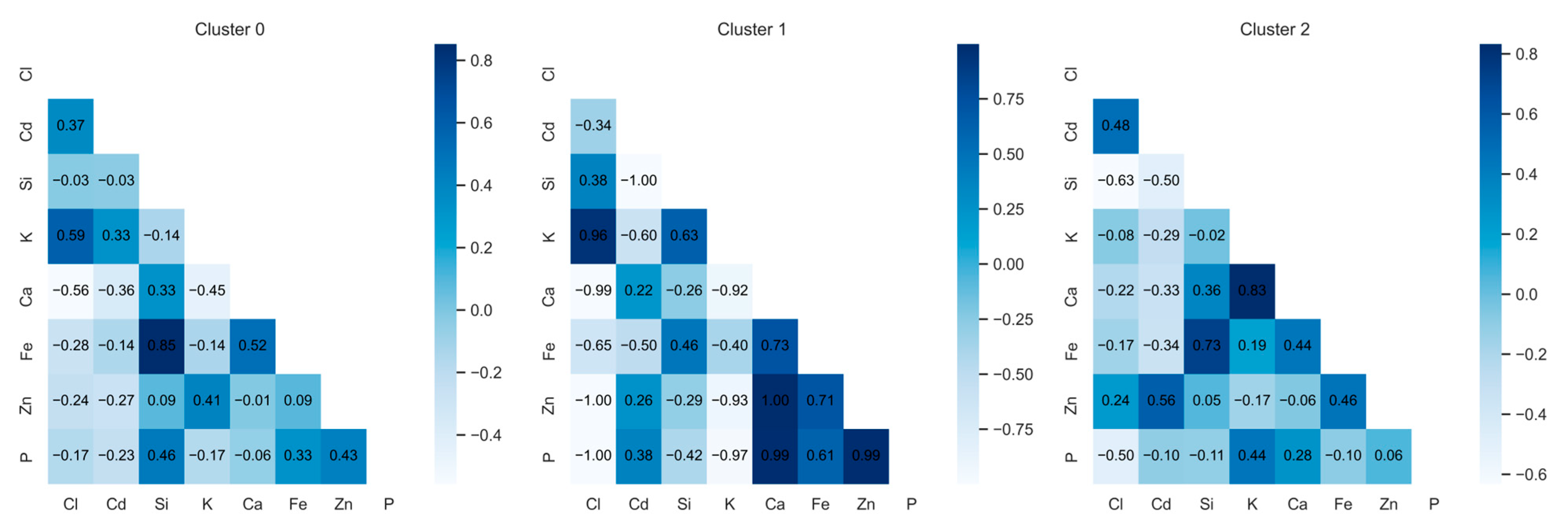
| Cluster | Cl | Cd | Si | K | Ca | Fe | Zn | P |
|---|---|---|---|---|---|---|---|---|
| 0 | 18,540.58 ± 11,528.49 | 16.44 ± 1.61 | 7692.49 ± 6126.84 | 43,217.17 ± 21,348.37 | 65,431.30 ± 19,973.15 | 1453.98 ± 1266.47 | 87.64 ± 74.76 | 2675.41 ± 1519.74 |
| 1 | 492.42 ± 118.81 | 6.64 ± 2.51 | 117,910.33 ± 25,123.66 | 12,797.76 ± 1611.16 | 12,437.86 ± 6349.21 | 9779.36 ± 1570.38 | 78.80 ± 66.60 | 1023.27 ± 302.28 |
| 2 | 11,232.99 ± 5090.17 | 16.69 ± 2.55 | 17,446.51 ± 15,066.89 | 34,056.66 ± 21,563.29 | 22,782.48 ± 4489.93 | 3535.04 ± 3630.28 | 46.01 ± 35.26 | 1323.74 ± 796.05 |
| Plant | Amount | Description |
|---|---|---|
| Iris pseudacorus | 9 | Perennial plant. It can be planted in wet coastal areas and in shallow water; it grows well both in the sun and in semi-shade. |
| Bergenia crass folia | 8 | A perennial plant with medicinal properties. The stem is strong and thick, and the leaves are glossy, massive, and remain green in winter. It grows best in fertile soil, not too dry, in shade or partial shade. |
| Primula veris | 6 | A perennial plant; it is best planted in a sunny place or in partial shade, in fertile, moderately moist soil. Dry, sandy soil is not suitable. |
| Geranium macrorrhizum L. | 12 | Perennial plant suitable for carpet planting in larger areas. Grows well under old trees in full shade, and it grows well in full sun, but the plants in full sun become smaller and finer. |
| Ajuga reptans | 8 | Perennial herbaceous carpet plant that grows well in a sunny place. |
| Ligularia | 2 | Perennial plant that likes fertilizer, moist soil, and semi-shade. It does not require care. |
| Hosta sieboldiana | Planting area: 2 × 110 cm × 14 cm | A perennial flower. It can grow in wet, moderately wet, and dry soils. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andriulaityte, I.; Valentukeviciene, M.; Chadysas, V.; Kalinichenko, A. Residual Chlorine Interaction with Microelements in Plants Applied for Phytoremediation in Rain Gardens. Plants 2025, 14, 1957. https://doi.org/10.3390/plants14131957
Andriulaityte I, Valentukeviciene M, Chadysas V, Kalinichenko A. Residual Chlorine Interaction with Microelements in Plants Applied for Phytoremediation in Rain Gardens. Plants. 2025; 14(13):1957. https://doi.org/10.3390/plants14131957
Chicago/Turabian StyleAndriulaityte, Ieva, Marina Valentukeviciene, Viktoras Chadysas, and Antonina Kalinichenko. 2025. "Residual Chlorine Interaction with Microelements in Plants Applied for Phytoremediation in Rain Gardens" Plants 14, no. 13: 1957. https://doi.org/10.3390/plants14131957
APA StyleAndriulaityte, I., Valentukeviciene, M., Chadysas, V., & Kalinichenko, A. (2025). Residual Chlorine Interaction with Microelements in Plants Applied for Phytoremediation in Rain Gardens. Plants, 14(13), 1957. https://doi.org/10.3390/plants14131957







